 Open Access Article
Open Access ArticleLignin biopolymer: the material of choice for advanced lithium-based batteries
Marya Baloch
and
Jalel Labidi
 *
*
Department of Chemical and Environmental Engineering, School of Engineering, Donostia-San Sebastian, Gipuzkoa, Spain. E-mail: jalel.labidi@ehu.es
First published on 5th July 2021
Abstract
Lignin, an aromatic polymer, offers interesting electroactive redox properties and abundant active functional groups. Due to its quinone functionality, it fulfils the requirement of erratic electrical energy storage by only providing adequate charge density. Research on the use of lignin as a renewable material in energy storage applications has been published in the form of reviews and scientific articles. Lignin has been used as a binder, polymer electrolyte and an electrode material, i.e. organic composite electrodes/hybrid lignin-polymer combination in different battery systems depending on the principal charge of quinone and hydroquinone. Furthermore, lignin-derived carbons have gained much popularity. The aim of this review is to depict the meticulous follow-ups of the vital challenges and progress linked to lignin usage in different lithium-based conventional and next-generation batteries as a valuable, ecological and low-cost material. The key factor of this new finding is to open a new path towards sustainable and renewable future lithium-based batteries for practical/industrial applications.
1. Introduction
The fundamental challenges for the future progress and wide-ranging use of electrochemical energy storage systems (EESs) are a better performance and safety measurements including the production and material costs, the implementation of environmentally friendly materials and manufacturing processes, as well as developing easy recyclability and up-scalability.1,2 However, EES devices run on the basis of inorganic and/or rare metal electrodes (Mn, Co, Ni and Fe), polymeric binders (such as PVDF mixed with toxic NMP solvents), and volatile non-aqueous electrolytes (organic carbonates such as DMC and DEC, or ethers such as DME and DIOX), which are fundamentally expensive and toxic with imperishable impacts on the environment.3–6 However, it is possible to replace them with a low-cost and non-toxic material that could provide properties and features that an EES technology demands. Organic compounds as battery components have gained much interest from scientific communities, due to their fast reaction kinetics and high rate capability;7 they could be a perfect match for high power energy storage applications. Naturally, organic materials show poor ionic and electrical conductivity that could critically hinder their credibility as a high-performance energy source.8 Thus, a natural abundant material that offers an electroactive redox reversible reaction and capacity that mitigates these drawbacks can be the material of choice leading the battery technology to be a promising candidate for an efficient, cheap and longer lifetime EESs.4Lignin, a biomass-derived organic polymer and a major by-product of the paper industry, is a carbonyl compound with quinone functionality that fulfils the requirement of a cheap and abundant material for erratic electrical energy storage by only providing suitable charge density.9 It offers a versatile chemical structure and functional groups, which can lead towards advanced molecular tailoring modifications suitable for the application. Lignin has been used for decades as a cheap carbon source within the industrial-level production processes.
The major aim of this review is to emphasize the use of lignin as an improvised battery material in accessible available lithium battery systems. However, there have been few reviews explaining the application of lignocellulosic biomass as an active component in different EESs;10–15 but, our focus is mainly on up-to-date advances related to the electrochemical performance of lignin in Li-based systems as an active electrode (cathode/anode), binder, electrolyte and a major carbon source. This review mainly attributes lignin as a replacement to well-known frequently used expensive and harsh battery materials. This is a meticulous follow-up of the role of lignin with respect to its functionality showing the evolving interest in biomass-derived lignin biopolymers.
1.1 Lignin, its functionality and redox activity
Lignin is a phenolic biopolymer that is produced in larger quantity of ∼50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons each year, and due to its aromatic and inhomogeneous complex chemical structure, it is habitually used for combustion of pulps and paper industry boilers.16–19 Traditionally, 15–33 wt% of lignin contents are present in any biomass;20,21 Table 1 shows the percentage of lignin in basic wood types. Usually, lignin is based on three aromatic alcohols, sinapyl, coniferyl, and p-coumaryl, as shown in Fig. 1, and their production is essentially depending on the position of methoxy group attachment on the phenol group of the lignin. These alcohols further produce monolignols, i.e. syringyl (S), guaiacyl (G) and p-hydroxyphenyl (H). Lignin as an amorphous polymer network differs in functionalities due to its original plant type, extraction methods and experimental conditions.22,23
000 tons each year, and due to its aromatic and inhomogeneous complex chemical structure, it is habitually used for combustion of pulps and paper industry boilers.16–19 Traditionally, 15–33 wt% of lignin contents are present in any biomass;20,21 Table 1 shows the percentage of lignin in basic wood types. Usually, lignin is based on three aromatic alcohols, sinapyl, coniferyl, and p-coumaryl, as shown in Fig. 1, and their production is essentially depending on the position of methoxy group attachment on the phenol group of the lignin. These alcohols further produce monolignols, i.e. syringyl (S), guaiacyl (G) and p-hydroxyphenyl (H). Lignin as an amorphous polymer network differs in functionalities due to its original plant type, extraction methods and experimental conditions.22,23
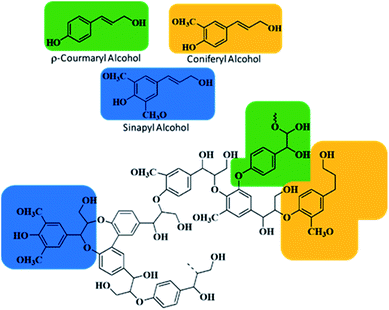 | ||
| Fig. 1 Chemical structure of the lignin molecule showing three alcoholic monolignols, p-coumaryl, coniferyl, and sinapyl. | ||
The complex heterogeneous chemical structure of lignin offers unique functionalities such as methoxy groups, hydroxyl (aliphatic and aromatic) groups, carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups, carboxyl groups and a large number of aromatic rings24 ready to be harnessed for their potential chemical functions, that lead further to production of chemicals and allows a vast number of chemical modifications for various applications, for instance, the –OH group of lignin can further result in a wide range of polymeric compounds, higher carbon content (∼60%, due to generous amount of C
O) groups, carboxyl groups and a large number of aromatic rings24 ready to be harnessed for their potential chemical functions, that lead further to production of chemicals and allows a vast number of chemical modifications for various applications, for instance, the –OH group of lignin can further result in a wide range of polymeric compounds, higher carbon content (∼60%, due to generous amount of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionalities), bio-degradability, antimicrobial behaviour, adhesive properties, eminent thermal stability, and relative abundance. It also shows additive properties, dust dispersant and blending properties.19,25 The irregular complex molecular structure of lignin with a highly condensed linkage provides higher mechanical strength and rigidity to resist exterior forces.18–20 In general, the behaviour of lignin is basically dependent on these functional groups and their positions. However, the understanding of lignin's chemical structure is rather complicated due to its 3D irregular network order. The nature and valorisation of lignin has been thoroughly discussed in assorted publications and reviews over the years.26–35
O functionalities), bio-degradability, antimicrobial behaviour, adhesive properties, eminent thermal stability, and relative abundance. It also shows additive properties, dust dispersant and blending properties.19,25 The irregular complex molecular structure of lignin with a highly condensed linkage provides higher mechanical strength and rigidity to resist exterior forces.18–20 In general, the behaviour of lignin is basically dependent on these functional groups and their positions. However, the understanding of lignin's chemical structure is rather complicated due to its 3D irregular network order. The nature and valorisation of lignin has been thoroughly discussed in assorted publications and reviews over the years.26–35
The interesting functional group for the application of energy storage is the quinone moiety of the lignin, and quinones are basically dyes in plants, whereas it can facilitate a fast and reversible two electron/proton redox reaction, thanks to its six-membered ring with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional group staging anthracene, benzene and naphthalene chains.36–38 This property can be sufficiently explored to present lignin as a bio-based energy conversion material. The oxidation of methoxy groups S and G monolignols activates quinone species (quinone and derivatives like hydroquinone), and the charge transfer reaction of quinone/hydroquinone has been noted upon repeated cycling within the electrolyte between the electrodes.39–42 However, it has been stated that in order to allow charge storage, a conductive additional component is needed to share the responsibility of providing electronic conductivity and electroactive redox activity.43,44
O functional group staging anthracene, benzene and naphthalene chains.36–38 This property can be sufficiently explored to present lignin as a bio-based energy conversion material. The oxidation of methoxy groups S and G monolignols activates quinone species (quinone and derivatives like hydroquinone), and the charge transfer reaction of quinone/hydroquinone has been noted upon repeated cycling within the electrolyte between the electrodes.39–42 However, it has been stated that in order to allow charge storage, a conductive additional component is needed to share the responsibility of providing electronic conductivity and electroactive redox activity.43,44
1.2 Lignin and quinone functionalities in energy storage systems
The investigation on lignin in the field of energy storage systems is indispensable, due to the fact that the research studies so far on the electrochemical/electrical behaviour of pure lignin are rather scarce. Meanwhile, lignins, due to their diverse functionalities, can play different roles in EESs, for example, lignosulfonate (LS) can provide sulphur doping agents in EES devices45,46 and alkali lignin proves itself to be quite useful for the application of building nanomaterials via electrospinning.47 Hydrolysed lignin has been used as a cathode,48 organosolv lignin and acetone lignin as anodes,49,50 Kraft lignin as a binder and a cathode51,52 and most of the lignins are widely used as carbon precursors.50,53–56Nonetheless, lignin has always been forecasted as an insulating material as other organic compounds, where prevailing sufficient electrochemical redox activity could be quite challenging.23 However, several studies provide the subsequent proof of reversible potential cycling and faster electron transfer kinetics of active redox of the quinone groups, which perceptibly is derived from electro-oxidation of lignins.
Quinone functional groups are mainly responsible for the redox activity of biopolymer materials via electron exchange. They consist of a conjugated cyclic structure with two ketone groups, where under redox reactions, the conjugated double bond undergoes cleavage/formation influencing the performance of the electrochemical battery.60–62 High content of quinone groups can be easily achieved by oxidation of the phenolic groups of lignin, which controls the redox activity of most quinones such as anthraquinone and napthaquinones, due to their property of undergoing a 2e−/H+ reversible redox transfer at low potential.63,64 The biggest issue with the usage of quinone biopolymers as a battery material is their solubility in the electrolyte solvents resulting in poor cycling life and performance. Research focused on this particular problem is crucial, and various methods have been applied to reduce the dissolution of organic electrode materials, for example, grafting onto the solid substrate to avoid mobilization within the electrolyte, but this affects the specific capacity of the battery. Polymerization of the organic material into a high-molecular weight polymer can help with the solubility issue, but again this affects the energy density negatively. The possible solution could also be providing an intermediate to help both cycling life and energy density. However, it has been proven that quinone functionalities perform best upon hybridization with a conductive material (organic or inorganic) for the energy storage applications.65–69
Lignin mixed with synthetic or bio-polymers can show promising properties such as the antioxidant property of the phenolic groups providing better resistance to oxidative degradation. However, to further exploit the properties of lignin, chemical modifications will be required. It is promising to use the lignin directly derived from biomass, but to allow charge storage/transfer, a hybrid material (synthetic or bio-polymer) is needed, where one component provides electronic conductivity and the other redox reactivity, respectively.43 When mixed with polyaniline, lignin represents two redox peaks around 0.7 and 0.9 V. The interaction of kraft lignin from Eucalyptus grandis with polyaniline was confirmed by an FTIR peak at 1146 cm−1 of the protonated amine vibration band. The value of electrical conductivity was totally dependent on the homogenous mixture of the PANI/lignin blends, and SEM shows the homogenous nature of the films without agglomeration of the globules of lignin, proving the miscibility of two polymers70 that could demonstrate the adoptability of lignin with synthetic polymers upon mixing.
Naturally derived lignin hybrid composites could provide sufficient charge transfer due to faradic processes of lignin activated via repeated electrochemical redox cycling. The hybrid composite of the LS lignin with polypyrrole (PPY/LS), where polypyrrole was electro-polymerised in the presence of lignin dopants, improves the charge storage ability of the hybrid composite electrode. The process was confirmed by in situ FTIR spectroscopy, giving a significantly visible IR band of quinone at ∼1705 cm−1 and hydroquinone at ∼1045 cm−1. The reversible redox reaction was confirmed by the vibrations bands of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O at 1706 and 1043 cm−1, respectively. This hybrid mixture of polypyrrole and the LS lignin electrode shows a discharge capacity of 72 mA h g−1 that is higher than that of the pure polypyrrole electrode (30 mA h g−1). Nonetheless, the cycling stability of this PPY/LS hybrid material electrode is poor with a dramatic loss of quinone redox capacity after 1000 cycles.72 It might be due to overconsumption/over oxidation of guaiacyl's quinone functionality, where possible covalent bonds cleave and facilitate a nucleophilic attack over quinone moieties, leading to poor performance of the PPY/LS hybrid composite.72
O and C–O at 1706 and 1043 cm−1, respectively. This hybrid mixture of polypyrrole and the LS lignin electrode shows a discharge capacity of 72 mA h g−1 that is higher than that of the pure polypyrrole electrode (30 mA h g−1). Nonetheless, the cycling stability of this PPY/LS hybrid material electrode is poor with a dramatic loss of quinone redox capacity after 1000 cycles.72 It might be due to overconsumption/over oxidation of guaiacyl's quinone functionality, where possible covalent bonds cleave and facilitate a nucleophilic attack over quinone moieties, leading to poor performance of the PPY/LS hybrid composite.72
Subsequently, 3,4-ethylenedioxythiophene (EDOT) is applied to obtain the PEDOT/LS hybrid material electrode, which displays a much better cycling stability with 83% capacity retention after 1000 charge–discharge cycles and a discharge capacity of 34 mA h g−1, with 140% capacity increase compared with PEDOT (14 mA h g−1).73 It has been established that to boost the performance of lignin in an electrochemical system, the contribution of the hybrid material's ionic and electronic charge transfer abilities is crucial.74
The carbonyl functional groups present in organic compounds could be a source for higher theoretical capacity,75 but added C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups might lack in active sites upon incorporation into the larger coordinated units, leading to a lower practical capacity.76,77 The units of p-quinone are used for they can provide sufficient active sites due to macrocyclic molecular structures and aid to achieve high efficiency.78,79 The macrocycle pillar[5]quinone (P5Q) has five methylene bridges linking quinone groups at the para position, and it undergoes oxidation process similarly to the quinone group39–41 of lignin to activate the charge transfer process within the electrolyte and electrode, as shown in Fig. 2a. It was cycled in an all-solid-state lithium organic battery to generate repeating 1,4-benzoquinone units, leading to the resulting final product that could intercalate ∼10 Li+ ions providing a high specific capacity of 418 mA h g−1, thanks to its active carbonyl sites (Fig. 2b).71
O groups might lack in active sites upon incorporation into the larger coordinated units, leading to a lower practical capacity.76,77 The units of p-quinone are used for they can provide sufficient active sites due to macrocyclic molecular structures and aid to achieve high efficiency.78,79 The macrocycle pillar[5]quinone (P5Q) has five methylene bridges linking quinone groups at the para position, and it undergoes oxidation process similarly to the quinone group39–41 of lignin to activate the charge transfer process within the electrolyte and electrode, as shown in Fig. 2a. It was cycled in an all-solid-state lithium organic battery to generate repeating 1,4-benzoquinone units, leading to the resulting final product that could intercalate ∼10 Li+ ions providing a high specific capacity of 418 mA h g−1, thanks to its active carbonyl sites (Fig. 2b).71
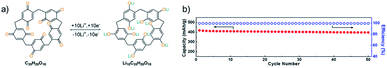 | ||
| Fig. 2 (a) Mechanism of electrochemical redox activity of P5Q. (b) All-solid-state lithium organic battery capacity and coulombic efficiency.71 | ||
The electrochemistry of modified lignin via electrochemical polymerization in thin layers was tested by electrocatalysis,39,41 proving the hypothesis that if lignin is mixed well with sufficiently good electronic/ionic conductive materials, it could permit charge transference back and forth from a quinone site using redox reactions.40,80,81
Phenol-formaldehyde condensation of homopolymers and copolymers of syringol (S) and guaiacol (G) leads to the polymerization of a well-defined synthetic model compound of lignin for the purpose to mimic the hardwood and softwood lignin conduct. The models were prepared to obtain a precise chemical structure with controlled phenol reactivity and purity for a well-defined hybrid composite as a final product, i.e. by incorporating synthetic lignin into single-wall carbon nanotubes (SWNTs) and polypyrrole (PPy) electron-conducting matrixes, whereas hydroquinone was used to augment the quinone functionalities to direct towards enhanced charge capacity (max. capacity 94 mA h g−1). It was demonstrated that well-defined synthetic lignin provides an efficient electronic transference due to the presence of hydroquinone, which helps reducing the amount of additional conductive materials (only 20 wt% of SWNTs).44
2. Lignin in lithium batteries
The possibility of lignin as an alternative battery component has already been explored in many rechargeable electrochemical systems, where lignin has played a significant role as a cathode, binder, electrolyte, and anode. Herein, we briefly summarise the use of lignin in different battery technologies in different manners.82,83Up to now, the major application of lignin in the EES was as a cheap carbon precursor, and lignin carbon proves to be an attractive choice due to its reduced energy consumption, cost and minimum CO2 emission, thus, it can be considered as a replacement for popular polyacrylonitrile (PAN ∼13 € per lb)-based carbons that require high treatment temperatures (≤1000 °C to ≥2000 °C), which could be a virulent process for the environment. Lignin has the vantage ample carbon content due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionality. Similarly, the highly rich aromatic structure of lignin allows a great deal in tuning the morphology of carbon, resulting in different forms, although the resulting shape is primarily influenced by the origin of lignin.84–86
O functionality. Similarly, the highly rich aromatic structure of lignin allows a great deal in tuning the morphology of carbon, resulting in different forms, although the resulting shape is primarily influenced by the origin of lignin.84–86
Extensive research has been carried out on lignin-derived carbons in the course of patents and reports since the 1960s. Lignin-based carbon materials have drawn much attention due to their use as anodes or as active carbon electrode materials12,51,87 in lithium/sodium-ion batteries85,88,89 and supercapacitors.87,90,91
Due to diverse heterogeneous morphology of lignin, the diffusion of lithium (Li+) ions is much easier leading it to be a low-cost electrode material in primary and rechargeable lithium batteries. Simultaneously, the distinctive lignin functional groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, OH, C–O–C) show strong electroactive redox properties towards Li+ ions. Owing to that, a primary Li/lignin cell could achieve the maximum capacity of ∼600 mA h g−1. Heretofore, this review presents a brief summary of lignin use in diverse Li-based systems.
O, OH, C–O–C) show strong electroactive redox properties towards Li+ ions. Owing to that, a primary Li/lignin cell could achieve the maximum capacity of ∼600 mA h g−1. Heretofore, this review presents a brief summary of lignin use in diverse Li-based systems.
2.1 Primary lithium batteries
Hydrolysed lignin as a cathode in primary lithium batteries shows a discharge capacity of 445 mA h g−1 due to the interaction of lithium ions with the ether group; however, its double the amount of specific capacity expected probably due to the presence of carbon black. Voltage plateaus at ∼1.8 and ∼1.1 V indicate the step-by-step discharge character of electrochemical reactions of lithium ions with oxygen of different characteristic groups present in the lignin characterized by XPS and IR.48 The incurred results conclude HL as a validated cathode material due to its low cost as compared to other available primary lithium batteries, whereas the Klason lignin (KL) cathode shows a specific capacity of 380 mA h g−1 for sun flower-derived lignin (SFDL) and 600 mA h g−1 for buckwheat-derived lignin (BWDL).23,92 The SEM study depicts that each lignin type has a different surface morphology, which seems to ease the Li+ ion diffusion within lignin particles, whereas EDS gave an estimate of oxygen content, i.e. SF-KL, BW-KL and HL contains 40, 35 and 23 wt% of oxygen, respectively. The presence and interaction of these oxygen groups help to allow the practical discharge capacity of 450 mA h g−1 versus Li-anodes.482.2 Lithium-ion batteries (LiBs)
Li-ion batteries (LiBs) are the most promising candidate of the EES for large-scale applications such as electric vehicles (EVs) and major electronic devices. The conventional lithium ion battery is composed of three basic components: the anode usually carbon, i.e. graphite, etc., the cathode is mainly a metal oxide with polyanion containing lithium and electrolyte an organic solvent containing lithium salts. This facilitates the redox reaction/transference of Li+ ions back and forth between the anode and the cathode (Fig. 3).93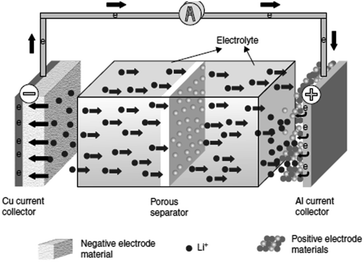 | ||
| Fig. 3 Working mechanism of a conventional Li-ion battery.93 | ||
LIBs can be the solution to some of our society's most pressing challenges such as high CO2 emission from the transport industry, energy poverty and as a green alternative to store renewable energy. However, Li-ion batteries are too expensive, and the most challenging problem in LiBs is the safety issue, beside the environmental effects of detrimental battery components. Thus, the replacement of these materials with bio-polymers like lignin can be a solution.
However, lignin has been used as an active electrode, binder and as an electrolyte, but it has gained much popularity as a hybrid composite, where a conductive polymer is mixed with a lignin biopolymer to attain the desired electroactive properties and coupled redox progressions.
The combination of 20% insulating lignin in bulk amount (80%) of conductive PEDOT (poly(3,4-ethylenedioxythiophene)) can improve the electronic conductivity and transport of lignin. The lignin/PEDOT hybrid composite, as shown in Fig. 4, was analysed in Li-ion batteries and the maximum delivered capacity of ∼50 mA h g−1 at C/20 cycling rate in LiPF6 and NaPF6 was achieved, higher than the capacity of lignin and PEDOT, individually. Furthermore, conductive carbon was added into the mixture of lignin/PEDOT to further increase the Li+ ion diffusion and electronic conductivity, and the capacity was increased up to 159 mA h g−1.94
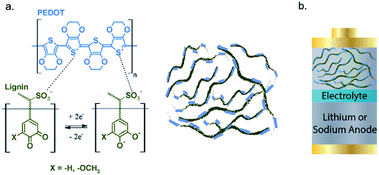 | ||
| Fig. 4 (a) Structural representation of lignin, PEDOT, and (b) assembled Li/PEDOT hybrid as cathode in the Li+/Na+-ion battery. Reproduced from ref. 94 with permission from The Royal Society of Chemistry. | ||
Carbon-anodes have been extensively used in LiBs and their demand has increased within time owing to their facile Li+ ion intercalation, existence in larger quantity with cheap prices and their high chemical stability. As established, lignin is one of the auspicious applicant for carbon production, though, mixing carbon with lignin itself can provide a hybrid L–C composite with enhanced reversible Li+ capacity and faster kinetics. The faradic and non-faradic charge storage studies of a hybrid composite of conductive carbon with commercially available unmodified kraft lignin displays a capacity of 80 mA h g−1, where the charge storage of conductive carbon has been boosted by the contribution of the additional redox chemical activity of lignin.51
Lignin has also been used to reduce the decomposition of electrolyte and help to increase the battery lifetime with a major –OH (phenolic) group, which behaves as a free radical scavenger. It has been proven quite beneficial in LIBs, where carbonate-based electrolytes are used, and they experience solvent polymerization and create cathode/electrolyte interphase (CES), due to alkyl free radicals decomposed through oxidation when the voltage exceeds 4.3 V. The organic liquid electrolytes in LIBs face a grave safety issue including fire hazard, leakage, and worst case scenario a blast. Therefore, gel polymer electrolytes (GPEs) gained quite a lot of popularity because of limited liquid presence within the electrolyte; however, some GPEs are not easily biodegradable such as polyacrylonitrile (PAN), polyvinyl acetate (PVA), polyvinylidene fluoride (PVDF), and polyethylene oxide (PEO). The continuous usage of GPEs in LIBs will result in a grand disaster “white pollution” in the natural environment; thence, lignin as an ample natural biopolymer could be a great replacement of synthetic polymer electrolytes.95 Lignin-based GPE (L-GPE) membranes can uphold liquid electrolytes as high as ∼230 wt% with an exceptional ionic conductivity of 3.73 mS cm−1 at RT. The LGPE membrane was prepared in water and collected after simple drying, which imposed the process as green and environment friendly. Nonetheless, the LGPE membrane shows thermal stability of 250 °C along with a higher Li+ ion transference of 0.85, thanks to the phenolic hydroxyl functional group of lignin interacting strongly with anions in lithium salts. The potential use of L-GPE could be proven by its electrochemical stability within the voltage range of 2.5–4.2 V and its good compatibility with the Li-anode during cycling. Nevertheless, the mechanical strength is poor as compared to the standard GPE membrane; to improve the mechanical strength, PVP has been introduced to compose a lignin/PVP membrane. PVP/lignin composite membrane exhibits an amended mechanical property and astonishing safety as severe requirements of LIBs. Additionally, after 100 cycles, the PVP/lignin composite Li-ion cell shows a phenomenal capacity retention of 95.3% with a capacity of 135 mA h g−1 at a current density of 0.2C.101
Conventional and widely used binder in LIBs, polyvinylidene fluoride (PVDF), reacts with the lithium metal anode during cycling that forms a stable LiF (lithium fluoride) compound affecting the cycling performance of the cell, while introducing lignin as a binder, in the well-known cathode (LiFePO4) versus graphite anode gives a comparatively high specific capacity and stability. Reversible capacities of 148 and 305 mA h g−1 have been shown for the lignin-bound cathode and anode at a C-rate of 0.1C, respectively. In comparison with a PVDF-bound cathode cell that shows a capacity retention of 46.2%, the lignin-bound cathode gives a capacity retention of 94.1% after 1000 cycles with a specific capacity of 110.8 mA h g−1 and a coulombic efficiency of 99.5%.102
Table 2 shows some recent developments of lignin as an anode and binder in LIBs; however, the lignin has been complimented with an active polymeric compound to enhance the electronic conductivity and redox activity during cycling. A lignin carbon nanofiber anode was prepared by mixing with polylactic acid (PLA) and thermoplastic elastomeric polyurethane (TPU). The morphology was controlled by concordance of lignin with PLA/TPU in the mix; however, PLA introduced the porous quality to the final carbonised product. The porosity of the lignin/PLA blend helps enhancing the performance of the LIB by showing 670 m2 g−1 for a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio of lignin/PLA, whereas 345 m2 g−1 for 50
50 ratio of lignin/PLA, whereas 345 m2 g−1 for 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 of lignin/TPU.50 To improve the cycling life and performance, lignin has been pyrolised with silicon to achieve carbon-coated silicon (Si@C), which gives a specific capacity of 955 mA h g−1 after 51 cycles. It shows even better results upon cycling at 800 °C (1515 mA h g−1).96 However, using alkali lignin (AL) and its derivative azo polymer (AL-azo-NO2) to fabricate Si@C shows that upon the addition of an azo group, the anode shows better performance with increased electronic conductivity due to N-doping. It gives a specific capacity of 882 mA h g−1 with a coulombic efficiency of 99% at 150 cycles.97 The controlled morphological hierarchical mesoporous carbon (HMPC) derived from lignosulfonate was later on incorporated into Ni(OH)2 to obtain NiO-HMPC. Further, the final product was thermally annealed without having any difference in the nanosphere morphology and shows a higher surface area 852 m2 g−1 that helps Li+ ion diffusion easier and exhibit a discharge capacity of 863 mA h g−1 at 0.1 A g−1 after 100 cycles.98
50 of lignin/TPU.50 To improve the cycling life and performance, lignin has been pyrolised with silicon to achieve carbon-coated silicon (Si@C), which gives a specific capacity of 955 mA h g−1 after 51 cycles. It shows even better results upon cycling at 800 °C (1515 mA h g−1).96 However, using alkali lignin (AL) and its derivative azo polymer (AL-azo-NO2) to fabricate Si@C shows that upon the addition of an azo group, the anode shows better performance with increased electronic conductivity due to N-doping. It gives a specific capacity of 882 mA h g−1 with a coulombic efficiency of 99% at 150 cycles.97 The controlled morphological hierarchical mesoporous carbon (HMPC) derived from lignosulfonate was later on incorporated into Ni(OH)2 to obtain NiO-HMPC. Further, the final product was thermally annealed without having any difference in the nanosphere morphology and shows a higher surface area 852 m2 g−1 that helps Li+ ion diffusion easier and exhibit a discharge capacity of 863 mA h g−1 at 0.1 A g−1 after 100 cycles.98
| Materials | Function | Performance | Ref. |
|---|---|---|---|
| Organosolv lignin and PLA and PTU | Anode | 611 mA h g−1 with 500 cycles | 50 |
| Lignin carbon/Si (Si@C) | Anode | 955 mA h g−1 with 51 cycles | 96 |
| Alkali lignin (AL)/SiNPs | Anode | 882 mA h g−1 after 150 cycles | 97 |
| Sodium lignosulfonate/NiO | Anode | 863 mA h g−1 after 100 cycles | 98 |
| LiNi0.5Mn1.5O4/lignin/graphite/acetylene black | Binder | 110.8 mA h g−1 after 1000 cycles | 99 |
| Sodium polyacrylate grafted on alkali lignin | Binder (silicone anode) | 1914 mA h g−1 after 100 cycles | 100 |
Similarly, the role of lignin as a binder has also been explored within the LIB cathode and anode, where it was introduced within the LiNi0.5Mn1.5O4 cathode99 and silicon micro particle anode as grafted on sodium polyacrylate (PAL-NaPAA),100 providing stable cycling life as compared to the conventional PVDF binder. It shows a capacity of 110.8 mA h g−1 with 99.5% of coulombic efficiency after 1000 cycles for the LiNi0.5Mn1.5O4 cathode99 and the PAL-NaPAA anode shows a lithiation capacity of 800 mA h g−1 for over 940 cycles.100 These examples anticipate the easy use of lignin within LIB systems as an active component. LIBs may be proven as the best choice for energy-related challenges, but due to high cost of cathode materials, next-generation low-cost batteries such as lithium–sulphur, lithium–oxygen and lithium–selenium batteries have been introduced. Our motive here is to give just a brief introduction of lignin usage in these beyond lithium-ion batteries.
2.3 Lithium–sulphur (Li–S) batteries
Li–S batteries could be an interesting choice owing to their inexpensiveness and abundance of the cathode materials. The working principle of Li–S batteries is similar to Li-ions, except that sulfur accepts 2 Li+ ions allowing a high specific capacity of 1675 mA h g−1. Sulphur, like lignin, is insulating itself, thus, it is usually impregnated into conductive carbon to provide the electrical conductivity. Conductive carbon submerging with sulphur also helps restrict the polysulphide diffusion, which leads to cycling stability of Li–S batteries. Li–S battery's prime problem is shuttling of polysulphide species within the liquid electrolyte, i.e. usually faster active sulphur mass consumption affecting the cycling life of the battery. To systematically suppress the shuttling of the polysulphide species within the electrolyte in Li–S, the multiwall carbon nanotubes (MWCNTs) were used, which are rather expensive. In order to replace expensive conductive carbon, lignin has been used as an alternative source of carbon. It can be carbonized at high temperatures; to avoid shuttling, MWCNTs were mixed and the impacts were investigated. When 25 wt% of lignosulphonate (LS) lignin was employed in MWCNTs as a protection layer, minimum capacity decay with a higher initial discharge capacity at a cycling rate of 0.5 and 1C was observed,104 whereas using LS as a binder to replace the conventional PVDF binder that cannot mitigate the shuttling effect leading to low electrochemical performance, a higher specific discharge capacity of ∼1307 mA h g−1 at a cycling rate of 0.1C was achieved in comparison to PVDF (706 mA h g−1) with a capacity retention of 71.9%.105Nonetheless, the encapsulation of sulphur within the micro-porous carbon network derived from commercially available lignin (Fig. 5) allows high surface area, reasonable pore size, and the outer surface to pretend as a functionalised matrix, due to the presence of oxygen functional groups. The encapsulation was controlled by time; the samples were collected after 6 hours (C-S-6) and 10 hours (C-S-10). The results indicate that prolonged duration of impregnation leads to better battery performance due to the negative impacts of polysulphide transition in the electrolyte, perhaps, owing to the micro/macro porous network of lignin carbon. The C-S-10 shows a discharge capacity of 1241 mA h g−1 with a specific capacity of 791 mA h g−1 at 100th cycle.103
 | ||
| Fig. 5 Graphic representation of the encapsulation of sulphur into carbon mixtures.103 | ||
Yet, in any Li-based system, it appears that lignin can be beneficial due to the presence of its oxygen functional groups when carbonised, whereas the mixture with GPE can demonstrate an astonishing ionic conductivity of 4.52 mS cm−1 at RT. Lignin-GPE (L-GPE) as a separator and electrolyte in Li–S batteries shows a capacity retention of 55.1% at 20 mA g−1 with discharge capacity in the range from 1186.3 to 653.5 mA h g−1 for 100 cycles, where the liquid electrolyte cell only attained a discharge capacity of 242.2 mA h g−1 with low capacity retention (21.4%), confirming the hindrance of the polysulphide diffusion leading to better electrochemical performance.106
Hydrothermal carbonization of activated lignin with KOH and further doping yield nitrogen-doped nanoporous carbon with a honeycomb structure (n-hC), where sulphur was impregnated within the well-organized micro/mesoporous honeycomb structure for better electrochemical and thermal stability among redox processes, which gives an initial discharge capacity of ∼1295 mA h g−1 at 0.1C after 600 cycles. Thus, a well-organized hierarchical structure helps the Li+ ion diffusion and the presence of nitrogen helps the faster kinetics, as shown in Fig. 6d, Tafel plots current density exchange. Thus, it is proven that the functionalities of the lignin and rich-carbon structure play an important role in the carbon morphology that later on helps confining the highest amount of sulphur (64.1 wt%) and reversible redox reactions.107 A separator plays an important role in Li–S batteries, and hence, lignin nanoparticle-coated Celgard (LC) was introduced to induce the chemical binding of polysulphide species using essential electron-donating groups and reduce the shuttling. It aids the cycling stability of the Li–S cell with a typical sulphur cathode (sulphur with acetylene black) for over 500 cycles as compared to the normal Celgard separator at 1C.108
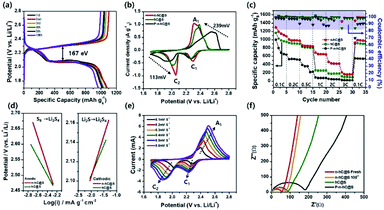 | ||
| Fig. 6 (a) Sulphur-confined nitrogen-doped activated carbon composite charge and discharge plateaus. (b) Cyclic voltammograms. (c) Rate capability cycling test of sulphur-confined nitrogen-doped activated carbon, sulphur-confined honeycomb-activated carbon and polysulphide-confined nitrogen-doped honeycomb-activated carbon. (d) Tafel comparison plots. (e) CV at different scanning rates of sulphur-confined nitrogen-doped activated carbon. (f) EIS plots before and after 100 cycles of sulphur-confined nitrogen-doped activated carbon in comparison with sulphur-confined honeycomb-activated carbon and polysulphide-confined nitrogen-doped honeycomb-activated carbon.107 | ||
Nonetheless, lignosulfonate sodium salt (LSS), an inexpensive biopolymer derived from the waste of wood mills was used as a binder that improved the capacity and cycling retention, due to the complex and distinctive chemical structure of lignin, as shown in Fig. 7, with comparison to other widely used binders. The amphiphilic functional groups of LSS and thanks to its negative charged sulphonate group, the polysulphide dissolution can be efficiently blocked and it improves the ionic conductivity. The Li–S cell with the LSS binder shows the sustained capacity of ∼661 mA h g−1.109
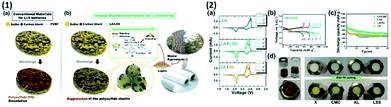 | ||
| Fig. 7 (1) (a) Cathode with a conventional binder before and after discharge and (b) cathode with an LSS binder before and after discharge with the chemical structure of the LSS binder. (2) (a) CV curves of the Li–S cell with different binders like LA133 (LA), LA/lignosulphonate sodium salt (LA/LSS) and LA/kraft lignin (LA/KL) between the range of 1.7 and 2.7 V with 0.1 mV s−1 scan rate, (b) galvanostatic 1st, 2nd, 5th, 20th, and 100th cycle profiles at 0.2C for LA/LSS, (c) cycling profile of the sulphur cathode with LA, LSS, LA/LSS, and LA/KL at 0.2C, and (d) image of polysulphide reduction via a glass fiber filter-soaked pristine (x), CMC, KL and LSS solutions.109 | ||
Thus, the inexpensive biomass-derived material has been used due to its variety of functional groups, which make it suitable for any role within Li-based batteries, particularly within Li–S batteries, where it can tackle the prime problem of the Li–S system by alleviation of the shuttle effect. We would like to address that besides Li–S as a next-generation battery system, and the exploiting of lignin in other Li-based systems has already been on the way. For example, lithium–oxygen (Li–O2) batteries have emerged as the most promising candidate within lithium-based batteries, where lignin has so far been used in the form of activated carbon as the cathode, where various activation methods have been used such as KOH, H3PO4 and steam activation methods. Due to these activation methods, the samples exhibited a higher surface area (over 1000 m2 g−1) along with defective area and vast functional groups. A higher discharge capacity of 2.8 mA h cm−2 at 0.02 mA cm−2 was observed with lignin activated via H3PO4 (LPAC) and steam (LSAC), where LPAC shows a capacity retention of 100% over 800 cycles. Meanwhile, lignin with KOH (LKAC) exhibited the highest discharge capacity of 7.2 mA h cm−2 with up to 300 cycles. The cathode based on lignin carbon activated by different methods shows promising results with the low-cost fabrication process for lithium–O2 batteries.110
Another promising system is lithium–selenium (Li–Se) batteries, which can be an alternative to the Li–S batteries, by replacing the cathode from sulphur to selenium, which avoids the polysulphide shuttling problem and can contribute significantly to improved electrical conductivity (Se = ∼10−5 S cm−1) with better electrochemical performance.
Thus far, alkaline lignin-derived porous carbon (LPC) has been used to produce Se/LPC composites by melt diffusion technique at 260 °C to achieve encapsulated selenium into porous carbonaceous frameworks. Se/LPC shows high specific surface area, large pore volume and good electron conductivity that allow a smooth electrochemical reversible reaction of selenium with Li. The SE/LPC electrode in the Li–Se cell shows a specific capacity of 596 mA h g−1 and at 0.5C with a capacity retention of 453 mA h g−1 over 300 cycles (Fig. 8).111
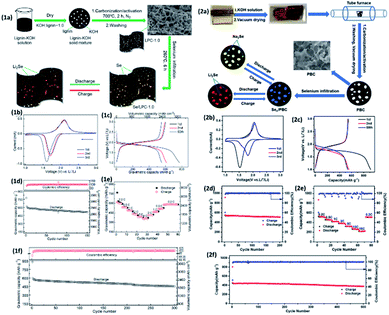 | ||
| Fig. 8 (1a) Experimental scheme of preparation and application of selenium with lignin porous carbon (Se/LPC-1.0). (1b) CV curves at 0.1 mV s−1. (1c) 1st, 2nd and 50th voltage profiles of Se/LPC-1.0 at 0.2C. (1d) Cycling performance of Se/LPC-1.0 at 0.2C. (1e) Rate capability profiles at different current densities. (1f) Galvanostatic cycling profile at 0.5C. (2a) Schematic of the experimental preparation process of Sen/PBC composites. (2b) CVs of Se50/PBC at 0.1 mV s−1 in the initial three cycles. (2c) Discharge–charge curves. (2d) Cycling performance of Se50/PBC at a current density of 0.2C. (2e) Rate performance and (2f) cycling performance at 0.5C of Se50/PBC for Li–Se batteries.113 | ||
The 3D lignin hierarchical porous carbon could take up the higher Se loading due to classified porous structures providing a capacity of 450 mA h g−1 at 0.5C after 500 cycles with an advantage of prolonged cycling life in the carbonate electrolyte. Due to the complex porous structure, selenium loss during charge–discharge would be reduced.112
However, the Li–Se system still faces the shuttle effect, and to help avoiding the shuttling of polyselenide species in the electrolyte that further demolishes the electrochemical performance, bamboo-derived porous carbon (PBC) was used as a framework for the encapsulation of elemental selenium, and the framework contains abundant meso/micropores. The Se/PBC cathode in Li–Se batteries shows an improved capacity of 509 mA h g−1 within 200 cycles at C-rate of 0.2C, which proves that due to the 3D structure, porosity and huge surface area of PBC facilitate the transport of Li+ ions thanks to the buffering of the electrolyte volume change, leading to improved conductivity. Nevertheless, bamboo-derived porous carbon is a cheap and effective technique to avoid the shuttling and better performance of the Li–Se batteries.113
3. Conclusions
In this review, we tried to follow the possible advances of lignin as a material of choice in renewable lithium-based conventional and next-generation battery systems. Lignin has been explored by means of potential quinone functionality-related electrochemical properties, possible carbon source, its advantages and behaviour. The waste product extracted from the paper industry proves itself to be an interesting candidate for the high-performance energy storage systems, not only because it is cheaper and more abundant in nature than other well-known used materials but also because its impact globally are less harmful to completely extinct. However, the insulating nature of lignin still restricts mundane use of lignin in battery systems. Besides, the combination with a conductive synthetic polymer doesn't aid the idea of economic final product due to higher prices. Nevertheless, research done in the past decades has been substantial; despite, lignin and lignin-derived materials still face some challenges in the practical applications of the batteries. In addition, only limited lignin spin-off (i.e. kraft, lignosulphonate, and organosolv lignin) has been investigated. Thus, we believe that the further investigation in this topic is rather crucial to fully understand and realise the replete properties and hidden occurrence of lignin for its commercialization.In general, although there are still many challenges to overcome before lignin can be applied commercially in any energy storage system, great progress has been made in recent decades. Important advances have been attained to improve performance and understand the mechanism of the use of lignin-derived materials. We believe that forthcoming research investigation through the scientific community will open a path to much more interesting outcomes and ultimately will make lignin a highly valuable material towards practical industrial applications.
It is becoming much clearer with recent studies that lignin as a low-cost and environmentally friendly material can be capable of being a future of battery systems. However, achieving lignin-based renewable biobatteries will have a high impact on improving world's economy and will also promote a greener environment.
Conflicts of interest
There are no conflicts to declare.References
- Z. Yang, J. Zhang, M. C. W. W. Kintner-Meyer, X. Lu, D. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 111, 3577–3613 CrossRef CAS PubMed.
- M. Armand and J.-M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- R. Holze, J. Solid State Electrochem., 2015, 19, 1253 CrossRef CAS.
- N.-S. Choi, Z. Chen, S. A. Freunberger, X. Ji, Y.-K. Sun, K. Amine, G. Yushin, L. F. Nazar, J. Cho and P. G. Bruce, Angew. Chem. Int. Ed., 2012, 51, 9994–10024 CrossRef CAS.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587–603 CrossRef CAS.
- J. B. Goodenough and K.-S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS.
- Y. Liang, Z. Tao and J. Chen, Adv. Energy Mater., 2012, 2, 742–769 CrossRef CAS.
- M. Miroshnikov, K. P. Divya, G. Babu, A. Meiyazhagan, L. M. Reddy Arava, P. M. Ajayan and G. John, J. Mater. Chem. A, 2016, 4, 12370–12386 RSC.
- L. Zhang, Z. Liu, G. Cui and L. Chen, Prog. Polym. Sci., 2014, 43, 136–164 CrossRef.
- X. Wu, J. Jiang, C. Wang, J. Liu, Y. Pu, A. Ragauskas, S. Li and B. Yang, Biofuels, Bioprod. Biorefin., 2020, 14, 650–672 CrossRef CAS.
- J. Zhu, C. Yan, X. Zhang, C. Yang, M. Jiang and X. Zhang, Prog. Energy Combust. Sci., 2020, 76, 100788 CrossRef.
- T. C. Nirmale, B. B. Kale and A. J. Varma, Int. J. Biol. Macromol., 2017, 103, 1032–1043 CrossRef CAS PubMed.
- D. Wang, S. H. Lee, J. Kim and C. B. Park, ChemSusChem, 2020, 13, 2807–2827 CrossRef CAS.
- J. L. Espinoza-Acosta, P. I. Torres-Chávez, J. L. Olmedo-Martínez, A. Vega-Rios, S. Flores-Gallardo and E. A. Zaragoza-Contreras, J. Energy Chem., 2018, 27, 1422–1438 CrossRef.
- S. Mehta, S. Jha and H. Liang, Renewable Sustainable Energy Rev., 2020, 134, 110345 CrossRef CAS.
- K. R. Hakeem, J. Mohammad and O. Alothman, Agricultural Biomass Based Potential Materials, 2015 Search PubMed.
- S. V. Obydenkova, P. D. Kouris, E. J. M. M. Hensen, H. J. Heeres and M. D. Boot, Bioresour. Technol., 2017, 243, 589–599 CrossRef CAS.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344, 709 CrossRef CAS.
- Q. Li, A. J. Ragauskas and J. S. Yuan, Tappi J., 2017, 16, 107–108 CAS.
- J. Becker and C. Wittmann, Biotechnol. Adv., 2019, 37, 107360 CrossRef CAS PubMed.
- J. Zhu, C. Yan, X. Zhang, C. Yang, M. Jiang and X. Zhang, Prog. Energy Combust. Sci., 2020, 76, 100788 CrossRef.
- E. I. Chupka and T. M. Rykova, Chem. Nat. Compd., 1983, 19, 78–80 CrossRef.
- S. V. Gnedenkov, D. P. Opra, L. A. Zemnukhova, S. L. Sinebryukhov, I. A. Kedrinskii, O. V. Patrusheva and V. I. Sergienko, J. Energy Chem., 2015, 24, 346–352 CrossRef.
- G. Torgovnikov, Dielectric Properties of Wood and Wood-Based Materials, 1993, vol. 1 Search PubMed.
- A. Naseem, S. Tabasum, K. M. Zia, M. Zuber, M. Ali and A. Noreen, Int. J. Biol. Macromol., 2016, 93, 296–313 CrossRef CAS.
- S. Laurichesse and L. Avérous, Prog. Polym. Sci., 2014, 39, 1266–1290 CrossRef CAS.
- V. K. Ponnusamy, D. D. Nguyen, J. Dharmaraja, S. Shobana, J. R. Banu, R. G. Saratale, S. W. Chang and G. Kumar, Bioresour. Technol., 2019, 271, 462–472 CrossRef CAS PubMed.
- M. N. Collins, M. Nechifor, F. Tanasă, M. Zănoagă, A. McLoughlin, M. A. Stróżyk, M. Culebras and C. A. Teacă, Int. J. Biol. Macromol., 2019, 131, 828–849 CrossRef CAS PubMed.
- O. Y. Abdelaziz, D. P. Brink, J. Prothmann, K. Ravi, M. Sun, J. García-Hidalgo, M. Sandahl, C. P. Hulteberg, C. Turner, G. Lidén and M. F. Gorwa-Grauslund, Biotechnol. Adv., 2016, 34, 1318–1346 CrossRef CAS PubMed.
- P. Azadi, O. R. Inderwildi, R. Farnood and D. A. King, Renewable Sustainable Energy Rev., 2013, 21, 506–523 CrossRef CAS.
- J. Sameni, S. A. Jaffer, J. Tjong and M. Sain, Curr. For. Rep., 2020, 6, 159–171 Search PubMed.
- A. Kumar, Anushree, J. Kumar and T. Bhaskar, J. Energy Inst., 2020, 93, 235–271 CrossRef CAS.
- O. Yu and K. H. Kim, Appl. Sci., 2020, 10, 4626 CrossRef CAS.
- A. Romaní, H. A. Ruiz, J. A. Teixeira and L. Domingues, Renewable Energy, 2016, 95, 1–9 CrossRef.
- H. Shaghaleh, X. Xu and S. Wang, RSC Adv., 2018, 8, 825–842 RSC.
- VTT TIEDOTTEITA – Research Notes 2558, ed. A. Harlin and M. Vikman, 2010 Search PubMed.
- B. Lee, Y. Ko, G. Kwon, S. Lee, K. Ku, J. Kim and K. Kang, Joule, 2018, 2, 61–75 CrossRef CAS.
- E. J. Son, J. H. Kim, K. Kim and C. B. Park, J. Mater. Chem. A, 2016, 4, 11179–11202 RSC.
- G. Milczarek, Langmuir, 2009, 25, 10345–10353 CrossRef CAS.
- G. Milczarek, Electrochim. Acta, 2009, 54, 3199–3205 CrossRef CAS.
- G. Milczarek, Electroanalysis, 2008, 20, 211–214 CrossRef CAS.
- F. N. Ajjan, M. Ambrogi, G. A. Tiruye, D. Cordella, A. M. Fernandes, K. Grygiel, M. Isik, N. Patil, L. Porcarelli, G. Rocasalbas, G. Vendramientto, E. Zeglio, M. Antonietti, C. Detrembleur, O. Inganäs, C. Jérôme, R. Marcilla, D. Mecerreyes, M. Moreno, D. Taton, N. Solin and J. Yuan, Polym. Int., 2017, 66, 1119–1128 CrossRef CAS.
- S. Admassie, F. N. Ajjan, A. Elfwing and O. Inganäs, Mater. Horiz., 2016, 3, 174–185 RSC.
- T. Rębiś, T. Y. Nilsson and O. Inganäs, J. Mater. Chem. A, 2016, 4, 1931–1940 RSC.
- J. Tian, Z. Liu, Z. Li, W. Wang and H. Zhang, RSC Adv., 2017, 7, 12089–12097 RSC.
- L. Li, L. Huang, R. J. Linhardt, N. Koratkar and T. Simmons, Sustainable Energy Fuels, 2018, 2, 422–429 RSC.
- C. Lai, Z. Zhou, L. Zhang, X. Wang, Q. Zhou, Y. Zhao, Y. Wang, X. F. Wu, Z. Zhu and H. Fong, J. Power Sources, 2014, 247, 134–141 CrossRef CAS.
- S. V. Gnedenkov, D. P. Opra, S. L. Sinebryukhov, A. K. Tsvetnikov, A. Y. Ustinov and V. I. Sergienko, J. Ind. Eng. Chem., 2014, 20, 903–910 CrossRef CAS.
- Z. Z. Chang, B. J. Yu and C. Y. Wang, Electrochim. Acta, 2015, 176, 1352–1357 CrossRef CAS.
- M. Culebras, H. Geaney, A. Beaucamp, P. Upadhyaya, E. Dalton, K. M. Ryan and M. N. Collins, ChemSusChem, 2019, 12, 4516–4521 CrossRef CAS PubMed.
- S. Chaleawlert-umpon, T. Berthold, X. Wang, M. Antonietti and C. Liedel, Adv. Mater. Interfaces, 2017, 4, 1–7 Search PubMed.
- T. Chen, Q. Zhang, J. Pan, J. Xu, Y. Liu, M. Al-Shroofy and Y. T. Cheng, ACS Appl. Mater. Interfaces, 2016, 8, 32341–32348 CrossRef CAS PubMed.
- D. I. Choi, J. N. Lee, J. Song, P. H. Kang, J. K. Park and Y. M. Lee, J. Solid State Electrochem., 2013, 17, 2471–2475 CrossRef CAS.
- T. Liu, S. Sun, W. Song, X. Sun, Q. Niu, H. Liu, T. Ohsaka and J. Wu, J. Mater. Chem. A, 2018, 6, 23486–23494 RSC.
- A. P. Nowak, J. Hagberg, S. Leijonmarck, H. Schweinebarth, D. Baker, A. Uhlin, P. Tomani and G. Lindbergh, Holzforschung, 2018, 72, 81–90 CrossRef CAS.
- W. Chen, M. Luo, K. Yang and X. Zhou, Microporous Mesoporous Mater., 2020, 300, 110178 CrossRef CAS.
- P. Salehian, K. Karimi, H. Zilouei and A. Jeihanipour, Fuel, 2013, 106, 484–489 CrossRef CAS.
- J. Xu, B. Liu, H. Hou and J. Hu, Bioresour. Technol., 2017, 234, 406–414 CrossRef CAS PubMed.
- O. Merino, V. Almazán, R. Martínez-Palou and J. Aburto, Waste Biomass Valorization, 2017, 8, 733–742 CrossRef CAS.
- O. I. Grzegorz Milczarek, Science, 2012, 335, 1468–1472 CrossRef PubMed.
- M. Quan, D. Sanchez, M. F. Wasylkiw and D. K. Smith, J. Am. Chem. Soc., 2007, 129, 12847–12856 CrossRef CAS PubMed.
- H. Chen, M. Armand, M. Courty, M. Jiang, C. P. Grey, F. Dolhem, J. Tarascon, P. Poizot and J. Verne, J. Am. Chem. Soc., 2009, 131(25), 8984–8988 CrossRef CAS.
- T. A. Enache and A. M. Oliveira-Brett, J. Electroanal. Chem., 2011, 655, 9–16 CrossRef CAS.
- T. Grygar, Š. Kučková, D. Hradil and D. Hradilová, J. Solid State Electrochem., 2003, 7, 706–713 CrossRef CAS.
- D. Schmidt, M. D. Hager and U. S. Schubert, Adv. Energy Mater., 2016, 6, 1500369 CrossRef.
- H. Wang, F. Li, B. Zhu, L. Guo, Y. Yang, R. Hao, H. Wang, Y. Liu, W. Wang, X. Guo and X. Chen, Adv. Funct. Mater., 2016, 26, 3472–3479 CrossRef CAS.
- P. Hu, H. Wang, Y. Yang, J. Yang, J. Lin and L. Guo, Adv. Mater., 2016, 28, 3486–3492 CrossRef CAS PubMed.
- K. Oyaizu, Y. Niibori, A. Takahashi and H. Nishide, J. Inorg. Organomet. Polym. Mater., 2013, 23, 243–250 CrossRef CAS.
- Y. Yang, H. Wang, R. Hao and L. Guo, Small, 2016, 12, 4683–4689 CrossRef CAS PubMed.
- P. C. Rodrigues, P. Cant and M. A. B. Gomes, Eur. Polym. J., 2002, 38, 2213–2217 CrossRef CAS.
- Z. Zhu, M. Hong, D. Guo, J. Shi, Z. Tao and J. Chen, J. Am. Chem. Soc., 2014, 136, 16461–16464 CrossRef CAS PubMed.
- F. N. Ajjan, M. J. Jafari, T. Rębiś, T. Ederth and O. Inganäs, J. Mater. Chem. A, 2015, 3, 12927–12937 RSC.
- M. Wagner, T. Rębis and O. Inganäs, J. Power Sources, 2016, 302, 324–330 CrossRef CAS.
- P. K. Kulkarni, A. G. Satayanarayana and K. G. Rohatgi, J. Mater. Sci. Lett., 1981, 16, 1719–1726 CrossRef.
- Y. Liang, P. Zhang and J. Chen, Chem. Sci., 2013, 4, 1330–1337 RSC.
- J. Geng, J.-P. Bonnet, S. Renault, F. Dolhem and P. Poizot, Energy Environ. Sci., 2010, 3, 1929–1933 RSC.
- S. Renault, J. Geng, F. Dolhem and P. Poizot, Chem. Commun., 2011, 47, 2414–2416 RSC.
- W. Huang, Z. Zhu, L. Wang, S. Wang, H. Li, Z. Tao, J. Shi, L. Guan and J. Chen, Angew. Chem. Int. Ed., 2013, 52, 9162–9166 CrossRef CAS PubMed.
- B. Genorio, K. Pirnat, R. Cerc-Korosec, R. Dominko and M. Gaberscek, Angew. Chem. Int. Ed., 2010, 49, 7222–7224 CrossRef CAS.
- L. Zhang, Z. Liu, G. Cui and L. Chen, Prog. Polym. Sci., 2015, 43, 136–164 CrossRef CAS.
- G. Milczarek, Electroanalysis, 2007, 19, 1411–1414 CrossRef CAS.
- C. A. S. Hill, Wood Modif., 2006, vol. 1–18 Search PubMed.
- M. Matrakova, T. Rogachev, D. Pavlov and B. O. Myrvold, J. Power Sources, 2003, 113, 345–354 CrossRef CAS.
- K. Xia, Q. Ouyang, Y. Chen, X. Wang, X. Qian and L. Wang, ACS Sustain. Chem. Eng., 2016, 4, 159–168 CrossRef CAS.
- W. E. Tenhaeff, O. Rios, K. More and M. A. McGuire, Adv. Funct. Mater., 2014, 24, 86–94 CrossRef CAS.
- H. Lu and X. S. Zhao, Sustainable Energy Fuels, 2017, 1, 1265–1281 RSC.
- A. Manuscript, S. Chaleawlert-Umpon and C. Liedel, J. Mater. Chem. A, 2017, 5, 24344–24352 RSC.
- S. Chatterjee, T. Saito, O. Rios and A. Johs, ACS Symp. Ser., 2014, 1186, 203–218 CrossRef CAS.
- S. Kubo and J. F. Kadla, J. Polym. Environ., 2005, 13, 97–105 CrossRef CAS.
- A. M. Navarro-Suárez, D. Saurel, P. Sánchez-Fontecoba, E. Castillo-Martínez, J. Carretero-González and T. Rojo, J. Power Sources, 2018, 397, 296–306 CrossRef.
- L. Zhang, T. You, T. Zhou, X. Zhou and F. Xu, ACS Appl. Mater. Interfaces, 2016, 8, 13918–13925 CrossRef CAS PubMed.
- S. V. V. V Gnedenkov, S. L. L. L. Sinebryukhov, D. P. P. P. Opra, L. A. A. A. Zemnukhova, A. K. K. Tsvetnikov, A. N. N. Minaev, A. A. A. Sokolov and V. I. I. Sergienko, Procedia Chem., 2014, 11, 96–100 CrossRef.
- H. Qiao and Q. Wei, Functional nanofibers in lithium-ion batteries, Woodhead Publishing Limited, 2012 Search PubMed.
- A. M. Navarro-Suárez, J. Carretero-González, N. Casado, D. Mecerreyes, T. Rojo and E. Castillo-Martínez, Sustainable Energy Fuels, 2018, 2, 836–842 RSC.
- M. Zhu, J. Wu, B. Liu, W. H. Zhong, J. Lan, X. Yang and G. Sui, J. Membr. Sci., 2019, 588, 117194 CrossRef CAS.
- C. Y. Chou, J. R. Kuo and S. C. Yen, ACS Sustain. Chem. Eng., 2018, 6, 4759–4766 CrossRef CAS.
- L. Du, W. Wu, C. Luo, H. Zhao, D. Xu, R. Wang and Y. Deng, Solid State Ionics, 2018, 319, 77–82 CrossRef CAS.
- Z. Zhou, F. Chen, T. Kuang, L. Chang, J. Yang, P. Fan, Z. Zhao and M. Zhong, Electrochim. Acta, 2018, 274, 288–297 CrossRef CAS.
- Y. Ma, K. Chen, J. Ma, G. Xu, S. Dong, B. Chen, J. Li, Z. Chen, X. Zhou and G. Cui, Energy Environ. Sci., 2019, 12, 273–280 RSC.
- C. Luo, L. Du, W. Wu, H. Xu, G. Zhang, S. Li, C. Wang, Z. Lu and Y. Deng, ACS Sustain. Chem. Eng., 2018, 6, 12621–12629 CrossRef CAS.
- B. Liu, Y. Huang, H. Cao, A. Song, Y. Lin, M. Wang and X. Li, J. Solid State Electrochem., 2018, 22, 807–816 CrossRef CAS.
- H. Lu, A. Cornell, F. Alvarado, M. Behm, S. Leijonmarck, J. Li, P. Tomani and G. Lindbergh, Materials, 2016, 9, 1–17 Search PubMed.
- F. Yu, Y. Li, M. Jia, T. Nan, H. Zhang, S. Zhao and Q. Shen, J. Alloys Compd., 2017, 709, 677–685 CrossRef CAS.
- Y. H. Lai, Y. T. Kuo, B. Y. Lai, Y. C. Lee and H. Y. Chen, Int. J. Energy Res., 2019, 43, 5803–5811 CrossRef CAS.
- X. Wu, C. Luo, L. Du, Y. Xiao, S. Li, J. Wang, C. Wang and Y. Deng, ACS Sustain. Chem. Eng., 2019, 7, 8413–8418 CrossRef CAS.
- A. Song, Y. Huang, X. Zhong, H. Cao, B. Liu, Y. Lin, M. Wang and X. Li, J. Membr. Sci., 2018, 556, 203–213 CrossRef CAS.
- J. S. Yeon, S. H. Park, J. Suk, H. Lee and H. S. Park, Chem. Eng. J., 2020, 382, 122946 CrossRef CAS.
- Z. Zhang, S. Yi, Y. Wei, H. Bian, R. Wang, Y. Min, H. L. S. Batteries, Z. Zhang, S. Yi, Y. Wei, H. Bian and R. Wang, Polymers, 2019, 11, 1946 CrossRef CAS PubMed.
- J. Jeon, J.-K. K. Yoo, S. Yim, K. Jeon, G. H. Lee, J. H. Yun, D. K. Kim and Y. S. Jung, ACS Sustain. Chem. Eng., 2019, 7, 17580–17586 CrossRef CAS.
- G. Zhang, Y. Yao, T. Zhao, M. Wang and R. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 16521–16530 CrossRef CAS.
- H. Zhang, D. Jia, Z. Yang, F. Yu, Y. Su, D. Wang and Q. Shen, Carbon, 2017, 122, 547–555 CrossRef CAS.
- P. Lu, F. Liu, F. Zhou, J. Qin, H. Shi and Z. S. Wu, J. Energy Chem., 2021, 55, 476–483 CrossRef.
- C. Ma, H. Wang, X. Zhao, X. Wang, Y. Miao, L. Cheng, C. Wang, L. Wang, H. Yue and D. Zhang, Energy Technol., 2020, 8, 1–8 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |


