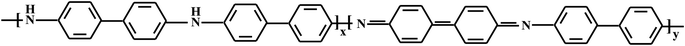Open Access Article
Open Access ArticleOne-step synthesis, characterization and properties of novel hybrid electromagnetic nanomaterials based on polydiphenylamine and Co–Fe particles in the absence and presence of single-walled carbon nanotubes
Sveta Zhiraslanovna Ozkan *a,
Galina Petrovna Karpachevaa,
Mikhail Nikolaevich Efimova,
Andrey Aleksandrovich Vasileva,
Dmitriy Gennad'evich Muratova,
Valeriy Alekseevich Petrova,
Petr Aleksandrovich Chernavskiiab and
Galina Viktorovna Pankinaab
*a,
Galina Petrovna Karpachevaa,
Mikhail Nikolaevich Efimova,
Andrey Aleksandrovich Vasileva,
Dmitriy Gennad'evich Muratova,
Valeriy Alekseevich Petrova,
Petr Aleksandrovich Chernavskiiab and
Galina Viktorovna Pankinaab
aA. V. Topchiev Institute of Petrochemical Synthesis, Russian Academy of Sciences, 29 Leninsky Prospect, Moscow, 119991, Russia. E-mail: ozkan@ips.ac.ru
bDepartment of Chemistry, Lomonosov Moscow State University, 1-3 Leninskie Gory, Moscow, 119991, Russia
First published on 15th July 2021
Abstract
A one-step preparation method for hybrid electromagnetic nanomaterials based on polydiphenylamine (PDPA) and bimetallic Co–Fe particles in the absence and presence of single-walled carbon nanotubes (SWCNT) was proposed. During IR heating of PDPA in the presence of Co(II) and Fe(III) salts in an inert atmosphere at T = 450–600 °C, the polycondensation of diphenylamine (DPA) oligomers and dehydrogenation of phenyleneamine units of the polymer with the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds and reduction of metals by evolved hydrogen with the formation of bimetallic Co–Fe particles dispersed in a polymer matrix occur simultaneously. When carbon nanotubes are introduced into the reaction system, a nanocomposite material is formed, in which bimetallic Co–Fe particles immobilized on SWCNT are distributed in the matrix of the polymer. According to XRD data, reflection peaks of bimetallic Co–Fe particles at diffraction scattering angles 2θ = 69.04° and 106.5° correspond to a solid solution based on the fcc-Co crystal lattice. According to SEM and TEM data, a mixture of particles with sizes of 8–30 nm and 400–800 nm (Co–Fe/PDPA) and 23–50 nm and 400–1100 nm (Co–Fe/SWCNT/PDPA) is formed in the nanocomposites. The obtained multifunctional Co–Fe/PDPA and Co–Fe/SWCNT/PDPA nanomaterials demonstrate good thermal, electrical and magnetic properties. The saturation magnetization of the nanomaterials is MS = 14.99–31.32 emu g−1 (Co–Fe/PDPA) and MS = 29.48–48.84 emu g−1 (Co–Fe/SWCNT/PDPA). The electrical conductivity of the nanomaterials reaches 3.5 × 10−3 S cm−1 (Co–Fe/PDPA) and 1.3 S cm−1 (Co–Fe/SWCNT/PDPA). In an inert medium, at 1000 °C the residue is 71–77%.
N bonds and reduction of metals by evolved hydrogen with the formation of bimetallic Co–Fe particles dispersed in a polymer matrix occur simultaneously. When carbon nanotubes are introduced into the reaction system, a nanocomposite material is formed, in which bimetallic Co–Fe particles immobilized on SWCNT are distributed in the matrix of the polymer. According to XRD data, reflection peaks of bimetallic Co–Fe particles at diffraction scattering angles 2θ = 69.04° and 106.5° correspond to a solid solution based on the fcc-Co crystal lattice. According to SEM and TEM data, a mixture of particles with sizes of 8–30 nm and 400–800 nm (Co–Fe/PDPA) and 23–50 nm and 400–1100 nm (Co–Fe/SWCNT/PDPA) is formed in the nanocomposites. The obtained multifunctional Co–Fe/PDPA and Co–Fe/SWCNT/PDPA nanomaterials demonstrate good thermal, electrical and magnetic properties. The saturation magnetization of the nanomaterials is MS = 14.99–31.32 emu g−1 (Co–Fe/PDPA) and MS = 29.48–48.84 emu g−1 (Co–Fe/SWCNT/PDPA). The electrical conductivity of the nanomaterials reaches 3.5 × 10−3 S cm−1 (Co–Fe/PDPA) and 1.3 S cm−1 (Co–Fe/SWCNT/PDPA). In an inert medium, at 1000 °C the residue is 71–77%.
1. Introduction
One of the promising growth areas of the modern nanoindustry is the creation of nanocomposite materials with required properties. Progress in the creation of novel multifunctional nanomaterials is due to the development of sustainable technologies and new methods for the synthesis of nanostructures. Ternary nanocomposites containing conjugated polymers, magnetic metal oxide nanoparticles and carbon nanomaterials take a special place in this class of nanocomposites.1–3Conjugated polymers are a special class of polymeric materials whose distinctive feature is the delocalization of π-electrons along the conjugation chain.4–6 The specific electronic structure of polymers with a system of polyconjugation determines their excellent electrophysical, optical and electrochemical properties.7–16
The primary advantages of ternary nanocomposites are the synergistic effect between the three components, resulting in excellent electronic, optical, sensing, catalytic, magnetic properties, environmental and thermal stability.17–28 Polymer–metal–carbon nanomaterials based on conjugated polymers can be used in magnetic information recording systems, for medical purposes, as electromagnetic radiation absorbing materials, to create sensors and nanoprobes, rechargeable batteries, and other electrochemical devices.
The strategy for the synthesis of ternary systems usually involves two stages. There are two most common approaches to the preparation of ternary nanocomposites. The first way is the in situ oxidative polymerization of aromatic amines in the presence of prefabricated binary metal–carbon nanocomposites.29–34 The second approach provides the in situ oxidative polymerization of aromatic amines in the presence of carbon nanomaterials followed by the deposition of magnetic nanoparticles on the surface of polymer–carbon nanocomposites.17,35–37 Aniline, pyrrole, thiophene and their derivatives are examples of aromatic amines monomers used to synthesize ternary nanocomposites. Fe3O4, γ-Fe2O3, α-Fe2O3, Co3O4, CoFe2O4, FeCoO can be used as magnetic metal oxide nanoparticles. Graphene, reduced graphene oxide (RGO), carbon nanotubes (CNT) can be used as carbonaceous nanofillers.1–3,30,33,34,38–43 The obtained ternary nanomaterials are superparamagnetic, with saturation magnetization MS ∼19.3–37.2 emu g−1.29,36,44
For instance, Fe3O4/RGO/polyaniline (PANI) ternary nanocomposites were prepared by in situ polymerization of aniline on the Fe3O4/RGO surface.30 Fe3O4 nanoparticles with sizes in the range of 5–10 nm were immobilized on a RGO sheet. The amount of RGO in the nanocomposite was 20–80 wt%. Graphene oxide was reduced with hydrazine. The obtained Fe3O4/RGO/PANI nanocomposites are superparamagnetic. Saturation magnetization and residual magnetization decrease, whereas coercive force grows with the increase of the Fe3O4/RGO ratio. The electromagnetic wave absorbing properties of composite materials were measured. The maximum reflection loss was −36.5 dB at 7.4 GHz with a thickness of 4.5 mm.
Ternary composites based on RGO, Fe3O4 porous nanospheres and PANI were synthesized.45 The uniform Fe3O4 porous nanospheres with the average size of 250–400 nm were grown on the RGO sheets and the PANI layer covered over the surface of RGO/porous Fe3O4 binary composite. The ternary composites showed ferromagnetic behavior. As the content of porous Fe3O4 nanoparticles decreases, the saturation magnetization of the ternary composites decreases, while the coercive force increases. The composites showed excellent microwave absorbing properties. The minimum reflection loss value reached −29.51 dB at 14.69 GHz with a thickness of 1.0 mm.
Polypyrrole (PPy)/Fe2O3/RGO ternary composites were obtained via facile two-step synthesis. Method includes the binary Fe2O3/RGO composite preparation under hydrothermal conditions, followed by oxidative polymerization of pyrrole on the Fe2O3/RGO surface. Testing of ternary nanocomposites as an electrode material revealed their high specific capacitance of 140 F g−1 and excellent capacitance stability of 93%.46
PPy/Fe3O4/CNT ternary nanocomposites were prepared via oxidative polymerization of pyrrole in the presence of carbon nanotubes decorated with Fe3O4 nanoparticles.23 Nanocomposites have both magnetic loss and dielectric loss resulting in a good electromagnetic wave absorbing properties. The maximum reflection loss value was −51.67 dB at 10.2 GHz with a thickness of 3.0 mm.
Ternary nanocomposites based on PANI, graphene and magnetite nanoparticles were synthesized via a two-step method.36 Graphene/PANI nanocomposite was prepared via oxidative polymerization of aniline in a dilute solution of sulfuric acid in the presence of graphene oxide reduced with hydrazine. The Fe3O4 nanoparticles with the size in the range of 5–20 nm were anchored via co-precipitation. The obtained graphene/PANI/Fe3O4 nanocomposites are superparamagnetic, with saturation magnetization MS of 19.3 emu g−1.
Nanocomposites containing PANI, multi-walled carbon nanotubes (MWCNT) and magnetite nanoparticles were prepared via a two-step method.35 First, in situ oxidative polymerization of aniline in the presence of MWCNT treated with concentrated nitric acid was performed. Then, the Fe3O4 nanoparticles modified with aniline dimer were added to the reaction solution.47 The resulting MWCNT/PANI/Fe3O4 ternary nanomaterials can be easily dispersed in water and separated under an external magnetic field.
Fe2O3/poly(3,4-ethylene-dioxythiophene) (PEDOT)/RGO ternary nanocomposites were synthesized by one-pot method.25 The reaction mixture was placed in an autoclave and kept at 170 °C for 4 h. Then, the mixture was centrifuged, washed with ethanol and water, and dried at 60 °C. An electrochemical sensor based on Fe2O3/PEDOT/RGO for the determination of caffeine was developed and applied successfully to the analysis of caffeine in beverage samples.
We have expanded the range of electroactive polymers as part of hybrid polymer–metal–carbon nanocomposites. A simple and effective method to obtain nanocomposite magnetic materials based on poly-3-amine-7-methylamine-2-methylphenazine (PAMMP) via oxidative polymerization of 3-amine-7-dimethylamine-2-methylphenazine hydrochloride on the surface of magnetite nanoparticles immobilized on single-walled carbon nanotubes (Fe3O4/SWCNT) was proposed.48 The prepared ternary nanomaterials are electrically conductive, with high saturation magnetization (MS up to 47.24 emu g−1) and thermal stability.
Hybrid ternary nanomaterials based on polydiphenylamine-2-carboxylic acid (PDPAC), Fe3O4 nanoparticles and SWCNT were obtained for the first time.49 Polymer–metal–carbon Fe3O4/SWCNT/PDPAC nanocomposites were synthesized via oxidative polymerization of diphenylamine-2-carboxylic acid (DPAC) by two different ways: in an acidic medium and in the interfacial process in an alkaline medium. Electrical conductivity of the nanomaterials depends strongly on the synthesis method as well as on the concentration both of carbon nanotubes and of Fe3O4 nanoparticles. Depending on the synthesis method, the electrical conductivity of the nanocomposites reaches 9.6 × 10−7 S cm−1 and 3.3 × 10−3 S cm−1. Higher electrical conductivity of nanomaterials synthesized in an acidic medium is due to the doping of the polymer matrix that occurs during the synthesis of nanocomposites. Regardless of the preparation method, the Fe3O4/SWCNT/PDPAC nanomaterials are superparamagnetic (κπ = MR/MS = 0). The saturation magnetization MS depends on the content of magnetite nanoparticles and reaches 31.6–39.4 emu g−1 depending on the synthesis method of nanocomposites. At the same time, the Fe3O4/SWCNT/PDPAC nanomaterials prepared in the interfacial process in an alkaline medium by one-pot method form high stability magnetic fluids in ethanol.50
The analysis of the literature data showed that polymer–metal–carbon composites have a high potential for practical use in industry, medicine, energy and human life due to their multifunctionality. However, their practical application is hindered by the complexity of the multi-stage synthesis of such ternary nanomaterials. The problem can be solved by developing a facile one-stage environmentally friendly synthesis method. According to our knowledge, no comprehensive systematic research has been conducted in this area. Ternary PEDOT/GO/Fe2O3 nanocomposites were obtained by electropolymerization of 3,4-ethylene-dioxythiophene monomer on a substrate in the presence of GO and iron sulfate.26 The study of electrochemical behavior of PEDOT/GO/Fe2O3 nanocomposites showed a high specific electrochemical capacity (221 F g−1) at a charge–discharge rate of 25 mV s−1 and long-term stability performance even after 1000 cycles owing to the high mechanical strength provided by GO. These performances make PEDOT/GO/Fe2O3 composites as promising material for supercapacitor. However, the fact that the resulting material exists on a substrate significantly limits the scope of its practical application. The task of developing facile and energy-saving methods for obtaining ternary nanomaterials remains urgent.
It should be noted that to obtain ternary nanomaterials, oxidative polymerization is usually carried out on the surface of prefabricated magnetic nanoparticles (Fe3O4, γ-Fe2O3, α-Fe2O3, Co3O4, CoFe2O4, FeCoO). Metal oxide nanoparticles are characterized by not too high magnetic characteristics depending on the synthesis conditions. Besides, the high surface energy of such nanoparticles results in their strong tendency for aggregation. One of the ways to prevent the aggregation of metal nanoparticles during synthesis is to stabilize them in a polymer matrix. The difficulty of the inclusion of other magnetic nanoparticles in the composition of nanocomposites is attributed to the need to develop a complex synthetic strategy that would make it possible to expand the range of magnetic nanoparticles in the composition of polymer–metal–carbon nanocomposites. It is more preferable to use bimetallic magnetic nanoparticles with higher values of magnetic characteristics, such as Co–Fe, Ni–Fe or Co–Ni, as a magnetic component in nanocomposites.51–54
Earlier we have synthesized hybrid magnetic nanocomposites based on polyphenoxazine (PPOA) and bimetallic Co–Fe nanoparticles under IR heating.55 The prepared metal–polymer nanomaterials are thermally stable and superparamagnetic. Saturation magnetization of Co–Fe/PPOA nanocomposites reaches MS = 27.3 emu g−1. As can be seen from Table 1, the use of iron or cobalt oxides as magnetic nanoparticles leads to low values of saturation magnetization and to an increase in the coercive force.56–59
We have previously shown that the size, shape, and phase state of the resulting particles depend on the nature of the polymer matrix. An important role belongs to the thermal stability of the polymer, the presence of functional groups capable of complexation with ions of metals, because acidic residues of salts are carried away by the argon flow under IR heating, and also the presence of a sufficient amount of hydrogen in the polymer structure capable of reducing metals during dehydrogenation. This was shown in the preparation of metal–polymer nanocomposites based on PPOA and polydiphenylamine (PDPA) in the presence of salts (FeCl3·6H2O or Co(OOCCH3)2·4H2O), when various cobalt- and iron-containing nanoparticles of different sizes and shapes were formed.56–60 Under the same synthesis conditions, IR heating of PDPA in the presence of Co(OOCCH3)2·4H2O leads to the formation of spherical α-Co nanoparticles with a hexagonal close-packed lattice and β-Co nanoparticles with a face-centered cubic lattice, their size ranging 2 < d < 8 nm. In the case of PPOA, only spherical β-Co nanoparticles are formed, their size ranging 4 < d < 14 nm. In the presence of FeCl3·6H2O in PPOA, iron-containing nanoparticles Fe2O3, Fe3O4, FeO, α-Fe, γ-Fe, as well as Fe3N, Fe4N and Fe3C are formed, their size and shape depend on the synthesis conditions.
PDPA, an aromatic derivative of PANI, is characterized by better solubility in organic solvents than PANI, redox activity, electrical and optical properties and high thermal stability. PDPA was synthesized by chemical oxidative polymerization or under conditions of electrosynthesis.61–65 Due to the low electrical conductivity, PDPA has not attracted much attention of researchers until recently. As part of binary nanocomposites with carbon nanotubes or polyvinyl fluoride, PDPA has been studied to create sensors and rechargeable lithium batteries, in particular as an additive to a polymer electrolyte.66–68 However, PDPA has a high intrinsic theoretical specific electrochemical capacity (160 W h kg−1) and can be considered as an independent cathode material for rechargeable batteries. Dedoped PDPA was proposed as a highly promising cathode material for lithium and potassium based dual-ion batteries. PDPA showed a record high value of energy density with an active material loading of 80% (418 W h kg−1, calculated for full cathode mass). It outperforms significantly the characteristics of all known polymeric cathodes for dual-ion batteries.69
The low electrical conductivity of PDPA proved to be in demand in the production of electrorheological suspensions. Electrorheological suspension based on silica/PDPA microspheres was prepared without dedoping step due to quite low conductivity of PDPA. Novel semiconductive nanoscale incapsulated spherical particles with core–shell structure were prepared via chemical oxidative polymerization DPA in the presence of silica particles. PDPA shell thickness is of approximately 30 nm. Superior sensitivity and reversibility were observed by the on–off test.70
In this study, a one-step synthesis method for metal–polymer and polymer–metal–carbon electromagnetic nanomaterials based on PDPA and bimetallic Co–Fe particles was developed for the first time. The nanocomposites were prepared by IR heating of precursors based on PDPA, cobalt(II) and iron(III) salts in an inert atmosphere. SWCNT were used as a carbonaceous component. A comparative analysis of the structure, morphology, thermal, magnetic, and electrical properties of Co–Fe/PDPA and Co–Fe/SWCNT/PDPA nanocomposites depending on the synthesis conditions was done.
2. Experimental
2.1. Materials
Diphenylamine (DPA) (analytical grade) was purified by double recrystallization from isopropyl alcohol, and ammonium persulfate (analytical grade) was purified by recrystallization from distilled water. Isopropyl alcohol (extra pure grade), toluene (analytical grade), aqueous ammonia (reagent grade), DMFA (Acros Organics, 99%), as well as Co(OOCCH3)2·4H2O (pure grade) and iron(III) chloride (pure grade) were used without additional purification. Aqueous solutions of reagents were prepared with the use of distilled water. SWCNT from Carbon Chg, Ltd. were produced using electric arc discharge technique with a Ni/Y catalyst (d = 1.4–1.6 nm, l = 0.5–1.5 μm).2.2. Synthesis of polydiphenylamine
Polydiphenylamine (PDPA) was synthesized under conditions of chemical oxidative polymerization in situ in the interfacial process according to the method developed by the authors in.62 To synthesize PDPA in the interfacial process, 0.2 mol L−1 (6.0 g) of DPA were dissolved in an organic solvent–toluene (90 mL), whereas 0.25 mol L−1 (10.26 g) of ammonium persulfate and 1.0 mol L−1 (15.0 mL) of hydrochloric acid were dissolved in distilled water (75.0 mL). The volume ratio of the aqueous and organic phases is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Solutions of the organic and aqueous phases were mixed immediately, without gradual dosing of reagents. The process was carried out under vigorous stirring using an Ika Werke RW 16 Basic electronic overhead stirrer in a narrow cylindrical round-bottom two-necked flask (to increase the stirring efficiency) at 0 °C for 4 h. When the synthesis was completed, the reaction mixture was precipitated in a fivefold excess of isopropyl alcohol (400 mL). The obtained product was filtered off and washed repeatedly with distilled water to remove residual reagents. The neutralization of PDPA was carried out in a 3% solution of NH4OH (200 mL) for 24 h; after that, the polymer was filtered off and washed repeatedly in the excess of distilled water until neutral reaction, and then vacuum-dried over KOH to constant weight. The PDPA yield is 5.15 g (85.8%).
1. Solutions of the organic and aqueous phases were mixed immediately, without gradual dosing of reagents. The process was carried out under vigorous stirring using an Ika Werke RW 16 Basic electronic overhead stirrer in a narrow cylindrical round-bottom two-necked flask (to increase the stirring efficiency) at 0 °C for 4 h. When the synthesis was completed, the reaction mixture was precipitated in a fivefold excess of isopropyl alcohol (400 mL). The obtained product was filtered off and washed repeatedly with distilled water to remove residual reagents. The neutralization of PDPA was carried out in a 3% solution of NH4OH (200 mL) for 24 h; after that, the polymer was filtered off and washed repeatedly in the excess of distilled water until neutral reaction, and then vacuum-dried over KOH to constant weight. The PDPA yield is 5.15 g (85.8%).
2.3. Synthesis of Co–Fe/PDPA
The following method was used to obtain Co–Fe/PDPA.71 A co-solution of PDPA, Co(OOCCH3)2·4H2O and FeCl3·6H2O in DMFA was prepared in a crystallization dish. The concentration of PDPA in the DMFA solution was 2 wt%, the content of cobalt(II) [Co] = 3–10 wt%, and iron(III) [Fe] = 5–22 wt% relative to the polymer weight. The ratios of metals are given in Table 2. After the solvent was removed at T = 60–85 °C, the precursor consisting of PDPA and cobalt(II) acetate and iron(III) chloride salts was IR-heated with an automated IR heating unit in an Ar atmosphere at different sample temperatures in the range of T = 400–600 °C for 2–10 min. The heating rate was 50 °C min−1. Depending on the selected synthesis conditions, the Co–Fe/PDPA yield is 58.3–76.0% (Table 2).| τ, min | T, °C | [Co], wt% | [Fe], wt% | [SWCNT]a, wt% | Yield, % | Co and Fe phase composition | cd110, nm (Co–Fe) | d111, nm (β-Co) | d200, nm | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Co–Fe/PDPA | Co–Fe/SWCNT/PDPA | (Co–Fe) | (β-Co) | ||||||||
| a [SWCNT] in Co–Fe/SWCNT/PDPA.b Fe3O4 and β-Co as trace amounts in Co–Fe/PDPA.c Miller indices interplanar distances according to the database: d110 = 0.2020 nm (Co–Fe), d111 = 0.2040 nm (β-Co), d200 = 0.1429 nm (Co–Fe) and 0.1770 nm (β-Co). d110 = 0.2023/0.2019 nm in Co–Fe/PDPA and Co–Fe/SWCNT/PDPA, respectively. | |||||||||||
| 10 | 400 | 5 | 10 | 10 | 76.0 | 77.4 | α-Co, β-Co, Fe3O4 | — | 0.2045/0.2046 | — | 0.1771/0.1770 |
| 450 | 69.6 | 71.3 | Co–Fe, β-Co, Fe3O4b | 0.2009/0.2012 | 0.2046/0.2048 | 0.1420/0.1422 | 0.1774/0.1769 | ||||
| 500 | 67.9 | 68.4 | Co–Fe, β-Co, Fe3O4b | 0.2011/0.2018 | 0.2046/– | 0.1422/0.1426 | 0.1773/– | ||||
| 550 | 65.5 | 66.2 | Co–Fe, β-Cob | 0.2020/0.2019 | — | 0.1426/0.1426 | — | ||||
| 600 | 63.6 | 64.3 | Co–Fe | 0.2023/0.2019 | — | 0.1428/0.1428 | — | ||||
| 2 | 600 | 5 | 10 | 10 | 65.5 | 71.4 | Co–Fe | 0.2021/0.2020 | — | 0.1428/0.1428 | — |
| 10 | 600 | 10 | 5 | 10 | 66.5 | 69.9 | Co–Fe, β-Co | 0.2014/0.2014 | 0.2052/0.2060 | 0.1422/0.1423 | 0.1776/0.1785 |
| 10 | 10 | 58.3 | 63.7 | Co–Fe, β-Co | 0.2015/0.2014 | 0.2052/0.2056 | 0.1422/0.1421 | 0.1777/0.1780 | |||
| 5 | 20 | 65.3 | 68.3 | Co–Fe | 0.2021/0.2020 | — | 0.1428/0.1427 | — | |||
| 3 | 22 | 74.1 | 70.8 | Co–Fe, Fe3O4 | 0.2023/0.2020 | — | 0.1431/0.1430 | — | |||
| 10 | 600 | 5 | 10 | 0 | — | 63.6 | Co–Fe | 0.2023/0.2019 | — | 0.1428/0.1428 | — |
| 1 | — | 61.1 | Co–Fe, β-Co | 0.2021/0.2016 | 0.2050/0.2054 | 0.1423/0.1426 | 0.1779/0.1781 | ||||
| 3 | — | 66.5 | Co–Fe, β-Co | 0.2019/0.2013 | 0.2055/0.2060 | 0.1421/0.1423 | 0.1782/0.1785 | ||||
| 5 | — | 67.1 | Co–Fe, β-Co | 0.2022/0.2019 | 0.2055/0.2061 | 0.1422/0.1426 | 0.1780/0.1783 | ||||
The IR heating unit is a laboratory quartz tube IR furnace.72,73 The halogen lamps (total maximum power of 24 kW) located outside of quartz reactor were implemented as a radiation source. The maximum wavelength of emission of these IR tubes is in the range of 0.85–1.2 μm. The rectangular graphite box with the samples covered with a graphite lid was placed in quartz reactor. The heating temperature was measured by a chromel–alumel thermocouple fixed in a similar graphite box next to the sample one and was regulated by the temperature controller via IR radiation intensity adjustment.
2.4. Preparation of Co–Fe/SWCNT/PDPA
To synthesize Co–Fe/SWCNT/PDPA a co-solution of PDPA, cobalt(II) acetate Co(OOCCH3)2·4H2O and iron(III) chloride FeCl3·6H2O in DMFA containing SWCNT was prepared.74 The concentration of PDPA in the DMFA solution was 2 wt%, the content of carbon nanotubes was [SWCNT] = 3 and 10 wt%, cobalt(II) [Co] = 3–10 wt%, and iron(III) [Fe] = 5–22 wt% relative to the polymer weight. The ratios of metals are given in Table 2. After the solvent was removed at T = 60–85 °C, the precursor consisting of PDPA, SWCNT, and cobalt(II) acetate and iron(III) chloride salts was IR-heated in an Ar atmosphere at various sample temperatures in the range of T = 400–600 °C for 2–10 min. Depending on the selected synthesis conditions, the Co–Fe/SWCNT/PDPA yield is 63.7–77.4% (Table 2).2.5. Characterization
The metal content in the nanocomposites was measured quantitatively by an inductively coupled plasma atomic emission spectroscopy method (ICP-AES) using a Shimadzu ICP emission spectrometer (ICPE-9000) (Kyoto, Japan).Attenuated Total Reflection (ATR) FTIR spectra of the samples in the attenuated total reflectance mode were recorded using a HYPERION-2000 IR microscope (Bruker, Karlsruhe, Germany) coupled with the Bruker IFS 66v FTIR spectrometer (Karlsruhe, Germany) in the range of 600–4000 cm−1 (150 scans, ZnSe crystal, resolution of 2 cm−1).
X-ray diffraction study was performed in ambient atmosphere using a Difray-401 X-ray diffractometer (Scientific Instruments Joint Stock Company, Saint-Petersburg, Russia) with Bragg–Brentano focusing on CrKα radiation, λ = 0.229 nm.
An electron microscopic study was performed using a JEM-2100 transmission electron microscope (accelerating voltage of 200 kV) (JEOL, Akishima, Tokyo, Japan) and a Hitachi TM 3030 scanning electron microscope (Hitachi High-Technologies Corporation, Fukuoka, Japan) with magnification up to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 30 nm resolution.
000 and 30 nm resolution.
Thermogravimetric analysis (TGA) was performed on a Mettler Toledo TGA/DSC1 (Giessen, Germany) in the dynamic mode in the range of 30–1000 °C in air and in the argon flow. The weight of the samples was 100 mg, the heating rate was 10 °C min−1, and the argon flow velocity was 10 mL min−1. Calcined aluminum oxide was used as a reference. The samples were analyzed in an Al2O3 crucible.
Differential Scanning Calorimetry (DSC) was performed on a Mettler Toledo DSC823e calorimeter (Giessen, Germany). The samples were heated at the rate of 10 °C min−1 in the nitrogen atmosphere, with the nitrogen flow rate of 70 mL min−1. The measurement results were processed with the service program STARe supplied with the device.
The ac conductivity was measured with an E7-20 precision LCR-meter (MC Meratest, Moscow, Russia) in the frequency range of 25.0 Hz to 1.0 MHz.
A vibration magnetometer was used to study the magnetic characteristics of the systems. The cell of the vibration magnetometer was designed as a flow quartz microreactor, which made it possible to study chemical transformations in the in situ mode.75 Specific magnetization depending on the magnetic field value was measured; magnetic characteristics of the samples at room temperature were determined.
3. Results and discussion
3.1. Characterization of nanomaterials
A one-step method was proposed to synthesize novel hybrid nanomaterials based on PDPA and bimetallic Co–Fe particles in the absence and presence of SWCNT. PDPA synthesized in the interfacial process for the first time62,63 is an aromatic polyamine with diphenylene units divided by amine groups and has the following structure:Molecular weight of the polymer reached Mw = (9–11) × 103. PDPA was chosen due to the simplicity of its synthesis under the conditions of oxidative polymerization and to the stability of the polymer under operating conditions, as well as its high thermal stability (up to 450 °C in air and up to 600–650 °C in an inert atmosphere64).
A specific feature of the selected polymer is that when PDPA is heated in the presence of metal salts, further growth of the polymer chain occurs due to the polycondensation reaction of crystalline diphenylamine oligomers, as well as dehydrogenation of phenyleneamine units with the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds included in the general system of conjugated double bonds. At the same time, the molecular weight of the polymer increases.
N bonds included in the general system of conjugated double bonds. At the same time, the molecular weight of the polymer increases.
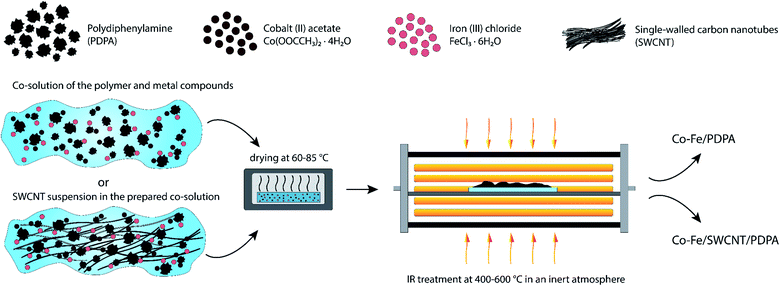
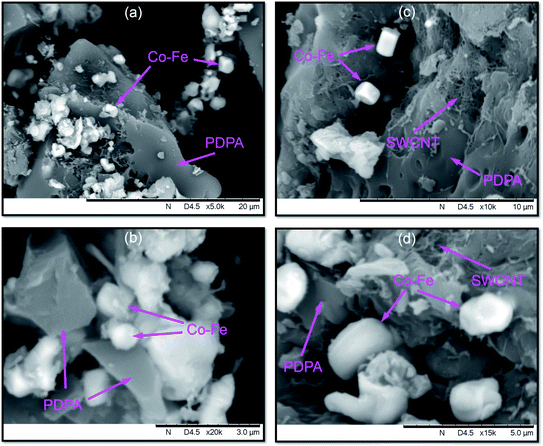
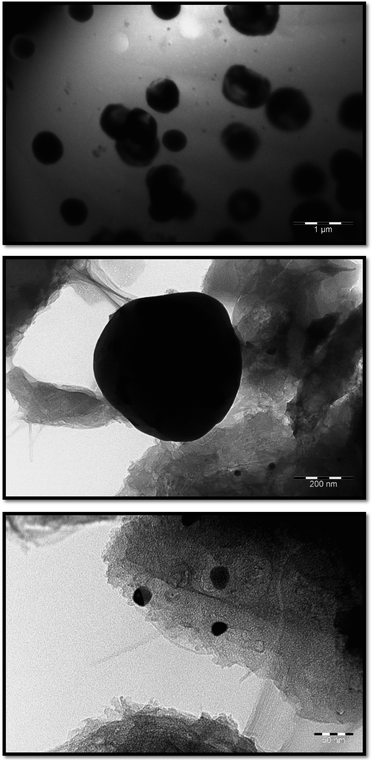
Nanocomposites were synthesized via chemical transformations of PDPA containing cobalt(II) acetate Co(OOCCH3)2·4H2O and iron(III) chloride FeCl3·6H2O salts in the absence and presence of SWCNT in an inert atmosphere under IR heating. As a result, hybrid metal–polymer and polymer–metal–carbon nanocomposites are formed, which are black powders insoluble in organic solvents. Fig. 1 shows a synthesis scheme of the Co–Fe/PDPA and Co–Fe/SWCNT/PDPA nanomaterials.
The formation of hybrid nanocomposites was confirmed by FTIR spectroscopy and X-ray diffraction analysis (XRD), transmission (TEM) and scanning (SEM) electron microscopy.
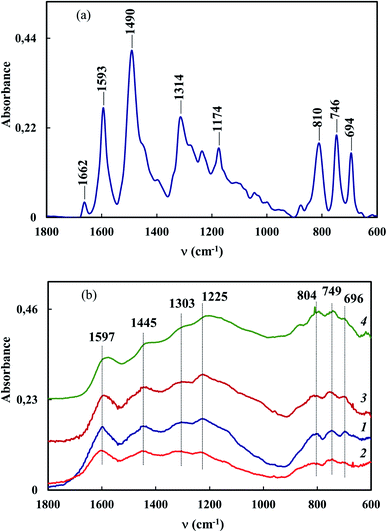
The fact that ATR FTIR spectra of Co–Fe/PDPA and Co–Fe/SWCNT/PDPA retain all the main absorption bands typical of the chemical structure of PDPA confirms the absence of polymer matrix degradation processes during IR heating. FTIR spectroscopy shows that IR heating of precursors leads to dehydrogenation of PDPA phenyleneamine units with the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds incorporated into the general conjugation system. The formation of C
N bonds incorporated into the general conjugation system. The formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds is evidenced by the shift and broadening of the bands at 1593 and 1490 cm−1, that correspond to the stretching vibrations of νC–C bonds in aromatic rings (Fig. 2). As the synthesis temperature rises, there is a decrease in the intensity of the absorption bands at 3380 and 3020 cm−1, corresponding to the stretching vibrations of νN–H and νC–H bonds in phenyleneamine structures.
N bonds is evidenced by the shift and broadening of the bands at 1593 and 1490 cm−1, that correspond to the stretching vibrations of νC–C bonds in aromatic rings (Fig. 2). As the synthesis temperature rises, there is a decrease in the intensity of the absorption bands at 3380 and 3020 cm−1, corresponding to the stretching vibrations of νN–H and νC–H bonds in phenyleneamine structures.
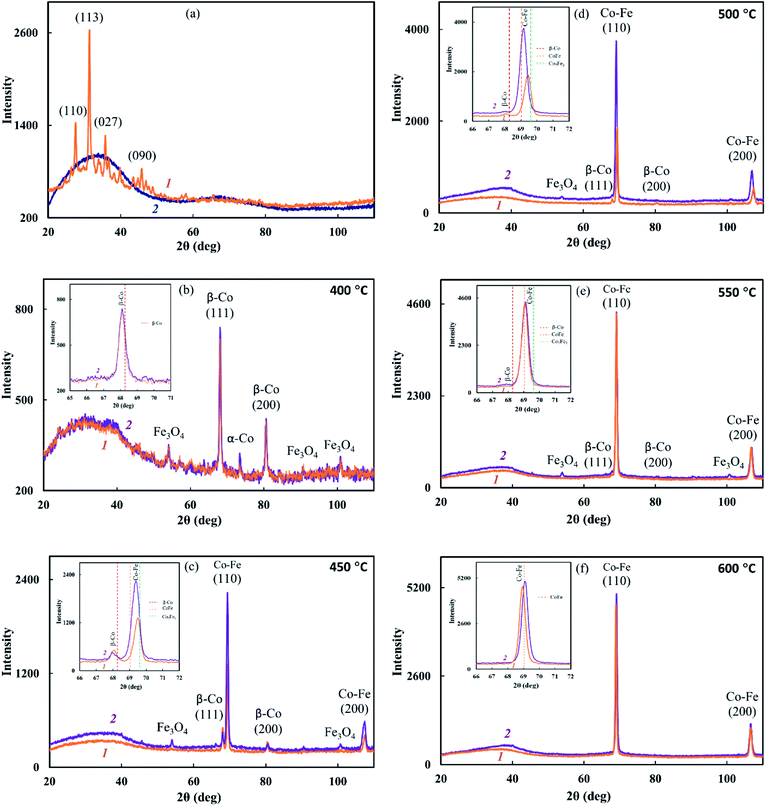
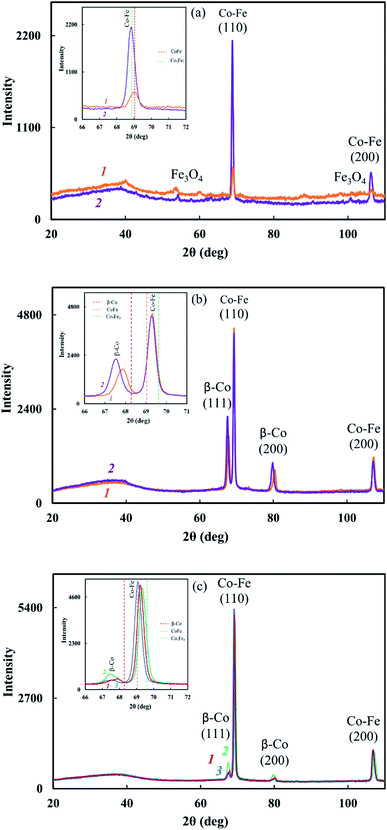
It was found that IR heating of precursors from PDPA, cobalt(II) acetate and iron(III) chloride salts in the absence and presence of SWCNT results in the growth of the polymer chain due to the polycondensation reaction of crystalline DPA oligomers contained in PDPA. This is confirmed by FTIR spectroscopy and XRD data. In the ATR FTIR spectra of Co–Fe/PDPA and Co–Fe/SWCNT/PDPA, an increase in the intensity of the absorption band at 810 cm−1, caused by non-planar deformation vibrations of δC–H 4,4/-substituted benzene rings,62 indicates the growth of the PDPA polymer chain. At the same time, the intensity of the absorption band at 694 cm−1 of the monosubstituted phenyl ring decreases, i.e. the number of end groups of the polymer drops considerably (Fig. 2). In addition, the diffraction pattern of the initial PDPA subjected to IR heating at 600 °C and the diffraction patterns of nanocomposites lack reflection peaks at scattering angles 2θ = 20–50°, which characterize the crystalline low-molecular phase. After processing neat PDPA at 600 °C, a wide halo is observed in this area, which confirms the amorphous structure of the polymer (Fig. 3a). Table 3 shows XRD data of the crystalline low-molecular phase of neat PDPA.
| 2θ, ° | d, Å | hkl | Intensity |
|---|---|---|---|
| 26.34 | 5.027 | 025 | 32 |
| 27.57 | 4.806 | 110 | 54 |
| 31.39 | 4.235 | 113 | 100 |
| 32.4 | 4.101 | 123 | 39 |
| 35.77 | 3.730 | 027 | 48 |
| 36.73 | 3.636 | 105 | 37 |
| 39.85 | 3.361 | 018 | 32 |
| 45.85 | 2.941 | 090 | 32 |
| 47.85 | 2.825 | 173 | 23 |
| 48.84 | 2.771 | 147 | 23 |
The PDF-2 database of the International Centre for Diffraction Data (ICDD) was used to identify the phases.76
At the same time, according to the elemental analysis data, the hydrogen content in the nanocomposites decreases in comparison with PDPA and depends on the IR heating temperature and the quantitative content of cobalt and iron salts in the precursor (Table 4). For instance, in the presence of Co(II) and Fe(III) salts along with SWCNT, the hydrogen content in PDPA decreases from 5.8% for the neat polymer to 1.3% when the IR heating temperature is increased to 600 °C. Elimination of hydrogen can occur both due to the dehydrogenation reaction of phenyleneamine units and due to the polycondensation reaction of DPA oligomers. The evolved hydrogen contributes to the reduction of metals.
| Materials | [Co]a, wt% | [Fe]a, wt% | Co, % | Fe, % | C, % | N, % | H, % | O, % | C/N | C/H |
|---|---|---|---|---|---|---|---|---|---|---|
| a [Co] and [Fe] wt% at the loading.b T = 400 °C, in other cases T = 600 °C.c [SWCNT] = 10 wt%. | ||||||||||
| PDPA | — | — | — | — | 83.0 | 8.4 | 5.8 | 2.8 | 9.9 | 14.2 |
| Co,Fe3O4/PDPA | 5 | 10 | 4.3 | 7.4 | 60.8 | 6.1 | 3.0b | 18.4 | 10.0 | 20.3 |
| Co–Fe/PDPA | 5 | 10 | 5.4 | 7.0 | 61.2 | 5.3 | 1.5 | 19.6 | 11.6 | 41.3 |
| 10 | 5 | 9.7 | 3.7 | 62.7 | 5.1 | 1.8 | 17.0 | 12.2 | 35.2 | |
| Co,Fe3O4/SWCNT/PDPAc | 5 | 10 | 3.8 | 8.4 | 61.0 | 5.6 | 2.9b | 18.3 | 10.9 | 21.2 |
| Co–Fe/SWCNT/PDPAc | 5 | 10 | 4.3 | 6.1 | 63.2 | 4.8 | 1.6 | 20.0 | 13.3 | 40.0 |
| 10 | 5 | 10.9 | 4.2 | 63.9 | 4.7 | 1.3 | 15.0 | 13.6 | 51.1 | |
| 10 | 10 | 9.0 | 6.0 | 60.3 | 5.3 | 1.5 | 17.9 | 11.3 | 39.4 | |
| 5 | 20 | 3.8 | 11.4 | 55.3 | 4.2 | 1.7 | 23.6 | 13.2 | 32.1 | |
The reduction of metals accompanied by the formation of bimetallic Co–Fe particles was confirmed by XRD. The diffraction patterns of nanocomposites show reflection peaks of bimetallic Co–Fe particles at diffraction scattering angles 2θ = 69.04°, 106.5° (Fig. 3c–f) that correspond to a solid solution based on the fcc-Co crystal lattice.55 The inserts show line diagrams for β-Co and Co–Fe.
Based on the XRD results shown in Fig. 3, the formation of a Co–Fe solid solution can be represented as follows. At 400 °C, the fcc phase of the Co–Fe solid solution on the basis of the β-Co crystal lattice with the cobalt content of 75–100% is formed. Then, as iron is reduced and dissolved in the fcc lattice, a polymorphic transformation of the fcc lattice of the solid solution into the bcc lattice with a predominant cobalt content occurs. Upon the complete reduction of iron and the interaction of a large number of metal particles with each other, particles of the Co–Fe solid solution are formed with the metal content close to Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co = 50
Co = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50.
50.
Comparison of the values of Miller indices of interplanar distances in the Co–Fe and β-Co phases with the database shows the formation of a solid solution during the synthesis of nanocomposites. According to the database Miller indices, interplanar distances are: d110 = 0.2020 nm (Co–Fe), d111 = 0.2040 nm (β-Co), d200 = 0.1429 nm (Co–Fe) and 0.1770 nm (β-Co). As seen from Table 2, regardless of the synthesis conditions of nanomaterials, the interplanar distances of the β-Co phase turned out to be larger in comparison with the tabular data, which indicates the dissolution of iron in the β-Co lattice.
According to SEM data, not only spherical, but also rectangular particles are formed in the Co–Fe/PDPA and Co–Fe/SWCNT/PDPA nanocomposites (Fig. 4). Fig. 5 shows TEM images of nanocomposites at different magnifications. A mixture of particles with sizes of 8–30 nm and 400–800 nm (Co–Fe/PDPA) and 23–50 nm and 400–1100 nm (Co–Fe/SWCNT/PDPA) is formed in nanocomposites. The formation of larger Co–Fe particles in the Co–Fe/SWCNT/PDPA nanocomposites is apparently due to the interaction of metal ions with the SWCNT surface during the formation of particles, which leads to a slower reduction of metals. Depending on the synthesis conditions, small peaks of Fe3O4 and β-Co remain on diffraction patterns of Co–Fe/SWCNT/PDPA nanocomposites (Fig. 3).
Thus, at IR heating of precursors from PDPA, cobalt(II) acetate and iron(III) chloride salts in the absence and presence of SWCNT in an inert atmosphere at a sample temperature T = 450–600 °C the following reactions occur simultaneously: the growth of the PDPA polymer chain due to the polycondensation reaction of crystalline oligomers of DPA; dehydrogenation of phenyleneamine units with the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds; reduction of metals due to the evolved hydrogen with the formation of bimetallic Co–Fe particles. This results in the formation of nanostructured Co–Fe/PDPA and Co–Fe/SWCNT/PDPA materials containing particles of Co–Fe solid solution dispersed in a polymer matrix, either free or immobilized on SWCNT.
N bonds; reduction of metals due to the evolved hydrogen with the formation of bimetallic Co–Fe particles. This results in the formation of nanostructured Co–Fe/PDPA and Co–Fe/SWCNT/PDPA materials containing particles of Co–Fe solid solution dispersed in a polymer matrix, either free or immobilized on SWCNT.
The fundamental novelty of the proposed approach is determined by the fact that in a self-organizing system within one stage under IR heating conditions, hybrid metal–polymer and polymer–metal–carbon nanocomposite materials are formed with a structure that contains bimetallic particles, free or anchored onto SWCNT, dispersed in the polymer PDPA matrix. Also, the method makes it possible to obtain bimetallic particles directly during the synthesis of nanocomposites, without subjecting the polymer matrix to degradation.
The impact of the synthesis conditions (sample temperature, heating time, concentration of Co and Fe, as well as SWCNT) on the yield and the phase composition of nanocomposites was studied. It was found that the yield of nanomaterials decreases with the increase in heating time and sample temperature; depending on the synthesis conditions, it is 58.3–76.0% (Co–Fe/PDPA) and 63.7–77.4% (Co–Fe/SWCNT/PDPA) (see Table 2). According to ICP-AES data, the content of Co = 3.8–10.9 wt%, and the content of Fe = 3.7–11.4 wt% (see Table 4).
The study of the sample temperature effect on the phase composition of Co–Fe/PPOA and Co–Fe/SWCNT/PDPA nanocomposites showed that the formation of bimetallic Co–Fe particles occurs at temperatures above 400 °C. At T = 450–500 °C and concentrations of [Co] = 5 wt% and [Fe] = 10 wt% at the loading, nanocomposites contain not only bimetallic Co–Fe particles, but also β-Co particles with a cubic face-centered lattice, which is confirmed by the presence of reflections at diffraction angles 2θ = 68.12°, 80.94°, as well as Fe3O4 particles with reflection peaks in the range of 2θ = 46.01°, 54.08°, 66.54°, 84.27°, 90.82°, 101.46° (Fig. 3). In an inert atmosphere at T = 600 °C under IR heating for 2–10 min at concentrations of [Co] = 5 wt% and [Fe] = 10–20 wt% at the loading, only bimetallic Co–Fe particles were registered. The increase in the concentration of iron to 22 wt% at the loading leads to the formation of Fe3O4 particles due to the excess of iron in relation to cobalt (Fig. 6a). On the contrary, at [Co] = 10 wt% and [Fe] = 5–10 wt%, the diffraction patterns of nanocomposites show reflection peaks not only of bimetallic Co–Fe particles, but also of β-Co particles (Fig. 6b).
An interesting feature was observed when studying the effect of SWCNT concentration on the phase composition of Co–Fe/SWCNT/PDPA nanocomposites. If the content of carbon nanotubes [SWCNT] = 1–5 wt% relative to the polymer weight, the diffractograms of the nanocomposites contain not only reflection peaks of the Co–Fe particles, but also reflection peaks of the fcc phase of the solid solution based on the β-Co crystal lattice with the maximum intensity at [SWCNT] = 3 wt% (Fig. 6c). The inset shows line diagrams for β-Co and Co–Fe. When the SWCNT content is increased up to 10 wt%, only Co–Fe particles are registered, as in Co–Fe/PDPA nanocomposites obtained in the absence of SWCNT (Fig. 3f).
3.2. Thermal properties of nanomaterials
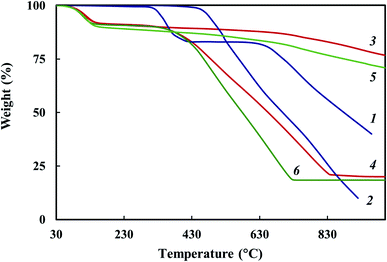
TGA and DSC methods were used to study thermal stability of the Co–Fe/PDPA and Co–Fe/SWCNT/PDPA nanocomposites prepared at 600 °C for 10 min at [Co] = 5 wt% and [Fe] = 10 wt% at the loading. Fig. 7 shows the temperature dependence on the decrease in the weight of nanocomposites compared to PDPA at heating up to 1000 °C in the nitrogen flow and in air. Table 5 shows the main thermal characteristics of materials.The Co–Fe/PDPA and Co–Fe/SWCNT/PDPA nanomaterials have high thermal stability. Approximately 9% weight loss occurs due to the presence of moisture in the nanocomposites, which is also confirmed by DSC data (Fig. 8). The DSC thermograms of the nanomaterials show an endothermic peak at 100 °C. In the second heating this peak is absent.
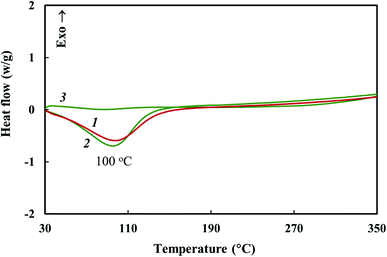 | ||
| Fig. 8 DSC thermograms of Co–Fe/PDPA (1) and Co–Fe/SWCNT/PDPA (2 and 3) at heating in the nitrogen flow to 350 °C at the rate of 10 °C min−1 (1 and 2 – first heating, 3 – second heating). | ||
After the moisture is removed, the weight of nanocomposites does not change until 350–380 °C in air. The processes of thermal oxidative destruction of Co–Fe/PDPA and Co–Fe/SWCNT/PDPA nanocomposites begin at 400 °C, in PDPA they begin at 470 °C. A 50% weight loss in air is observed at 698 °C in PDPA. The Co–Fe/PDPA and Co–Fe/SWCNT/PDPA nanomaterials lose half of their initial weight in air at 660 and 580 °C, respectively. The higher thermal stability of PDPA in air is associated with the fact that, as the temperature rises, in the neat polymer there is the process of oligomer polycondensation induced by atmospheric oxygen.64 At the same time, an increase in the polymerization degree of PDPA and a sharp decrease in the content of the crystalline low-molecular fraction are observed. For the Co–Fe/PDPA and Co–Fe/SWCNT/PDPA nanocomposites in air at 1000 °C, the residue is 20 and 18%, respectively. At the same time, according to ICP-AES data, the metal content in Co–Fe/PDPA is 5.4% Co and 7.0% Fe, which is slightly higher than in Co–Fe/SWCNT/PDPA – 4.3% Co and 6.1% Fe (Table 4).
In an inert medium in neat PDPA in the temperature range of 300–400 °C, the decomposition of diphenylamine oligomers (∼15%) occurs, followed by some stabilization. PDPA loses half of its initial weight in an inert atmosphere at 880 °C, whereas a gradual weight loss is observed in the Co–Fe/PDPA and Co–Fe/SWCNT/PDPA nanocomposites; at 1000 °C, the residue is 77 and 71%, respectively.
3.3. Electrical properties of nanomaterials
Frequency dependence on ac conductivity of the Co–Fe/PDPA and Co–Fe/SWCNT/PDPAC nanocomposites obtained at 600 °C during 10 min at [Co] = 5 wt% and [Fe] = 10 wt% at the loading compared to neat PDPA and PDPA treated at 600 °C was studied (Fig. 9). The composition of the metallic phase corresponds to the Co–Fe phase (Fig. 3f).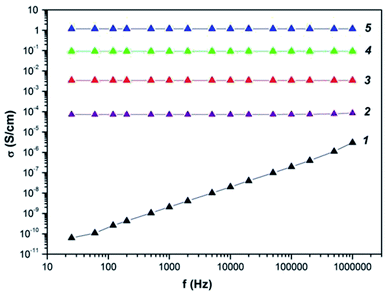 | ||
| Fig. 9 Frequency dependence on the conductivity for PDPA (1) and PDPA at 600 °C (2), Co–Fe/PDPA (3) and Co–Fe/SWCNT/PDPA, prepared at [SWCNT] = 3 (4) and 10 wt% (5). | ||
According to,77–79 the frequency dependence on the conductivity (σac) is described by equation:
| σac = σdc + Aωn, |
Table 6 shows the values of conductivity (σac) of materials at 25 Hz and 1 MHz. Neat PDPA demonstrates a low conductivity value. σdc for PDFA is 5.2 × 10−11 S cm−1. As frequency grows, the electrical conductivity σac of the polymer increases linearly from 6.3 × 10−1 S cm−1 to 3.1 × 10−6 S cm−1. The approximation by the equation of the frequency dependence on the electrical conductivity gives the value of n = 0.99, which indicates the hopping conductivity mechanism (0 ≤ n ≤ 1), typical of most conductive polymers.77–80
| Materials | aσac, S cm−1 | σdc, S cm−1 | n | ||
|---|---|---|---|---|---|
| a σ – The ac conductivity at 25 Hz and 1.0 MHz.b [SWCNT] = 3 and 10 wt%. | |||||
| PDPA | Neat | 6.3 × 10−11 | 3.1 × 10−6 | 5.2 × 10−11 | 0.99 |
| Heated | 7.3 × 10−5 | 8.5 × 10−5 | 7.0 × 10−5 | 0.98 | |
| Co–Fe/PDPA | 3.4 × 10−3 | 3.5 × 10−3 | 2.7 × 10−3 | 0.002 | |
| bCo–Fe/SWCNT/PDPA | 3 | 9.2 × 10−2 | 9.3 × 10−2 | 8.5 × 10−2 | 0.001 |
| 10 | 1.2 | 1.3 | 9.7 × 10−1 | 0.001 | |
Under heat treatment of PDPA at 600 °C, electrical conductivity σac increases significantly to (7.3–8.5) × 10−5 S cm−1 (f = 25–106 Hz), while σdc = 7 × 10−5 S cm−1, n = 0.98. Thus, the mechanism of electrical conductivity has not changed. The observed increase in the conductivity σdc is caused by the increase in the size of the conducting regions, which are areas of continuous conjugation resulting from dehydrogenation reactions with the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds and polycondensation of DPA oligomers. As shown above, IR treated at 600 °C, PDPA is amorphous (Fig. 3a). Thus, heat treatment of PDFA leads to amorphization of the polymer due to phase and morphological transformations. The formation of an extended conjugated system during heat treatment causes an increase in the degree of percolation and leads to an increase in electrical conductivity.
N double bonds and polycondensation of DPA oligomers. As shown above, IR treated at 600 °C, PDPA is amorphous (Fig. 3a). Thus, heat treatment of PDFA leads to amorphization of the polymer due to phase and morphological transformations. The formation of an extended conjugated system during heat treatment causes an increase in the degree of percolation and leads to an increase in electrical conductivity.
The presence of bimetallic Co–Fe particles in the matrix of heat-treated PDPA increases the degree of percolation even more, because metals have a high inherent electrical conductivity. As a result, σac of the Co–Fe/PDPA nanocomposite increases to 3.4 × 10−3 S cm−1 and demonstrates weak dependency on frequency (n = 0.002). Apparently, the presence of metal particles helps the formation of large conducting regions, which leads to exceeding the percolation threshold. In this case, no change in the conductivity of the Co–Fe/PDPA nanocomposite in the high-frequency region is observed.
When metal particles and SWCNT are simultaneously introduced into the structure of nanocomposites, an even greater increase in electrical conductivity is observed. For a nanocomposite containing 3 wt% of SWCNT, σac = (9.2–9.3) × 10−2 S cm−1 in the frequency range 25–106 Hz (n = 0.001), and for a nanocomposite containing 10 wt% of SWCNT, σac = 1.2–1.3 S cm−1 (n = 0.001). Just as for metal–polymer nanocomposites, a very weak frequency dependence on the conductivity is observed in Co–Fe/SWCNT/PDPA, which indicates that the percolation threshold is exceeded and a common conducting region is formed. An increase in the SWCNT content provides a decrease in resistance due to an increase in the number of conduction channels.
3.4. Magnetic properties of nanomaterials
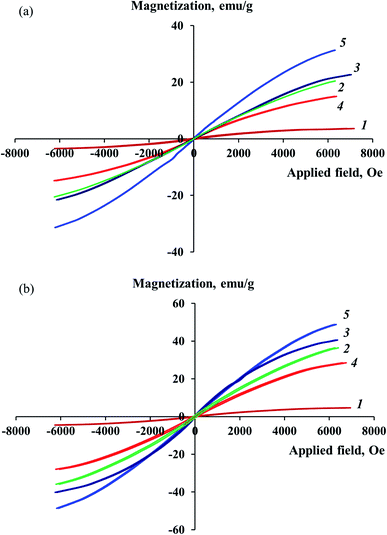
The study of magnetic properties at room temperature showed that the obtained Co–Fe/PDPA and Co–Fe/SWCNT/PDPA nanocomposites demonstrate a hysteresis character of remagnetization. The magnetization dependency on the value of the applied magnetic field is shown in Fig. 10. Table 7 shows the values of the main magnetic properties of nanocomposites. As seen in Fig. 10, the saturation magnetization of nanomaterials grows with the increase in the concentration of both cobalt and iron, and reaches MS = 14.99–31.32 emu g−1 (Co–Fe/PDPA) and MS = 29.48–48.84 emu g−1 (Co–Fe/SWCNT/PDPA) at H = 7000 Oe, depending on the synthesis conditions. The values of the coercive force HC and residual magnetization MR are nonzero for the studied Co–Fe/PDPA and Co–Fe/SWCNT/PDPA nanomaterials: MR is 0.06–0.18 emu g−1 and 0.39–0.85 emu g−1, and HC reaches 5–42 Oe and 27–80 Oe, respectively. This indicates the contribution of large (400–800 nm for Co–Fe/PDPA and 400–1100 nm for Co–Fe/SWCNT/PDPA, according to TEM data) ferromagnetic particles to the magnetization processes. Small nanoparticles formed during the synthesis of nanocomposites (8–30 nm and 23–50 nm for Co–Fe/PDPA and Co–Fe/SWCNT/PDPA, respectively) have a superparamagnetic nature. The MR/MS ratio is 0.003–0.011 for Co–Fe/PDPA and 0.011–0.018 for Co–Fe/SWCNT/PDPA, which indicates the formation of both superparamagnetic and ferromagnetic particles. Higher values of the coercive force HC and residual magnetization MR in the Co–Fe/SWCNT/PDPA nanocomposites seem to be associated with the formation of larger Co–Fe particles. Nanocomposites obtained at 400 °C are characterized by low saturation magnetization MS values and high coercive force (curves 1 in Fig. 10). This is due to the fact that at temperatures below 450 °C, Co–Fe particles have not formed. As shown above, nanomaterials containing α-Co, β-Co and Fe3O4 particles are formed at 400 °C.| Nanomaterials | [Co]a, wt% | [Fe]a, wt% | Cob, % | Feb, % | Co and Fe phase composition | HC, Oe | MS, emu g−1 | MR, emu g−1 | MR/MS |
|---|---|---|---|---|---|---|---|---|---|
| a [Co] and [Fe] wt% at the loading.b According to ICP-AES data.c T = 400 °C, in other cases T = 600 °C. [SWCNT] = 10 wt%. HC – coercive force, MS – saturation magnetization, MR – residual magnetization. | |||||||||
| Co,Fe3O4/PDPAc | 5 | 10 | 4.3 | 7.4 | α-Co, β-Co, Fe3O4 | 156 | 3.67 | 0.18 | 0.049 |
| Co–Fe/PDPA | 5 | 10 | 5.4 | 7.0 | Co–Fe | 5 | 20.43 | 0.06 | 0.003 |
| 5 | 20 | 4.6 | 12.0 | Co–Fe | 25 | 23.07 | 0.16 | 0.006 | |
| 10 | 5 | 9.7 | 3.7 | Co–Fe, β-Co | 42 | 14.99 | 0.17 | 0.011 | |
| 10 | 10 | 9.2 | 7.5 | Co–Fe, β-Co | 11 | 31.32 | 0.18 | 0.006 | |
| Co,Fe3O4/SWCNT/PDPAc | 5 | 10 | 3.8 | 8.4 | α-Co, β-Co, Fe3O4 | 80 | 4.63 | 0.14 | 0.030 |
| Co–Fe/SWCNT/PDPA | 5 | 10 | 4.3 | 6.1 | Co–Fe | 73 | 36.48 | 0.67 | 0.018 |
| 5 | 20 | 3.8 | 11.4 | Co–Fe | 35 | 40.59 | 0.55 | 0.013 | |
| 10 | 5 | 10.9 | 4.2 | Co–Fe, β-Co | 80 | 29.48 | 0.55 | 0.018 | |
| 10 | 10 | 9.0 | 6.0 | Co–Fe, β-Co | 27 | 48.84 | 0.57 | 0.011 | |
Thus, the magnetic behavior of the studied nanocomposites is determined by the presence of small superparamagnetic and large ferromagnetic particles in their structure.
4. Conclusions
The one-stage method for the formation of hybrid electromagnetic nanomaterials based on PDPA and Co–Fe particles in the absence and presence of SWCNT under IR heating conditions makes it possible, without subjecting the polymer matrix to degradation, to obtain magnetic bimetallic particles directly during the synthesis of nanocomposites, and thereby to expand the range of magnetic nanoparticles and to obtain novel magnetic and electrically conductive nanomaterials. The originality of the proposed approach is determined by the fact that the Co–Fe/PDPA and Co–Fe/SWCNT/PDPA nanomaterials are formed in situ with simultaneous reactions of polycondensation of DPA oligomers, dehydrogenation of phenyleneamine units of the polymer matrix and the reduction of metals by evolved hydrogen under IR heating. The saturation magnetization of nanomaterials increases with an increase in the concentration of both cobalt and iron, and at H = 7000 Oe it is MS = 14.99–31.32 emu g−1 (Co–Fe/PDPA) and MS = 29.48–48.84 emu g−1 (Co–Fe/SWCNT/PDPA). In this case, the MR/MS ratio is 0.003–0.011 (Co–Fe/PDPA) and 0.011–0.018 (Co–Fe/SWCNT/PDPA). The formation of a mixture of superparamagnetic and ferromagnetic particles is associated with the fact that both small and large Co–Fe particles are formed during the synthesis of nanomaterials. Nanocomposites are characterized by a weak frequency dependence on electrical conductivity. An increase in the electrical conductivity of nanomaterials occurs due to the presence of both bimetallic Co–Fe particles and SWCNT and reaches 3.5 × 10−3 S cm−1 (Co–Fe/PDPA) and 1.3 S cm−1 (Co–Fe/SWCNT/PDPA). In an inert medium, a gradual weight loss is observed in nanocomposites, and at 1000 °C the residue is 71–77%. Prepared electromagnetic nanomaterials have potential applications in the manufacture of electrochemical devices, such as sensors and nanoprobes, rechargeable batteries, supercapacitors, as well as at fabrication of electromagnetic shielding and corrosion resistant coatings, etc.Author contributions
Conceptualization, investigation and writing – original draft, S. Z. O.; supervision, writing – review & editing, G. P. K.; investigation, M. N. E, A. A. V., D. G. M., V. A. P., P. A. Ch., G. V. P.Funding
This research received no external funding.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was carried out within the State Program of TIPS RAS. This work was performed using the equipment of the Shared Research Center «Analytical center of deep oil processing and petrochemistry of TIPS RAS.References
- F. S. A. Khan, N. M. Mubarak, Y. H. Tan, R. R. Karri, M. Khalid, R. Walvekar, E. Ch. Abdullah, Sh. A. Mazari and S. Nizamuddin, Environ. Sci. Pollut. Res., 2020, 27, 43526–43541 CrossRef PubMed.
- L. Zhang, W. Y. Du, A. Nautiyal, Z. Liu and X. Y. Zhang, Sci. China Mater., 2018, 61, 303–352 CrossRef CAS.
- D. W. Jiang, V. Murugadoss, Y. Wang, J. Lin, T. Ding, Z. C. Wang, Q. Shao, C. Wang, H. Liu and N. Lu, Polym. Rev., 2019, 59, 280–337 CrossRef CAS.
- I. Sapurina and J. Stejskal, Polym. Int., 2008, 57, 1295–1325 CrossRef CAS.
- I. Yu. Sapurina and J. Stejskal, Russ. Chem. Rev., 2010, 79, 1123–1143 CrossRef CAS.
- E. Song and J. W. Choi, Nanomaterials, 2013, 3, 498–523 CrossRef CAS PubMed.
- E. Song and J. Choi, Nanomaterials, 2013, 3, 498–523 CrossRef CAS.
- S. Ghosh, T. Maiyalagan and R. N. Basu, Nanoscale, 2016, 8, 6921–6947 RSC.
- M. Sh. Zoromba, A. A. Alshehri, A. F. Al-Hossainy and M. H. Abdel-Aziz, Opt. Mater., 2021, 111, 110621 CrossRef CAS.
- M. Sh. Zoromba, M. H. Abdel-Aziz, M. Bassyouni, A. Attar and A. F. Al-Hossainy, J. Mol. Struct., 2021, 1225, 129131 CrossRef CAS.
- N. Almutlaq, A. F. Al-Hossainy and M. Sh. Zoromba, J. Mol. Struct., 2021, 1227, 129712 CrossRef CAS.
- E. Tomsík, O. Kohut, I. Ivanko, M. Pekarek, I. Bieloshapka and P. Dallas, J. Phys. Chem., 2018, 122, 8022–8030 Search PubMed.
- A. F. Al-Hossainy and M. Sh. Zoromba, Appl. Phys. A, 2021, 127, 278 CrossRef CAS.
- A. Bourezgui, A. F. Al-Hossainy, I. H. El. Azab, F. Alresheedi, S. A. Mahmoud, M. Bassyouni, M. H. Abdel-Aziz and M. Sh. Zoromba, J. Mater. Sci.: Mater. Electron., 2021, 32, 5489–5503 CrossRef CAS.
- Y. Wang, H. D. Tran, L. Liao, X. Duan and R. B. Kaner, J. Am. Chem. Soc., 2010, 132, 10365–10373 CrossRef CAS PubMed.
- M. Sh Zoromba, A. F. Al-Hossainy, M. Rzaigui, A. Abdelkader, F. Alresheedi, I. H. El. Azab and F. M. Eissa, Opt. Mater., 2021, 112, 110758 CrossRef.
- A. Ibrahim, M. H. Abdel-Aziz, M. S. Zorombam and A. F. Al-Hossainy, Synth. Met., 2018, 238, 1–13 CrossRef CAS.
- H. Lin, Q. Huang, J. Wang, J. Jiang, F. Liu, Y. Chen, C. Wang, D. Lu and S. Han, Electrochim. Acta, 2016, 191, 444–451 CrossRef CAS.
- Z. He, Y. Fang, X. Wang and H. Pang, Synth. Met., 2011, 161, 420–425 CrossRef CAS.
- R. B. Yang, P. M. Reddy, C. J. Chang, P. A. Chen, J. K. Chen and C. C. Chang, Chem. Eng. J., 2016, 285, 497–507 CrossRef CAS.
- G. Ren, Y. Li, Z. Guo, G. Xiao, Y. Zhu, L. Dai and L. Jiang, Nano Res., 2015, 8, 3461–3471 CrossRef CAS.
- P. Xiong, H. Huang and X. Wang, J. Power Sources, 2014, 245, 937–946 CrossRef CAS.
- K. Zhang, X. Zhang, X. Zhao, X. Gai, W. An, G. Fang, A. Zhang and X. Chen, J. Mater. Sci.: Mater. Electron., 2019, 30, 17333–17341 CrossRef CAS.
- M. Alsultan, J. Choi, P. Wagner and G. F. Swiegers, ChemCatChem, 2020, 12, 1580–1584 CrossRef CAS.
- L. Gao, R. Yue, J. Xu and Z. Liu, Int. J. Electrochem. Sci., 2018, 13, 6791–6802 CrossRef CAS.
- N. H. N. Azman, M. S. Mamat, H. N. Lim and Y. Sulaiman, J. Mater. Sci.: Mater. Electron., 2018, 29, 6916–6923 CrossRef CAS.
- S. F. Hojati, A. Amiri, N. MoeiniEghbali and S. Mohamadi, Appl. Organomet. Chem., 2018, 32, E423 CrossRef.
- J. Dalal, S. Lather, A. Gupta, R. Tripathi, A. S. Maan, K. Singh and A. Ohlan, Adv. Mater. Technol., 2019, 4, 1900023 CrossRef CAS.
- Y. J. Zhang, Y. W. Lin, C. C. Chang and T. M. Wu, Synth. Met., 2011, 161, 937–942 CrossRef CAS.
- T. Chen, J. Qiu, K. Zhu, Y. Che, Y. Zhang, J. Zhang, H. Li, F. Wang and Z. Wang, J. Mater. Sci.: Mater. Electron., 2014, 25, 3664–3673 CrossRef CAS.
- T. M. Wu, S. J. Yen, E. C. Chen and R. K. Chiang, J. Polym. Sci., Part B: Polym. Phys., 2008, 46, 727–733 CrossRef CAS.
- S. Radhakrishnan, K. Krishnamoorthy, C. Sekar, J. Wilson and S. J. Kim, Chem. Eng. J., 2015, 259, 594–602 CrossRef CAS.
- L. Kong, X. Lu and W. Zhang, J. Solid State Chem., 2008, 181, 628–636 CrossRef CAS.
- M. S. Cao, J. Yang, W. L. Song, D. Q. Zhang, B. Wen, H. B. Jin, Z. L. Hou and J. Yuan, ACS Appl. Mater. Interfaces, 2012, 4, 6949–6956 CrossRef CAS PubMed.
- H. Zhou, X. Wang, K. Yu, C. Zhang, H. Li and Z. Du, Integr. Ferroelectr., 2014, 154, 159–165 CrossRef CAS.
- P. Liu, Y. Huang and X. Zhang, J. Alloys Compd., 2014, 596, 25–31 CrossRef CAS.
- P. Liu, Y. Huang, L. Wang and W. Zhang, Synth. Met., 2013, 177, 89–93 CrossRef CAS.
- A. P. Singh, M. Mishra, P. Sambyal, B. K. Gupta, B. P. Singh, A. Chandra and S. K. Dhawan, J. Mater. Chem. A, 2014, 2, 3581–3593 RSC.
- Y. Liu, J. Li, F. Li, W. Li, H. Yang, X. Zhang, Y. Liu and J. Ma, J. Mater. Chem. A, 2016, 4, 4472–4478 RSC.
- S. Giri, D. Ghosh and C. K. Das, J. Electroanal. Chem., 2013, 697, 32–45 CrossRef CAS.
- C. Zhao, Y. Jin, X. Du and W. Du, J. Power Sources, 2018, 399, 337–342 CrossRef CAS.
- S. Wang, H. Bao, P. Yang and G. Chen, Anal. Chim. Acta, 2008, 612, 182–189 CrossRef CAS.
- A. Zhu, P. Shi, S. Sun and M. Rui, Prog. Org. Coat., 2019, 133, 117–124 CrossRef CAS.
- Y. Ma, Y. Zhou, Y. Sun, H. Chen, Z. Xiong, X. Li, L. Shen and Y. Liu, J. Alloys Compd., 2019, 796, 120–130 CrossRef CAS.
- J. Luo, Y. Xu, W. Yao, C. Jiang and J. Xu, Compos. Sci. Technol., 2015, 117, 315–321 CrossRef CAS.
- A. Moyseowicz, A. Sliwak, E. Miniach and G. Gryglewicz, Composites, Part B, 2017, 109, 23–29 CrossRef CAS.
- X. Lu, H. Mao, D. Chao, W. Zhang and Y. Wei, J. Solid State Chem., 2006, 179, 2609–2615 CrossRef CAS.
- S. Zh. Ozkan, G. P. Karpacheva, P. A. Chernavskii, E. L. Dzidziguri, G. N. Bondarenko and G. V. Pankina, Polymers, 2018, 10, 544 CrossRef.
- S. Zh. Ozkan, A. I. Kostev, G. P. Karpacheva, P. A. Chernavskii, A. A. Vasilev and D. G. Muratov, Polymers, 2020, 12, 1568 CrossRef CAS.
- S. Zh. Ozkan, A. I. Kostev and G. P. Karpacheva, Polym. Bull., 2021 DOI:10.1007/s00289-021-03558-4.
- S. Koutsopoulos, R. Barfod, K. M. Eriksen and R. Fehrmann, J. Alloys Compd., 2017, 725, 1210–1216 CrossRef CAS.
- K. Zehani, R. Bez, A. Boutahar, E. K. Hlil, H. Lassri, J. Moscovici, N. Mliki and L. Bessais, J. Alloys Compd., 2014, 591, 58–64 CrossRef CAS.
- E. V. Yakushko, D. G. Muratov, L. V. Kozhitov, E. Y. Korovin, A. A. Lomov and A. V. Popkova, Russ. Phys. J., 2021, 63, 2226–2235 CrossRef CAS.
- D. G. Muratov, A. A. Vasilev, M. N. Efimov, G. P. Karpacheva, E. L. Dzidziguri and P. A. Chernavskiy, Inorg. Mater., 2019, 10, 666–672 CrossRef.
- S. Zh. Ozkan, G. P. Karpacheva, P. A. Chernavskii, E. L. Dzidziguri, G. N. Bondarenko, M. N. Efimov and G. V. Pankina, Polym. Bull., 2017, 74, 3043–3060 CrossRef CAS.
- S. Zh. Ozkan, G. P. Karpacheva, E. L. Dzidziguri, P. A. Chernavskii, G. N. Bondarenko and G. V. Pankina, J. Res. Updates Polym. Sci., 2017, 5, 137–148 CrossRef CAS.
- S. Zh. Ozkan, G. P. Karpacheva, E. L. Dzidziguri, M. N. Efimov, G. N. Bondarenko, G. A. Shandryuk, P. A. Chernavskii and G. V. Pankina, J. Polym. Res., 2019, 26, 176 CrossRef.
- S. Zh. Ozkan, E. L. Dzidziguri, G. P. Karpacheva, P. A. Chernavskii, M. N. Efimov and G. N. Bondarenko, Russ. Chem. Bull., 2015, 64, 196–201 CrossRef CAS.
- S. Zh. Ozkan, E. L. Dzidziguri, P. A. Chernavskii, G. P. Karpacheva, M. N. Efimov and G. N. Bondarenko, Nanotechnol. Russ., 2013, 8, 452–460 CrossRef.
- G. P. Karpacheva, S. Zh. Ozkan, E. L. Dzidziguri, P. A. Chernavskii, I. S. Eremeev, M. N. Efimov, M. I. Ivantsov and G. N. Bondarenko, Eur. Chem. Bull., 2015, 4, 135–141 Search PubMed.
- H. Yang and A. J. Bard, J. Electroanal. Chem. Interfacial Electrochem., 1991, 306, 87–109 CrossRef CAS.
- A. V. Orlov, S. Zh. Ozkan, G. N. Bondarenko and G. P. Karpacheva, Polym. Sci., Ser. B, 2006, 48, 5–10 CrossRef.
- A. V. Orlov, S. Zh. Ozkan and G. P. Karpacheva, Polym. Sci., Ser. B, 2006, 48, 11–17 CrossRef.
- S. Zh. Ozkan, G. P. Karpacheva, A. V. Orlov and M. A. Dzyubina, Polym. Sci., Ser. B, 2007, 49, 36–41 CrossRef.
- M. H. Kim, D. H. Bae, H. J. Choi and Y. Sen, Polymer, 2017, 119, 40–49 CrossRef CAS.
- A. I. Gopalan, K.-P. Lee, K. M. Manesh and P. Santhosh, J. Membr. Sci., 2008, 318, 422–428 CrossRef CAS.
- M. Baibarac, I. Baltog, S. Lefrant and P. Gomez-Romero, Mater. Sci. Eng., B, 2011, 176, 110–120 CrossRef CAS.
- T. Permpool, A. Sirivat and D. Aussawasathien, Sens. Actuators, B, 2015, 220, 91–100 CrossRef CAS.
- F. A. Obreskov, A. F. Shestakov, S. G. Vasil'ev, K. J. Stevenson and P. A. Troshin, J. Mater. Chem. A, 2021, 9, 2864 RSC.
- H. Jang, S. Kwon, J. Lee and H. Choi, Polymer, 2019, 182, 121851 CrossRef.
- S. Zh. Ozkan and G. P. Karpacheva, RU patent for the invention, Utility patents, No 2724251 C1 from 22. 06. 2020.
- M. N. Efimov, A. A. Vasilev, D. G. Muratov, A. E. Baranchikov and G. P. Karpacheva, J. Environ. Chem. Eng., 2019, 7, 103514 CrossRef CAS.
- M. N. Efimov, V. E. Sosenkin, Y. M. Volfkovich, A. A. Vasilev, D. G. Muratov, S. A. Baskakov, O. N. Efimov and G. P. Karpacheva, Electrochem. Commun., 2018, 96, 98–102 CrossRef CAS.
- S. Zh. Ozkan and G. P. Karpacheva, RU patent for the invention, Utility patents, No 2737184 C1 from 25.11.2020.
- P. A. Chernavskii, G. V. Pankina and V. V. Lunin, Russ. Chem. Rev., 2011, 80, 579–604 CrossRef CAS.
- PDF-2, The International Centre for Diffraction Data, http://www.icdd.com/index.php/pdf-2/, 2021 [accessed 08 April 2021] Search PubMed.
- A. K. Jonscher, Nature, 1977, 267, 673–679 CrossRef CAS.
- J. C. Dyre and T. B. Schrøder, Rev. Mod. Phys., 1999, 72, 873–892 CrossRef.
- P. Xie, Y. Li, Q. Hou, K. Sui, C. Liu, X. Fu, J. Zhang, V. Murugadoss, J. Fan, Y. Wang, R. Fan and Z. Guo, J. Mater. Chem. C, 2020, 8, 3029–3039 RSC.
- S. Yoshimoto, F. Ohashi and T. Kameyama, Macromol. Rapid Commun., 2004, 25, 1687–1691 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |