Open Access Article
Open Access ArticleCopper-catalyzed thioketalization of enones featuring trifluoromethyl groups†
Tongfei Zhang and
Zhenbo Gao *
*
College of Sciences, Nanjing Agricultural University, Nanjing 210095, China. E-mail: zgao@njau.edu.cn
First published on 1st June 2021
Abstract
Synthetic methods for the preparation of thioketals featuring CF3 groups are rare. Here, we have developed a copper-catalyzed thioketalization of enones bearing CF3 groups and various mercaptans. 24 thioketal molecules have been obtained with moderate to excellent yield. Meanwhile, a preparative scale experiment has been performed giving over 95% yield. This work allows the straightforward formation of thioketals containing CF3 groups and unsaturated double bonds.
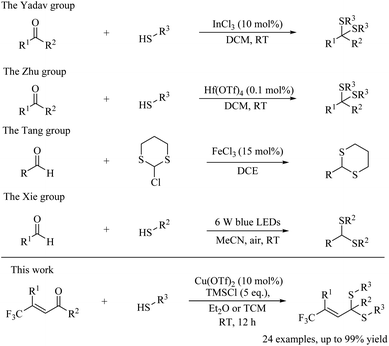
Thioacetals and thioketals are widely used as building blocks for the synthesis of various chemicals including a series of natural products.1 In most cases, the thioacetal and thioketal were obtained by addition of a thiol, especially 1,3-propanedithiol, to aldehydes and ketones.2 Few works on addition of diverse mercaptans to enones to create various thioketals have been reported.
Most methods have adopted Bronsted or Lewis acid catalysis to activate ketone and obtained thioketal.3–17 Researchers have discovered catalysts, such as I2,3 InI3,4 TiCl4,5 InCl3,6,7 InBr3,8 BF3·OEt2,9 AgOTf,10 Hf(OTf)4,11 In(OTf)3,12 TMSCl,13 NH2SO3H,14 DBSA,15 Cu(DS)2,16 and (COOH)2 (ref. 17) working well in thioketalization. The Lee group18 and Xie group19 later reported two photoredox-catalyzed thioacetalizations respectively (Scheme 1b and c). The Tang group discovered a Fe-catalyzed direct dithioacetalization of aldehydes with 2-chloro-1,3-dithiane (Scheme 1d).20 However, most methods were developed only for aldehyde or ketone. Approaches for more complicated substrates like enone need to be advanced.
Fluorine have played an important role in chemistry due to the wide applications of fluorinated molecules in pharmaceuticals and materials.21,22 The incorporation of fluorine atom into a drug molecule would significantly advance its medicinal properties.23 Therefore, synthesis of compounds featuring fluorine atoms has been a hot area in organic chemistry.24–27
Among the syntheses of various fluorinated molecules, the installation of trifluoromethyl group is a very important and challenging task, since plenty of drug molecules contain this moiety.28–34 Syntheses of compounds featuring both of F and S atoms would be of interest to synthetic chemists.35 Here, we have discovered a copper-catalyzed approach achieving a variety of thioketals featuring CF3 groups in order to further advance both of thioketalization and fluorinated molecule synthesis (Scheme 1e). This method is realized by adding abundant thiols to various CF3 substituted enones36 with good to excellent yields.
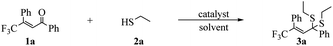
We started the exploration from intensive optimizations. We chose substrate (E)-4,4,4-trifluoro-1,3-diphenylbut-2-en-1-one, 1a and ethanethiol, 2a as thioketalization partners (Table 1).
| Entry | Catalyst | Solvent | Time | Yieldg [%] |
|---|---|---|---|---|
| a Reaction conditions: enone 1.0 eq., thiol (2.0 eq.), catalyst (10 mol%).b Additive TMSCl (5 eq.) was added.c 5 mol% catalyst Cu(OTf)2 was used.d 15 mol% catalyst Cu(OTf)2 was used.e 0.5 eq. TMSCl was used.f 3.0 eq. TMSCl was used.g Yield of isolated product. | ||||
| 1 | — | DCM | 12 h | 0 |
| 2b | — | DCM | 12 h | 14 |
| 3b | CuCl | DCM | 12 h | 6 |
| 4b | CuBr2 | DCM | 12 h | 7 |
| 5b | Cu(OAc)2 | DCM | 12 h | Trace |
| 6b | CuSCN | DCM | 12 h | Trace |
| 7b | Cu(OTf)2 | DCM | 12 h | 51 |
| 8b | Cu(OTf)2 | DCE | 12 h | 51 |
| 9b | Cu(OTf)2 | MeCN | 12 h | Trace |
| 10b | Cu(OTf)2 | Et2O | 12 h | Trace |
| 11b | Cu(OTf)2 | THF | 12 h | Trace |
| 12b | Cu(OTf)2 | MTBE | 12 h | Trace |
| 13b | Cu(OTf)2 | DMF | 12 h | 0 |
| 14b | Cu(OTf)2 | TCM | 12 h | 70 |
| 15b | Cu(OTf)2 | TCM | 20 h | 70 |
| 16b,c | Cu(OTf)2 | TCM | 12 h | 58 |
| 17b,d | Cu(OTf)2 | TCM | 12 h | 70 |
| 18e | Cu(OTf)2 | TCM | 12 h | 16 |
| 19f | Cu(OTf)2 | TCM | 12 h | 55 |
First, we confirmed that this transformation could not proceed spontaneously without catalyst and additive (Table 1, entry 1). With the activation by Lewis acid TMSCl, we collected some product (entry 2). We then remained the additive and screened copper catalysts. No improvement was observed by applying catalyst CuCl, CuBr2, Cu(OAc)2 and CuSCN (entries 3–6) until Cu(OTf)2 (51% yield, entry 7). To further advance the result, we optimized solvents. We tried DCE, MeCN, Et2O, THF, MTBE, DMF and chloroform (TCM), and finally enhanced the yield to 70% by using TCM (entries 8–14). Further extension of reaction time to 20 hours showed no improvement to the result (entry 15). We also studied the optimal amount of catalyst Cu(OTf)2 and additive TMSCl. The yield reduced to 58% with 5 mol% catalyst (entry 16), while the yield maintained at 70% using 15 mol% catalyst (entry 17). In terms of TMSCl, we found that decreasing its amount would strongly deteriorate the reaction (entries 18 and 19). Only 16% yield was achieved using TMSCl (0.5 eq.) compared to 70% yield with TMSCl (5.0 eq.). Finally, we obtained our best result using TCM, Cu(OTf)2 (10 mol%), TMSCl (5.0 eq.) under room temperature after 12 hours.
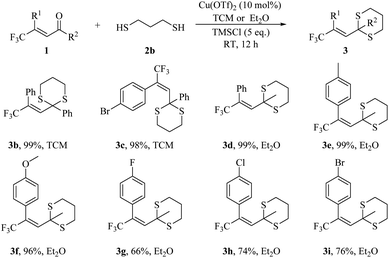
We next started scope of enone using propane-1,3-dithiol, 2b, since it is widely used in previous thioketalization. To our delight, dithiol has shown much better results than monothiol in our thioketalization. We first studied enone 1a, and obtained 99% yield with Cu(OTf)2 (10 mol%) and TMSCl (5 eq.) in TCM (3b). We then investigated bromine substituted enone 1b with 2b, and received 3c with 98% yield. We next studied enone substrates with a methyl group instead of benzyl group attached to the carbonyl group (R2 is Me instead of Ph). In the reaction of methyl group substituted enones, we replaced solvent TCM with Et2O, which could afford better results. We found that Et2O suits methyl group substituted enones, such as 1c, while TCM perfomed better in most phenyl group substituted enones, like 1a. We next investigated the influence of substituent groups in enone. We found that electron donating groups, methyl and methoxyl have no influence on results, giving 99% and 96% yield respectively (3e and 3f). The electron withdrawing groups like F, Cl, Br deteriorated the yield, leading to a nearly 20% decrease (3g, 3h, and 3i, Scheme 2).
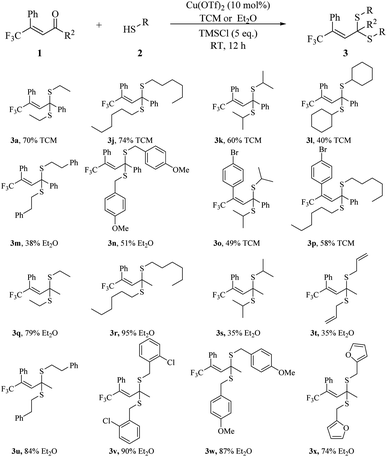
We decided to apply both substrate 1a and (E)-5,5,5-trifluoro-4-phenylpent-3-en-2-one 1c to further explore the scope of thiol in our methodology, since α-phenyl-substituted enone, 1a and α-methyl-substituted enone, 1c have different favourable conditions to afford corresponding thioketals. Using conditions mentioned in entry 14, Table 1, we obtained product 3a with 70% yield (Scheme 3). We then replaced ethanethiol with more complicated thiols. We introduced hexane-1-thiol, propane-2-thiol, cyclohexanethiol, 2-phenylethane-1-thiol and (4-methoxyphenyl)methanethiol successively into enone 1a affording different thioketals (3j–3n). We found that the size of thiol would affect the result. With an increasing size of alkyl group on thiol, the yield dropped from 74% to 40% (3j–3l). As for aromatic ring substituted thiols, few products were collected in TCM, and moderate results were received (3m, 38% and 3n, 51%) in Et2O. Adding some electron donating groups, e.g. MeO to the aromatic rings would improve the result a bit (3n). We also tried other α-phenyl-substituted enone and got medium results (3o, 49% and 3p, 58%).
We next explored more thiol on α-methyl-substituted enone, 1c in Et2O. With an increasing carbon chain on thiol, the result enhanced from 79% to 95% (3q and 3r). In terms of bulky thiol, propane-2-thiol, we could also obtain the desired thioketal 3s in 35% yield. We then examined thiols featuring functional groups. We obtained thioketals bearing unsaturated C–C double bond, phenyl ring, 2-chlorobenzyl, 4-methoxybenzyl, furan ring respectively (3t to 3x). In most cases, excellent yields were achieved, especially for 3v, 90% yield and 3w, 87% yield.
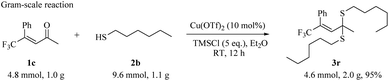
We then attempted the preparative scale reaction to synthesize CF3-substituted thioketal. We got 2.0 g product 3r with 95% yield using 1b (4.8 mmol, 1.0 g) and thiol 2b (9.6 mmol, 1.1 g) catalyzed by Cu(OTf)2 (10 mol%) and TMSCl (5.0 eq.) in Et2O after 12 hours reaction under room temperature (Scheme 4).
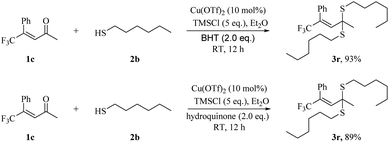
We also attempted to understand the mechanism of our approach. We did two radical trapping experiments using BHT and hydroquinone as radical scavengers respectively. We excluded free radical reaction mechanism, since no interruption was observed after the addition of these radical trapping reagents (Scheme 5). The product 3r was obtained in 93% and 89% yield respectively with BHT and hydroquinone, compared to 95% yield without the radical scavenger. The high yield of 3r for two radical trapping experiments excluded the radical pathway of our approach.
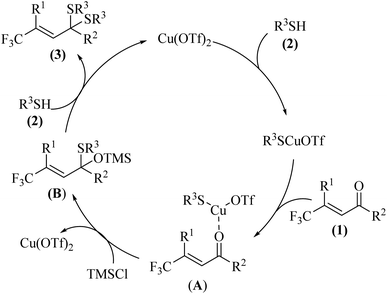
Considering no disulfide by-product was observed in our method, we proposed a plausible mechanism in Scheme 6. We supposed the catalysis started from the combination of Cu(OTf)2 and thiol giving the copper species. After coordination of copper and enone 1, intermediate A was formed. Then the thiol would leave the copper and attack the carbonyl group. With the help of additive TMSCl, intermediate B was obtained and Cu(OTf)2 was recycled. In the final stage, substitution, the OTMS group would leave the intermediate B and a second thiol would attack the intermediate to afford the desired product thioketal 3.
Conclusions
We have reported a new copper-catalyzed method to add various thiols to a series of β-CF3-substituted-enones affording plenty of thioketals in moderate to excellent yield. 24 thioketals featuring CF3 groups and different SR groups were synthesized. We also proved the efficiency of preparative scale reaction and got 2.0 g product 3r. Thioketals are widely used in organic synthesis, while few CF3 containing thioketals were reported. Our work opens a new door in this area, and would promote more applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support by 2019 Jiangsu Shuang Chuang Doctoral Grants and Nanjing Agricultural University Research Start-up Funding.Notes and references
- A. B. Smith and C. M. Adams, Acc. Chem. Res., 2004, 37, 365–377 CrossRef CAS PubMed.
- M. Yus, C. Nájera and F. Foubelo, Tetrahedron, 2003, 59, 6147–6212 CrossRef CAS.
- H. Firouzabadi, N. Iranpoor and H. Hazarkhani, J. Org. Chem., 2001, 66, 7527–7529 CrossRef CAS PubMed.
- K. Nishino, K. Minato, T. Miyazaki, Y. Ogiwara and N. Sakai, J. Org. Chem., 2017, 82, 3659–3665 CrossRef CAS PubMed.
- V. Kumar and S. Dev, Tetrahedron Lett., 1983, 24, 1289–1292 CrossRef CAS.
- J. S. Yadav, B. V. Subba Reddy and S. K. Pandey, Synth. Commun., 2002, 32, 715–719 CrossRef CAS.
- S. Muthusamy, S. A. Babu and C. Gunanathan, Tetrahedron Lett., 2001, 42, 359–362 CrossRef CAS.
- M. A. Ceschi, L. De Araujo Felix and C. Peppe, Tetrahedron Lett., 2000, 41, 9695–9699 CrossRef CAS.
- I. Miranda and J. A. Soderquist, Tetrahedron Lett., 1986, 27, 6305–6306 CrossRef.
- R. Wu, S. Gao, G. Yang, L. Pan, M. Liu, K. Hu, W. Zhong and C. Yu, Lett. Org. Chem., 2015, 12, 299–305 CrossRef CAS.
- Y. C. Wu and J. Zhu, J. Org. Chem., 2008, 73, 9522–9524 CrossRef CAS PubMed.
- S. Muthusamy, S. Arulananda Babu and C. Gunanathan, Tetrahedron, 2002, 58, 7897–7901 CrossRef CAS.
- G. Zhao, L. Z. Yuan, M. Alami and O. Provot, Adv. Synth. Catal., 2018, 360, 2522–2536 CrossRef CAS.
- A. Leitemberger, L. M. C. Böhs, M. L. B. Peixoto, C. H. Rosa, G. R. Rosa and M. Godoi, ChemistrySelect, 2020, 5, 8253–8257 CrossRef CAS.
- D. Dong, Y. Ouyang, H. Yu, Q. Liu, J. Liu, M. Wang and J. Zhu, J. Org. Chem., 2005, 70, 4535–4537 CrossRef CAS PubMed.
- S. S. Weng, S. C. Chang, T. H. Chang, J. P. Chyn, S. W. Lee, C. A. Lin and F. K. Chen, Synthesis, 2010, 1493–1499 CrossRef CAS.
- H. Miyake, Y. Nakao and M. Sasaki, Chem. Lett., 2007, 36, 104–105 CrossRef CAS.
- K. Du, S. C. Wang, R. S. Basha and C. F. Lee, Adv. Synth. Catal., 2019, 361, 1597–1605 CrossRef CAS.
- Z. Xing, M. Yang, H. Sun, Z. Wang, P. Chen, L. Liu, X. Wang, X. Xie and X. She, Green Chem., 2018, 20, 5117–5122 RSC.
- J. Lai, W. Du, L. Tian, C. Zhao, X. She and S. Tang, Org. Lett., 2014, 16, 4396–4399 CrossRef CAS PubMed.
- J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. Del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed.
- Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Acenã, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422–518 CrossRef CAS PubMed.
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC.
- P. Shah and A. D. Westwell, J. Enzyme Inhib. Med. Chem., 2007, 22, 527–540 CrossRef CAS PubMed.
- M. Bassetto, S. Ferla and F. Pertusati, Future Med. Chem., 2015, 7, 527–546 CrossRef CAS PubMed.
- D. E. Yerien, S. Bonesi and A. Postigo, Org. Biomol. Chem., 2016, 14, 8398–8427 RSC.
- N. A. Meanwell, J. Med. Chem., 2018, 61, 5822–5880 CrossRef CAS PubMed.
- H. L. Yale, J. Med. Pharm. Chem., 1959, 1, 121–133 CrossRef CAS PubMed.
- G. Landelle, A. Panossian and F. R. Leroux, Curr. Top. Med. Chem., 2014, 14, 941–951 CrossRef CAS PubMed.
- W. Zhu, J. Wang, S. Wang, Z. Gu, J. L. Aceña, K. Izawa, H. Liu and V. A. Soloshonok, J. Fluorine Chem., 2014, 167, 37–54 CrossRef CAS.
- X. Yang, T. Wu, R. J. Phipps and F. D. Toste, Chem. Rev., 2015, 2, 826–870 CrossRef PubMed.
- X. Pan, H. Xia and J. Wu, Org. Chem. Front., 2016, 3, 1163–1185 RSC.
- M. Khalid and S. Mohammed, Orient. J. Chem., 2018, 34, 2708–2715 CAS.
- Y. Wang, Z. Ye, H. Zhang and Z. Yuan, Adv. Synth. Catal., 2021, 363, 1–21 CrossRef.
- C. Ni, M. Hu and J. Hu, Chem. Rev., 2015, 115, 765–825 CrossRef CAS PubMed.
- A. Sanz-Marco, G. Blay, C. Vila and J. R. Pedro, Org. Lett., 2016, 18, 3538–3541 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra03222d |
| This journal is © The Royal Society of Chemistry 2021 |