Open Access Article
Open Access ArticleOn-surface synthesis of π-conjugated ladder-type polymers comprising nonbenzenoid moieties†
José I. Urgel‡
 *a,
Julian Bock
*a,
Julian Bock bc,
Marco Di Giovannantonio§
bc,
Marco Di Giovannantonio§
 a,
Pascal Ruffieux
a,
Pascal Ruffieux a,
Carlo A. Pignedolia,
Milan Kivala
a,
Carlo A. Pignedolia,
Milan Kivala *bc and
Roman Fasel
*bc and
Roman Fasel *ad
*ad
aEmpa, Swiss Federal Laboratories for Materials Science and Technology, 8600 Dübendorf, Switzerland. E-mail: jose-ignacio.urgel@imdea.org; roman.fasel@empa.ch
bInstitute of Organic Chemistry, Ruprecht-Karls-University Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany. E-mail: milan.kivala@oci.uni-heidelberg.de
cCentre for Advanced Materials, Ruprecht-Karls-University Heidelberg, Im Neuenheimer Feld 225, 69120 Heidelberg, Germany
dDepartment of Chemistry and Biochemistry, University of Bern, 3012 Bern, Switzerland
First published on 2nd July 2021
Abstract
On-surface synthesis provides a powerful approach toward the atomically precise fabrication of π-conjugated ladder polymers (CLPs). We report herein the surface-assisted synthesis of nonbenzenoid CLPs from cyclopenta-annulated anthracene monomers on Au(111) under ultrahigh vacuum conditions. Successive thermal annealing steps reveal the dehalogenative homocoupling to yield an intermediate 1D polymer and the subsequent cyclodehydrogenation to form the fully conjugated ladder polymer. Notably, neighbouring monomers may fuse in two different ways, resulting in six- and five-membered rings, respectively. The structure and electronic properties of the reaction products have been investigated via low-temperature scanning tunneling microscopy and spectroscopy, complemented by density-functional theory calculations. Our results provide perspectives for the on-surface synthesis of nonbenzenoid CLPs with the potential to be used for organic electronic devices.
Conjugated ladder polymers (CLPs) represent an attractive type of π-conjugated polymers which consist of a sequence of fused rings with adjacent rings sharing two or more atoms.1 The restriction of bond rotations inherent to CLPs represent an important approach that has been systematically pursued since the 1990s.2,3 Such rigidification generally provides enhanced intermolecular π–π stacking interactions and high thermal stability, which may lead to application-relevant properties (or improved materials properties),4–10 with prospects in organic field-effect transistors,11 organic light emitting diodes,12 gas separation13,14 or optically pumped lasers.15 While the majority of CLPs reported to date have been obtained through solution chemistry,16,17 the potential of on-surface synthesis to overcome some of the intrinsic challenges of solution-based approaches, including low solubility, high reactivity of reaction products or precursors and limited diversity of reaction designs, toward the effective fabrication of tailored ladder polymers18–20 has increasingly come to forth in the last decade only.21
In this context, graphene nanoribbons (GNRs)22 represent the most acknowledged class of CLPs. Despite the recent expansion of the GNR catalogue, the controlled incorporation of nonbenzenoid rings into the GNR backbone to modulate the structural23,24 and electronic25,26 properties remains comparably scarce. Theoretical calculations have anticipated the existence of several nonbenzenoid carbon allotropes which are expected to exhibit pronouncedly different (opto)-electronic properties,27,28 mechanical stability,29,30 or electron transport properties.31 Only recently, the on-surface synthesis of several CLPs containing nonbenzenoid rings has been reported.32–38 Nevertheless, systematic investigations of the structural and electronic properties resulting from selective incorporation of nonbenzenoid rings into CLPs are still lacking.
In this work, we introduce an exemplary approach toward the on-surface formation of nonbenzenoid π-conjugated ladder-type polymers on a coinage metal surface under ultrahigh vacuum (UHV) conditions. To this end, we synthesized in solution 2,7-dibromocyclopenta[hi]aceanthrylene as a precursor (1),39 which features electron acceptor properties due to the presence of the 5-membered rings.39,40 Compound 1 can be sublimed intactly and converted to nonbenzenoid CLPs (3) by thermal activation on the metal surface (see ESI† for the precursor synthesis and characterization). The reaction route toward the formation of the CLP 3, illustrated in Scheme 1, follows two surface-assisted steps including dehalogenative homocoupling to yield an intermediate one-dimensional (1D) polymer (2) and cyclodehydrogenation to form 3. Interestingly, two different carbon–carbon (C–C) bonds between adjacent monomers can be formed in the cyclodehydrogenation step, resulting in six- or five-membered ring formation between monomers (3a and 3b, respectively). All the synthesis steps are investigated by scanning tunneling microscopy (STM) and complemented by density-functional theory (DFT).
The chemical structure of 2 and 3 are unambiguously identified by ultrahigh-resolution STM (UHR-STM) and their electronic properties studied by scanning tunneling spectroscopy (STS).
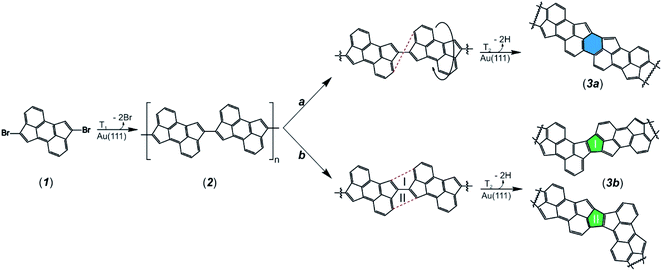
To study the on-surface reactions illustrated in Scheme 1, we sublimed precursor 1 onto an atomically clean Au(111) surface held at room temperature and subsequently annealed at 330 °C. High-resolution STM images (Fig. 1a and b) reveal the predominant presence of 1D polymers together with rounded protrusions between the chains, which we assign to bromine atoms detached from 1. To confirm the chemical structure of 2, ultrahigh-resolution STM (UHR-STM) images acquired with a CO-functionalized tip recorded in the Pauli repulsion regime41,42 were performed (Fig. 1c). Hereby, intramolecular features corresponding to the cyclopenta[hi]aceanthrylene molecular backbone linked via C–C bonds through the pentagons of each molecular subunit are distinguished (see Fig. S1† for a detailed STS and voltage dependent differential conductance spectra characterization of a segment of 2). Additionally, UHR-STM images allow us to discern single (s)-trans and -cis isomers within the same polymer chain (see Scheme S1† for the reaction pathway between adjacent s-trans and s-cis isomers). The DFT-optimized equilibrium geometry of 2 shown in Fig. 1d indicates that the polymer adopts a planar configuration, with an adsorption height of 3.3 Å with respect to the underlying gold surface.
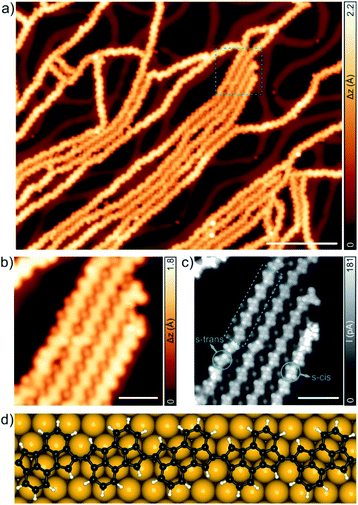
Annealing the sample at 400 °C induced minor variations in the molecular structure of 2 and only small segments of the 1D polymer (∼8%), attributed to the onset of the cyclodehydrogenation reaction, present a different appearance (see Fig. S2† for STM and UHR-STM images). Only after a further annealing step at 420 °C, the formation of the targeted CLP 3, coexisting with a few laterally fused segments, is achieved (Fig. 2a). A zoom-in STM image (Fig. 2b), allows us to discern two different C–C coupling motifs in the formation of 3. Herein, the kinked/linear segments observed in Fig. 2b are attributed to the formation of a hexagon/pentagon (3a/3b) between two molecular units (Fig. 2c and d). Importantly, statistics over the coupling motifs observed for more than 500 molecular units indicates that the coupling through the six-membered ring is favoured over the five-membered one (3a = 58%, 3b = 32%, non-cyclodehydrogenated = 10%). This can be rationalized on the basis of simple energetic considerations. On the one hand, gas phase calculations for 3a and 3b reveal that 3a is ≈1 eV more energetically favoured compared to 3b. This energy difference is further enhanced on the substrate and 3a (Fig. 2f) is ≈1.7 eV more stable compared to 3b on Au(111). Configurations of the polymer units that would lead to the formation of 3b motifs are hindered by the fact that the polymer should undergo a torsion (deviating from linearity) that is unlikely to occur for long strands. Therefore, cyclodehydrogenation between adjacent s-trans/s-cis and s-cis/s-trans isomers (synthetic pathway (a) in Scheme S1†) implies the formation of a new hexagon (3a), while cyclodehydrogenation between adjacent s-trans/s-trans and s-cis/s-cis isomers (synthetic pathway (b) in Scheme 1) affords the formation of a new pentagon (3b, with 3bI, 3bII ≈ 50%). In addition, it is expected that the reaction mechanisms in the formation of 3a and 3b involve a rotation of cyclopenta[hi]aceanthrylene molecular units on the surface substrate, at temperatures typical for the annealing, prior to cyclization (see synthetic pathway (a) in Scheme 1 and synthetic pathway (b) in Scheme S1†).32,43,44 Finally, Fig. 2e and f display the DFT equilibrium geometry of the formed pentagon (in green in Fig. 2e) and hexagon (in blue in Fig. 2f), respectively. Both adopt a planar configuration, with an adsorption height of 3.2 Å with respect to the underlying surface.
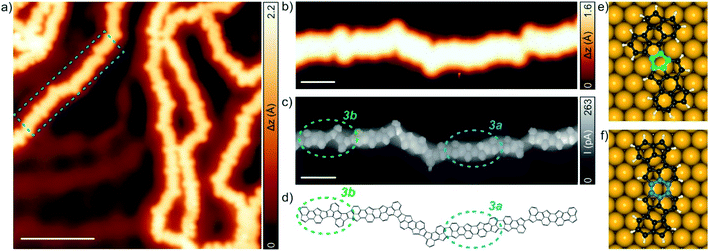
Next, we performed STS measurements on a 3a segment (six consecutive units), whose formation is favoured over 3b, to probe its electronic structure. Voltage-dependent differential conductance spectra (dI/dV vs. V) feature characteristic peaks at −1.12 eV and +0.65 eV (Fig. 3a), which are assigned to the valence band minimum (VBM) and the conduction band maximum (CBM), respectively, corresponding to an electronic bandgap of 1.77 eV on Au(111), matching very well with the calculated band structure for an infinite 3a segment (Fig. 3b). Constant-current maps of the dI/dV signal of a 3a acquired with a CO-functionalized tip at the VBM (top) and CBM (bottom) energetic positions are shown in Fig. 3c (see Fig. S3 and S4† for comparison with the constant-height dI/dV maps of 3a as well as dI/dV vs. V and constant-current dI/dV maps of the minority 3b segment, respectively). Finally, the experimental dI/dV maps are well reproduced by the DFT-calculated local density of states (LDOS) maps for the CBM and VBM (Fig. 3d), calculated at 3 Å above the molecular plane.
In conclusion, we have demonstrated a novel synthetic pathway toward the on-surface fabrication of nonbenzenoid π-conjugated ladder-type polymers on Au(111) by a combination of high-resolution STM and STS complemented with DFT calculations. A first annealing step at 330 °C induces debromination, leading to the formation of 1D polymers (2). A subsequent annealing step at 420 °C promotes the cyclodehydrogenation and the consequent formation of nonbenzenoid CLPs (3). Interestingly, two types of C–C coupling motifs, comprising the formation of a hexagon (3a) or a pentagon (3b) between cyclopenta[hi]aceanthrylene subunits, are observed. Such C–C couplings arises from the presence of s-trans and s-cis isomers at the polymer step, though the formation of a hexagon, which implies cyclodehydrogenation between adjacent s-trans/s-cis and s-cis/s-trans isomers, is favoured over the formation of the pentagon (cyclodehydrogenation between adjacent s-trans/s-trans and s-cis/s-cis isomers). We expect that the synthetic strategy presented in this work is of general relevance for the synthesis of novel conjugated ladder-type polymers, and will contribute to expand the knowledge in this field with notable potential for device applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Swiss National Science Foundation (200020_182015), the European Union's Horizon 2020 research and innovation programme (GrapheneCore2 785219), the Office of Naval Research (N00014-18-1-2708), and the Swiss National Centre for Computational Design and Discovery of Novel Materials (MARVEL). The Swiss National Supercomputing Centre (CSCS) under project ID s904 is acknowledged for computational resources. M.K. gratefully acknowledges the generous funding by the Deutsche Forschungsgemeinschaft (DFG) – Project number 182849149 – SFB 953 “Synthetic Carbon Allotropes”.Notes and references
- W. Metanomski, R. Bareiss, J. Kahovec, K. Loening, L. Shi and V. Shibaev, Pure Appl. Chem., 1993, 65, 1561–1580 CAS
.
- A.-D. Schlüter, Adv. Mater., 1991, 3, 282–291 CrossRef
.
- L. Yu, M. Chen and L. R. Dalton, Chem. Mater., 1990, 2, 649–659 CrossRef CAS
.
- A. C. Grimsdale and K. Müllen, Macromol. Rapid Commun., 2007, 28, 1676–1702 CrossRef CAS
.
- P. Bornoz, M. S. Prévot, X. Yu, N. Guijarro and K. Sivula, J. Am. Chem. Soc., 2015, 137, 15338–15341 CrossRef CAS PubMed
.
- W. Huang, H. Zhang, J. Ma, M. Chen, H. Zhu and W. Wang, J. Mater. Chem. C, 2015, 3, 6200–6208 RSC
.
- S. Che, J. Pang, A. J. Kalin, C. Wang, X. Ji, J. Lee, D. Cole, J.-L. Li, X. Tu, Q. Zhang, H.-C. Zhou and L. Fang, ACS Mater. Lett., 2020, 2, 49–54 CrossRef CAS
.
- J. Chen, K. Yang, X. Zhou and X. Guo, Chem.–Asian J., 2018, 13, 2587–2600 CrossRef CAS PubMed
.
- F. Trilling, M.-K. Ausländer and U. Scherf, Macromolecules, 2019, 52, 3115–3122 CrossRef CAS
.
- J. Lee, A. J. Kalin, C. Wang, J. T. Early, M. Al-Hashimi and L. Fang, Polym. Chem., 2018, 9, 1603–1609 RSC
.
- M. M. Durban, P. D. Kazarinoff, Y. Segawa and C. K. Luscombe, Macromolecules, 2011, 44, 4721–4728 CrossRef CAS
.
- M. D. Watson, A. Fechtenkötter and K. Müllen, Chem. Rev., 2001, 101, 1267–1300 CrossRef CAS PubMed
.
- M. A. Abdulhamid, H. W. H. Lai, Y. Wang, Z. Jin, Y. C. Teo, X. Ma, I. Pinnau and Y. Xia, Chem. Mater., 2019, 31, 1767–1774 CrossRef CAS
.
- T. Corrado and R. Guo, Mol. Syst. Des. Eng., 2020, 5, 22–48 RSC
.
- M. Morales-Vidal, P. G. Boj, J. M. Villalvilla, J. A. Quintana, Q. Yan, N.-T. Lin, X. Zhu, N. Ruangsupapichat, J. Casado, H. Tsuji, E. Nakamura and M. A. Díaz-García, Nat. Commun., 2015, 6, 8458 CrossRef CAS PubMed
.
- J. Lee, A. J. Kalin, T. Yuan, M. Al-Hashimi and L. Fang, Chem. Sci., 2017, 8, 2503–2521 RSC
.
- Y. C. Teo, H. W. H. Lai and Y. Xia, Chem.–Eur. J., 2017, 23, 14101–14112 CrossRef CAS PubMed
.
- B. de la Torre, A. Matěj, A. Sánchez-Grande, B. Cirera, B. Mallada, E. Rodríguez-Sánchez, J. Santos, J. I. Mendieta-Moreno, S. Edalatmanesh, K. Lauwaet, M. Otyepka, M. Medveď, Á. Buendía, R. Miranda, N. Martín, P. Jelínek and D. Écija, Nat. Commun., 2020, 11, 4567 CrossRef CAS PubMed
.
- K. Biswas, J. I. Urgel, A. Sánchez-Grande, S. Edalatmanesh, J. Santos, B. Cirera, P. Mutombo, K. Lauwaet, R. Miranda, P. Jelínek, N. Martín and D. Écija, Chem. Commun., 2020, 56, 15309–15312 RSC
.
- M. Di Giovannantonio, Q. Chen, J. I. Urgel, P. Ruffieux, C. A. Pignedoli, K. Müllen, A. Narita and R. Fasel, J. Am. Chem. Soc., 2020, 142, 12925–12929 CrossRef CAS PubMed
.
- Q. Shen, H.-Y. Gao and H. Fuchs, Nano Today, 2017, 13, 77–96 CrossRef CAS
.
- J. Cai, P. Ruffieux, R. Jaafar, M. Bieri, T. Braun, S. Blankenburg, M. Muoth, A. P. Seitsonen, M. Saleh, X. Feng, K. Müllen and R. Fasel, Nature, 2010, 466, 470–473 CrossRef CAS PubMed
.
- K. Kawasumi, Q. Zhang, Y. Segawa, L. T. Scott and K. Itami, Nat. Chem., 2013, 5, 739–744 CrossRef CAS PubMed
.
- S. Mishra, M. Krzeszewski, C. A. Pignedoli, P. Ruffieux, R. Fasel and D. T. Gryko, Nat. Commun., 2018, 9, 1714 CrossRef PubMed
.
- H. Terrones, M. Terrones, E. Hernández, N. Grobert, J.-C. Charlier and P. M. Ajayan, Phys. Rev. Lett., 2000, 84, 1716–1719 CrossRef CAS PubMed
.
- J. Lahiri, Y. Lin, P. Bozkurt, I. I. Oleynik and M. Batzill, Nat. Nanotechnol., 2010, 5, 326–329 CrossRef CAS PubMed
.
- S. Bravo, J. Correa, L. Chico and M. Pacheco, Sci. Rep., 2018, 8, 11070 CrossRef PubMed
.
- H. Xin and X. Gao, ChemPlusChem, 2017, 82, 945–956 CrossRef CAS PubMed
.
- R. Grantab, V. B. Shenoy and R. S. Ruoff, Science, 2010, 330, 946–948 CrossRef CAS PubMed
.
- Y. Wei, J. Wu, H. Yin, X. Shi, R. Yang and M. Dresselhaus, Nat. Mater., 2012, 11, 759–763 CrossRef CAS PubMed
.
- N. M. R. Peres, F. Guinea and A. H. Castro Neto, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 125411 CrossRef
.
- M. Di Giovannantonio, J. I. Urgel, U. Beser, A. V. Yakutovich, J. Wilhelm, C. A. Pignedoli, P. Ruffieux, A. Narita, K. Müllen and R. Fasel, J. Am. Chem. Soc., 2018, 140, 3532–3536 CrossRef CAS PubMed
.
- M. Di Giovannantonio, K. Eimre, A. V. Yakutovich, Q. Chen, S. Mishra, J. I. Urgel, C. A. Pignedoli, P. Ruffieux, K. Müllen, A. Narita and R. Fasel, J. Am. Chem. Soc., 2019, 141, 12346–12354 CrossRef CAS PubMed
.
- J. I. Urgel, M. D. Giovannantonio, K. Eimre, T. G. Lohr, J. Liu, S. Mishra, Q. Sun, A. Kinikar, R. Widmer, S. Stolz, M. Bommert, R. Berger, P. Ruffieux, C. A. Pignedoli, K. Müllen, X. Feng and R. Fasel, Angew. Chem., 2020, 132, 13383–13389 CrossRef
.
- J. I. Urgel, M. Di Giovannantonio, G. Gandus, Q. Chen, X. Liu, H. Hayashi, P. Ruffieux, S. Decurtins, A. Narita, D. Passerone, H. Yamada, S. Liu, K. Müllen, C. A. Pignedoli and R. Fasel, ChemPhysChem, 2019, 20, 2360–2366 CrossRef CAS PubMed
.
- L. Lafferentz, F. Ample, H. Yu, S. Hecht, C. Joachim and L. Grill, Science, 2009, 323, 1193–1197 CrossRef CAS PubMed
.
- C. Sánchez-Sánchez, T. Dienel, A. Nicolaï, N. Kharche, L. Liang, C. Daniels, V. Meunier, J. Liu, X. Feng, K. Müllen, J. R. Sánchez-Valencia, O. Gröning, P. Ruffieux and R. Fasel, Chem.–Eur. J., 2019, 25, 12074–12082 CrossRef PubMed
.
- I. C.-Y. Hou, Q. Sun, K. Eimre, M. Di Giovannantonio, J. I. Urgel, P. Ruffieux, A. Narita, R. Fasel and K. Müllen, J. Am. Chem. Soc., 2020, 142, 10291–10296 CrossRef CAS PubMed
.
- J. D. Wood, J. L. Jellison, A. D. Finke, L. Wang and K. N. Plunkett, J. Am. Chem. Soc., 2012, 134, 15783–15789 CrossRef CAS PubMed
.
- S. R. Bheemireddy, M. P. Hautzinger, T. Li, B. Lee and K. N. Plunkett, J. Am. Chem. Soc., 2017, 139, 5801–5807 CrossRef CAS PubMed
.
- C. Weiss, C. Wagner, C. Kleimann, M. Rohlfing, F. S. Tautz and R. Temirov, Phys. Rev. Lett., 2010, 105, 086103 CrossRef CAS PubMed
.
- G. Kichin, C. Weiss, C. Wagner, F. S. Tautz and R. Temirov, J. Am. Chem. Soc., 2011, 133, 16847–16851 CrossRef CAS PubMed
.
- Q. Sun, O. Gröning, J. Overbeck, O. Braun, M. L. Perrin, G. Borin Barin, M. El Abbassi, K. Eimre, E. Ditler, C. Daniels, V. Meunier, C. A. Pignedoli, M. Calame, R. Fasel and P. Ruffieux, Adv. Mater., 2020, 1906054 CrossRef CAS PubMed
.
- M. Ammon, T. Sander and S. Maier, J. Am. Chem. Soc., 2017, 139, 12976–12984 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra03253d |
| ‡ Present address: IMDEA Nanoscience, C/ Faraday 9, Campus de Cantoblanco, 28049 Madrid, Spain. |
| § Present address: Istituto di Struttura della Materia-CNR (ISM-CNR), via Fosso del Cavaliere 100, 00133 Roma, Italy. |
| This journal is © The Royal Society of Chemistry 2021 |