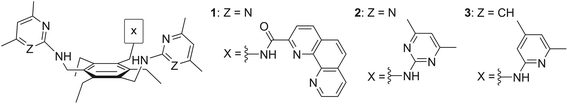
Open Access Article
Open Access ArticleBinding modes of methyl α-D-glucopyranoside to an artificial receptor in crystalline complexes†
Linda Köhler,
Conrad Hübler,
Wilhelm Seichter and
Monika Mazik *
*
Institut für Organische Chemie, Technische Universität Bergakademie Freiberg, Leipziger Strasse 29, 09599 Freiberg, Germany. E-mail: monika.mazik@chemie.tu-freiberg.de; Web: https://tu-freiberg.de/fakultaet2/orgch Fax: +49 3731393170; Tel: +49 3731392389
First published on 24th June 2021
Abstract
Compared to the numerous X-ray crystal structures of protein-carbohydrate complexes, the successful elucidation of the crystal structures of complexes between artificial receptors and carbohydrates has been very rarely reported in the literature. In this work, we describe the binding modes of two complexes formed between methyl α-D-glucopyranoside and an artificial receptor belonging to the class of compounds consisting of a 1,3,5-trisubstituted 2,4,6-trialkylbenzene scaffold. It is particularly noteworthy that these two complexes are present in one crystal structure, as was observed by us for the first time in the case of the recently reported three crystal structures of the complexes with methyl β-D-glucopyranoside, each containing two different receptor–carbohydrate complexes. The noncovalent interactions stabilizing the new complexes are compared with those observed in the aforementioned crystalline complexes with methyl β-D-glucopyranoside.
1 Introduction
Recently we have reported the crystal structures of complexes formed between methyl β-D-glucopyranoside (MeβGlc) and artificial receptors1 belonging to the class of compounds consisting of a 1,3,5-trisubstituted 2,4,6-trialkylbenzene scaffold2 (compounds 1–3, as given in Fig. 1 and 2), the representatives of which we have systematically examined for their ability to bind carbohydrates over the past few years.3–5 Especially noteworthy is that each of the described crystal structures is characterized by the presence of two different receptor–sugar complexes, as illustrated in Fig. 1. In contrast to these results, the presence of only one type of receptor–sugar complex could be observed in the crystal structures reported by us earlier.6 It should be emphasized that the crystal structures of complexes formed between artificial receptors and sugars have rarely been reported until now. Beside the above mentioned crystalline complexes of acyclic receptors,1,6 the crystal structures of foldamers7a–d and of a macrocyclic receptor8 with a bound sugar molecule are described in the literature and contribute to a better understanding of the basic molecular features of carbohydrate recognition (for recent examples of binding studies in solution, see ref. 9). In contrast, a large number of X-ray crystal structures of proteins bound to various sugar substrates has been described in the literature10,11 and represents an important source of information about the noncovalent interactions that contribute to the selective and effective binding of carbohydrates by proteins.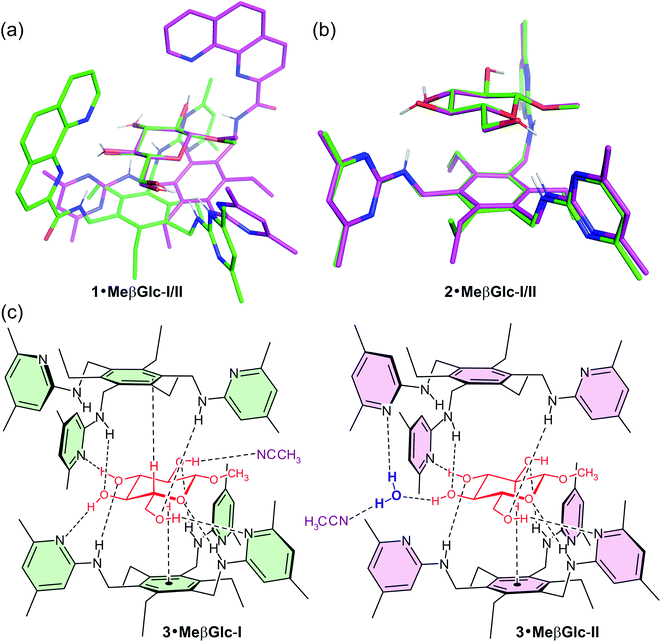 | ||
Fig. 1 A view of the superposition of complex I (green lines) and complex II (pink lines) observed in the crystal structure 1·MeβGlc (a) and in the crystal structure 2·MeβGlc (b) fitted on the carbohydrate atoms C1–C5 and O5 (N atoms are colored blue and O atoms red; all H atoms, which are not involved in hydrogen bonds or C–H⋯π interactions are omitted for clarity). (c) Schematic view of the crystalline 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor–sugar complexes 3·MeβGlc-I (green) and 3·MeβGlc-II (pink).1 For structures of compounds 1–3, see Fig. 2. 1 receptor–sugar complexes 3·MeβGlc-I (green) and 3·MeβGlc-II (pink).1 For structures of compounds 1–3, see Fig. 2. | ||
The above mentioned complexes of the artificial systems contain such bound carbohydrates as methyl1,6 and octyl β-D-glucosides,6 β-D-/β-L-glucopyranose,8 β-D-/α-L-mannopyranose, β-D-/β-L-fructopyranose, α-D-/α-L-xylopyranose,7a,c,d and α-1,4-xylobiose.7b To the best of our knowledge, no crystalline complexes with α-D-glucopyranosides have been reported in the literature so far.
In this paper we describe the binding modes of complexes formed between methyl α-D-glucopyranoside (MeαGlc) and the triethylbenzene derivative 2 bearing three aminopyrimidine-based recognition units. It is particularly remarkable that again two different complexes are present in the crystal structure 2·MeαGlc (assigned as 2·MeαGlc-I and 2·MeαGlc-II), as previously observed by us for the crystal structures of the receptor–carbohydrate complexes containing methyl β-D-glucopyranoside.1
In addition to the detailed analysis of the noncovalent interactions stabilizing the new complexes, their comparison with those observed in the aforementioned crystalline complexes with methyl β-D-glucopyranoside1 is also the subject of this work.
2 Results and discussion
2.1 Crystal structure 2·MeαGlc: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1 receptor–sugar complexes 2·MeαGlc-I and 2·MeαGlc-II
1 receptor–sugar complexes 2·MeαGlc-I and 2·MeαGlc-II
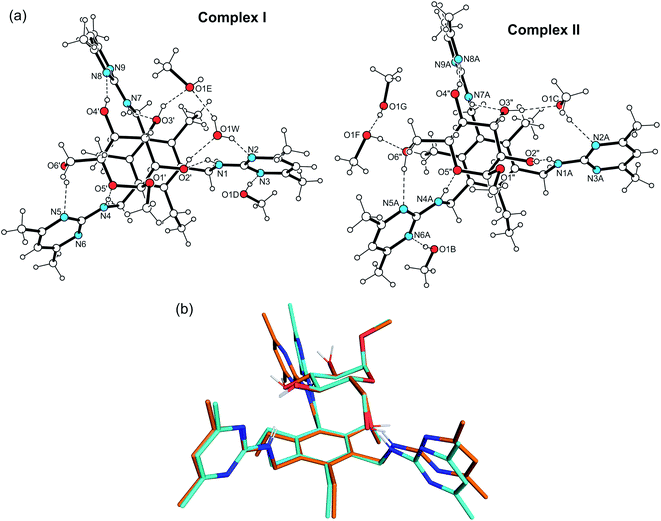
The crystal structure 2·MeαGlc was solved in the space group P1 with the asymmetric unit containing two receptor molecules, two molecules of the carbohydrate, one water and six methanol molecules. These components are connected to two 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor–sugar complexes, designated as complex I and II, as shown in Fig. 3. Under the chosen experimental conditions the methanol molecules marked as F and G (see Fig. S1†) display large displacement parameters, so that their anisotropic refinement was dispensed with.
1 receptor–sugar complexes, designated as complex I and II, as shown in Fig. 3. Under the chosen experimental conditions the methanol molecules marked as F and G (see Fig. S1†) display large displacement parameters, so that their anisotropic refinement was dispensed with.
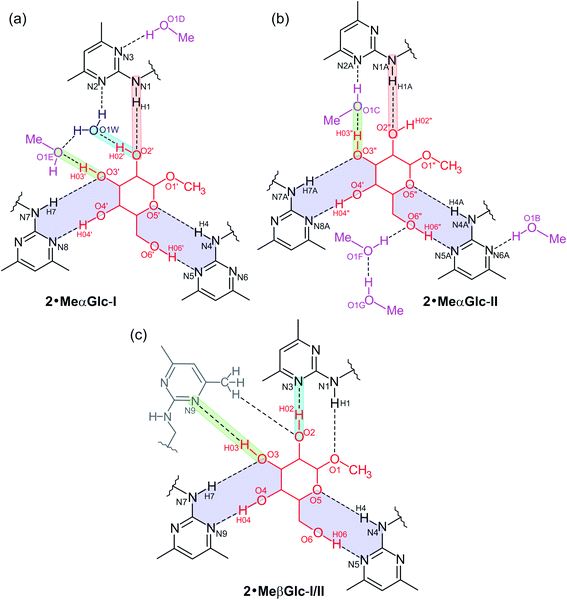
The two complexes display structural similarities which become particularly evident when looking at their superposition viewed in Fig. 3b. In both complexes the substituents of the receptor molecule are arranged in an alternating order above and below the plane of the benzene ring [ab′ab′ab′ arrangement, a = above, b = below (a′/b′ = Et above/below)12]. The sugar molecule is located in the receptor cavity created by the three functionalized side-arms. The space-filling model of complex I showing the spatial fit of the sugar component towards the receptor molecule is presented in Fig. S2.†
The functionalized side-arms of the receptor molecules exist in an elongated conformation with torsion angles of 160.6–179.9° for the atomic sequences Cbenzene–C–N–Cpyrimidine. The inclination angles of the heterocyclic units with reference to the benzene plane are 79.8, 72.1 and 87.5° for complex I, whereas for complex II they amount to 74.0, 88.1 and 76.7° (for further details, see Table S2†).
The structural differences between the complexes are essentially due to the coordination behavior of the solvent molecules, which result in different connection patterns between the complex components. These are shown schematically in Fig. 4.
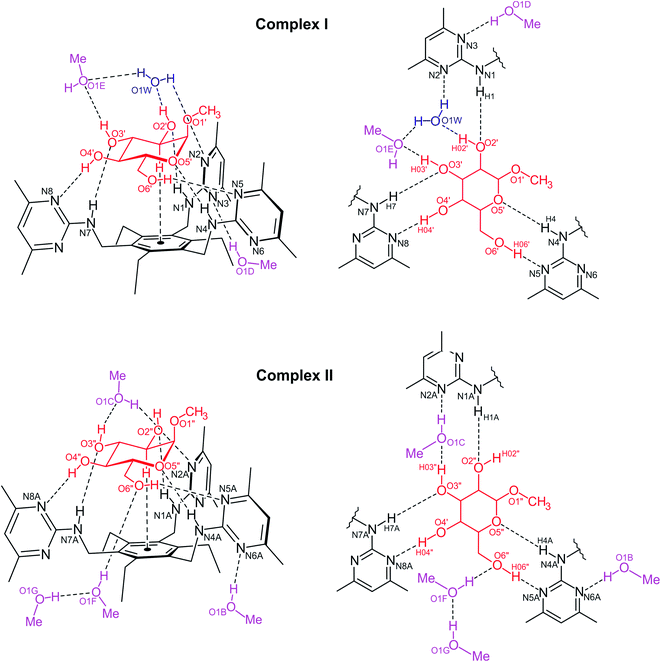
In each of the complexes, the 3-, 4- and 6-OH groups as well as the ring O atom contribute to the formation of bidentate hydrogen bonds13a with two pyrimidin-2-yl-amino units thus forming two cyclic synthons of the graph set14 R22(9). These bidentate hydrogen bonds include the interactions 4-OH⋯Npyrimidine/N–H⋯OH-3 and 6-OH⋯Npyrimidine/N–H⋯Oring.
In complex I (2·MeαGlc-I; see Fig. 4), the water molecule and one of the alcohol molecules (E) prevent the 3-OH group of the glucopyranoside to act as hydrogen bond donor to the receptor. Instead, two fused synthons of structures R33(8) and R23(9) emerge from the connection of these guest (solvent) molecules with the 2-OH and 3-OH groups of the sugar molecule and the remaining pyrimidin-2-yl-amino moiety of the receptor. The atom N(3) of this heterocyclic unit acts as an acceptor for an O–H⋯N bond with the second methanol molecule (D). The hydrogen bond lengths in complex I are 1.96–2.09 Å for O–H⋯N, 1.94–2.65 Å for N–H⋯O, and 1.84–1.92 Å for O–H⋯O interactions (see Tables 1 and S3†).
| 2·MeαGlc-I | 2·MeαGlc-II | ||||||
|---|---|---|---|---|---|---|---|
| XH⋯Y interactions Complex I | XH⋯Y (Å) | X⋯Y (Å) | XH⋯Y angle (deg) | XH⋯Y interactions Complex II | XH⋯Y (Å) | X⋯Y (Å) | XH⋯Y angle (deg) |
| a O(1W): water oxygen atom. b O(1C), O(1E): methanol oxygen atoms. c N(5), N(8): pyrimidine nitrogen atoms. d Centroid (centre of gravity) of the central benzene ring. e N(5A), N(8A): pyrimidine nitrogen atoms. | |||||||
| NH⋯OH-2 | 1.94 | 2.82 | 172 | NH⋯OH-2 | 2.06 | 2.91 | 158 |
| NH⋯OH-3 | 1.99 | 2.88 | 173 | NH⋯O5 | 2.53 | 3.34 | 152 |
| NH⋯O5 | 2.65 | 3.45 | 151 | NH⋯OH-3 | 2.05 | 2.93 | 169 |
| 2-OH⋯O(1W)a | 1.92 | 2.73 | 165 | 3-OH⋯O(1C)b | 1.96 | 2.77 | 158 |
| 3-OH⋯O(1E)b | 1.84 | 2.64 | 158 | 4-OH⋯N(8A)e | 2.14 | 2.97 | 173 |
| 4-OH⋯N(8)c | 2.09 | 2.92 | 166 | 6-OH⋯N(5A)e | 1.99 | 2.79 | 162 |
| 6-OH⋯N(5)c | 1.96 | 2.79 | 168 | 2-CH⋯πd | 2.75 | 3.70 | 158 |
| 2-CH⋯πd | 2.74 | 3.66 | 154 | ||||
In contrast to complex I, the complex II (2·MeαGlc-II) does not contain a bound water molecule, but four methanol molecules. While three of the alcohol molecules are located in the peripheral region of the complex and therefore do not significantly affect the binding mode between the sugar and receptor, the remaining solvent molecule prevents the direct connection of the 3-OH group of MeαGlc with the pyrimidine atom N(2A) of the receptor thus forming a 11-membered synthon of the graph set R33(11).
A common structural feature of the binding mode in both complexes is the presence of a C–H⋯π contact15 between the sugar 2-CH and the central benzene ring of the receptor molecule with distances of 2.74 Å (complex I) and 2.75 Å (complex II). As a result of the axial arrangement of the methoxy group of MeαGlc, the oxygen atom of this group is excluded from the interactions with the receptor molecule.
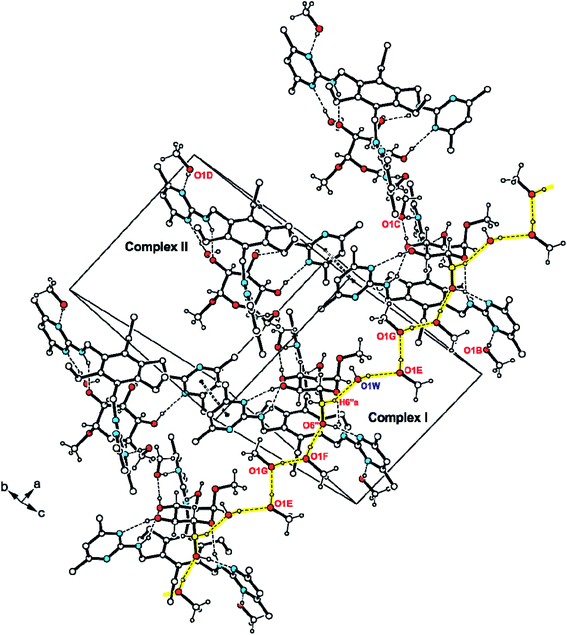
The excerpt of 2·MeαGlc displayed in Fig. 5 reveals a strand-like linkage of sugar, alcohol and water molecules that creates an infinite pattern of O–H⋯O bonds in direction of the crystallographic a-axis. The hydroxymethyl group of the sugar molecule of complex II as well as the methanol molecules E, F and G contribute in the formation of this supramolecular strand-like structure. Only one hydrogen atom of each water molecule is included in this hydrogen bond pattern. The crystallographically independent receptor molecules interact via offset π⋯π arene interactions16 with a centroid⋯centroid (Cg⋯Cg) distance of 3.53 Å and a slippage of 0.56 Å between the pyrimidine rings involved in this interaction. Moreover, C–H⋯O interactions17 [d(H⋯O) 2.47–2.50 Å] and C–H⋯π contacts [d(H⋯Cg) 2.80–2.95 Å; see Table S3†] connect the complexes to a three-dimensional supramolecular network.
It should also be noted that as in the case of the previously investigated crystalline complexes of the triethylbenzene-based receptors with β-D-glucosides,1,6 the binding motifs observed in the complexes with methyl α-D-glucoside show a similarity to those observed in protein–carbohydrate complexes. To mention is, for example, the use of bidentate hydrogen bonds (Fig. 6c), the presence of water-mediated (methanol-mediated) hydrogen bonds (Fig. 6a and b) and the additional stabilization of the complex by CH⋯π interactions, as shown for the 2-CH of α-D-glucose (natural complex) and methyl α-D-glucoside (2·MeαGlc-I/II) in Fig. 6d. In the formation of the aforementioned bidentate hydrogen bonds in the complexes 2·MeαGlc-I/-II are not only involved two adjacent hydroxy groups (4-OH⋯Npyrimidine/N–H⋯OH-3), but also the ring oxygen and the 6-OH of the methyl α-D-glucoside (6-OH⋯Npyrimidine/N–H⋯Oring). A similar situation is observed in natural complexes, and is exemplarily illustrated in Fig. 6c for the case where ring oxygen and the 6-OH of D-glucose are involved in the formation of bidentate hydrogen bonds (6-OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/N–H⋯Oring).
C/N–H⋯Oring).
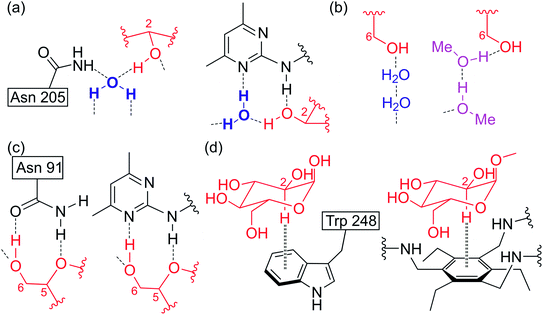 | ||
| Fig. 6 Examples of hydrogen bonds (a–c) and C–H⋯π interactions (d) in the complexes formed by carbohydrate-binding proteins (left) and by the artificial receptor (right) described in this work (complexes 2·MeαGlc-I and 2·MeαGlc-II). In the case of the protein–carbohydrate complexes, the binding motifs observed in the complexes of D-galactose-binding protein with D-glucose (a, c)10e and α-glucose-binding protein with α-D-glucose (b, d)10h are illustrated. | ||
The importance of the above mentioned hydrogen bonds and CH⋯π interactions has also been demonstrated by binding studies in solution using both anomers of the glucopyranoside, in which, however, the compound 2 was shown to be a more powerful receptor for β-D-glucopyranoside than for the α-anomer.4d
2.2 Comparison of the crystal structures 2·MeαGlc and 2·MeβGlc (complexes 2·MeαGlc-I/2·MeαGlc-II and 2·MeβGlc-I/2·MeβGlc-II)
The pronounced donor/acceptor properties of the solvent species present in the crystal structure 2·MeαGlc only permit a limited comparison with the recently reported solvent-free structure of the same receptor with β-glucopyranoside,1 since in the latter case the binding behavior of the carbohydrate towards the receptor is unaffected by a strongly coordinating solvent. Therefore, the almost identical 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor–sugar complexes (2·MeβGlc-I and 2·MeβGlc-II, see Fig. 1b) of this crystal structure represent an ideal case in which the recognition process between the complex components proceeds undisturbed resulting in formation of an approximately symmetrical pattern of hydrogen bonds in which all OH groups and the ring O atom of the glucopyranoside are included. This gives rise to formation of three 9-membered cyclic hydrogen bond motifs of identical structures (Fig. 7).
1 receptor–sugar complexes (2·MeβGlc-I and 2·MeβGlc-II, see Fig. 1b) of this crystal structure represent an ideal case in which the recognition process between the complex components proceeds undisturbed resulting in formation of an approximately symmetrical pattern of hydrogen bonds in which all OH groups and the ring O atom of the glucopyranoside are included. This gives rise to formation of three 9-membered cyclic hydrogen bond motifs of identical structures (Fig. 7).
The crystal structure 2·MeαGlc also consists of two complexes in which, however, different numbers of solvent molecules are associated with the receptor–sugar unit. Analogous to the solvent-free structure 2·MeβGlc, the 4-OH, 5-OH and 6-OH groups as well as the ring O atom of the sugar are involved in the interactions with two aminopyrimidine-based recognition units of the receptor. Thus the difference is restricted to the binding behavior of the sugar molecule with the third recognition unit of the respective receptor molecule, which is disrupted by solvent molecules. The water and one of the alcohol molecules present in complex I prevent the direct linkage of the 3-OH group of the sugar molecule with a pyrimidine N atom of this recognition unit by creating a complex H-bond pattern consisting of two ring synthons. Similarly, one of the methanol molecules present in complex II affects the binding behavior between receptor and sugar by inserting its OH group in the binding pattern thus creating an 11-membered supramolecular synthon.
The solvent molecules mediate hydrogen bonds between the sugar and the receptor molecule in a similar way, as it is realised by the water-mediated hydrogen bonds in protein-carbohydrate complexes.
The structural difference between the α- and β-anomer of the sugar molecule is limited to the spatial arrangement of the methoxy group at the anomeric center of the ring. The equatorial position of this group in the β-anomer enables the formation of a strong hydrogen bond between its O-atom and an amino H-atom of the receptor molecule and thus has a stabilizing influence on the complex formation. This applies to both complexes of the crystal structure 2·MeβGlc. The axial arrangement of the methoxy group in the α-anomer of the glucopyranoside prevents this interaction with the receptor. Instead, in both complexes of the crystal structure 2·MeαGlc, the spatial environment of the methoxy group is determined by solvent molecules. Moreover, the presence of solvent molecules prevents unfavorable steric interactions within the complexes, so that the six-membered ring of each sugar molecule adopts an almost ideal 4C1 conformation.
3 Conclusion
Compared to numerous X-ray crystal structures of protein-sugar complexes, the crystalline complexes of artificial receptors have rarely been published so far, therefore the new complexes with methyl α-D-glucopyranoside described here make an important contribution to this field of research and to the understanding of the basic principles of the molecular recognition of carbohydrates. It should be emphasized that the presence of two different complexes in one crystal structure represents an especially interesting result and, to the best of our knowledge, has been observed for the first time for artificial carbohydrate receptors in the crystal structures of the complexes with methyl β-D-glucopyranoside we have recently reported.18The detailed analysis of the non-covalent interactions in the receptor–glucopyranoside complexes gives a deeper insight into the process of molecular recognition of carbohydrates and allows a comparison with the results obtained in solution. The crystallographic investigations confirmed the binding strategy, which was predicted from the receptor design and indicated by the binding studies in solution.
4 Experimental section
4.1 Synthesis of 1,3,5-tris[(4,6-dimethylpyrimidin-2-yl)aminomethyl]-2,4,6-triethylbenzene (2)
Compound 2 was prepared by the reaction of 1,3,5-tris(bromomethyl)-2,4,6-triethylbenzene with 2-amino-4,6-dimethylpyrimidine, as reported by us previously4d (see also ESI†).4.2 Crystallographic investigations
The crystals were grown by isothermal evaporation of the solvent from a solution of the receptor in the presence of glucopyranoside. The sugar/receptor stoichiometry was varied between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and as solvent was used a mixture of water-containing methanol and acetonitrile.
10 and as solvent was used a mixture of water-containing methanol and acetonitrile.
Crystal data for 2·MeαGlc were recorded at 113 K on a STOE diffractometer (MoKα radiation, λ = 0.71073 Å) equipped with an image plate detector (IPDS-2T). The software used for data collection and cell refinement was X-AREA.19 Absorption correction was carried out using X-AREA.19 Preliminary structural models were created using direct methods.20 The structures were refined using a full-matrix least-squares calculation based on F2 values for all reflexes.21 With the exception of two of the methanol molecules, all other non-hydrogen atoms were refined anisotropically. The positions of the OH hydrogen atoms of the carbohydrate molecules were identified in the difference Fourier maps. All other hydrogen atoms were included in the model in calculated positions and were refined as constrained to bonding atoms.
Crystallographic data for the structures in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication numbers CCDC 2072720.†
Conflicts of interest
There are no conflicts to declare.References
- L. Köhler, W. Seichter and M. Mazik, Eur. J. Org. Chem., 2020, 7023–7034 CrossRef.
- For a review on 1,3,5-substituted 2,4,6-triethylbenzene derivatives, see: G. Hennrich and E. V. Anslyn, Chem.–Eur. J., 2002, 8, 2219–2224 Search PubMed.
- (a) M. Mazik, Chem. Soc. Rev., 2009, 38, 935–956 RSC; (b) M. Mazik, RSC Adv., 2012, 2, 2630–2642 RSC; for first examples reported by our group, see: (c) M. Mazik, W. Radunz and W. Sicking, Org. Lett., 2002, 4, 4579–4582 CrossRef CAS PubMed; (d) M. Mazik, W. Radunz and R. Boese, J. Org. Chem., 2004, 69, 7448–7462 CrossRef CAS PubMed.
- For recent examples reported by our group, see: (a) M. Stapf, W. Seichter and M. Mazik, Eur. J. Org. Chem., 2020, 4900–4915 CrossRef CAS; (b) S. Kaiser, C. Geffert and M. Mazik, Eur. J. Org. Chem., 2019, 7555–7562 CrossRef CAS; (c) N. Koch, W. Seichter and M. Mazik, Synthesis, 2016, 48, 2757–2767 CrossRef CAS; (d) J. Lippe, W. Seichter and M. Mazik, Org. Biomol. Chem., 2015, 13, 11622–11632 RSC.
- For triethylbenzene-based macrocyclic systems from our laboratory, see: (a) J. Lippe and M. Mazik, J. Org. Chem., 2013, 78, 9013–9020 CrossRef CAS PubMed; (b) J. Lippe and M. Mazik, J. Org. Chem., 2015, 80, 1427–1439 CrossRef CAS PubMed; (c) F. Amrhein, J. Lippe and M. Mazik, Org. Biomol. Chem., 2016, 14, 10648–10659 RSC.
- M. Mazik, H. Cavga and P. G. Jones, J. Am. Chem. Soc., 2005, 127, 9045–9052 CrossRef CAS PubMed.
- (a) P. Mateus, N. Chandramouli, C. D. Mackereth, B. Kauffmann, Y. Ferrand and I. Huc, Angew. Chem., Int. Ed., 2020, 59, 5797–5805 (Angew. Chem., 2020, 132, 5846–5854) CrossRef CAS PubMed; (b) S. Saha, B. Kauffmann, Y. Ferrand and I. Huc, Angew. Chem., Int. Ed., 2018, 57, 13542–13546 CrossRef CAS PubMed; (c) P. Mateus, B. Wicher, Y. Ferrand and I. Huc, Chem. Commun., 2018, 54, 5078–5081 RSC; (d) N. Chandramouli, Y. Ferrand, G. Lautrette, B. Kauffmann, C. D. Mackereth, M. Laguerre, D. Dubreuil and I. Huc, Nat. Chem., 2015, 7, 334–341 CrossRef CAS PubMed.
- P. K. Mandal, B. Kauffmann, H. Destecroix, Y. Ferrand, A. P. Davis and I. Huc, Chem. Commun., 2016, 52, 9355–9358 RSC.
- For recent examples of binding studies with artificial carbohydrate receptors operating via noncovalent interactions, see ref. 4 and: (a) M. F. Bravo, K. Palanichamy, M. A. Shlain, F. Schiro, Y. Naeem, M. Marianski and A. B. Braunschweig, Chem.–Eur. J., 2020, 26, 11782–11795 CrossRef CAS PubMed; (b) M. F. Bravo, M. A. Lema, M. Marianski and A. B. Braunschweig, Biochemistry, 2021, 60, 999–1018 CrossRef CAS PubMed; (c) K. Palanichamy, M. F. Bravo, M. A. Shlain, F. Schiro, Y. Naeem, M. Marianski and A. B. Braunschweig, Chem.–Eur. J., 2018, 24, 13971–13982 CrossRef CAS; (d) R. A. Tromans, T. S. Carter, L. Chabanne, M. P. Crump, H. Li, J. V. Matlock, M. G. Orchard and A. P. Davis, Nat. Chem., 2019, 11, 52–56 CrossRef CAS PubMed; (e) P. Stewart, C. M. Renney, T. J. Mooibroek, S. Ferheen and A. P. Davis, Chem. Commun., 2018, 54, 8649–8652 RSC; (f) P. Rios, T. J. Mooibroek, T. S. Carter, C. Williams, M. R. Wilson, M. P. Crump and A. P. Davis, Chem. Sci., 2017, 8, 4056–4061 RSC; (g) O. Francesconi, M. Martinucci, L. Badii, C. Nativi and S. Roelens, Chem.–Eur. J., 2018, 24, 6828–6836 CrossRef CAS PubMed; (h) Y. Ohishi, K. Masuda, K. Kudo, H. Abe and M. Inouye, Chem.–Eur. J., 2021, 27, 785–793 CrossRef CAS PubMed.
- (a) H. Lis and N. Sharon, Lectins, Kluwer Academic Publishers, Dordrecht, The Netherlands, 2003 Search PubMed; (b) H. Lis and N. Sharon, Chem. Rev., 1998, 98, 637–674 CrossRef CAS PubMed; (c) N. Sharon, Trends Biochem. Sci., 1993, 18, 221–226 CrossRef CAS PubMed; (d) W. I. Weiss and K. Drickamer, Annu. Rev. Biochem., 1996, 65, 441–473 CrossRef PubMed; (e) F. A. Quiocho, Pure Appl. Chem., 1989, 61, 1293–1306 CAS; (f) A. Quiocho and D. K. Wilson, Nature, 1989, 340, 404–407 CrossRef; (g) R. U. Lemieux, Acc. Chem. Res., 1996, 29, 373–380 CrossRef CAS; (h) M. Chandravanshi, P. Gogoi and S. P. Kanaujia, FEBS J., 2020, 287, 1576–1597 CrossRef CAS PubMed.
- (a) H. J. Gabius, The Sugar Code – Fundamentals of Glycoscience, Wiley-Blackwell, 2009 Search PubMed; (b) H.-J. Gabius, S. André, J. Jiménez-Barbero, A. Romero and D. Solis, Trends Biochem. Sci., 2011, 36, 298–313 CrossRef CAS PubMed.
- For a discussion on the conformations of triethylbenzene-based compounds, see: (a) N. Koch, W. Seichter and M. Mazik, CrystEngComm, 2017, 19, 3817–3833 RSC; (b) M. Schulze, A. Schwarzer and M. Mazik, CrystEngComm, 2017, 19, 4003–4016 RSC.
- (a) For a description of bidentate hydrogen bonds in protein-carbohydrate complexes, see ref. 10d–f; ; (b) For a description of cooperative hydrogen bonds, see ref. 10e and 10f..
- (a) J. Grell, J. Bernstein, G. Tinhofer, Acta Cryst. B: Struct. Sci., 2000, 56, 400166 (Erratum); Search PubMed; Acta Cryst. B: Struct. Sci., 1999, 55, 1030–1043 Search PubMed; (b) M. C. Etter, J. C. MacDonald and J. Bernstein, Acta Cryst. B: Struct. Sci., 1990, 46, 256–262 CrossRef PubMed; (c) M. C. Etter, J. Phys. Chem., 1991, 95, 4601–4610 CrossRef CAS; (d) J. Bernstein, R. E. Davis, L. Shimoni and N.-L. Chang, Angew. Chem., Int. Ed., 1995, 34, 1555–1573 CrossRef CAS.
- For discussions on the importance of carbohydrate-arene interactions, see: (a) J. Houser, S. Kozmon, D. Mishra, Z. Hammerová, M. Wimmerová and J. Koća, Chem.–Eur. J., 2020, 26, 10769–10780 CrossRef CAS PubMed; (b) J. L. Asensio, A. Ardá, F. J. Caňada and J. Jiménez-Barbero, Acc. Chem. Res., 2013, 46, 946–954 CrossRef CAS PubMed; (c) S. Tsuzuki, T. Uchimaru and M. Mikami, J. Phys. Chem. B, 2009, 113, 5617–5621 CrossRef CAS PubMed; (d) G. Terraneo, D. Potenza, A. Canales, J. Jiménez-Barbero, K. K. Baldridge and A. Bernardi, J. Am. Chem. Soc., 2007, 129, 2890–2900 CrossRef CAS PubMed; (e) M. I. Chávez, C. Andreu, P. Vidal, N. Aboitiz, F. Freire, P. Groves, J. L. Asensio, G. Asensio, M. Muraki, F. J. Caňada and J. Jiménez-Barbero, Chem.–Eur. J., 2005, 11, 7060–7074 CrossRef PubMed; (f) J. Screen, E. C. Stanca-Kaposta, D. P. Gamblin, B. Liu, N. A. Macleod, L. C. Snoek, B. G. Davis and J. P. Simons, Angew. Chem., Int. Ed., 2007, 46, 3644–3648 CrossRef CAS PubMed; (g) S. H. Kiehna, Z. R. Laughrey and M. L. Waters, Chem. Commun., 2007, 4026–4028 RSC.
- (a) S. L. James, in Encyclopedia of Supramolecular Chemistry, ed. J. L. Atwood and J. W. Steed, CRC Press, Boca Raton, 2004, pp. 1093–1099 Search PubMed; (b) C. R. Martinez and B. L. Iverson, Chem. Sci., 2012, 3, 2191–2201 RSC.
- (a) G. R. Desiraju and T. Steiner, The Weak Hydrogen Bond in Structural Chemistry and Biology, Oxford University Press, New York, 1999 Search PubMed; (b) G. R. Desiraju, Chem. Commun., 2005, 2995–3001 RSC; for examples of discussions on the role of CH⋯O hydrogen bonds in the molecular recognition of various substrates by proteins, see: (c) S. Sarkhel and G. R. Desiraju, Proteins, 2004, 54, 247 CrossRef CAS PubMed; (d) A. C. Pierce, K. L. Sandretto and G. W. Bemis, Proteins, 2002, 49, 567 CrossRef CAS PubMed.
- Meant are different complexes containing the same carbohydrate substrate in the receptor cavity. In the case of the macrocycle described in ref. 8, β-D- and β-L-glucopyranose are involved in complex formation.
- Stoe & Cie, X-RED and X-AREA, Stoe & Cie, Darmstadt, Germany, 2009 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: ORTEP-plot of the crystal structure 2·MeαGlc (Fig. S1). Space filling representation of complex I in the crystal structure 2·MeαGlc (Fig. S2). Views of the superimposed complex structures of 2·MeαGlc-I and 2·MeαGlc-II with 2·MeβGlc-I fitted on the carbohydrate atoms C1–C5 and O5 (Fig. S3). Crystallographic and structure refinement data of 2·MeαGlc (Table S1). Selected geometric parameters of 2·MeαGlc (Table S2). Geometrical parameters of hydrogen bonds and arene interactions in the crystal structure 2·MeαGlc (Table S3). Synthesis of compound 2. CCDC 2072720. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra03390e |
| This journal is © The Royal Society of Chemistry 2021 |