Open Access Article
Open Access ArticlePreparation of Mn2O3/MIL-100(Fe) composite and its mechanism for enhancing the photocatalytic removal of rhodamine B in water†
Nguyen Trung Dung*a,
Tran Thi Huea,
Vu Dinh Thaoa and
Nguyen Nhat Huy *bc
*bc
aFaculty of Physical and Chemical Engineering, Le Quy Don Technical University, 236 Hoang Quoc Viet St., Bac Tu Liem District, Hanoi, Vietnam. E-mail: nguyentrungdung1980@gmail.com
bFaculty of Environment and Natural Resources, Ho Chi Minh City University of Technology (HCMUT), 268 Ly Thuong Kiet Street, District 10, Ho Chi Minh City, Vietnam. E-mail: nnhuy@hcmut.edu.vn
cVietnam National University Ho Chi Minh City, Linh Trung Ward, Thu Duc City, Ho Chi Minh City, Vietnam
First published on 24th August 2021
Abstract
In this study, Mn2O3/MIL-100(Fe) composite was successfully synthesized by the hydrothermal method and applied for photocatalytic removal of rhodamine B (RhB) in water. The physical and chemical properties of the synthesized materials were characterized by XRD, FTIR, SEM, UV-visible, and BET analyses. Experimental results showed a great enhancement in the photocatalytic ability of the Mn2O3/MIL-100(Fe) composite as compared to individual Mn2O3 or MIL-100(Fe) under visible light and persulfate activation. The affecting factors such as pH, photocatalyst dose, RhB concentration, and Na2S2O8 concentration were investigated to find out the best conditions for efficient photocatalysis. By conducting a radical quenching test, all radicals of HO˙, SO4˙−, 1O2, and O2˙− were found to be important in photocatalytic decomposition. The mechanism was proposed for the enhancement of photocatalytic RhB removal via band potential calculation, charge separation, surface redox reaction, and key reactive oxidation species. With its durability, reusability, and high efficiency, the Mn2O3/MIL-100(Fe) composite emerges as a potential photocatalyst working under visible light for application in wastewater treatment.
Introduction
The textile and dyeing industry is one of the main contributors to the economies of developing countries such as Vietnam. Dyes have become widely produced and used not only in the textile industry but also in the food industry and in biotechnology. Rhodamine B (RhB) is an organic dye that is representative of the group of xanthene dyes. It is widely used for coloring in the fiber industry, laboratory staining, and cytological testing because of its stable properties, fast coloring, and low cost as compared to other industrial color products. Therefore, the amount of wastewater from the textile industry in general, and RhB in particular, discharging into the water environment is very large, which brings many risks to ecosystems and human health.1,2 Wastewater containing dyes is difficult to treat due to the persistent and complex structure of the dyes, which resist conventional biological treatment such as aerobic and anaerobic processes. As alternatives to biological treatment, physicochemical processes for the treatment of RhB have been studied extensively, including adsorption,3,4 photocatalysis,5–10 electrochemical-Fenton,11 biological treatment, and filtration.12 Among them, advanced oxidation processes, particularly photocatalysis, are usually considered as some of the most suitable techniques for the treatment of RhB and other dyes due to their being highly efficient, low-cost, and environment-friendly techniques. Persulfate-based photocatalysis has attracted much research attention since sulfate radical (SO4˙−) has advantages over hydroxyl radical in the degradation of organics in water such as faster reaction rate (106–109 M−1 s−1), higher oxidation potential (2.5–3.1 V), longer lifetime (30–40 μs as compared to 1 μs of HO˙), broader pH range, wider application, and higher degradation efficiency.13,14 Sulfate radical can be generated by activation of persulfate via traditional methods such as using UV, heat, ultrasonication, transition metals, and photocatalysts.15,16 Among them, the activation of persulfate by photocatalysis is usually considered as a green and promising route due to its low energy consumption, high recyclability, and lower photoexcited electron–hole recombination.In recent years, manganese oxides have attracted more attention due to their practical application in many fields of study such as photocatalysis, fluorescence, gas sensors, electrochemical films, and lithium batteries. Among the manganese oxides, Mn2O3 is the most widely used photocatalyst under visible light, which exists in different morphologies17–19 and can be prepared by different methods such as hydrothermal and precipitation.19–21 Mn2O3 nanomaterials were synthesized and applied as photocatalysts for environment treatment such as degradation of ciprofloxacin,21,22 decompositions of ammonium and nitrate to N2,23 reduction of CO2,24 and elimination of heavy metals such as As(III) and As(VI).25 MIL-100(Fe), a type of metal–organic framework, has received lots of attention due to its hollow cage structure with a large specific surface area and high pore volume. Among preparation methods, the hydrothermal method is the preferred choice because of its simplicity, ease of implementation, and high efficiency.26–31 In the environmental field, MIL-100(Fe) is also capable of removing many pollutants, such as heavy metals (Cr6+),29,31 RhB,32,33 tetracycline antibiotic,31 and microcystin-LR.34 Although both Mn2O3 and MIL-100(Fe) can be used as photocatalysts under visible light due to their low bandgaps (i.e., 1.44 eV and 2.6–2.7 eV, respectively), the high recombination of photoexcited electrons and holes in these two individual materials hinders their practical application for photocatalysis. Therefore, a composite of Mn2O3 and MIL-100(Fe) could be a potential material for enhancing photocatalytic activity via effective charge separation, which has not been reported in the literature.
In this study, Mn2O3/MIL-100(Fe) was synthesized by the hydrothermal method and applied for photocatalytic degradation of RhB under visible light irradiation and persulfate activation. Factors that affect the RhB degradation efficiency such as solution pH, material dosage, RhB concentration, and Na2S2O8 concentration were investigated. Besides, durability and reusability tests were also conducted for evaluating the applicability of the material.
Experimental
Material synthesis and characterization
RhB was imported directly from Macklin (China). The chemicals for material synthesis (e.g., KMnO4, polyvinylpyrrolidone (PVP), iron powder, benzene-1,3,5-tricarboxylic acid (H3BTC), and ethanol) and for photocatalytic experiments (e.g., sodium persulfate, NaCl, NaOH, HNO3, HCl, HF, and H2SO4) were all pure chemicals (≥99%) from China.Mn2O3 was prepared by the hydrothermal method using KMnO4 precursor.21 At first, PVP solution was prepared by dissolving 1 g of PVP in 10 mL of ethanol and KMnO4 solution was prepared by stirring 0.4789 g of KMnO4 in 15 mL of double-distilled water. The two solutions were then mixed and vigorously stirred at room temperature for 10 min and transferred to a 100 mL Teflon autoclave containing 40 mL of hot double-distilled water at 90 °C. After that, the mixture was hydrothermally treated in the Teflon autoclave at 180 °C for 3 h and then allowed to cool to room temperature. The collected brown powder was centrifuged and rinsed several times with each of double-distilled water and ethanol. Finally, the material was subsequently dried at 80 °C for 12 h and heated at 700 °C for 4 h at a heating rate of 2 °C min−1 to obtain Mn2O3 black powder.
Mn2O3/MIL-100(Fe) materials with different weight ratios of MIL-100(Fe) (i.e., 50, 60, 70, 80, and 90%) were synthesized by the hydrothermal method.31 A pre-calculated amount of Mn2O3 was dispersed in a glass beaker containing 10 mL of double-distilled water and ultrasonically treated for 30 min. The suspension then had added to it 0.082 g of iron powder and 0.206 g of H3BTC and stirred for 30 min. The suspension was subsequently transferred to the Teflon autoclave and hydrothermally treated at 150 °C for 4 days. After cooling down to room temperature, the material was centrifuged and rinsed with double-distilled water at 80 °C and ethanol at 60 °C before drying at 150 °C for 24 h. MIL-100(Fe) material was synthesized using the same procedure but without the addition of Mn2O3. The Mn2O3/MIL-100(Fe) composite was denoted as M100Mn. For comparison purposes, a physically mixed Mn2O3/MIL-100(Fe) material with a MIL-100(Fe)/Mn2O3 weight ratio of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 was prepared by directly mixing pre-determined amounts of Mn2O3 and MIL-100(Fe) in ethanol under ultrasonication and stirring at room temperature for 48 h, following by centrifugation and drying at 80 °C for 12 h.
40 was prepared by directly mixing pre-determined amounts of Mn2O3 and MIL-100(Fe) in ethanol under ultrasonication and stirring at room temperature for 48 h, following by centrifugation and drying at 80 °C for 12 h.
The synthesized materials were characterized by X-ray diffraction (XRD, D8 Advance, Bruker, Germany) for studying the crystalline structure and Fourier-transform infrared (FTIR) spectroscopy (Spectrum Two, PerkinElmer, USA) for exploring the surface bonding and functional groups. The morphology and the surface elemental composition of the materials were examined by scanning electron microscopy (SEM, S-4800, Hitachi, Japan) coupled with energy dispersive X-ray spectroscopy (EDX) and EMSA/MAS spectral data file. The surface area and porous structure of the materials were analyzed by the Brunauer–Emmett–Teller (BET) method using a surface area and porosity analyzer (TriStar II Plus 2.03, Micromeritics, USA). The UV-visible absorption spectra and the bandgap energy of the material were obtained by UV-visible diffuse reflectance spectroscopy using a spectrophotometer (V670, Jasco, Japan).
The pHpzc of the material was determined by the titration method. Six conical flasks containing 12.5 mL of 0.1 M NaCl were prepared and the initial pH of these solutions (pHi) was adjusted in the range of 2–11 by adding 0.1 M HCl or NaOH solutions. Each flask then had added to it 0.025 mg of material and was shaken for 48 h. After that, the material was removed by settling and filtering and the pH of the filtered solution was measured (pHf). The pHpzc was estimated at the pHi value that does not change with the addition of the material (pHi = pHf).
Photocatalytic degradation of RhB
The photocatalytic activity of the material was evaluated through its ability for persulfate activation and photocatalytic degradation of RhB at room temperature (25 ± 2 °C) using a batch reactor. The light source in this experiment was a 40 W L4X LED, with the highest intensity at a wavelength of 446 nm. The distance from the lamp to the solution in a water-jacketed beaker was kept constant at 9 cm in all experiments. The pH of the RhB solution was adjusted by adding 0.05 M NaOH or H2SO4 solutions. Before photocatalytic tests, the photocatalyst was added into the RhB solution and stirred in the dark for 30 min to reach the adsorption equilibrium. After that, Na2S2O8 was added to the solution and continuously aerated with air. The light was then turned on for photocatalytic reaction. During the experiment, 4 mL of the sample was taken, centrifuged, and sent for RhB analysis by measuring the absorbance at 554 nm using a spectrophotometer (Libra SP60, Biochrom, UK).To determine the suitable conditions for photocatalytic degradation of RhB using M100Mn, the affecting factors were investigated in a range of solution pH (2–11), photocatalyst dosage (0–1000 mg L−1), RhB concentration (15–50 mg L−1), and Na2S2O8 concentration (100–500 mg L−1). To evaluate the reusability of the material, the photocatalyst was separated from the dye solution by centrifugation after each reaction cycle and then washed with water and ethanol to remove the RhB on the surface of the catalyst. Next, the material was dried in a vacuum oven at 80 °C for 5 h before using it for the next cycle. Each cycle of the photocatalytic reaction was performed in the presence of Na2S2O8 and under aeration conditions. To determine the major reactive oxygen species, a quenching test was conducted by adding radical scavenging agents with a concentration of 10 mM, namely ethylenediaminetetraacetic acid disodium salt (EDTA-2Na, for photoexcited holes h+), tert-butanol (TBA, for HO˙), p-benzoquinone (p-BQ, for O2˙−), furfuryl alcohol (FFA, for 1O2 and HO˙), and phenol (PheOH, for SO4˙− and HO˙), before turning on the light for the photocatalytic reaction.
All the photocatalytic experiments were repeated 3 times and the average values were reported in this study. The RhB degradation efficiency (H, %) and the rate constant of the pseudo-first-order kinetic model (kapp, min−1) were determined by the following formulas:
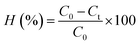 | (1) |
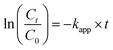 | (2) |
Results and discussion
Characteristics of the synthesized materials
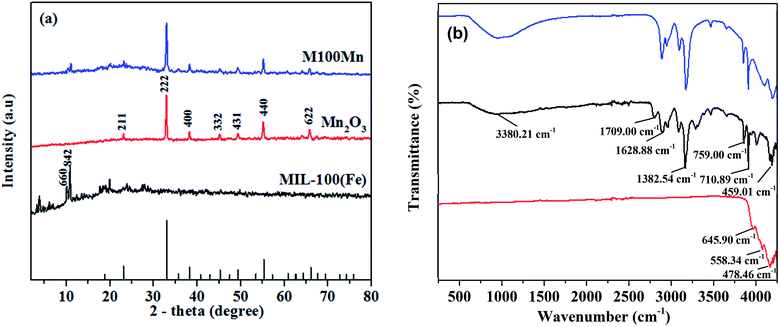
XRD patterns of Mn2O3, MIL-100(Fe), and M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) materials are plotted in Fig. 1(a). In the XRD pattern of Mn2O3, diffraction peaks are observed at 2θ of 23.2, 33.0, 38.3, 45.2, 49.4, 55.2, and 65.9°, corresponding to the planes of (211), (222), (400), (332), (431), (440), and (622) of Mn2O3 crystals (JCPDS card no. 41-1442). Diffraction peaks of MIL-100(Fe) are located at 2θ of 3.4, 4.0, 5.9, 6.8, 7.0, 10.2, 10.8, and 11.0°, assigned to the planes (220), (311), (511), (660), (842), (440), (600), and (422).29,35,36 In addition, no other impurity peaks were detected, which demonstrates that the synthesized MIL-100(Fe) and Mn2O3 are of high purity and well crystallized. The XRD peaks of the M100Mn(60
40) materials are plotted in Fig. 1(a). In the XRD pattern of Mn2O3, diffraction peaks are observed at 2θ of 23.2, 33.0, 38.3, 45.2, 49.4, 55.2, and 65.9°, corresponding to the planes of (211), (222), (400), (332), (431), (440), and (622) of Mn2O3 crystals (JCPDS card no. 41-1442). Diffraction peaks of MIL-100(Fe) are located at 2θ of 3.4, 4.0, 5.9, 6.8, 7.0, 10.2, 10.8, and 11.0°, assigned to the planes (220), (311), (511), (660), (842), (440), (600), and (422).29,35,36 In addition, no other impurity peaks were detected, which demonstrates that the synthesized MIL-100(Fe) and Mn2O3 are of high purity and well crystallized. The XRD peaks of the M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) material are in good agreement with the respective peaks of Mn2O3 and MIL-100(Fe), demonstrating the successful synthesis of Mn2O3/MIL-100(Fe) with a good combination between the two materials.
40) material are in good agreement with the respective peaks of Mn2O3 and MIL-100(Fe), demonstrating the successful synthesis of Mn2O3/MIL-100(Fe) with a good combination between the two materials.
As seen in Fig. 1(b), there are two bands at 645.9 and 558.34 cm−1 in the FTIR spectrum of Mn2O3, which are assigned to the characteristic Mn–O stretching vibration of Mn(III) oxide.37,38 In the MIL-100(Fe) spectrum, a peak at 3380.21 cm−1 is attributed to the vibration of the O–H group, showing the presence of adsorbed water. The strong peaks at 812 and 710.89 cm−1 are characteristic of the 1,3,5-tri-substitution of the benzene ring. The peaks observed at 1628.86, 1450.31, and 1382.54 cm−1 are assigned to asymmetrical and symmetrical vibrations of the corresponding carboxyl group. The high-intensity peak at 1709 cm−1 corresponds to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in 1,3,5-BTC. There is also a peak at 459.01 cm−1 associated with Fe–O stretching vibrations. The above results showed that the MIL-100(Fe) structure contains the 1,3,5-BTC base frame while losing the bond of the carboxyl group of 1,3,5-BTC, proving the bond formation of Fe ions and organic ligand of 1,3,5-H3BTC, which is consistent with previous studies.28,31 The decrease of Mn2O3 peak intensity and the presence of MIL-100(Fe) peak in the spectrum of the M100Mn(60
O group in 1,3,5-BTC. There is also a peak at 459.01 cm−1 associated with Fe–O stretching vibrations. The above results showed that the MIL-100(Fe) structure contains the 1,3,5-BTC base frame while losing the bond of the carboxyl group of 1,3,5-BTC, proving the bond formation of Fe ions and organic ligand of 1,3,5-H3BTC, which is consistent with previous studies.28,31 The decrease of Mn2O3 peak intensity and the presence of MIL-100(Fe) peak in the spectrum of the M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) material proved the successful synthesis of Mn2O3/MIL-100(Fe) composite by the hydrothermal method (Fig. 1(b)).
40) material proved the successful synthesis of Mn2O3/MIL-100(Fe) composite by the hydrothermal method (Fig. 1(b)).
Fig. 2 shows the SEM results of the synthesized materials. As seen in Fig. 2(a), Mn2O3 has a relatively uniform particle size in the range of 100–150 nm, which is consistent with previous publications.38 Regarding MIL-100(Fe) (Fig. 2(b)), it has irregular polyhedral morphologies with particle size from 150 to 600 nm, which is also consistent with previous results from the literature.34,39 The morphology of M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) is similar to that of Mn2O3, but with a rougher surface (Fig. 2(c)), resulting from the combined structure of Mn2O3 and MIL-100(Fe). EDX result reveals that the main components of the M100Mn(60
40) is similar to that of Mn2O3, but with a rougher surface (Fig. 2(c)), resulting from the combined structure of Mn2O3 and MIL-100(Fe). EDX result reveals that the main components of the M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) material are Fe, Mn, C, and O (Fig. S1 of ESI†), proving that the M100Mn material was successfully synthesized with a combination of MIL-100(Fe) and Mn2O3 without other impurities.
40) material are Fe, Mn, C, and O (Fig. S1 of ESI†), proving that the M100Mn material was successfully synthesized with a combination of MIL-100(Fe) and Mn2O3 without other impurities.
The N2 adsorption–desorption isotherms, as well as the pore size distributions, of Mn2O3, MIL-100(Fe), and M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) materials are presented in Fig. 3(a) and S2.† Mn2O3 shows a slightly porous structure and its isotherm can be classified as a type III adsorption isotherm. It has a low surface area of 12.51 m2 g−1, a low pore volume of 0.051 cm3 g−1, and a large pore size of 20.28 nm. In contrast, MIL-100(Fe) shows a highly porous structure with a type II adsorption isotherm, a very high surface area of 1160.73 m2 g−1, a high pore volume of 0.67 cm3 g−1, and a very low pore size of 1.94 nm. Hence, the porous structure of Mn2O3 was significantly increased when combined with highly porous MIL-100(Fe) material, making M100Mn(60
40) materials are presented in Fig. 3(a) and S2.† Mn2O3 shows a slightly porous structure and its isotherm can be classified as a type III adsorption isotherm. It has a low surface area of 12.51 m2 g−1, a low pore volume of 0.051 cm3 g−1, and a large pore size of 20.28 nm. In contrast, MIL-100(Fe) shows a highly porous structure with a type II adsorption isotherm, a very high surface area of 1160.73 m2 g−1, a high pore volume of 0.67 cm3 g−1, and a very low pore size of 1.94 nm. Hence, the porous structure of Mn2O3 was significantly increased when combined with highly porous MIL-100(Fe) material, making M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) exhibit a type II adsorption isotherm with a high surface area of 766.45 m2 g−1, a pore volume of 0.30 cm3 g−1, and a pore size of 3.22 nm. Therefore, the structure of M100Mn(60
40) exhibit a type II adsorption isotherm with a high surface area of 766.45 m2 g−1, a pore volume of 0.30 cm3 g−1, and a pore size of 3.22 nm. Therefore, the structure of M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) is not only highly crystalline (from XRD results) but also highly porous with uniform pore size distribution (from BET results), which could be promising for adsorption and photocatalytic applications.
40) is not only highly crystalline (from XRD results) but also highly porous with uniform pore size distribution (from BET results), which could be promising for adsorption and photocatalytic applications.
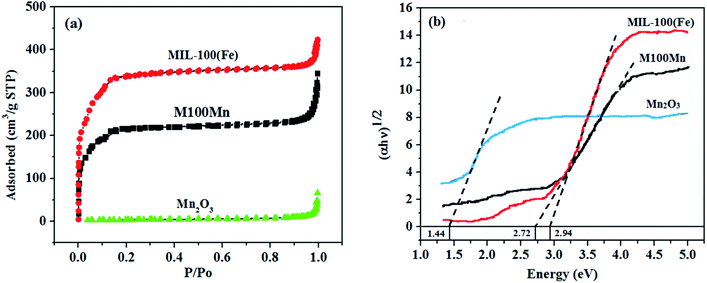 | ||
Fig. 3 (a) The N2 adsorption–desorption isotherms and (b) plots of (αhν)1/2 versus hν for Mn2O3, MIL-100(Fe), and M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) photocatalysts. 40) photocatalysts. | ||
The results from UV-visible light absorption of the materials are presented in Fig. 3(b). The bandgap energies of Mn2O3, MIL-100(Fe), and M100Mn were calculated via the Kubelka–Munk equation to be 1.44, 2.94, and 2.72 eV, respectively, which are consistent with previous results in the literature.21–23,29,32,40 From the UV-visible absorption spectra, the absorption edges of the Mn2O3, MIL-100(Fe), and M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) materials were determined to be 861, 420, and 454 nm, respectively. The visible light absorption of M100Mn was improved as compared to MIL-100(Fe) due to the combination with Mn2O3. This proved the successful combination of Mn2O3 and MIL-100(Fe) in the composite structure of the M100Mn material. The enhancement of visible light absorption can improve the light-harvesting ability, suggesting an improvement of the photocatalytic activity of the M100Mn material under visible light conditions.
40) materials were determined to be 861, 420, and 454 nm, respectively. The visible light absorption of M100Mn was improved as compared to MIL-100(Fe) due to the combination with Mn2O3. This proved the successful combination of Mn2O3 and MIL-100(Fe) in the composite structure of the M100Mn material. The enhancement of visible light absorption can improve the light-harvesting ability, suggesting an improvement of the photocatalytic activity of the M100Mn material under visible light conditions.
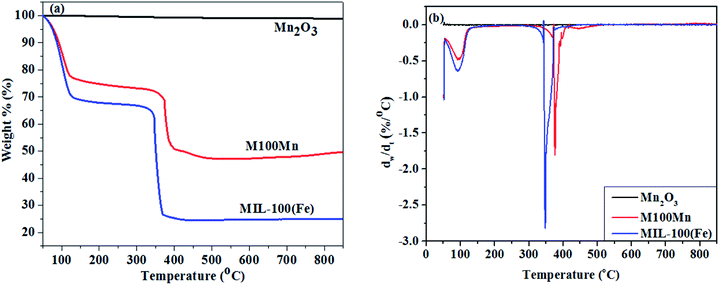
The thermal stability of Mn2O3, MIL-100(Fe), and M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) was examined by TGA from 50 to 900 °C, and the results are shown in Fig. 4. When the temperature increases, the weight of the Mn2O3 material is almost unchanged, indicating that the Mn2O3 material is stable in the temperature range up to 900 °C. When the temperature is raised above 900 °C, Mn2O3 would be converted to Mn3O4.41,42 Regarding MIL-100(Fe), there are 3 stages of weight loss observed. The first stage of 31.9% weight loss in the temperature range of 50–150 °C corresponds to the removal of physically adsorbed water molecules trapped inside the pore of MIL-100(Fe). The second stage with a very low weight loss of 3.20% at 150–350 °C is attributed to the removal of chemically adsorbed water molecules and carboxylic groups, proving that MIL-100(Fe) is stable in this temperature range. Significant weight loss of 74.403% is observed in the third stage with a temperature range of 350–410 °C. At above 350 °C, the structural collapse of MIL-100(Fe) upon ligand decomposition involves H3BTC combustion, MOF structure destruction, and organic matter evaporation. A significant weight loss then begins at 400 °C, due to the continuing breakdown of the framework accompanied by a reduction in the amount of iron present in the structure. The degradation of the third stage ends at 410 °C, indicating that MIL-100(Fe) is completely decomposed to Fe2O3. Hence, the overall thermal stability of the synthesized MIL-100(Fe) sample is determined at below 350 °C, which should be activated at a temperature between 150 and 350 °C before application for adsorption and catalysis.43–46
40) was examined by TGA from 50 to 900 °C, and the results are shown in Fig. 4. When the temperature increases, the weight of the Mn2O3 material is almost unchanged, indicating that the Mn2O3 material is stable in the temperature range up to 900 °C. When the temperature is raised above 900 °C, Mn2O3 would be converted to Mn3O4.41,42 Regarding MIL-100(Fe), there are 3 stages of weight loss observed. The first stage of 31.9% weight loss in the temperature range of 50–150 °C corresponds to the removal of physically adsorbed water molecules trapped inside the pore of MIL-100(Fe). The second stage with a very low weight loss of 3.20% at 150–350 °C is attributed to the removal of chemically adsorbed water molecules and carboxylic groups, proving that MIL-100(Fe) is stable in this temperature range. Significant weight loss of 74.403% is observed in the third stage with a temperature range of 350–410 °C. At above 350 °C, the structural collapse of MIL-100(Fe) upon ligand decomposition involves H3BTC combustion, MOF structure destruction, and organic matter evaporation. A significant weight loss then begins at 400 °C, due to the continuing breakdown of the framework accompanied by a reduction in the amount of iron present in the structure. The degradation of the third stage ends at 410 °C, indicating that MIL-100(Fe) is completely decomposed to Fe2O3. Hence, the overall thermal stability of the synthesized MIL-100(Fe) sample is determined at below 350 °C, which should be activated at a temperature between 150 and 350 °C before application for adsorption and catalysis.43–46
The overall trend of weight loss in the M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) material is similar to that in MIL-100(Fe). The weight loss is attributed to the loss of water molecules and solvents below 250 °C. Framework breakdown of both MIL-100(Fe) and M100Mn(60
40) material is similar to that in MIL-100(Fe). The weight loss is attributed to the loss of water molecules and solvents below 250 °C. Framework breakdown of both MIL-100(Fe) and M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) composite material occurs in the range 250–381 °C due to the carboxyl breakdown of trimesic acid. Therefore, the new composite material is stable below 250 °C. The structure collapses after being heated at temperatures greater than 381 °C, indicating that M100Mn(60
40) composite material occurs in the range 250–381 °C due to the carboxyl breakdown of trimesic acid. Therefore, the new composite material is stable below 250 °C. The structure collapses after being heated at temperatures greater than 381 °C, indicating that M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) is more thermally stable than MIL-100(Fe) because of its thermal stability enhancement from combination with Mn2O3 and that the effect of temperature on the stability of the material can be ignored during the photocatalytic process.
40) is more thermally stable than MIL-100(Fe) because of its thermal stability enhancement from combination with Mn2O3 and that the effect of temperature on the stability of the material can be ignored during the photocatalytic process.
Photocatalytic degradation of RhB using the M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 40) material
40) material
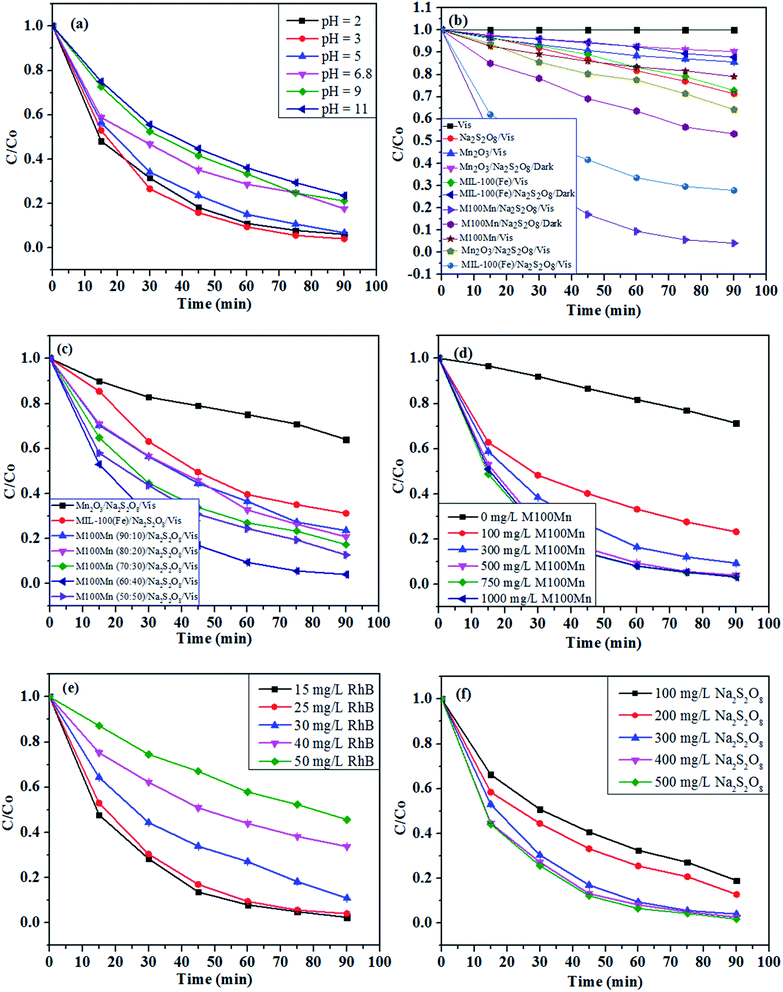
The pH value is of significance and has an impact on the adsorption capacity and reaction rate of heterogeneous catalytic reactions, thus affecting the entire photocatalytic process as well as the efficiency of the reaction. Fig. 5(a) presents the effect of pH on the photocatalytic degradation of RhB using the M100Mn/Na2S2O8/RhB/Vis system with RhB concentration of 25 mg L−1, M100Mn dosage of 500 mg L−1, Na2S2O8 concentration of 300 mg L−1, and temperature of 25 °C. The RhB degradation efficiency after 90 min of reaction increased from 93.92% at pH 2 to 95.91% at pH 3, but then gradually decreased to 76.42% with a further increase of pH up to 11. The better performance of the M100Mn material can be explained by its isoelectric point, which was determined as pHpzc of 4.65 (Fig. S3†). When the solution pH is less than pHpzc, the surface of the material is positively charged and promotes the adsorption of persulfate anion (S2O82−) onto the surface, and thereby enhancing persulfate activation ability to form reactive oxygen species. When the solution pH is greater than pHpzc, the negative surface of the material reduces the persulfate anion adsorption and thus reduces the treatment efficiency. However, at pH 2, the amount of proton H+ exceeds a certain threshold, which leads to a decrease in the reaction rate. Therefore, the acidic condition is favorable for photocatalytic reaction and pH 3 (with RhB degradation rate constant of 0.0393 min−1; Fig. S4†) was then chosen for further investigations.
The degradation of RhB was studied under different conditions of visible light, Na2S2O8, and catalyst presence, and the results are presented in Fig. 5(b). Photolysis alone (i.e., Vis: visible light only, without Na2S2O8 and photocatalyst) led to very low RhB degradation, which can be negligible during the reaction. Adding Na2S2O8 under visible light irradiation (i.e., Na2S2O8/Vis) enhanced the RhB degradation efficiency to 28.69%. Although it is a notable improvement, this photolytic oxidation cannot be used due to its still low degradation efficiency. Without Na2S2O8, the RhB photocatalytic degradation efficiencies after 90 min using Mn2O3, MIL-100(Fe), and M100Mn were 14.38, 27.21, and 21.59%, respectively, under visible light. In the presence of Na2S2O8 but without visible light, the chemical degradation efficiencies using these materials were 9.71, 12.23, and 46.74%, respectively. The photocatalytic RhB degradation efficiencies under visible light and persulfate activation of pure Mn2O3 and MIL-100(Fe) were low at 35.97 and 68.77%, respectively, which is due to the rapid recombination of photoexcited electron and hole pairs in these two materials. Meanwhile, the RhB degradation efficiency was greatly enhanced to 95.91% when using the M100Mn composite. The RhB degradation rate constant using M100Mn was 7.72 and 2.67 times higher than when using Mn2O3 and MIL-100(Fe), respectively (Fig. S5†). This proves the enhancement of electron transfer between single materials in the composite, thus reducing the electron–hole recombination potential and improving the photocatalytic activity. Meanwhile, the presence of Na2S2O8 would increase the formation of reactive oxygen species, thus significantly improving the photocatalytic efficiency of the material, proving the important role of the simultaneous presence of photocatalyst, visible light, and Na2S2O8 in RhB degradation.
The effect of MIL-100(Fe) content (i.e., 50–90%) on the photocatalytic performance of M100Mn is illustrated in Fig. 5(c). The degradation efficiency increased from 87.26 to 95.91% with an increase of MIL-100(Fe) content from 50 to 60% but gradually decreased to 76.49% with a further increase of MIL-100(Fe) content up to 90%. The M100Mn composite with the MIL-100(Fe)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn2O3 weight ratio of 60
Mn2O3 weight ratio of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 had the highest RhB degradation efficiency with a rate constant of 2.28, 2.11, 1.95, and 1.70 higher than those of M100Mn material with MIL-100(Fe)
40 had the highest RhB degradation efficiency with a rate constant of 2.28, 2.11, 1.95, and 1.70 higher than those of M100Mn material with MIL-100(Fe)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn2O3 weight ratio of 90
Mn2O3 weight ratio of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 80
10, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 70
20, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, and 50
30, and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, respectively (Fig. S6†).
50, respectively (Fig. S6†).
The photocatalytic activities of M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) composites synthesized by the hydrothermal and physical mixing methods were also compared. As seen in Fig. S7,† the RhB degradation efficiency after 90 min of reaction was 95.91% for M100Mn(60
40) composites synthesized by the hydrothermal and physical mixing methods were also compared. As seen in Fig. S7,† the RhB degradation efficiency after 90 min of reaction was 95.91% for M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) prepared by the hydrothermal method and 80.53% for that prepared by the physical mixing method. The RhB degradation rate constant of M100Mn(60
40) prepared by the hydrothermal method and 80.53% for that prepared by the physical mixing method. The RhB degradation rate constant of M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) prepared by the hydrothermal method was 1.9 times higher than that of M100Mn(60
40) prepared by the hydrothermal method was 1.9 times higher than that of M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) prepared by the mixing method. The physical mixing method has the advantages of simplicity and ease of operation. However, it is hard to achieve the highly homogeneous dispersion of small Mn2O3 on the surface of the MIL-100(Fe) material. Moreover, the physical links between the two materials in the physical mixing method are not as good as those in the hydrothermal method. These issues could result in high electron–hole recombination in each material, which decreases the photocatalytic activity.47,48
40) prepared by the mixing method. The physical mixing method has the advantages of simplicity and ease of operation. However, it is hard to achieve the highly homogeneous dispersion of small Mn2O3 on the surface of the MIL-100(Fe) material. Moreover, the physical links between the two materials in the physical mixing method are not as good as those in the hydrothermal method. These issues could result in high electron–hole recombination in each material, which decreases the photocatalytic activity.47,48
The UV-visible absorption spectrum of RhB in Fig. S8† shows that the characteristic absorption peak at 554 nm of RhB did not shift during the reaction period. The spectral intensity decreased sharply after the first 15 min and almost disappeared after 90 min. The M100Mn composite is effective for the activation of Na2S2O8 to form reactive oxygen species, which can completely break down the structure of RhB.49
The effect of M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) dosage (in the range of 0–1000 mg L−1) on RhB degradation efficiency is demonstrated in Fig. 5(d). At low dosages, the degradation of RhB increased from 28.69 to 95.91% with an increase of the catalyst dosage from 0 to 500 mg L−1 (Fig. 5(d)), corresponding to an increase of rate constant from 0.0038 to 0.0363 min−1 (Fig. S9†). However, when the catalyst dosage increased from 500 to 1000 mg L−1, the RhB degradation changed insignificantly. It can be explained in that an increase in the catalyst dosage results in an increase in surface active sites for photocatalysis as well as persulfate activation and thus reactive oxygen species formation for faster RhB degradation.8,50 However, when the content of M100Mn catalyst material is in excess, there is not sufficient persulfate activation at a constant Na2S2O8 concentration, resulting in the unchanged degradation efficiency. Therefore, 500 mg L−1 was chosen as a suitable M100Mn dosage for further experiments.
40) dosage (in the range of 0–1000 mg L−1) on RhB degradation efficiency is demonstrated in Fig. 5(d). At low dosages, the degradation of RhB increased from 28.69 to 95.91% with an increase of the catalyst dosage from 0 to 500 mg L−1 (Fig. 5(d)), corresponding to an increase of rate constant from 0.0038 to 0.0363 min−1 (Fig. S9†). However, when the catalyst dosage increased from 500 to 1000 mg L−1, the RhB degradation changed insignificantly. It can be explained in that an increase in the catalyst dosage results in an increase in surface active sites for photocatalysis as well as persulfate activation and thus reactive oxygen species formation for faster RhB degradation.8,50 However, when the content of M100Mn catalyst material is in excess, there is not sufficient persulfate activation at a constant Na2S2O8 concentration, resulting in the unchanged degradation efficiency. Therefore, 500 mg L−1 was chosen as a suitable M100Mn dosage for further experiments.
Fig. 5(e) presents the degradation efficiency of RhB under different initial RhB concentrations from 15 to 50 mg L−1. It can be seen that the degradation efficiency decreased continuously with an increase of RhB concentration. As provided in Fig. S10,† the rate constant gradually decreased from 0.0404 to 0.0086 min−1 when the RhB concentration increased from 15 to 50 mg L−1. This can be explained by the fact that an increase in the number of dye molecules leads to a longer time to complete the RhB decomposition, resulting in a decrease in treatment efficiency.51 Besides, the rate of the photocatalytic reaction depends largely on the formation of reactive oxygen species on the photocatalytic surface and on the types of reactive oxygen species that are produced to attack and weaken the structure of RhB.51,52 The increase in the initial concentration of RhB causes a large amount of adsorbed RhB on the active centers of the catalyst, thus interfering with the adsorption of S2O82− on the surface and reducing the rate of formation of reactive oxygen species (e.g., sulfate and hydroxyl radicals) and therefore the degradation of RhB. Besides, an increase of RhB concentration could prevent the transmission of light in the solution, thus reducing the ability of the photocatalyst to absorb photons, resulting in a significant reduction in photocatalytic degradation. Therefore, an RhB concentration of 25 mg L−1 was then chosen for subsequent experiments.
The effect of the Na2S2O8 concentration on the degradation of RhB is shown in Fig. 5(f). It is obvious that the degradation of RhB was proportional to the Na2S2O8 concentration. When the concentration of Na2S2O8 increased from 100 to 300 mg L−1, the RhB degradation efficiency after 90 min increased from 81.01 and 95.91% (Fig. 5(f)), and the rate constant increased from 0.0172 to 0.0363 min−1 (Fig. S11†). This is because the increase in Na2S2O8 concentration enhances the persulfate activation to produce more reactive oxygen species, which leads to a corresponding increase in RhB degradation. However, when using high Na2S2O8 concentrations of 400 and 500 mg L−1, both the degradation efficiency and rate constant did not change significantly. The reason may be due to the side effect of excessive Na2S2O8 forming a weaker oxidizing radical (S2O8˙−) as in Re. (3). Therefore, a Na2S2O8 concentration of 300 mg L−1 was selected for the subsequent experiments.
| S2O82− + SO4˙− → SO42− + S2O8˙− | (3) |
The reusability and stability of the catalyst are significant indicators of its feasibility and applicability in practice. The reusability of the prepared M100Mn was evaluated via five cycles of reaction. As shown in Fig. 6(a), the RhB degradation efficiency slightly decreased from 95.91 to 91.78% after the first three cycles, and to 77.96% after the two next cycles, proving its relatively good recyclability and stability. The reduction in the RhB degradation efficiency can be attributed to the partial destruction of the photocatalyst structure and the loss of some photocatalyst mass during washing for reuse. Also, the adsorbed RhB or intermediates and by-products on the photocatalyst during the previous test were not completely eliminated, causing a decrease in the RhB degradation in the subsequent cycles.
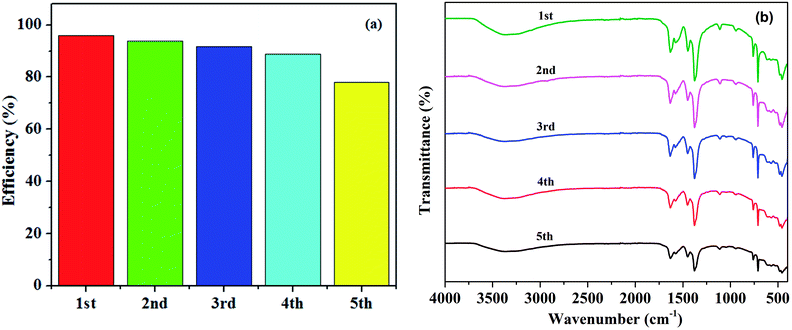 | ||
Fig. 6 (a) Degradation efficiency of RhB during five times of reusing the M100Mn material and (b) FTIR spectrum of M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) after each of the five cycles. 40) after each of the five cycles. | ||
Besides, the durability of M100Mn after five cycles was evaluated by FTIR (Fig. 6(b)). The FTIR characteristic peaks of the M100Mn material were not significantly changed after five cycles of reuse, showing that the material was not poisoned by intermediates of the process of RhB degradation. However, the intensity of the characteristic peak slightly decreased after the first three cycles and obviously decreased after the fourth and fifth cycles, signifying the loss of metal ions from the surface of the photocatalyst. This is evidenced by the significant decrease in the peak at a wavenumber of 459.01 cm−1 corresponding to the Fe–O bond after the fifth cycle.
Proposed mechanism for photocatalytic degradation of RhB by the M100Mn material
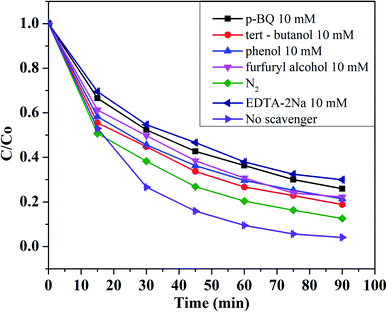
In a regular photocatalytic reaction, photoexcited holes (h+), superoxide radical (O2˙−), and hydroxyl radical (HO˙) are usually the main reactive species for the degradation of organic pollutants in water.53 In this study, radical scavengers TBA, PheOH, FFA, p-BQ, and EDTA-2Na with a concentration of 10 mM were used for quenching the oxidation activity of HO˙, SO4˙−/HO˙, 1O2/HO˙, O2˙−, and h+, respectively. There was a decrease in the RhB degradation rate constant (see Fig. S12†) and efficiency (see Fig. 7) from 95.91% (without scavengers) to 81.14, 78.10, 77.77, 74.04, and 70.01% in the presence of TBA, PheOH, FFA, p-BQ, and EDTA-2Na, respectively. However, these insignificant reductions indicate that HO˙, SO4˙−, 1O2, O2˙−, and h+ play important roles in the photocatalytic degradation of RhB under visible light and persulfate activation. Besides, a decrease in RhB degradation efficiency after 90 min of reaction from 95.91% (under air aeration) to 87.39% (with N2 aeration) proved the important role of oxygen in the photocatalytic reaction, suggesting the contribution of O2 as an electron scavenger for reducing the electron–hole recombination and as a source for the formation of O2˙− radicals.A comparison of M100Mn with other materials for persulfate activation for RhB removal is summarized in Table 1. Although the tested conditions were different for each study, the M100Mn/PS/Vis system at the optimum conditions in this study shows many advantages such as using environment-friendly Fe and Mn, removal of high RhB concentration (25 mg L−1) within a short time (90 min), low persulfate consumption (1.26 mM), and especially use of low-cost LED light, all of which are promising for large-scale application.
| Catalyst | Reaction conditions | Performance | Reference |
|---|---|---|---|
| Mn2O3/MIL-100(Fe) (60%) | RhB: 25 mg L−1; catalyst: 500 mg L−1; persulfate: 1.26 mM; pH 3.0; temperature: 25 °C; lamp: LED (40 W) | 95.91% of RhB was removed in 90 min with kapp = 0.0363 min−1; HO˙, SO4˙−, 1O2, O2˙−, and h+ play important roles in the photocatalytic degradation of RhB | This work |
| MIL-88A | RhB: 10 mg L−1; catalyst: 500 mg L−1; persulfate: 1.68 mM; pH 3.0; temperature: 40 °C; lamp: UVA (9 W) | 80% of RhB was removed in 120 min with kapp = 0.131 min−1; SO4˙− and HO˙ were the main reactive oxygen species determining the RhB oxidation | 60 |
MIL-53(Fe)/BiOCl (0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
RhB: 20 mg L−1; catalyst: 500 mg L−1; persulfate: 2.1 mM; pH 3.0; temperature: 25 °C; lamp: xenon (350 W) with a 420 nm cut-off filter | 99.5% of RhB was removed in 30 min with kapp = 0.157 min−1; SO4˙− and HO˙ were the main reactive oxygen species, along with electrons and holes (h+), determining the RhB oxidation | 61 |
| Fe0/C3N4 | RhB: 20 mg L−1; catalyst: 400 mg L−1; persulfate: 3 mM; pH 3.5; temperature: 30 °C; lamp: metal halide–xenon (350 W) with a 400 nm cut-off filter | 97% of RhB was removed in 40 min with kapp = 0.156 min−1; h+ and SO4˙− played important roles in the oxidation process | 8 |
| CeO2@LDH | RhB: 10 mg L−1; catalyst: 400 mg L−1 persulfate: 6 mM; pH 7.0; temperature: 30 °C; lamp: xenon (50 W) with a 400 nm cut-off filter | 96.9% of RhB was removed in 30 min with kapp = 0.0809 min−1; SO4˙−, O2˙−, and HO˙ were the main reactive oxygen species determining the RhB oxidation | 62 |
BiOI/Fe3O4 (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
RhB: 20 mg L−1; catalyst: 500 mg L−1 persulfate: 1 mM; pH 4.6; temperature: 25 °C; lamp: xenon (500 W) with a 420 nm cut-off filter | 98.4% of RhB was removed in 30 min with kapp = 0.130 min−1; SO4˙− and HO˙ were the main reactive oxygen species, along with photoexcited holes (h+), determining the RhB oxidation | 58 |
| TiO2/FeOCl (20%) | RhB: 5 mg L−1; catalyst: 400 mg L−1; persulfate: 1.48 mM; no pH adjustment; temperature: 25 °C; lamp: LED (50 W) | 64.6% of RhB was removed in 90 min with kapp = 0.0378 min−1; SO4˙−, O2˙−, and HO˙ were the main reactive oxygen species, along with photoexcited holes (h+), determining the RhB oxidation | 63 |
| TiO2/carbon dots | RhB: 5 mg L−1; catalyst: 400 mg L−1; persulfate: 1.48 mM; no pH adjustment; temperature: 25 °C; lamp: LED (50 W) | 67% of RhB was removed in 240 min with kapp = 0.0439 min−1; SO4˙− and O2˙− were the main reactive oxygen species, along with photoexcited holes (h+), determining the RhB oxidation | 64 |
| ZnO/CuBi2O4 (5%) | RhB: 5 mg L−1; catalyst: 400 mg L−1; persulfate: 1.48 mM; no pH adjustment; temperature: 25 °C; lamp: LED (50 W) | 100% of RhB was removed in 210 min with kapp = 0.0178 min−1; SO4˙−, O2˙−, and HO˙ were the main reactive oxygen species, along with photoexcited holes (h+), determining the RhB oxidation | 65 |
| ZnS/ZnFe2O4 | RhB: 20 mg L−1; catalyst: 400 mg L−1; persulfate: 0.37 mM; no pH adjustment; temperature: 25 °C; lamp: low-pressure mercury UV (6 W, 254 nm) | 97.67% of RhB was removed in 90 min with kapp = 0.03815 min−1; SO4˙−, O2˙−, and HO˙ were the main reactive oxygen species determining the RhB oxidation | 66 |
The mechanism for photocatalytic removal of RhB under visible light and persulfate activation relates to the charge transfer on the surface of the M100Mn material. The energy levels of valance band (EVB) and conduction band (ECB) are calculated via bandgap energy (Eg) by the following equations.54,55
| EVB = X − Ee + 0.5 × Eg | (4) |
| ECB = EVB − Eg | (5) |
From UV-visible diffuse reflectance spectroscopy results, the bandgap energies of MIL-100(Fe) and Mn2O3 materials were determined to be 2.94 eV and 1.44 eV, respectively. The EVB and ECB values were then calculated to be 2.44 and −0.5 eV, respectively, for MIL-100(Fe), and 0.34 and −1.1 eV, respectively, for Mn2O3. Based on these band energy values, the combination of Mn2O3 and MIL-100(Fe) in M100Mn material as well as its mechanism for removal of RhB is proposed in Fig. 8.
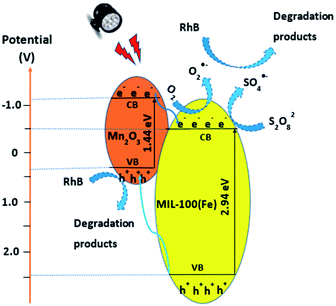 | ||
Fig. 8 The proposed mechanism for photocatalytic degradation of RhB by M100Mn(60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) under visible light and persulfate activation. 40) under visible light and persulfate activation. | ||
Under visible light irradiation, the electrons (e−) from the valance bands of Mn2O3 and MIL-100(Fe) jump to the corresponding conduction bands of the materials and leave holes (h+) in the valance bands (Re. (6)). These photoexcited electron–hole pairs are easily recombined together with a very short lifetime unless they are separated and consumed. In the M100Mn material, the electromagnetic field at the interface of Mn2O3 and MIL-100(Fe) helps to move the electrons and holes in opposite ways.56 Specifically, the electrons from the conduction band of Mn2O3 transfer to the conduction band of MIL-100(Fe) with higher potential. In contrast, the holes from the valence band of MIL-100(Fe) transfer to the valance band of Mn2O3 with lower potential. Hence, the recombination of electrons and holes is effectively limited and they can easily move to the surface of the corresponding materials for participating in the reduction and oxidation reactions. Since the ECB of MIL-100(Fe) is more negative than the potential of the O2/O2˙− redox couple (−0.33 V), the electrons are oxidized by dissolved oxygen (O2) and persulfate (S2O82−) to form superoxide radicals (O2˙−) (Re. (7)) and sulfate radicals (SO4˙−) (Re. (8)). This process fosters the electron transfer from Mn2O3 to MIL-100(Fe) and inhibits photoexcited electron–hole recombination. On the other hand, the EVB of Mn2O3 is lower than the potential of the H2O/HO˙ redox couple (2.4 V); thus the photoexcited holes on Mn2O3 cannot react with H2O to produce hydroxyl radical (HO˙), which is consistent with the above described h+ quenching test by using EDTA-2Na. Here, h+ directly oxidize RhB into products and intermediates. Besides, the sulfate radicals in the solution could react with water to produce hydroxyl radical (HO˙) (Re. (9)). There is also the production of singlet oxygen (1O2) via the interaction between superoxide and hydroxyl radicals (Re. (10)). The presence of metal ions such as Fe2+ and Mn3+ on the surface of M100Mn activates S2O82− to become Fe3+, Mn4+, and sulfate ion (SO42−) (Re. (11) and (12)). Moreover, Fe3+ and Mn4+ are also reduced by S2O82− to form Fe2+, Mn3+, and persulfate (S2O8˙−) with weak oxidation property (Re. (13) and (14)).57 Furthermore, Fe3+ and Mn4+ are reduced to Fe2+ and Mn3+ by photoexcited electrons (Re. (15) and (16)), which enhances the charge separation.8,58 In addition, since the potentials of Fe3+/Fe2+ and Mn4+/Mn3+ redox couples are 0.77 V and 0.15 V, respectively, the reactions of Fe3+ and Mn3+ thermodynamically occurred, which boosts the internal charge transfer (Re. (17)).59 The RhB adsorbed on the material surface would be easily oxidized by reactive oxidative species (e.g., O2˙−, 1O2, SO4˙−, and HO˙) and photoexcited holes (h+) to form mineralized products of CO2 and water (Re. (18)).
| MIL-100(Fe)/Mn2O3 + hν → MIL-100(Fe) (e−) + Mn2O3 (h+) | (6) |
| e− + O2 → O2˙− | (7) |
| e− + S2O82− → SO4˙− + SO42− | (8) |
| SO4˙− + H2O → SO42− + HO˙ + HO− | (9) |
| O2˙− + HO˙ → HO− + 1O2 | (10) |
| Fe2+ + S2O82− → Fe3+ + SO4˙− + SO42− | (11) |
| Mn3+ + S2O82− → Mn4+ + SO4˙− + SO42− | (12) |
| Fe3+ + S2O82− → Fe2+ + S2O8˙− | (13) |
| Mn4+ + S2O82− → Mn3+ + S2O8˙− | (14) |
| Fe3+ + e− → Fe2+ | (15) |
| Mn4+ + e− → Mn3+ | (16) |
| Mn3+ + Fe3+ → Mn4+ + Fe2+ | (17) |
| [h+, O2˙−, 1O2, SO4˙−, HO˙] + RhB → mineralized products + CO2 + H2O + SO42− | (18) |
Conclusions
The study was successful in the synthesis of a highly pure M100Mn composite with both advantages of highly crystalline and porous structure from Mn2O3 and MIL-100(Fe) for effective photocatalytic degradation of RhB in water under visible light and persulfate activation. The suitable material was found to be MIL-100(Fe)/Mn2O3 with a weight ratio of 60/40 and reaction conditions of pH 3, M100Mn dosage of 500 mg L−1, RhB concentration of 25 mg L−1, and Na2S2O8 concentration of 300 mg L−1 leading to high RhB degradation of 95.91% after 90 min of the experiment. The radical quenching test showed that HO˙, SO4˙−, 1O2, O2˙−, and h+ are all important reactive species and oxygen has a significant role in the photocatalytic reaction. A mechanism was also proposed for the oxidation of RhB catalyzed by the M100Mn composite. Besides, the stability and reusability of M100Mn were evaluated over 5 experimental cycles, proving its practicability for wastewater applications.Conflicts of interest
There are no conflicts to declare.Notes and references
- B. Cuiping, X. Xianfeng, G. Wenqi, F. Dexin, X. Mo, G. Zhongxue and X. Nian, Desalination, 2011, 278, 84–90 CrossRef.
- E. Forgacs, T. Cserhati and G. Oros, Environ. Int., 2004, 30, 953–971 CrossRef CAS PubMed.
- J. Wu, J. Yang, G. Huang, C. Xu and B. Lin, J. Cleaner Prod., 2020, 251, 119717 CrossRef CAS.
- W. Xiao, Z. N. Garba, S. Sun, I. Lawan, L. Wang, M. Lin and Z. Yuan, J. Cleaner Prod., 2020, 253, 119989 CrossRef CAS.
- C. Huang, Y. Wang, M. Gong, W. Wang, Y. Mu and Z.-H. Hu, Sep. Purif. Technol., 2020, 230, 115877 CrossRef CAS.
- X. Li, X. Yan, X. Hu, R. Feng and M. Zhou, J. Environ. Manage., 2020, 262, 110299 CrossRef CAS PubMed.
- Y. Pang, L. Kong, D. Chen, G. Yuvaraja and S. Mehmood, J. Hazard. Mater., 2020, 384, 121447 CrossRef CAS PubMed.
- H. Heidarpour, M. Padervand, M. Soltanieh and M. Vossoughi, Chem. Eng. Res. Des., 2020, 153, 709–720 CrossRef CAS.
- J. Zhang, Z. Zhang, W. Zhu and X. Meng, Appl. Surf. Sci., 2020, 502, 144275 CrossRef CAS.
- J. Zhao, M. Ji, J. Di, Y. Zhang, M. He, H. Li and J. Xia, J. Photochem. Photobiol., A, 2020, 391, 112343 CrossRef CAS.
- Y. Yang, Y. Liu, X. Fang, W. Miao, X. Chen, J. Sun, B.-J. Ni and S. Mao, Chemosphere, 2020, 243, 125423 CrossRef CAS PubMed.
- K. Paździor, A. Klepacz-Smółka, S. Ledakowicz, J. Sójka-Ledakowicz, Z. Mrozińska and R. Żyłła, Chemosphere, 2009, 75, 250–255 CrossRef PubMed.
- G. Chen, Y. Yu, L. Liang, X. Duan, R. Li, X. Lu, B. Yan, N. Li and S. Wang, J. Hazard. Mater., 2021, 408, 124461 CrossRef CAS PubMed.
- Z. Zhou, X. Liu, K. Sun, C. Lin, J. Ma, M. He and W. Ouyang, Chem. Eng. J., 2019, 372, 836–851 CrossRef CAS.
- R. Xiao, Z. Luo, Z. Wei, S. Luo, R. Spinney, W. Yang and D. D. Dionysiou, Curr. Opin. Chem. Eng., 2018, 19, 51–58 CrossRef.
- U. Ushani, X. Lu, J. Wang, Z. Zhang, J. Dai, Y. Tan, S. Wang, W. Li, C. Niu, T. Cai, N. Wang and G. Zhen, Chem. Eng. J., 2020, 402, 126232 CrossRef CAS.
- X. Zhang, H. Li, F. Hou, Y. Yang, H. Dong, N. Liu, Y. Wang and L. Cui, Appl. Surf. Sci., 2017, 411, 27–33 CrossRef CAS.
- X. Gu, J. Yue, L. Li, H. Xue, J. Yang and X. Zhao, Electrochim. Acta, 2015, 184, 250–256 CrossRef CAS.
- Y. Hai, Z. Zhang, H. Liu, L. Liao, P. Fan, Y. Wu, G. Lv and L. Mei, Front. Chem., 2019, 7, 437 CrossRef CAS PubMed.
- Y. Shao, B. Ren, H. Jiang, B. Zhou, L. Liping, J. Ren, L. Dong, J. Li and Z. Liu, J. Hazard. Mater., 2017, 333, 222–231 CrossRef CAS PubMed.
- J. Zhao, Z. Zhao, N. Li, J. Nan, R. Yu and J. Du, Chem. Eng. J., 2018, 353, 805–813 CrossRef CAS.
- J. Zhao, J. Nan, Z. Zhao and N. Li, Catal. Commun., 2017, 102, 5–8 CrossRef CAS.
- J. Zhao, N. Li, R. Yu, Z. Zhao and J. Nan, Chem. Eng. J., 2018, 349, 530–538 CrossRef CAS.
- M. M. Kandy and V. G. Gaikar, Renewable Energy, 2019, 139, 915–923 CrossRef CAS.
- H. Eslami, M. H. Ehrampoush, A. Esmaeili, A. A. Ebrahimi, M. H. Salmani, M. T. Ghaneian and H. Falahzadeh, Chemosphere, 2018, 207, 303–312 CrossRef CAS PubMed.
- P. Horcajada, S. Surblé, C. Serre, D.-Y. Hong, Y.-K. Seo, J.-S. Chang, J.-M. Grenèche, I. Margiolaki and G. Férey, Chem. Commun., 2007, 2820–2822 RSC.
- S. Duan, J. Li, X. Liu, Y. Wang, S. Zeng, D. Shao and T. Hayat, ACS Sustainable Chem. Eng., 2016, 4, 3368–3378 CrossRef CAS.
- M. Rezaei, A. Abbasi, R. Varshochian, R. Dinarvand and M. Jeddi-Tehrani, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 1390–1401 CrossRef CAS PubMed.
- X. Du, X. Yi, P. Wang, J. Deng and C.-c. Wang, Chin. J. Catal., 2019, 40, 70–79 CrossRef.
- G. Chaturvedi, A. Kaur, A. Umar, M. A. Khan, H. Algarni and S. K. Kansal, J. Solid State Chem., 2020, 281, 121029 CrossRef CAS.
- D.-D. Chen, X.-H. Yi, C. Zhao, H. Fu, P. Wang and C.-C. Wang, Chemosphere, 2020, 245, 125659 CrossRef CAS PubMed.
- S. Abdpour, E. Kowsari, M. R. A. Moghaddam, L. Schmolke and C. Janiak, J. Solid State Chem., 2018, 266, 54–62 CrossRef CAS.
- P. Hu, C. Chen, Y. Wang, L. Pan and C. Lu, ChemistrySelect, 2019, 4, 9703–9709 CrossRef CAS.
- H. Tian, T. Araya, R. Li, Y. Fang and Y. Huang, Appl. Catal., B, 2019, 254, 371–379 CrossRef CAS.
- H. Tian, J. Peng, Q. Du, X. Hui and H. He, Dalton Trans., 2018, 47, 3417–3424 RSC.
- M.-J. Chang, W.-N. Cui, X.-J. Chai, J. Liu, K. Wang and L. Qiu, J. Mater. Sci.: Mater. Electron., 2019, 30, 1009–1016 CrossRef CAS.
- Y. Li, J. Gong, G. He and Y. Deng, Synth. Met., 2011, 161, 56–61 CrossRef CAS.
- M. Salavati-Niasari, F. Mohandes, F. Davar and K. Saberyan, Appl. Surf. Sci., 2009, 256, 1476–1480 CrossRef CAS.
- Y. Luo, B. Tan, X. Liang, S. Wang, X. Gao, Z. Zhang and Y. Fang, Ind. Eng. Chem. Res., 2019, 58, 7801–7807 CrossRef CAS.
- K. Guesh, C. A. D. Caiuby, Á. Mayoral, M. Díaz-García, I. Díaz and M. Sanchez-Sanchez, Cryst. Growth Des., 2017, 17, 1806–1813 CrossRef CAS.
- M. Y. Nassar, A. S. Amin, I. S. Ahmed and S. Abdallah, J. Taiwan Inst. Chem. Eng., 2016, 64, 79–88 CrossRef CAS.
- Y. Zhao, D.-B. Ji, P. Wang, Y.-D. Yan, Y. Xue, H.-B. Xu, Y. Liang, H.-J. Luo, M.-L. Zhang and W. Han, Chem. Eng. J., 2018, 349, 613–621 CrossRef CAS.
- M. A. Simon, E. Anggraeni, F. E. Soetaredjo, S. P. Santoso, W. Irawaty, T. C. Thanh, S. B. Hartono, M. Yuliana and S. Ismadji, Sci. Rep., 2019, 9, 1–11 CAS.
- F. Zhang, J. Shi, Y. Jin, Y. Fu, Y. Zhong and W. Zhu, Chem. Eng. J., 2015, 259, 183–190 CrossRef CAS.
- G. Song, Z. Wang, L. Wang, G. Li, M. Huang and F. Yin, Chin. J. Catal., 2014, 35, 185–195 CrossRef CAS.
- H. Wang, R. Zhao, J. Qin, H. Hu, X. Fan, X. Cao and D. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 44249–44262 CrossRef CAS PubMed.
- Y. Xu, M. Lv, H. Yang, Q. Chen, X. Liu and W. Fengyu, RSC Adv., 2015, 5, 43473–43479 RSC.
- X. Jia, R. Dai, Y. Sun, H. Song and X. Wu, J. Mater. Sci.: Mater. Electron., 2016, 27, 3791–3798 CrossRef CAS.
- Y. Zhang, J. Zhou, J. Chen, X. Feng and W. Cai, J. Hazard. Mater., 2020, 392, 122315 CrossRef CAS PubMed.
- H. Zhu, B. Yang, J. Yang, Y. Yuan and J. Zhang, Chemosphere, 2021, 276, 130217 CrossRef CAS PubMed.
- S. Parvaz, M. Rabbani and R. Rahimi, Mater. Sci. Eng., B, 2021, 263, 114863 CrossRef CAS.
- Y. Tian, L. Ma, X. Tian, Y. Nie, C. Yang, Y. Li, L. Lu and Z. Zhou, Chemosphere, 2021, 269, 128717 CrossRef CAS PubMed.
- Y. Nosaka and A. Y. Nosaka, Chem. Rev., 2017, 117, 11302–11336 CrossRef CAS PubMed.
- B. Zhang, H. Shi, Y. Yan, C. Liu, X. Hu, E. Liu and J. Fan, Colloids Surf., A, 2021, 608, 125598 CrossRef CAS.
- Y. Chen, Q. Hu, M. Yu, X. Gong, S. Li, S. Wang, H. Yu and Z. Li, CrystEngComm, 2021, 23, 5070–5077 RSC.
- P. Hu, C. Chen, Y. Wang, L. Pan and C. Lu, ChemistrySelect, 2019, 4, 9703–9709 CrossRef CAS.
- J. Li, R. Guo, Q. Ma, L.-c. Nengzi and X. Cheng, Sep. Purif. Technol., 2019, 227, 115669 CrossRef CAS.
- Y. Liu, H. Guo, Y. Zhang, X. Cheng, P. Zhou, G. Zhang, J. Wang, P. Tang, T. Ke and W. Li, Sep. Purif. Technol., 2018, 192, 88–98 CrossRef CAS.
- Z. Dong, Q. Zhang, B.-Y. Chen and J. Hong, Chem. Eng. J., 2019, 357, 337–347 CrossRef CAS.
- K.-Y. Andrew Lin, H.-A. Chang and C.-J. Hsu, RSC Adv., 2015, 5, 32520–32530 RSC.
- S. Miao, Z. Zha, Y. Li, X. Geng, J. Yang, J. Yang and S. Cui, J. Photochem. Photobiol., A, 2019, 380, 111862 CrossRef CAS.
- C. Yang, G. Zhang, Y. Meng, G. Pan, Z. Ni and S. Xia, J. Hazard. Mater., 2021, 408, 124908 CrossRef CAS PubMed.
- M. Sabri, A. Habibi-Yangjeh, H. Chand and V. Krishnan, Sep. Purif. Technol., 2020, 250, 117268 CrossRef CAS.
- M. Sabri, A. Habibi-Yangjeh and S. Vadivel, J. Mater. Sci.: Mater. Electron., 2019, 30, 12510–12522 CrossRef CAS.
- M. Sabri, A. Habibi-Yangjeh and S. Ghosh, J. Photochem. Photobiol., A, 2020, 391, 112397 CrossRef CAS.
- B. Zhu, H. Cheng, J. Ma, Y. Kong and S. Komarneni, Chemosphere, 2019, 237, 124547 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra03496k |
| This journal is © The Royal Society of Chemistry 2021 |