Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A dual-mode visual detector for toxic hydrazine†
Brahmjot Kaur,
Rameez Raza and
Neil R. Branda *
*
4D LABS, Department of Chemistry, Simon Fraser University, 8888 University Drive, Burnaby, BC V5A 1S6, Canada. E-mail: nbranda@sfu.ca
First published on 28th June 2021
Abstract
Hydrazine (N2H4) is one of the commonly used chemical reagents in numerous industries and applications but its toxicity to humans poses a need to develop simple visual detection methods. Herein, we demonstrate a novel dual-mode system to detect and simultaneously consume hydrazine in vapour and solution by using a small photoresponsive molecule that has altered optical response (both colourimetric and fluorescent) after reacting with hydrazine.
1. Introduction
Hydrazine (N2H4) is classified as a frequently used industrial substrate with applications in sectors including the aerospace industry as rocket fuel propellants,1,2 the pharmaceutical industry,3 and chemical industries that produce textile dyes and pesticides.4,5 Despite hydrazine's growing demand for these and other industries, it is categorized as a likely carcinogen and is toxic for the liver, kidneys and the central nervous system of humans.5–7 Humans can be exposed to hydrazine by inhaling its vapours or ingesting water contaminated with it, which can lead to symptoms including irritation of eyes, nose, and throat, dizziness, and nausea.5 Hydrazine and its derivatives are also considered as genotoxic impurities that can form methyl adducts with nucleotide bases leading to DNA damage and gene mutations.8,9 All of these severe effects have resulted in the US Environmental Protection Agency's (EPA) suggesting a low threshold limit value (∼10 ppb).7,8 Even the residual fuel-based debris falling into the oceans after satellite launches has led to severe environmental concerns globally because of hydrazine.10 This fact and the steady increase in supply and demand of hydrazine makes it essential to develop simple, user-friendly, and cost-effective methods for detecting this toxic substance.11,12Existing detection methods that employ potentiometry,13 ion-selective electrodes,14 capillary electrophoresis15 metal and metal oxide-based nanostructures16–18 rely on the fabrication of tools, technical processes and time-consuming detection. Chromatography-based techniques4,19–21 rely on the low volatility and highly polar nature of hydrazine.4 These methods also suffer from fundamental problems associated with sophisticated equipment and experiment design, long processing times and greater equipment costs, which limit their use in public places.
Small molecule-based probes can detect hydrazine due to the toxin's nucleophilic behavior by reacting with it to produce a visual change in colour making them potentially more cost-effective and user-friendly.6,22–36 While fluorescence-based methods have the advantage of being highly sensitive, an optical response based on a simple change in the colour of a material can be more suitable for ‘naked-eye’ detection. A method that combines both optical techniques would provide a heightened level of reliability.3,37,38
Photochromic molecular systems reversibly transform into two or more easily recognizable, differently coloured forms when exposed to different colours of light and have been successfully used as detectors for small analytes.39 Those based on the dithienylethene backbone are of specific interest due to their noteworthy optical and thermal properties, and fast response times.40–44 These small organic molecules can easily be integrated in bulk materials to act as surface coatings on construction materials and textiles, and would not require any additional electronic controls when making the user aware of the presence of target analytes.
The reported examples of dithienylethene-based photochromic detectors have targeted metal ions,45–47 anions,48,49 biomolecules50 and toxic gases.51–53 The example we describe in this report detects hydrazine. Our photoresponsive compound offers a straightforward, easy-to-read visual colour change when exposed to hydrazine. A ‘turn-on’ emission output signal complements the change in colour and provides an additional way to detect this toxic analyte.
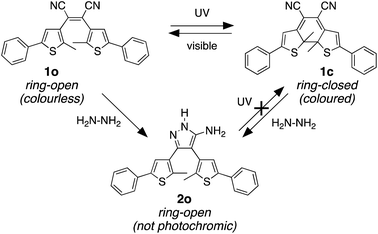
Our molecular design was inspired by the reaction of molecular backbones containing nitrile functional groups with hydrazine and the photoresponsive behaviour of the dithienylethene architecture as shown in Scheme 1. Compound 1o contains a photoreactive hexatriene common to all dithienylethenes and undergoes a ring-closing reaction when exposed to UV light to produce isomer 1c. While the ring-open isomer (1o) is colourless, its ring-closed counterpart is coloured due to the extended π-conjugated system running along the backbone of the molecule. Visible light is absorbed by 1c and drives the equilibrium back to 1o resetting the system.
It is well-known that hydrazine readily reacts with substituted cyanoethylenes to produce aminopyrazoles.54–56 In our case, both isomers (1o and 1c) react with hydrazine to produce the same aminopyrazole (2o), which is predicted to be non-photoresponsive as already demonstrated for similar pyrazole containing dithienylethenes57 and, therefore, is colourless. While the ring-open isomer (1o) is also colourless, it has limited appeal as a visual detector of hydrazine, although as will be shown, it does provide a means of detection using changes in ‘turn-on’ emission. The ring-closed isomer offers a convenient way to detect hydrazine because the blue colour of 1c should disappear when it is converted to 2o. In this manuscript, we describe how both visual changes in colour and emission offer facile methods to detect an important toxin. An additional appeal is that our detector traps hydrazine and removes it from the environment.
2. Results and discussion
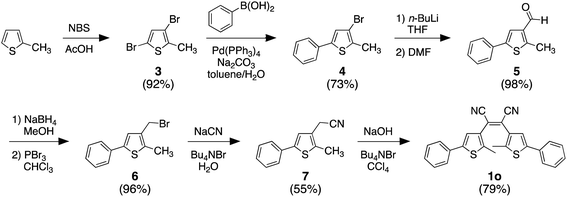
2.1 Synthesis of compounds 1o and 1c
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH2Cl2/hexanes as the eluant and is stable as long as it is kept away from light.
1 CH2Cl2/hexanes as the eluant and is stable as long as it is kept away from light.
2.2 Treatment of 1c with hydrazine to produce 2o
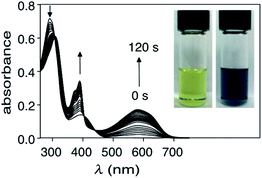
When a solution of ring-closed isomer 1c in DMSO is treated with hydrazine monohydrate, there is a rapid change in the absorption spectrum and an obvious change in colour of the solution (Fig. 2). In the spectrum, the broad band centred at 580 nm characteristic for the ring-closed isomer disappears and the band at 290 nm increases. From the pseudo-first order plot of these changes, a rate constant of 5.5 × 10−3 s−1 for the disappearance of 1c can be estimated (see ESI† for details).The product of the reaction of 1c with hydrazine can be isolated by column chromatography using silica gel and 15% EtOAc in hexanes as the eluant and confirmed as 2o using 1H and 13C NMR spectroscopy, and FT-IR spectroscopy (ESI†). The most characteristic change when 1c reacts with hydrazine is the disappearance of the band corresponding to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching at
N stretching at ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 2210 cm−1 in the FT-IR spectrum with the simultaneous appearance of a new set of bands corresponding to the amines (N–H stretching at
= 2210 cm−1 in the FT-IR spectrum with the simultaneous appearance of a new set of bands corresponding to the amines (N–H stretching at ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 3319–3076 cm−1, N–H bending at
= 3319–3076 cm−1, N–H bending at ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 1625 cm−1, and C–N stretching at
= 1625 cm−1, and C–N stretching at ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 1160 cm−1). In case of 1H NMR spectroscopy, the peak corresponding to the thiophene protons of 1c shift downfield from δ = 6.72 ppm to δ = 7.10 ppm supporting the restoration of aromaticity of the thiophene π-system as a result of ring-opening (for comparison, the thiophene proton in 1o appears at 6.91 ppm in the 1H NMR spectrum). The 13C NMR results show the disappearance of the peak at δ = 115.62 ppm corresponding to the carbon atoms of the nitrile groups and appearance of peaks at δ = 117.81 ppm for the bridging carbons of central pyrazole ring and δ = 153.24 ppm for carbon atom connected to the NH2 group. Tautomer 3o can be ruled out as the product based on density functional theory (DFT) computational methods based, which estimates that it is less stable than 2o (ESI†).
= 1160 cm−1). In case of 1H NMR spectroscopy, the peak corresponding to the thiophene protons of 1c shift downfield from δ = 6.72 ppm to δ = 7.10 ppm supporting the restoration of aromaticity of the thiophene π-system as a result of ring-opening (for comparison, the thiophene proton in 1o appears at 6.91 ppm in the 1H NMR spectrum). The 13C NMR results show the disappearance of the peak at δ = 115.62 ppm corresponding to the carbon atoms of the nitrile groups and appearance of peaks at δ = 117.81 ppm for the bridging carbons of central pyrazole ring and δ = 153.24 ppm for carbon atom connected to the NH2 group. Tautomer 3o can be ruled out as the product based on density functional theory (DFT) computational methods based, which estimates that it is less stable than 2o (ESI†).
We propose the mechanism shown in Scheme 3 to explain the conversion of the ring-closed isomer (1c) to pyrazole 2o. Initially hydrazine acts as a nucleophile in an addition reaction opening the cyclohexadiene ring system. Elimination followed by cyclization produces the aminopyrazole as described in the literature. The conversion of ring-open 1o to pyrazole 2o follows what has already been reported for cyanoethene derivatives.54–56
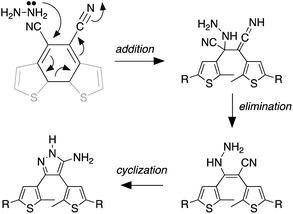 | ||
| Scheme 3 The mechanism that accounts for the ring-opening reaction in the presence of hydrazine. For the full mechanism, see ref. 54–56. | ||
2.3 Limit of detection (LoD) of hydrazine in solution
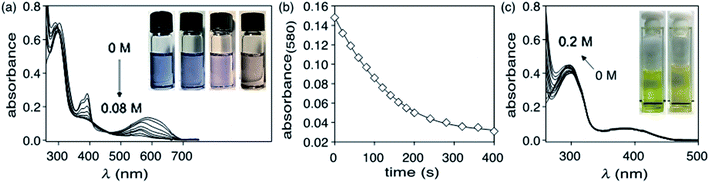
The limit of detection (the lowest concentration of hydrazine that can be measured with reasonable certainty)60 using the ring-closed isomer (1c) can be estimated by treating a solution of 1c (4.8 × 10−5 M) in DMSO with a stock solution of hydrazine monohydrate (1.7 × 10−4 M) in DMSO in incremental amounts (5 μL) for a total of 110 μL (corresponding to a total concentration of 1.7 × 10−5 M N2H4), and monitoring the decrease in the absorbance at 580 nm (ESI†). The LoD of hydrazine obtained from linear regression is estimated to be 9.0 × 10−7 M (29 ppb) (ESI†). The value of limit of detection obtained for probe 1c is much lower than the permissible exposure limit (PEL) for hydrazine determined by the federal Occupational Safety and Health Administration (OSHA) (1 ppm, 8 h time-weighted average),61 and the non-disabling and transient acute exposure guideline level (AEGL-1) established by US EPA (0.1 ppm, 8 h time-weighted average).62 However, the value obtained remains higher than the TLV recommended by American Conference of Governmental Industrial Hygienists (ACGIH) (0.01 ppm, 8 h time-weighted average).622.4 Changes in emission
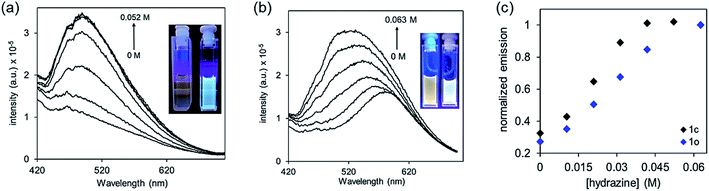
In addition to the conveniently observed change in colour, the photoisomerization process also causes changes in the electronic structure of the molecule that can, in turn, influence the luminescence of 1o and 1c.63 Unlike the majority of dithienylethenes, which are weakly emissive in both their ring-open and ring-closed forms, compound 1o is observably emissive due to a localized donor–acceptor intramolecular charge transfer from electron-rich thiophenes to strongly electron-poor nitrile groups (Fig. 3).64,65 On the other hand, ring-closed isomer 1c has π-electrons delocalized over the entire molecular backbone and is only weakly emissive.66,67 Treating DMSO solutions of either isomer with hydrazine monohydrate results in a substantial increase in emission (Fig. 3), which can also be used as an output signal for the detection.2.5 Detection of hydrazine vapours
Visual detection of hydrazine vapour can be readily demonstrated by exposing a piece of filter paper treated with a few drops of a solution of 1c (1 mg) dissolved in 0.1 mL of DMSO, drying the paper in a vacuum oven, placing it elevated in a vial containing 20 drops of hydrazine monohydrate and gently heating to generate hydrazine vapour. The originally blue and non-emissive paper loses its blue colour after a few minutes of exposure and becomes highly emissive under 365 nm light (Fig. 4). When exposed to light of wavelength 365 nm the filter paper remains pale yellow and emissive demonstrating that the non-photoresponsive 2o was generated.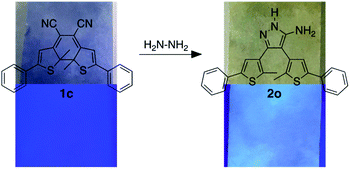 | ||
| Fig. 4 Changes in colour (top) and emission (bottom, λex = 365 nm) of a piece of filter paper soaked in compound 1c and exposed to vapours of hydrazine. | ||
2.6 Competitive analytes
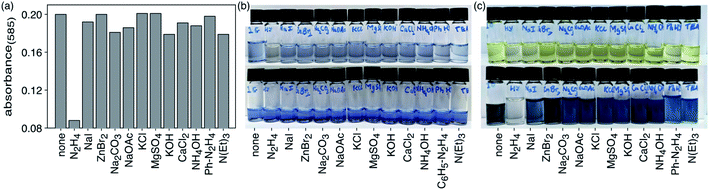
The selectivity of compound 1c to hydrazine over other possible interfering analytes such as cations (K+, Ca2+, Zn2+, Na+, Mg2+, NH4+), anions (I−, Br−, CO32−, OAc−, Cl−, SO42−), triethylamine (NEt3) and phenylhydrazine can be established by carrying out parallel UV-vis absorption experiments. When DMSO solutions of ring-closed isomer 1c (4.8 × 10−5 M) are treated with 20 molar equivalents of various analytes in ultrapure water (1.0 × 10−3 M) and incubated in dark at 24 °C for 30 min to ensure complete reaction, only the solution treated with hydrazine loses its deep blue colour and has a significant decrease in the intensity of the absorption band centred at 585 nm in the UV-vis absorption spectrum (Fig. 4). Only small changes are observed for the other analytes.When the identical experiment is carried out with ring-open isomer 1o the solutions containing all the potentially interfering analytes retained their bright yellow colour while the solution containing hydrazine became fainter. When exposed to UV light (312 nm) for 60 s, all the solutions turned blue except for the one containing hydrazine, which demonstrates the selectivity of the detection system (Fig. 5).
3. Experimental
3.1 Materials and methods
1H and 13C NMR characterizations were performed on a Bruker Avance-400 instrument with a 5 mm inverse probe operating at 400.13 MHz for 1H NMR and 100.61 MHz for 13C NMR unless stated otherwise. Chemical shifts (δ) are reported in parts per million (ppm) relative to tetramethylsilane using the residual solvent peak as a reference, and splitting patterns are designated as s (singlet), d (doublet), dd (doublet of doubles), t (triplet) and m (multiplet). Coupling constants (J) are reported in Hertz. UV-vis absorption spectra were recorded on a Shimadzu UV-3600 Plus spectrophotometer. Fluorescence measurements were performed on a PTI Quantamaster spectrofluorometer. A JDS Uniphase 980 nm laser diode (device type L4-9897510-100M) coupled to a 105 μm (core) fibre was employed as the excitation source. The output of the diode laser was collimated and directed on the 110 samples using a Newport F-91-C1-T Multimode Fiber Coupler. The visible emissions were collected from the samples at π/2 from the incident beam in the plane of the spectrometer. All of the samples were held in a square quartz cuvette (path length of 1 cm). All spectra were corrected for instrument sensitivity. IR spectroscopy measurements were conducted on PerkinElmer Spectrum Two™ IR spectrometer equipped with universal ATR accessory with a 9-bounce diamond top-plate. High Resolution Mass Spectroscopy (HRMS) measurements were performed using an Agilent 6210 TOF LC/MS in ESI-(+) mode. Microanalysis (C, H, N) was carried out using a Carlo Erba EA 1110 CHN Elemental Analyser. Melting points were measured using a Gallenkamp melting point apparatus and are uncorrected.
3.2 Synthetic methods
Only the final step in Scheme 2 is described below. All other compounds were prepared according to the literature without modifications.57,58 The analysis of all compounds matched those in the literature.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexanes/EtOAc). Upon completion, the reaction mixture was poured into water and extracted with chloroform (2 × 10 mL), dried over MgSO4, filtered and concentrated under reduced pressure. Purification by column chromatography using silica gel (10
1 hexanes/EtOAc). Upon completion, the reaction mixture was poured into water and extracted with chloroform (2 × 10 mL), dried over MgSO4, filtered and concentrated under reduced pressure. Purification by column chromatography using silica gel (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexanes/EtOAc) afforded 735 mg (79%) of the product as a yellow solid.
1 hexanes/EtOAc) afforded 735 mg (79%) of the product as a yellow solid.1H NMR (400 MHz, CDCl3): δ 7.40 (d, J = 8.1 Hz, 3H), 7.33 (d, J = 7.2 Hz, 2H), 7.29 (d, J = 7.1 Hz, 1H), 6.91 (s, 1H), 2.31 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 142.4, 133.1, 131.8, 129.4, 128.6, 127.5, 126.0, 121.1, 116.4, 15.0.
FT-IR (diamond ATR): ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (cm−1) 2924–2851 (C–H stretch, aromatic), 2210 (C
(cm−1) 2924–2851 (C–H stretch, aromatic), 2210 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretch), 1445 (C–C stretch, aromatic), 755 (C–H bend, oop), 690 (C–S stretch, thiophene).
N stretch), 1445 (C–C stretch, aromatic), 755 (C–H bend, oop), 690 (C–S stretch, thiophene).
Melting point: 132–133 °C.
HRMS (ESI): m/z (M + H) calculated for C26H18N2S2: 423.098417, found: 423.097986.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH2Cl2/hexanes).
1 CH2Cl2/hexanes).1H NMR (400 MHz, CDCl3): δ 7.58 (d, J = 6.8 Hz, 2H), 7.46 (d, J = 6.8 Hz, 2H), 7.44 (d, J = 6.7 Hz, 1H), 6.72 (s, 1H), 2.17 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 170.72, 144.69, 130.82, 129.20, 127.25, 115.62, 108.90, 99.58, 57.19, 29.87.
FT-IR (diamond ATR): ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (cm−1) 2924–2851 (C–H stretch, aromatic), 2210 (C
(cm−1) 2924–2851 (C–H stretch, aromatic), 2210 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretch), 1445 (C–C stretch, aromatic), 755 (C–H bend, oop), 690 (C–S stretch, thiophene).
N stretch), 1445 (C–C stretch, aromatic), 755 (C–H bend, oop), 690 (C–S stretch, thiophene).
1H NMR (600 MHz, CDCl3): δ 7.68–7.64 (m, 2H), 7.42 (d, J = 9.0 MHz, 3H), 7.10 (s, 1H), 2.11 (s, 3H).
13C NMR (151 MHz, CDCl3): δ 153.24, 133.77, 132.27, 129.14, 129.07, 128.71, 128.62, 127.87, 125.75, 125.60, 117.81, 14.72.
FT-IR (diamond ATR): ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (cm−1) 3319–3076 (N–H stretch), 2924 (C–H stretch, aromatic), 1625 (N–H bend), 1445 (C–C stretch, aromatic), 1160 (C–N stretch), 755 (C–H bend, oop), 690 (C–S stretch, thiophene).
(cm−1) 3319–3076 (N–H stretch), 2924 (C–H stretch, aromatic), 1625 (N–H bend), 1445 (C–C stretch, aromatic), 1160 (C–N stretch), 755 (C–H bend, oop), 690 (C–S stretch, thiophene).
Melting point: 110–113 °C.
Elemental analysis: C = 68.22%, H = 5.32%, N = 8.84%, S = 14.47%.
Conclusions
In this manuscript, we have described the use of a coloured photoresponsive molecule to conveniently detect toxic levels of hydrazine. The combination of the disappearance of colour and generation of bright emission provides two ways to ensure reliability. Our detector also consumes the hydrazine as it changes its optoelectronic properties, which can be considered as a hydrazine trap if applied to large surface area materials. The advantages of our system are based on a distinct colour change with excellent selectivity (see ESI† for a table of comparative systems).Author contributions
B. K. conducted all the experimentation, characterization, prepared the original draft and worked on the revisions. R. R. worked on investigation and validation of the photochemistry experiments and preparation of the first draft. B. K. formulated the idea of the project and developed the methodology. N. R. B. worked on all drafts of the manuscript and supervised the overall project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada and the Canada Research Chairs Program. This work made use of 4D LABS shared facilities supported by the Canada Foundation for Innovation (CFI), British Columbia Knowledge Development Fund (BCKDF) and Simon Fraser University.Notes and references
- R. Fletcher-Wood, Magnificent molecules: Hydrazine, https://edu.rsc.org/magnificent.molecules/hydrazine/2000023.article, 2016 Search PubMed.
- L. Cui, C. Ji, Z. Peng, L. Zhong, C. Zhou, L. Yan, S. Qu, S. Zhang, C. Huang, X. Qian and Y. Xu, Anal. Chem., 2014, 86, 4611–4617 CrossRef CAS PubMed.
- D. P. Elder, D. Snodin and A. Teasdale, J. Pharm. Biomed. Anal., 2011, 54, 900–910 CrossRef CAS PubMed.
- B. Roy and S. Bandyopadhyay, Anal. Methods, 2018, 10, 1117–1139 RSC.
- K. H. Nguyen, Y. Hao, W. Chen, Y. Zhang, M. Xu, M. Yang and Y. N. Liu, Luminescence, 2018, 33, 816–836 CrossRef.
- U.S.E.P. Agency, Hydrazine, 2016 Search PubMed.
- U.S. Department of Health and Human Services, Occupational Safety and Health Guideline for Hydrazine Potential Human Carcinogen, 1988 Search PubMed.
- S. Parodi, S. De Flora, M. Cavanna, A. Pino, L. Robbiano, C. Bennicelli and G. Brambilla, Cancer Res., 1981, 41, 1469–1482 CAS.
- M. J. Zeilmaker, M. J. Horsfall, J. B. van Helten, B. W. Glickman and G. R. Mohn, Mol. Carcinog., 1991, 4, 180–188 CrossRef CAS PubMed.
- M. Byers and C. Byers, Polar Rec., 2017, 53, 580–591 CrossRef.
- S. Satsangi, Hydrazine Market by Application (Pharmaceuticals, Water Treatment, Blowing Agents, Agrochemicals, and Others) – Global Opportunity Analysis and Industry Forecast, 2014–2022, www.alliedmarketresearch.com/hydrazine-market, accessed 13 April 2021 Search PubMed.
- Hydrazine Market Size, Share & Trends Analysis Report By Application, Regional Outlook, Competitive Strategies, And Segment Forecasts, 2019 to 2025, www.grandviewresearch.com/industry-analysis/hydrazine-market, accessed 13 April 2021 Search PubMed.
- J. R. Stetter, K. F. Blurton, A. M. Valentine and K. A. Tellefsen, J. Electrochem. Soc., 1978, 125, 1804–1807 CrossRef CAS.
- N. M. Ratcliffe, Anal. Chim. Acta, 1990, 239, 257–262 CrossRef CAS.
- J. Liu, W. Zhou, T. You, F. Li, E. Wang and S. Dong, Anal. Chem., 1996, 68, 3350–3353 CrossRef CAS.
- C. Batchelor-McAuley, C. E. Banks, A. O. Simm, T. G. J. Jones and R. G. Compton, Analyst, 2006, 131, 106–110 RSC.
- A. Umar, M. M. Rahman, S. H. Kim and Y. B. Hahn, Chem. Commun., 2008, 166–168 RSC.
- J. Liu, Y. Li, J. Jiang and X. Huang, Dalton Trans., 2010, 39, 8693–8697 RSC.
- M. Sun, L. Bai and D. Q. Liu, J. Pharm. Biomed. Anal., 2009, 49, 529–533 CrossRef CAS PubMed.
- H. Bhutani, S. Singh, S. Vir, K. K. Bhutani, R. Kumar, A. K. Chakraborti and K. C. Jindal, J. Pharm. Biomed. Anal., 2007, 43, 1213–1220 CrossRef CAS PubMed.
- T. Kean, J. H. M. B. Miller, G. G. Skellern and D. Snodin, Pharmeuropa Scientific Notes, 2006, pp. 23–33 Search PubMed.
- T. Miura, Y. Urano, K. Tanaka, T. Nagano, K. Ohkubo and S. Fukuzumi, J. Am. Chem. Soc., 2003, 125, 8666–8671 CrossRef CAS.
- X. Jiang, M. Shangguan, Z. Lu, S. Yi, X. Zeng, Y. Zhang and L. Hou, Tetrahedron, 2020, 76, 1–6 Search PubMed.
- X. Jin, C. Liu, X. Wang, H. Huang, X. Zhang and H. Zhu, Sens. Actuators, B, 2015, 216, 141–149 CrossRef CAS.
- S. W. Thomas and T. M. Swager, Adv. Mater., 2006, 18, 1047–1050 CrossRef CAS.
- X. Guo, S. Li, S. Mu, Y. Zhang, X. Liu and H. Zhang, Spectrochim. Acta, Part A, 2020, 226, 117625 CrossRef CAS PubMed.
- M. Li, H. Chen, X. Liu, Y. Wang, N. Zhang and K. Zheng, Tetrahedron Lett., 2019, 60, 151219 CrossRef CAS.
- Y. Li, X. Wu, S. Yang, S. Liang, H. Tian and B. Sun, Anal. Sci., 2020, 36, 323–327 CrossRef CAS PubMed.
- L. Xiao, J. Tu, S. Sun, Z. Pei, Y. Pei, Y. Pang and Y. Xu, RSC Adv., 2014, 4, 41807–41811 RSC.
- B. Roy, S. Halder, A. Guha and S. Bandyopadhyay, Anal. Chem., 2017, 89, 10625–10636 CrossRef CAS PubMed.
- J. Fan, W. Sun, M. Hu, J. Cao, G. Cheng, H. Dong, K. Song, Y. Liu, S. Sun and X. Peng, Chem. Commun., 2012, 48, 8117–8119 RSC.
- S. Goswami, S. Paul and A. Manna, RSC Adv., 2013, 3, 18872–18877 RSC.
- H. Xu, Z. Huang, Y. Li, B. Gu, Z. Zhou, R. Xie, X. Pang, H. Li and Y. Zhang, Analyst, 2018, 143, 4354–4358 RSC.
- J. Ma, J. Fan, H. Li, Q. Yao, J. Xia, J. Wang and X. Peng, Dyes Pigm., 2017, 138, 39–46 CrossRef CAS.
- W. Xu, X. Li, J. Yin, M. Han, W. Liu, Y. Yang and W. Li, J. Photochem. Photobiol., A, 2020, 390, 112262 CrossRef CAS.
- Y. Jung, I. G. Ju, Y. H. Choe, Y. Kim, S. Park, Y. M. Hyun, M. S. Oh and D. Kim, ACS Sens., 2019, 4, 441–449 CrossRef CAS PubMed.
- K. Vijay, C. Nandi and S. D. Samant, RSC Adv., 2014, 4, 30712–30717 RSC.
- J. Liu, T. Li, S. Wang, Q. Qi, H. Song, Z. Li, L. Yang and W. Huang, RSC Adv., 2020, 10, 5572–5578 RSC.
- M. Qin, Y. Huang, F. Li and Y. Song, J. Mater. Chem. C, 2015, 3, 9265–9275 RSC.
- M. Irie, Chem. Rev., 2000, 100, 1685–1716 CrossRef CAS PubMed.
- B. L. Feringa in Molecular Switches ed. B. Feringa, Wiley-VCH Verlag GmbH, Weinheim, 2001 Search PubMed.
- M. Irie in Organic Photochromic and Thermochromic Compounds Main Photochromic Families ed. J. C. Crano and R. J. Guglielmetti, Springer US, New York, 2002, pp. 207–222 Search PubMed.
- Z. Li, Y. Song, Y. Dai, Y. Pei, Z. Lu and H. Guo, Opt. Mater., 2019, 95, 109235 CrossRef CAS.
- Z. Li, Y. Pei, S. Hou, Y. Dai, D. Liu, J. Zhu, Y. P. Zhu and X. Liu, Dyes Pigm., 2020, 179, 108419 CrossRef CAS.
- Q. Zou, X. Li, J. Zhang, J. Zhou, B. Sun and H. Tian, Chem. Commun., 2012, 48, 2095–2097 RSC.
- Q. Zou, J. Jin, B. Xu, L. Ding and H. Tian, Tetrahedron, 2011, 67, 915–921 CrossRef CAS.
- S. Huang, Z. Li, S. Li, J. Yin and S. Liu, Dyes Pigm., 2012, 92, 961–966 CrossRef CAS.
- Z. Li, H. Zhang, H. Zhang, Y. Xie, H. Chen, C. He, Y. Wang, H. Ya and H. Guo, J. Chem. Res., 2018, 42, 305–308 CrossRef CAS.
- Z. Li, Y. Song, Y. Dai, Y. Pei, Z. Lu and H. Guo, Opt. Mater., 2019, 95, 109235 CrossRef CAS.
- K. Liu, Y. Wen, T. Shi, Y. Li, F. Li, Y. Zhao, C. Huang and T. Yi, Chem. Commun., 2014, 50, 9141–9144 RSC.
- F. Nourmohammadian, T. Wu and N. R. Branda, Chem. Commun., 2011, 47, 10954–10956 RSC.
- G. Vamvounis and N. Sandery, Aust. J. Chem., 2015, 68, 1723–1726 CrossRef CAS.
- V. Valderrey, A. Bonasera, S. Fredrich and S. Hecht, Angew. Chem., Int. Ed., 2017, 56, 1914–1918 CrossRef CAS.
- C. L. Dickinson, J. K. Williams and B. C. McKusic, J. Org. Chem., 1964, 29, 1915–1919 CrossRef CAS.
- S. M. Hecht, D. Werner, D. Traficante, M. Sundaralingam, P. Prusiner, T. Ito and T. Sakurai, J. Org. Chem., 1975, 40, 1815–1822 CrossRef CAS.
- A.-S. S. H. Elgazwy and M. R. M. Rafaee, Org. Chem.: Curr. Res., 2013, 2, 1–27 Search PubMed.
- M. M. Krayushkin, B. V. Lichitskii, A. P. Mikhalev, B. V. Nabatov, A. A. Dudinov and S. N. Ivanov, Russ. J. Org. Chem., 2006, 42, 860–864 CrossRef CAS.
- M. Ohsumi, T. Fukaminato and M. Irie, Chem. Commun., 2005, 3921–3923 RSC.
- B. Kaur, R. Raza, M. J. Stashick and N. R. Branda, Org. Chem. Front., 2019, 6, 1253–1256 RSC.
- A. D. McNaught and A. Wilkinson, IUPAC Compendium of Chemical Terminology (the ‘Gold Book’), Blackwell Scientific Publications, Oxford, 2nd edn, 1997 Search PubMed.
- U.S. Department of Health and Human Services, Occupational Safety and Health Guideline for Hydrazine Potential Human Carcinogen, 1988 Search PubMed.
- National Research Council in Acute Exposure Guideline Levels for Selected Airborne Chemicals, The National Academies Press, Washington, DC, 2010, vol. 8, DOI:10.17226/12770.
- T. B. Norsten and N. R. Branda, J. Am. Chem. Soc., 2001, 123, 1784–1785 CrossRef CAS PubMed.
- T. Kawai, T. Koshido, Y. Kaneuchi and K. Yoshino, Thin Solid Films, 1996, 273, 195–198 CrossRef CAS.
- M. Irie, T. Fukaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174–12277 CrossRef CAS PubMed.
- K. Kasatani, S. Kambe and M. Irie, J. Photochem. Photobiol., A, 1999, 122, 11–15 CrossRef CAS.
- K. Matsuda and M. Irie, J. Photochem. Photobiol., C, 2004, 5, 169–182 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra03677g |
| ‡ WARNING: Hydrazine vapour are toxic upon ingestion or inhalation. Extreme caution must be exercised while handling. |
| This journal is © The Royal Society of Chemistry 2021 |