Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Retracted Article: Boric acid in magnetized water: clean and powerful media for synthesis of 3,4-dihydropyrimidin-2(1H)-ones†
Vahid Khakyzadeh *a,
Ahmad Reza Moosavi-Zare
*a,
Ahmad Reza Moosavi-Zare b,
Sahra Sheikhaleslamia,
Amir Ehsania,
Salbin Sediqia,
Mohammad Rezaei-Goharb and
Zahra Jalilianc
b,
Sahra Sheikhaleslamia,
Amir Ehsania,
Salbin Sediqia,
Mohammad Rezaei-Goharb and
Zahra Jalilianc
aDepartment of Chemistry, K. N. Toosi University of Technology, P.O. Box 16315-1618, 15418 Tehran, Iran. E-mail: v.khakyzadeh@kntu.ac.ir
bHamedan University of Technology, Department of Chemistry, Hamedan, 65155, Iran
cChemistry Department, College of Science, University of Kurdistan, Pasdaran Street, Sanandaj, 66177-15177, Iran
First published on 28th June 2021
Abstract
Water was magnetized via an external magnetic field and employed, for the first time, as a solvent in green preparation of 3,4-dihydropyrimidin-2(1H)-ones by the one-pot three-component condensation reaction using boric acid as a catalyst. Shorter reaction times, higher yields, and cleaner reaction profiles were some advantages of using magnetic water.
It is well-known that water is the most crucial matter in nature, thus in numerous papers, physical and chemical properties of water and also their changes under actions of external factors like electromagnetic fields have been investigated,1 although the principle of magnetic field (MF) treatment on the water is still a controversial subject, and no clear mechanisms of this effect have been reported in the literature.2 Many studies have indicated that the physicochemical properties of water change by passing through the MF,3 for example, inter- and intra-cluster hydrogen bonds, coagulation, polymorphism of the crystals, the zeta potential of the precipitated or dispersed particles, viscosity, diffusivity, surface tension, friction coefficient, pH values, electrical conductivity, and some physical properties of water, including specific heat, evaporation amount and boiling point.4 Owing to the changes in physicochemical properties, magnetic water has been utilized mainly for agriculture, wastewater treatment, industry, and construction.5 The magnetization of water and its effectiveness on the physical and chemical properties of water depends on several factors including, magnetization and exhibition of a magnetic field, magnetic gradient, Lorentz force, and magnetic memory.6 Three mechanisms have been proposed for how the magnetic field affects water particles: (I) the spontaneous formation and collapse of cationic metal colloidal units in the interaction of magnetism and water, which leads to increase sedimentation in water; (II) the change in polarization of the dissolved ions in the water, the shape and size of the hydration layer cause some variations in the water properties; and (III) due to the polarity of the water molecules, the magnetic field will directly affect the nature of the water molecules, the third mechanism is largely consistent with the experiments performed.7 Recently, few research groups such as Alabi, Zuniga, and Rodgers have started to examine the magnetic field effects on chemical reactions and their consequences in yield and rate of reaction or product distribution. It was considered that these effects might be performed via the radical pair mechanism.8
Encouraged by previous works in the application of magnetic water, we decided to produce and employ magnetic water as a solvent in some interesting organic reactions.9 The multi-component condensation reactions are valuable protocols for synthesizing desired compounds in one step without the separation of any intermediates in organic chemistry.10 In this chemical strategy, some beneficial features are high yields, shorter reaction times, high atomic economy, and low waste materials and energy consumption.11 So staying in this direction can bring us closer to the goals of green chemistry.12 Preparation of 3,4-dihydropyrimidin-2(1H)-one (DHPMs) is significant due to their therapeutic and pharmacological properties, for example; Batzelladine, found in marine natural alkaloids, as an HIV inhibitor,13a also monastrol,13b and SQ 32926,13c have been explained as synthetic or natural DHPs with biological properties (Scheme 1). The Biginelli reaction was reported for the first time by Pietro Biginelli.14 In this reaction, 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) were prepared by a three-component condensation reaction of aldehyde, urea, and β-ketoester as interesting compounds with a potential for pharmaceutical applications. Various catalysts were reported for this reaction such as, [bmim][MeSO4],15 FeCl3,16 RuCl3,17 TMSCl,18 ZrSA19 and [H-NMP][CH3SO3].20 Due to the important properties of these compounds, finding the new protocols for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones are still needed.
Nowadays, many reactions need catalysts to carry out. Among many kinds of them, organocatalysts have attracted much attention. They are well-known as artificial catalysts for these plans. In comparison with inorganic catalysts and enzymes, they are not complicated, regularly less toxic, and widely available.21 Boric acid, as an organocatalyst, aqueous solution system, is one of the efficient and green catalytic systems in organic chemistry. Boric acid was successfully utilized in the preparation of different organic compounds such as, pyrano[2,3-d]pyrimidine diones,22 tetrahydrobenzo[b] pyrans,23 N,N′-alkylidene bisamides,24 4H-isoxazol-5-ones,25 2-arylamino-2-phenylacetamide,26 bis(indolyl)methanes.27 In these mild conditions, H+, which is abstracted from water by the interaction with B(OH)3, efficiently catalyzes the reaction,22,23 and water as a solvent plays a key role in these reactions. In water molecules, the non-uniform distribution of electrons leads to the creation of positive and negative particle charges. Based on points mentioned earlier, we wondered to evaluate the effect of magnetized water as a solvent and also using boric acid as a catalyst, on the synthesis of 3,4-dihydropyrimidin-2(1H)-one derivative (Scheme 2).
 | ||
| Scheme 2 The one-pot three-component preparation of 3,4-dihydropyrimidin-2(1H)-ones in magnetized water/B(OH)3 system. | ||
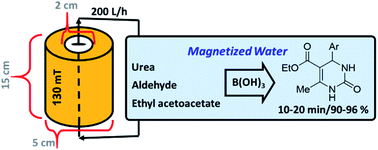
To prepare magnetic water, a cylindrical system with an approximate capacity of 100 mL was filled with neodymium super-magnets (Nd), which generated a magnetic field of 130 mT at the center and diameter of the tube, was used to construct the reactor of magnetic water. The water used in the system was distilled twice by the Prima30 machine and circulated in the system by a (SEBO P-280) pump with a power of 200 liters per hour, and after 8 hours, the water became magnetic. After the preparation of magnetic water, some main physicochemical properties, such as viscosity, gas solubility, refractive index, surface tension, pH, UV-visible and FT-IR analysis, were evaluated.
Initially, the oxygen gas solubility in magnetic and ordinary water was measured by Winkler method based on standard method procedure.28 After photometric testing, it was found that the solubility of oxygen in magnetic water was slightly higher than in ordinary water (MW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-MW = 8
n-MW = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 ppm). We suggested that probably the paramagnetism of oxygen structure, as well as the molecular orbital of oxygen, can be effective in presenting this result. A paramagnetic structure with magnetic properties is more soluble in a magnetized solvent.29 Next, the viscosity, as an essential factor in the efficiency of organic reaction, was measured at different temperatures according to the reference method.30 The results determined that the viscosity of magnetic water was generally slightly lower than ordinary distilled water. Based on our observations, it was found that the viscosity differences were higher at low temperatures, indicating that at high temperatures, the hydrogen bonds are almost weakened and results in no significant differences between the viscosity of magnetic water and ordinary water. In contrast, at low temperatures, because of the high number of intra-cluster bonds in ordinary water, they form a colloidal bulk structure that increases its viscosity. The intermolecular structure of the magnetic water molecules is hexagonal layers that are interconnected by inter-cluster hydrogen bonds, which form a graphite-like structure and gives a particular softness to the water and reducing its viscosity (Fig. 1a).31 This property could be effective in running the organic reaction. Moreover, the refractive index of magnetic water and nonmagnetic water was also evaluated by a refractometer at different temperatures. It was found that at low temperatures, the refractive index of magnetic water was approximately the same as distilled water, whereas, at higher temperatures, the refractive index of magnetic water was lower than ordinary water. To justify this observation, it can be noted that magnetic water has a more stable structure than ordinary water, and this somewhat stable structure limited waste fractures when the photons traveled through the water (Fig. 1b). Interestingly, our results were contrary to Hosoda et al. who showed magnetic water has a higher refractive index.32 They explained that it is because of the lifetime of the H-bonds. pH measurements can also be evaluated as a criterion for investigating hydrogen bond clusters. For this issue in mind, the pH changes of the water were measured at different temperatures. It was found that the acidity of the magnetic water was lower than ordinary water, and the difference between their pH enhanced with increasing temperature (Fig. 1c). Surface tension analysis for magnetized water and distilled water showed that ordinary water has a higher surface tension in different temperatures rather than magnetic water which was in good agreement with Cho's report that magnetic field reduced the surface tension of natural water by about 8% (Fig. 1d).33
7 ppm). We suggested that probably the paramagnetism of oxygen structure, as well as the molecular orbital of oxygen, can be effective in presenting this result. A paramagnetic structure with magnetic properties is more soluble in a magnetized solvent.29 Next, the viscosity, as an essential factor in the efficiency of organic reaction, was measured at different temperatures according to the reference method.30 The results determined that the viscosity of magnetic water was generally slightly lower than ordinary distilled water. Based on our observations, it was found that the viscosity differences were higher at low temperatures, indicating that at high temperatures, the hydrogen bonds are almost weakened and results in no significant differences between the viscosity of magnetic water and ordinary water. In contrast, at low temperatures, because of the high number of intra-cluster bonds in ordinary water, they form a colloidal bulk structure that increases its viscosity. The intermolecular structure of the magnetic water molecules is hexagonal layers that are interconnected by inter-cluster hydrogen bonds, which form a graphite-like structure and gives a particular softness to the water and reducing its viscosity (Fig. 1a).31 This property could be effective in running the organic reaction. Moreover, the refractive index of magnetic water and nonmagnetic water was also evaluated by a refractometer at different temperatures. It was found that at low temperatures, the refractive index of magnetic water was approximately the same as distilled water, whereas, at higher temperatures, the refractive index of magnetic water was lower than ordinary water. To justify this observation, it can be noted that magnetic water has a more stable structure than ordinary water, and this somewhat stable structure limited waste fractures when the photons traveled through the water (Fig. 1b). Interestingly, our results were contrary to Hosoda et al. who showed magnetic water has a higher refractive index.32 They explained that it is because of the lifetime of the H-bonds. pH measurements can also be evaluated as a criterion for investigating hydrogen bond clusters. For this issue in mind, the pH changes of the water were measured at different temperatures. It was found that the acidity of the magnetic water was lower than ordinary water, and the difference between their pH enhanced with increasing temperature (Fig. 1c). Surface tension analysis for magnetized water and distilled water showed that ordinary water has a higher surface tension in different temperatures rather than magnetic water which was in good agreement with Cho's report that magnetic field reduced the surface tension of natural water by about 8% (Fig. 1d).33
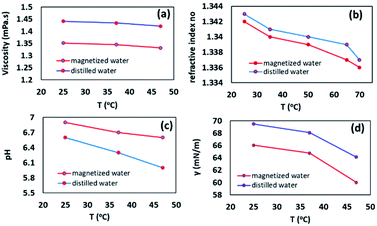 | ||
| Fig. 1 The viscosity, refractive index, pH measurements, and surface tension analysis for magnetic and distilled water in different temperatures. | ||
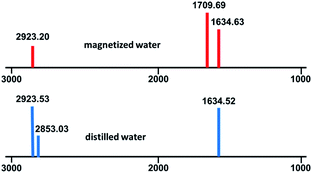
Eventually, for examining the optical properties of magnetic and ordinary water, IR and UV analyses were performed. No significant difference was detected in the UV spectra (ESI, S1†), but, interestingly, in the IR spectra, some peaks were weakened or disappeared, and some new peaks were observed (ESI, S2†). There are two differences in comparing ordinary and magnetic water IR spectra: (I) the detection of Peaks 2923.53 and 2853.03 is probably due to internal structural patterns of H2O molecules and/or hydrogen bond chain in ordinary water which in magnetic water these peaks are very weakened or not seen due to the new structural patterns of water molecules; (II) in the 1709.69 region, a new peak was detected in the magnetic water spectrum compared to ordinary water, which was attributed to the transition of the water phase from a complex irregular spherical structure to a regular hexagonal ring structure in magnetic water (Fig. 3).
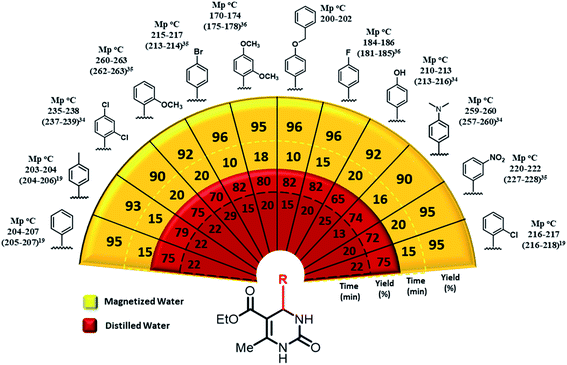 | ||
| Fig. 2 Scope of the synthesis of 3,4-dihydropyrimidin-2(1H)-ones in magnetized and distilled water as solvent under optimized condition.19,34–36 | ||
After preparation and characterization of magnetic water, we wonder to investigate the preparation of 3,4-dihydropyrimidin-2(1H)-ones using boric acid, in nonmagnetic water and also magnetic water as solvent under reflux conditions. To find optimized conditions, we have chosen the reaction of ethyl acetoacetate (1 mmol), urea (1 mmol), and benzaldehyde (1 mmol) as a model reaction (Table 1).
| Entrya | Catalyst (mol%) | Solvent | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction was caried out at 80 °C.b Isolated yield.c At = 25 °C.d At = 50 °C.e At = 100 °C. | ||||
| 1 | — | — | 180 | Trace |
| 2 | 3 | H2O | 55 | 45 |
| 3 | 5 | H2O | 40 | 50 |
| 4 | 10 | H2O | 30 | 60 |
| 5 | 15 | H2O | 22 | 75 |
| 6 | 15 | H2Oc | 60 | 25 |
| 7 | 15 | H2Od | 40 | 45 |
| 8 | 15 | H2Oe | 22 | 78 |
| 9 | 15 | m-H2O | 15 | 95 |
| 10 | 15 | n-Hexane | 40 | 15 |
| 11 | 15 | CH2Cl2 | 40 | 35 |
| 12 | 15 | Ethanol | 35 | 80 |
This reaction was investigated in the presence of different amounts of boric acid at temperatures ranging between 25 °C and 100 °C under aqueous conditions (entry 1–12). At first, in the model reaction without catalyst and solvent at 80 °C, a trace yield was observed (entry 1). Then we checked different amounts of the boric acid as a catalyst (entry 2–5, 3–15 mol%) in water, and 75% of desired product was seen when we employed a 15 mol% catalyst. After that, the reaction was carried out at different temperatures (entry 6–8). No improvement was recognized at 100 °C. Next, magnetized water was employed as a solvent in the model reaction with a similar condition of entry 5. Interestingly, shorter reaction time and higher yield were obtained (entry 9, 15 min: 95%), compared to distilled water and ethanol. Moreover, the model reaction was evaluated in some organic solvents such as n-hexane, dichloromethane, and ethanol under reflux conditions compared with water, no better results were seen (entry 10–12).
To investigate the efficacy and generality of boric acid in the synthesis of 3,4-dihydropyrimidin-2(1H)-ones, various aryl aldehydes were reacted with ethyl acetoacetate and urea using boric acid aqueous solution (magnetized water and distilled water) system under the optimal reaction conditions to obtain the desired products in high yields and in short reaction times. The highest reaction yields have been detected in para-substituted aryl aldehydes with halogen [–F (96%), –Cl (96%), –Br (96%)], whereas disubstituted aryl aldehyde with halogen has shown the lowest reaction yield (90%). Also, aryl aldehydes with electron-donating substitutions displayed lower reaction yields [–OH (92%), –OCH3 (92%)] (Fig. 2).
The acidity of the aqueous boric acid solution is exclusively due to the reaction of boric acid with water to prepare B(OH)4−. It can release of H+ in the aqueous solution.22,23 In the proposed mechanism, which is supported by the literature,19,20,30–32 at first, ethyl acetoacetate was reacted with aldehyde which was activated by produced H+ and afforded an intermediate as a Michael acceptor after removing one molecule of H2O. Then, the desired product was generated by the reaction of urea with aforesaid intermediate.
An interesting observation during the reaction was the lower solubility of the desired products when the solvent was magnetized water. This feature led to better purification of products.
General Procedure for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones
A mixture of ethyl acetoacetate (1 mmol), urea (1 mmol), aryl aldehyde (1 mmol), B(OH)3 (15 mol%) and water/magnetized water (3 mL) was added in a 25 mL round-bottomed flask connected to a reflux condenser and stirred under reflux condition. After completion of the reaction, as monitored by TLC, the reaction mixture was cooled to room temperature and desired product recrystallized from ethanol.Conclusions
Herein, we have investigated the employment of magnetized water as a reaction media to prepare 3,4-dihydropyrimidin-2(1H)-ones by a one-pot three-component condensation reaction using a boric acid catalyst and compared the results with non-magnetic media for the first time. Interestingly, applying magnetized water under reflux conditions resulted in higher yields and shorter reaction times.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank K. N. Toosi University of Technology, Tehran, Iran and Hamedan University of Technology, for financial support of the presented work.Notes and references
- (a) X. F. Pang and B. Deng, Phys. B, 2008, 403, 3571–3577 CrossRef CAS; (b) X. Han, Y. Peng and Z. Ma, Optik, 2016, 127, 6371–6376 CrossRef CAS; (c) L. Holysz, A. Szczes and E. Chibowski, J. Colloid Interface Sci., 2007, 316, 996–1002 CrossRef CAS PubMed; (d) M. C. Amiri and A. A. Dadkhah, Colloids Surf., A, 2006, 278, 252–255 CrossRef CAS; (e) Y. Wang, B. Zhang, Z. Gong, K. Gao, Y. Ou and J. Zhang, J. Mol. Struct., 2013, 1052, 102–104 CrossRef CAS.
- (a) R. Cai, H. Yang, J. He and W. Zhu, J. Mol. Struct., 2009, 938, 15–19 CrossRef CAS; (b) I. Ignatov and O. Mosin, Eur. J. Mol. Biotechnol., 2014, 4, 72–85 CrossRef.
- Y. Wang, H. Wei and Z. Li, Results Phys., 2018, 8, 262–267 CrossRef.
- (a) E. Chibowski, A. Szcześ and L. Hołysz, Colloids Interfaces, 2018, 2, 68 CrossRef CAS; (b) E. J. Toledo, T. C. Ramalho and Z. M. Magriotis, J. Mol. Struct., 2018, 888, 409–415 CrossRef; (c) K. T. Chang and C. I. Weng, J. Appl. Phys., 2006, 100, 043917 CrossRef.
- (a) S. Kobe, G. Dražić, P. J. McGuiness and J. Stražišar, J. Magn. Magn. Mater., 2001, 236, 71–76 CrossRef CAS; (b) M. E. Maffei, Front. Plant Sci., 2014, 5, 445 Search PubMed; (c) H. Wei, Y. Wang and J. Luo, Constr. Build. Mater., 2017, 147, 91–100 CrossRef CAS; (d) I. Vaskina, I. Roi, L. Plyatsuk, R. Vaskin and O. Yakhnenko, Journal of Ecological Engineering, 2020, 21, 251–260 CrossRef.
- (a) N. S. Zaidi, J. Sohaili, K. Muda and M. Sillanpä, Sep. Purif. Rev., 2014, 43, 206–240 CrossRef CAS; (b) T. Panczyk and P. J. Camp, J. Mol. Liq., 2021, 330, 115701 CrossRef CAS; (c) M. Colic and D. Morse, Colloids Surf., A, 1999, 154, 167–174 CrossRef CAS; (d) Y. Z. Guo, D. C. Yin, H. L. Cao, J. Y. Shi, C. Y. Zhang, Y. M. Liu, H. H. Huang, Y. Liu, Y. Wang, W. H. Guo and A. R. Qian, Int. J. Mol. Sci., 2012, 13, 16916–16928 CrossRef CAS PubMed.
- E. Chibowski and A. Szcześ, Chemosphere, 2018, 203, 54–67 CrossRef CAS PubMed.
- (a) C. T. Rodgers, Pure Appl. Chem., 2009, 81, 19–43 CAS; (b) A. Alabi, M. Chiesa, C. Garlisi and G. Palmisano, Environ. Sci.: Water Res. Technol., 2015, 1, 408–425 RSC; (c) L. Lin, W. Jiang, X. Xu and P. Xu, npj Clean Water, 2020, 3, 1–19 CrossRef; (d) O. Zúñiga, J. A. Benavides, D. I. Ospina-Salazar, C. O. Jiménez and M. A. Gutiérrez, Ing. Compet., 2016, 18, 217–232 Search PubMed.
- V. Khakyzadeh, R. Luque, M. A. Zolfigol, H. R. Vahidian, H. Salehzadeh, V. Moradi, A. R. Soleymani, A. R. Moosavi-Zare and K. Xu, RSC Adv., 2015, 5, 3917–3921 RSC.
- (a) A. Khazaei, A. R. Moosavi-Zare, H. Afshar-Hezarkhani and V. Khakyzadeh, RSC Adv., 2014, 4, 32142 RSC; (b) A. Khazaei, F. Gholami, V. Khakyzadeh, A. R. Moosavi-Zare and J. Afsar, RSC Adv., 2015, 5, 14305 RSC.
- A. R. Moosavi-Zare, M. A. Zolfigol and M. Daraei, Synlett, 2014, 25, 1173 CrossRef.
- A. R. Moosavi-Zare, M. A. Zolfigol, F. Derakhshan-Panah and S. Balalaie, Mol. Catal., 2018, 449, 142 CrossRef CAS.
- (a) A. D. Patil, N. V. Kumar, W. C. Kokke, M. F. Bean, A. J. Freyer, C. D. Brosse, S. Mai, A. Truneh and B. Carte, J. Org. Chem., 1995, 60, 1182 CrossRef CAS; (b) D. Russowsky, R. F. Canto, S. A. Sanches, M. G. D’oca, A. De Fatima, R. A. Pilli, L. K. Kohn, M. A. Antônio and J. E. De Carvalho, Bioorg. Chem., 2006, 34, 173 CrossRef CAS PubMed; (c) K. S. Atwal, B. N. Swanson, S. E. Unger, D. M. Floyd, S. Moreland, A. Hedberg and B. C. O'Reilly, J. Med. Chem., 1991, 34, 806 CrossRef CAS PubMed.
- C. O. Kappe, The Biginelli Reaction, in Multicomponent Reactions, ed. J. Zhu and H. Bienaymé, Wiley-VCH, Weinheim, ISBN 978-3-527-30806-4, 2005 Search PubMed.
- S. R. Roy, P. S. Jadhavar, K. Seth, K. K. Sharma and A. K. Chakraborti, Synthesis, 2011, 2261 CAS.
- I. Cepanec, M. Litvic, A. Bartolincic and M. Lovric, Tetrahedron, 2005, 61, 4275 CrossRef CAS.
- J. H. Schauble, E. A. Trauffer, P. P. Deshpande and R. D. Evans, Synthesis, 2005, 1333 CrossRef CAS.
- S. V. Ryabukhin, A. S. Plaskon, E. N. Ostapchuk, D. M. Volochnyuk and A. A. Tolmachev, Synthesis, 2007, 417, 417–427 Search PubMed.
- M. M. Hosseini, E. Kolvari, N. Koukabi, M. Ziyaei and M. A. Zolfigol, Catal. Lett., 2016, 146, 1040 CrossRef CAS.
- H. Naeimi and Z. S. Nazifi, Iran. J. Catal., 2018, 8, 249 CAS.
- M. P. van der Helm, B. Klemm and R. Eelkem, Nat. Rev. Chem., 2019, 3, 491–508 CrossRef CAS.
- A. Khazaei, H. A. Alavi Nik and A. R. Moosavi-Zare, J. Chin. Chem. Soc., 2015, 62, 675 CrossRef CAS.
- A. Khazaei, H. A. A. Nik, A. R. Moosavi-Zare and H. Afshar-Hezarkhani, Z. Naturforsch., B: J. Chem. Sci., 2018, 73, 707 CrossRef CAS.
- G. Harichandrana, S. David Amalraj and P. Shanmugam, J. Iran. Chem. Soc., 2011, 8, 298 CrossRef.
- H. Kiyani and F. Ghorbani, Res. Chem. Intermed., 2015, 41, 2653 CrossRef CAS.
- A. Kumar, D. Saxena and M. K. Gupta, RSC Adv., 2013, 3, 4610 RSC.
- J. S. Yadav, M. K. Gupta, R. Jain, N. N. Yadav and B. V. S. Reddy, Monatsh. Chem., 2010, 141, 1001 CrossRef CAS.
- APHA, Standard Methods for the Experimentation of Water and Wastewater, American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC, USA, 21st edn, 2005 Search PubMed.
- V. Vacek, Thermochim. Acta, 1980, 35, 181–185 CrossRef CAS.
- R. Biswas, D. Saha and R. Bandyopadhyay, Phys. Fluids, 2021, 33, 013103 CrossRef CAS.
- H. J. Lee and M. H. Kang, Nutr. Res. Pract., 2013, 7, 34–42 CrossRef CAS PubMed.
- H. Hosoda, H. Mori, N. Sogoshi, A. Nagasawa and S. Nakabayashi, J. Phys. Chem. A, 2004, 108, 1461–1464 CrossRef CAS.
- Y. I. Cho and S.-H. Lee, Int. Commun. Heat Mass Transfer, 2005, 32, 1–9 CrossRef CAS.
- H. Khabazzadeh, E. T. Kermani and T. Jazinizadeh, Arabian J. Chem., 2012, 5, 485–488 CrossRef CAS.
- J. Javidi, M. Esmaeilpour and F. N. Dodeji, RSC Adv., 2015, 5, 308–315 RSC.
- A. Ghorbani-Choghamarani and P. Zamani, Chin. Chem. Lett., 2013, 24, 804–808 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra03769b |
| This journal is © The Royal Society of Chemistry 2021 |