Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
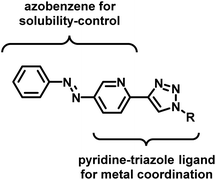
A photoresponsive palladium complex of an azopyridyl-triazole ligand: light-controlled solubility drives catalytic activity in the Suzuki coupling reaction†
Attila Kunfia,
István Jablonkai*a,
Tamás Gazdagab,
Péter J. Mayerac,
Péter Pál Kalaposa,
Krisztina Némethbd,
Tamás Holczbauer e and
Gábor London
e and
Gábor London *a
*a
aMTA TTK Lendület Functional Organic Materials Research Group, Institute of Organic Chemistry, Research Centre for Natural Sciences, Magyar tudósok krt. 2., 1117 Budapest, Hungary. E-mail: jablonkai.istvan@ttk.hu; london.gabor@ttk.hu
bInstitute of Chemistry, Eötvös Loránd University, Pázmány Péter stny. 1/A, 1117 Budapest, Hungary
cInstitute of Chemistry, University of Szeged, Rerrich tér 1., 6720 Szeged, Hungary
dMS Metabolomics Research Group, Instrumentation Center, Research Centre for Natural Sciences, Magyar tudósok krt. 2, 1117 Budapest, Hungary
eCentre for Structural Science and Institute of Organic Chemistry, Research Centre for Natural Sciences, Magyar tudósok krt. 2., 1117 Budapest, Hungary
First published on 9th July 2021
Abstract
Herein, the design and synthesis of a click-derived Pd-complex merged with a photoswitchable azobenzene unit is presented. While in the trans-form of the switch the complex showed limited solubility, the photogenerated cis-form rendered the molecule soluble in polar solvents. This light-controllable solubility was exploited to affect the catalytic activity in the Suzuki coupling reaction. The effect of the substrate and catalyst concentration and light intensity on the proceeding and outcome of the reaction was studied. Dehalogenation of the aryl iodide starting material was found to be a major side reaction; however, its occurrence was dependent on the applied light intensity.
1 Introduction
The combination of light-responsive molecular machines with catalytic transformations led to the rapidly developing field of photoswitchable catalysis.1–4 Modulating the catalytic activity with light as an input signal can impact the development of catalysis in different ways. Controlling the occurrence of chemical transformations temporally and spatially in molecular systems that approach cellular complexity can be foreseen. Furthermore, the light-induced actuation of different forms of the same catalyst will help us to integrate the aims of green chemistry5 such as activity, selectivity and recycling, within a single system through controlled structural changes.6 The remarkable progress that has been made in the field of light-driven molecular machines provides a variety of established photochromes to serve as control units.7 Among these, azobenzenes, dithienylethenes and overcrowded alkenes are the most frequently exploited units in photoswitchable catalysis. Within the vast set of molecular catalysts, organometallic complexes are arguably the most widely studied systems and used in a myriad of organic transformations. Accordingly, several organometallic complexes with photoswitchable activity have been reported, although the field is still in its infancy.8,9 The key feature of these systems is to affect the catalytic event through steric and/or electronic control of the environment around the metal center. Generally, electronic effects are best influenced by dithienylethenes10–14 due to changes in the conjugation pattern upon their light-triggered electrocyclization. The levels of steric control are mostly associated with isomerization around double bonds.15–21 As the focus in most examples is on the smart incorporation of steric and/or electronic control into the catalyst, more simple possibilities for activity modulation are overlooked.9 Among these, a straightforward way to control the occurrence of a reaction could be through manipulating the solubility of the organometallic complex. This type of control could be valuable in cascade reactions, where the insoluble catalyst would stay in a dormant state until UV-light irradiation occurs, upon which the metal-catalysed chemistry would be activated. The Suzuki cross-coupling reaction is among the most used reactions in current organic chemistry; hence, it can be foreseen that these reactions could be part of more complex reaction networks controlled by light-switchable catalysts. However, there are several characteristics of Suzuki coupling such as its heterogeneity (for the solubility of starting materials, inorganic bases are often used), the light absorption of the starting materials and products, and Pd loss via Pd black formation, which make its combination with photoswitching processes a challenge. It is probably due to these challenges that cross-coupling reactions in general, Suzuki coupling in particular, are not in the focus of explorations in the context of photoswitchable catalysis. In this contribution, we explored the design of an organometallic catalyst having photoswitchable solubility and its applicability and limitations in the Suzuki coupling reaction.2 Results and discussion
2.1 Synthesis and characterization of ligands and Pd complexes
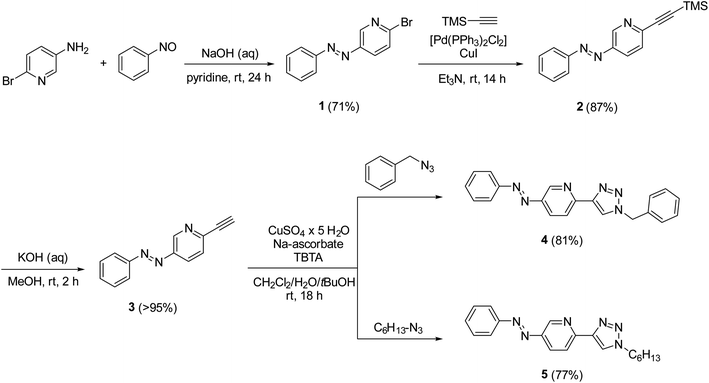
In the design of an organometallic catalyst with photocontrollable solubility, several requirements have to be fulfilled. First, a photochromic unit that is responsible for solubility alteration has to be introduced. Second, the light-induced switching of the photochrome has to be preserved in the presence of the coordinating metal. Finally, the complex has to remain stable, without the loss of metal, at the time scale of the process. In our design (Fig. 1), we considered azobenzene22 a promising choice of photochrome due to the relatively large change in its dipole moment upon trans-to-cis isomerization,23 which could be associated with different solubilities.24 Among the Pd ligands that are suitable for the Suzuki cross-coupling reaction, we turned to the click-derived pyridine–triazole structure,25–27 due to its easy synthesis procedure and strong interaction with Pd to ensure homogeneous organometallic catalysis.28,29To test the hypothesis, we synthesized azobenzene derivatives 4 and 5 with incorporated pyridine-triazole ligands bearing a benzyl and a hexyl side chain, respectively (Scheme 1). The first step of the synthesis was a Mills reaction between 5-amino-2-bromo-pyridine and nitrosobenzene affording 2-bromo-5-(phenylazo)pyridine 1.30 A subsequent Sonogashira coupling between 1 and TMS-acetylene followed by the deprotection of alkyne 2 yielded compound 3. The terminal alkyne of compound 3 was transformed into triazole rings via azide–alkyne cycloadditions with benzyl azide and hexyl azide leading to ligands 4 and 5, respectively (for detailed synthetic procedures, see ESI†).
Ligand 4 could be crystallized for X-ray crystallographic analysis (Fig. 2a), while the crystallization of ligand 5 having a more lipophilic sidechain was unsuccessful.
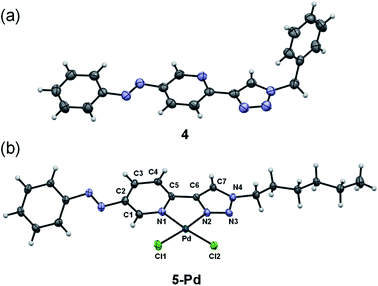 | ||
| Fig. 2 X-ray crystal structures of ligand 4 (a) and complex 5-Pd (b). ORTEP representations are drawn at the 50% probability level. | ||
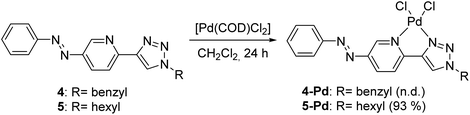
Both ligands showed reversible photoisomerization in solutions (Fig. S3, ESI†), while no apparent change in their solubility was observed upon irradiation with UV-light (365 nm). Pd-complexes were prepared from ligands 4 and 5 using Pd(COD)Cl2 as the metal source (Scheme 2).25
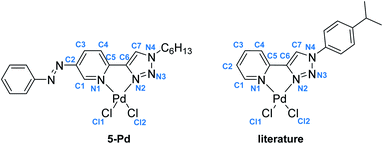
In the case of ligand 4 with a benzyl side chain, a yellow solid precipitate was formed that could not be dissolved in any common solvents (solubility was fairly good in DMSO; however, using this solvent led to the loss of coordinated Pd), which prevented its structural characterization. Complex 5-Pd, although precipitated from CH2Cl2 upon formation, was found to be moderately soluble in common organic solvents. The molecular structure of 5-Pd was confirmed by X-ray crystallography (Fig. 2b). The Pd binds to the ligand moiety through the pyridine nitrogen (N1) and the N2 nitrogen of the triazole ring. The ring N-atoms and the two chlorides provide a square planar coordination mode for the metal. The structure of the pyridine–triazole complex is in agreement with literature examples;25,28 hence, the pendant phenylazo group has negligible influence on the metal coordination (Table 1).
| Bond length (Å) (5-Pd) | Bond length (Å) (lit) | Bond angle (°) (5-Pd) | Bond angle (°) (lit) | ||
|---|---|---|---|---|---|
| Pd–Cl1 | 2.285 | 2.285 | N1–Pd–N2 | 80.26 | 80.6 |
| Pd–Cl2 | 2.289 | 2.264 | N1–Pd–Cl1 | 93.83 | 94.0 |
| Pd–N1 | 2.050 | 2.055 | N2–Pd–Cl2 | 94.79 | 94.4 |
| Pd–N2 | 2.005 | 2.007 | Cl1–Pd–Cl2 | 91.08 | 91.01 |
| N1–C5 | 1.353 | n.d. | |||
| C5–C6 | 1.453 | 1.464 | |||
| C6–C7 | 1.363 | 1.359 | |||
| C7–N4 | 1.338 | 1.343 | |||
| N4–N3 | 1.345 | 1.349 | |||
| N3–N2 | 1.294 | 1.312 | |||
| N2–C6 | 1.374 | 1.364 | |||
Regarding photochemical properties, no pronounced difference was observed between the free ligand and the complex (Fig. 3). Such minor changes in the absorption properties following Pd complexation were unexpected, but are in agreement with previous reports on Pd and Pt complexes of click-derived ligands.25
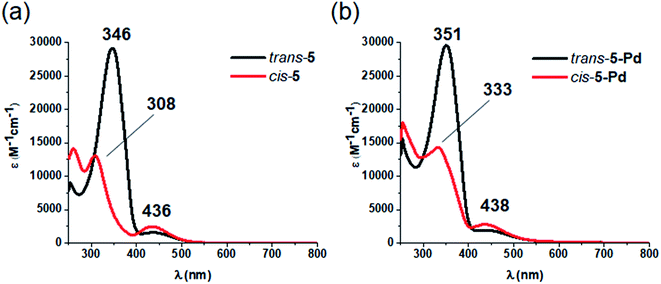 | ||
Fig. 3 UV-Vis spectra of the cis- and trans-form of ligand 5 (a) and complex 5-Pd (b) in DMF/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
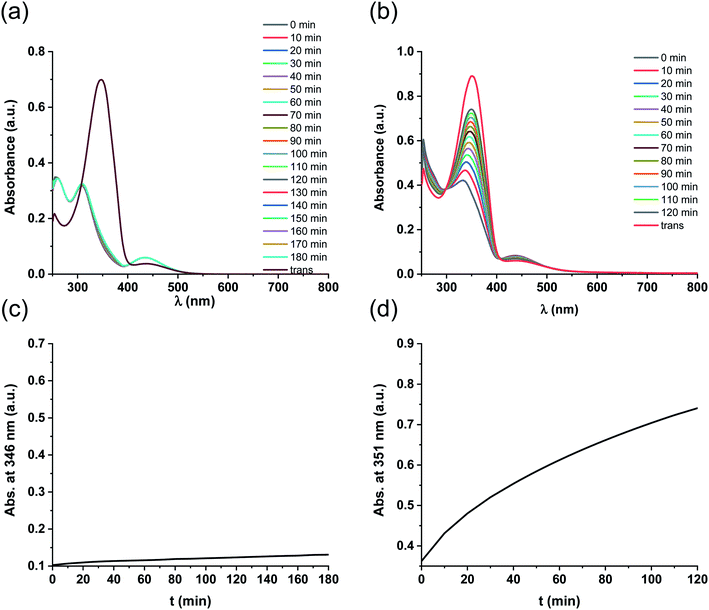
Like ligand 5, complex 5-Pd underwent light-induced isomerization to its cis-form. The thermal behavior of the photogenerated species, however, was markedly different (Fig. 4) as cis-5 exhibited increased stability compared to cis-5-Pd. At room temperature, the free ligand showed almost no reverse isomerization in 3 hours (Fig. 4a and c), while most of cis-5-Pd isomerized back to its trans-form within 2 hours (Fig. 4b and d). This suggests the potential contribution of Pd complexation to a decreased double-bond character of the azo group that leads to lower thermal stability.
Further experiments revealed that a mixture of DMF/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
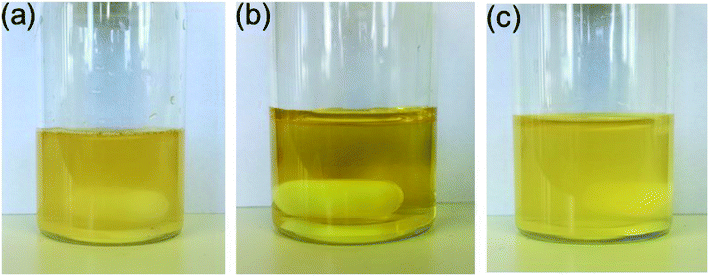
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was suitable for testing the catalytic activities28 of the different isomers in the Suzuki coupling reaction, since cis-5-Pd and trans-5-Pd showed different solubilities in this solvent system. While trans-5-Pd in the solvent mixture (0.15 mg/1 mL) was not fully dissolved forming an inhomogeneous mixture, irradiation of the system with 365 nm light for 20 min led to a clear solution (Fig. 5a and b). Irradiation of the solution with 440 nm light led to the re-formation of the cloudy mixture (Fig. 5c).
1) was suitable for testing the catalytic activities28 of the different isomers in the Suzuki coupling reaction, since cis-5-Pd and trans-5-Pd showed different solubilities in this solvent system. While trans-5-Pd in the solvent mixture (0.15 mg/1 mL) was not fully dissolved forming an inhomogeneous mixture, irradiation of the system with 365 nm light for 20 min led to a clear solution (Fig. 5a and b). Irradiation of the solution with 440 nm light led to the re-formation of the cloudy mixture (Fig. 5c).
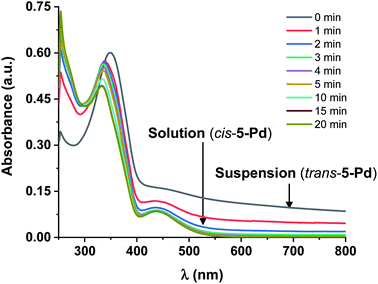
Further UV-Vis measurements regarding the solubility of the isomers were conducted. Increasing the concentration of trans-5-Pd from 10−5 M up to 3 × 10−4 M in DMF/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a cloudy mixture was formed showing a UV-Vis spectrum (Fig. 6) similar to that of trans-5-Pd at a lower concentration (Fig. 3). However, it was characterized by lower intensity absorptions and shifted baseline, indicating the inhomogeneity of the sample. Upon irradiation of the suspension, the catalyst was gradually dissolved and after about 20 min a spectrum corresponding to that of cis-5-Pd was obtained.
1, a cloudy mixture was formed showing a UV-Vis spectrum (Fig. 6) similar to that of trans-5-Pd at a lower concentration (Fig. 3). However, it was characterized by lower intensity absorptions and shifted baseline, indicating the inhomogeneity of the sample. Upon irradiation of the suspension, the catalyst was gradually dissolved and after about 20 min a spectrum corresponding to that of cis-5-Pd was obtained.
It is noted, that upon irradiation no apparent Pd black formation was observed, indicating the photostability of 5-Pd. The photostability of the complex was also confirmed by 1H NMR spectroscopy (Fig. 7 and S1, ESI†). Upon irradiation of a sample of trans-5-Pd for 2 hours, a clean mixture of trans-5-Pd and cis-5-Pd was formed in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 without any sign of degradation.
1.7 without any sign of degradation.
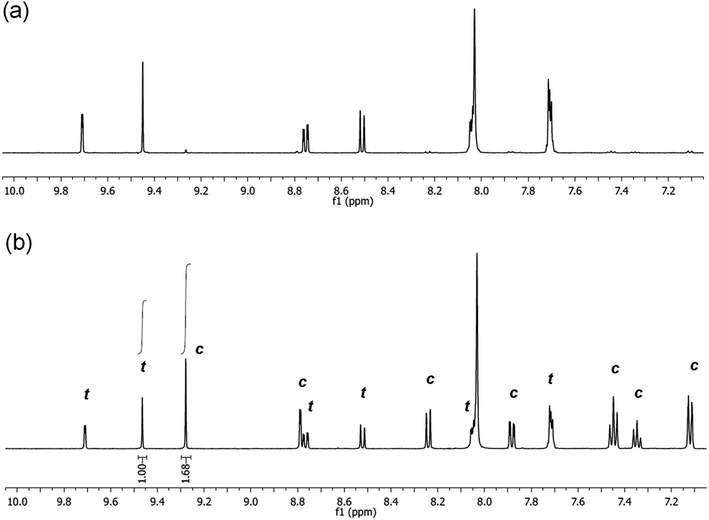 | ||
| Fig. 7 Partial 1H NMR spectra (DMF-d7, 500 MHz, 21 °C) of 5-Pd before (a) and after (b) irradiation with UV-light (365 nm, P = 10 W, 2 h, rt) (t – trans-5-Pd, c – cis-5-Pd). | ||
2.2 Evaluation of catalytic activity
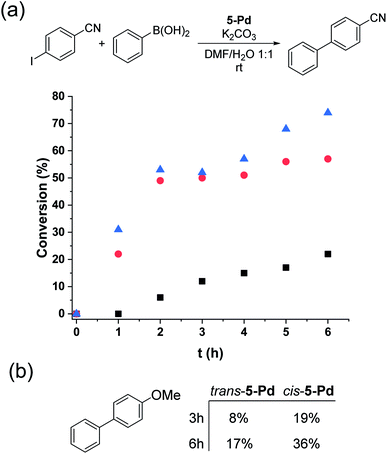
Trans-5-Pd (1.5 mol%) showed low activity in the Suzuki coupling reaction of phenylboronic acid and 4-iodobenzonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
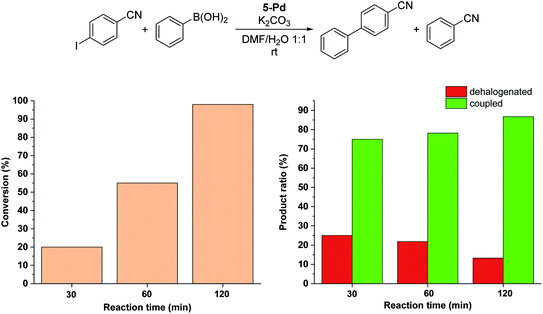
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) that is consistent with its moderate solubility in the reaction medium. After 6 hours in the darkness, only about 20% conversion into 4-cyanobiphenyl was observed (Fig. 8a). In all the reactions, the cis-form of the catalyst was generated by the irradiation (λmax = 365 nm, P = 10 W) of a solution of parent 5-Pd for 30 min at room temperature prior to catalysis. Following catalyst addition, the reaction mixture was continuously irradiated to maintain the concentration of the cis-form high. Gratifyingly, when the soluble cis-rich 5-Pd (1.5 mol%) was used as a catalyst, a remarkable increase in the activity was observed. In 2 hours, 50% conversion was obtained as compared to 6% by trans-5-Pd (Fig. 8a). Furthermore, the catalyst loading could be decreased to 0.6 mol% without any loss of activity.
1) that is consistent with its moderate solubility in the reaction medium. After 6 hours in the darkness, only about 20% conversion into 4-cyanobiphenyl was observed (Fig. 8a). In all the reactions, the cis-form of the catalyst was generated by the irradiation (λmax = 365 nm, P = 10 W) of a solution of parent 5-Pd for 30 min at room temperature prior to catalysis. Following catalyst addition, the reaction mixture was continuously irradiated to maintain the concentration of the cis-form high. Gratifyingly, when the soluble cis-rich 5-Pd (1.5 mol%) was used as a catalyst, a remarkable increase in the activity was observed. In 2 hours, 50% conversion was obtained as compared to 6% by trans-5-Pd (Fig. 8a). Furthermore, the catalyst loading could be decreased to 0.6 mol% without any loss of activity.
Time-course studies of the reaction catalyzed by cis-5-Pd up to 6 hours revealed that a steep increase in conversion in the first 2 h was followed by a plateau when 1.5 mol% catalyst was used, or a slight further increase up to 70% at a catalyst loading of 0.6 mol% (Fig. 8a). Along with the proceeding of the cross-coupling, the reaction mixture became increasingly dark, indicating gradual loss of metal. Probably, at higher catalyst concentrations, enhanced Pd aggregation led to loss of activity, while at low catalyst loading slower aggregation preserved activity. No such darkening of the reaction mixture was observed in the case of the less active trans-5-Pd catalyst. Based on the observed photostability of the complex, the metal loss likely occurs in the catalytic cycle, upon contact with the substrate molecules. Nevertheless, it is clear that the increased catalytic activity of cis-5-Pd is strongly dependent on its light-induced dissolution during the reaction allowing a more efficient reaction to take place. Employing 4-iodoanisole in the coupling, similar activity trends of the isomers were found (Fig. 8b).
To exclude a potential photoredox mechanism in the reaction, cis-5-Pd was generated in DMF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), then the clear catalyst solution was added to the reactants and the mixture was stirred at room temperature in the darkness. Samples were taken after 1 h and 2 h reaction times. After 1 h, 11%, while after 2 h 18%, conversion was obtained. These values are significantly higher than those obtained with trans-5-Pd, while much lower than with continuously irradiated reaction mixtures. The lower conversion can be explained with the favoured thermal cis-to-trans isomerization of the complex leading to catalyst precipitation and hence to the loss of activity.
1), then the clear catalyst solution was added to the reactants and the mixture was stirred at room temperature in the darkness. Samples were taken after 1 h and 2 h reaction times. After 1 h, 11%, while after 2 h 18%, conversion was obtained. These values are significantly higher than those obtained with trans-5-Pd, while much lower than with continuously irradiated reaction mixtures. The lower conversion can be explained with the favoured thermal cis-to-trans isomerization of the complex leading to catalyst precipitation and hence to the loss of activity.
In an attempt to increase the yield of the Suzuki coupling reaction by reducing the metal loss, the concentration of the starting materials was decreased by 50% (0.05 mmol/3 mL) compared to what was used initially (0.1 mmol/3 mL), while the concentration of the catalyst was kept at 0.6 mol%. This dilution resulted in slower product formation (1 h: 7%; 2 h: 24%) compared to the original conditions (1 h: 31%; 2 h: 53%), while the metal loss was visibly decreased.
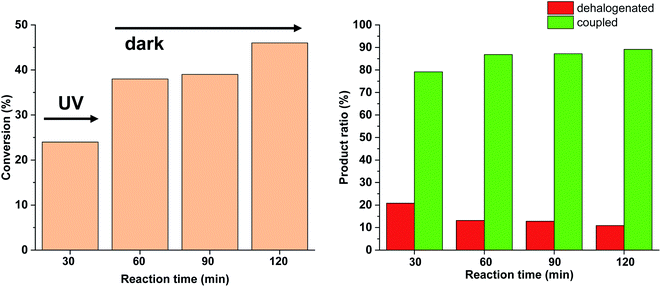
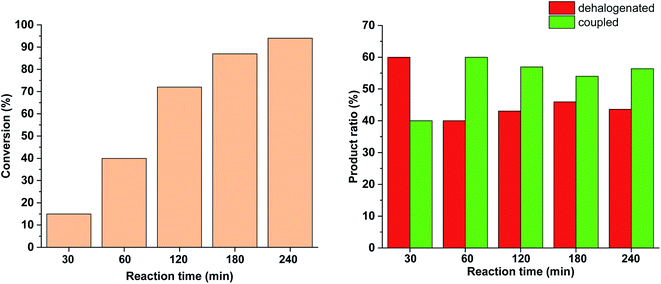
Since no considerable Pd loss was observed at lower reactant concentrations, we explored the possibility of increasing the reaction rate by increasing the intensity of the light source from 10 W to 30 W, while keeping the conditions otherwise identical. Gratifyingly, increased light intensity promoted the conversion to completion (Fig. 9), likely due to the shift of the photoequilibrium towards higher cis-contents. However, the selectivity of the conversion toward the coupled product somewhat decreased, as the dehalogenation of starting 4-iodobenzonitrile to benzonitrile took place. The light-induced dehalogenation of the aryl iodide was proved by the irradiation of the starting material for 2 h, which yielded benzonitrile (22%). Such photo-induced dehalogenations are precedented in the literature and likely proceed via the formation of an aryl radical that abstracts one hydrogen from the solvent.31–33
Due to the larger conversions in response to increased light intensity along with decreased selectivity toward the production of the coupled product, we explored how shorter irradiation times would affect the conversion and the product ratio. For this, after the addition of the cis-rich 5-Pd solution, the reaction mixture was irradiated for 30 min (30 W), and then the light was switched off for the rest of the reaction time. The conversion profile (Fig. 10) resembled that observed previously under similar conditions with 10 W light source. The conversion was increased initially and then slowed down due to isomerization to the catalytically less active trans-5-Pd. When compared to the situation of continuous irradiation (Fig. 9), the conversions were obviously lower when only 30 min irradiation was applied, however, the selectivities did not change substantially (approximately 10–20% dehalogenated and 80–90% coupled in both cases). This suggests that initially dehalogenation takes place more efficiently (most of the dehalogenated product forms in this period), then, after a short initiation period, the Suzuki coupling reaction predominates.
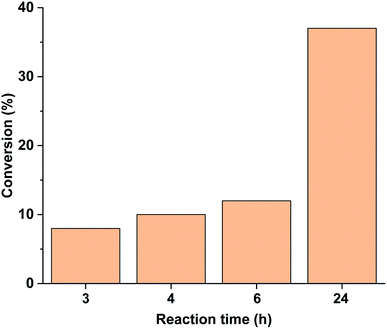
Performing the reaction in the darkness using trans-5-Pd led to a slow reaction with a conversion of 37% after 24 h (Fig. 11). No dehalogenation was detected in this case. For comparison, the trans-5-Pd-catalysed reaction at 40 °C led to 32% conversion (>99% selectivity) after 2 h due to the increased solubility of the catalyst at this temperature. Notably, this conversion is comparable to that obtained with cis-5-Pd applying 30 min initial irradiation (Fig. 10), while lower than the conversion upon continuous irradiation of the reaction mixture (Fig. 9).
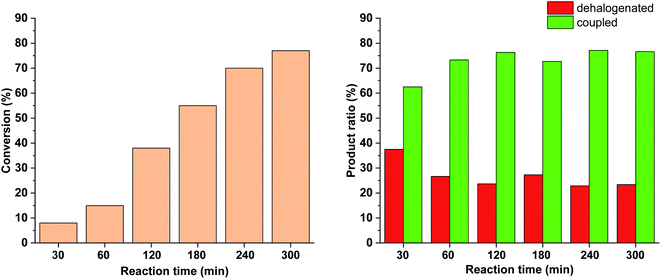
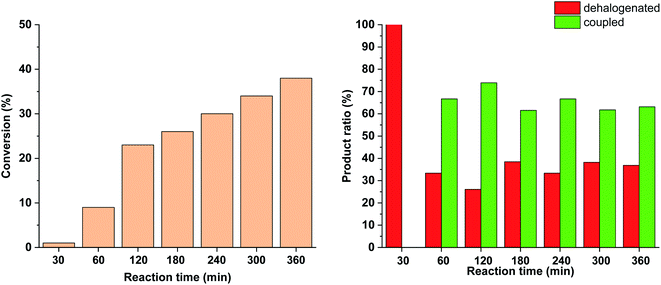
To further tune the conditions towards selective and high-yielding Suzuki coupling, the light intensity was decreased to 15 W (Fig. 12). Compared to the continuous irradiation with 30 W light, in this case the reaction rate, the conversion and the selectivity toward the Suzuki product were lower. Probably, the slower Suzuki reaction due to lower amount of cis-5-Pd facilitated the formation of an increased amount of side products. As previously (Fig. 9 and 10), the relative amount of the dehalogenated side product exhibited maximum at the early stage of the reaction.
Similar effect was observed when the catalyst concentration was decreased to 0.3 mol% keeping the light intensity at 30 W (Fig. 13). At the reduced catalyst concentration, regardless of the ratio of photogenerated cis-5-Pd, the rate of the Suzuki coupling step slowed down allowing the formation of an increased amount of dehalogenated products.
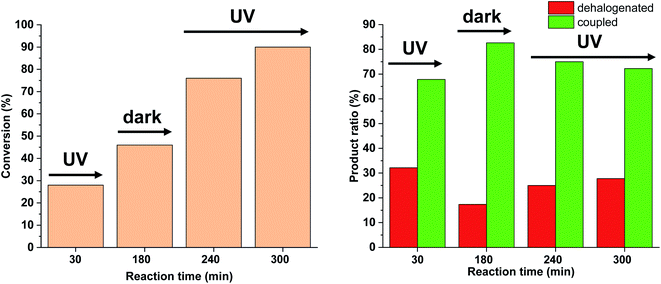
Based on the above-mentioned measurements it is clear that the rate of dehalogenation is highest under continuous irradiation of the reaction mixture with 30 W light intensity, while the activity of cis-5-Pd in this side reaction is negligible (Fig. 10). Furthermore, higher amount of cis-5-Pd can be generated using 30 W light, which is active in Suzuki coupling even in the darkness until back isomerization takes place (Fig. 10). Combining these observations, a feasible approach to facilitate Suzuki coupling along with suppressed dehalogenation could be the occasional switching of the catalyst under the reaction conditions. Regular, relatively short-time irradiations of the mixture could keep the catalyst mainly in its cis-form, while the dehalogenation would be somewhat less pronounced due to the non-continuous irradiation.
We tested this hypothesis by irradiating the reaction mixture initially for 30 min, and then turned off the light for the next 150 min (Fig. 14). As previously, under continuous irradiation in the first 30 min, we observed the formation of both products. However, when the light was switched off, as expected, the Suzuki coupling reaction was the primary transformation, while the amount of the dehalogenated product did not increase. Turning on the light again, the amount of both products was increased leading to decreased selectivity towards the coupling product.
We tested the coupling of 4-iodoanisole with phenylboronic acid under irradiation using a 30 W light source and lower initial reactant concentrations (Fig. 15). Compared to the initial conditions (Fig. 8b), higher conversions were obtained; however, as in the case of the reaction of 4-iodobenzonitrile, a large amount of dehalogenated product was detected.
Apart from aryl iodides, we attempted the coupling of aryl bromides (4-bromoacetophenone, 4-bromonitrobenzene) with phenylboronic acid. Unfortunately, these substrates showed no reactivity towards the Suzuki product in the presence of 5-Pd, and only the photodehalogenation products were obtained under continuous irradiation.
3 Discussion
The above-mentioned experimental results point towards the feasibility of impacting catalytic activity in the Suzuki cross-coupling reaction by photoswitching catalyst solubility. However, care should be taken if one considers integrating this chemistry into more complex systems, such as cascade transformations or reaction networks. Light intensity seems to be an important factor in faster/more complete photoswitching between the two forms of the catalyst. Increased light intensity, in the case of the model Suzuki reaction, led to higher conversions and faster response times, with the downside of initiating a considerable amount of side products through photodehalogenation. This latter problem could be a general disadvantage in coupling reactions beyond the Suzuki reaction. The amount of product and side product was found to be dependent also on the catalyst/substrate ratio. It is also clear that a faster Suzuki reaction (electron poor 4-iodobenzonitrile) is preferred if better selectivities are targeted, as photodehalogenation takes place mainly in the initial stage of the reaction. With further catalyst engineering, the light-induced side reaction could be eliminated if a molecule with thermally very stable and soluble cis-form could be synthesized (note that the impact of the reaction components on the stability of the cis-form is unknown). However, the soluble, catalytically active, but thermally less stable cis-catalyst could be exploited in dissipative molecular systems.34,35It is important to note that although tuning product formation in the Suzuki reaction via photoswitching solubility is demonstrated through this work, the expansion of this control to a wide substrate scope is not trivial. Solubility control is demonstrated in DMF/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture, which might not be an ideal solvent mixture for many substrates due to their limited solubility. This is illustrated by the lack of conversion in the case of 4-iodonitrobenzene, due to its poor solubility in DMF/H2O 1
1 mixture, which might not be an ideal solvent mixture for many substrates due to their limited solubility. This is illustrated by the lack of conversion in the case of 4-iodonitrobenzene, due to its poor solubility in DMF/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Moreover, the light absorption of several substrates/coupled products might interfere with the irradiation wavelength that decreases (or even eliminates) the possibility of catalyst isomerization. Hence, expanding the substrate scope could lead to individual optimization of conditions (and catalysts) for each starting material. The same holds for expanding the scope of cross-coupling reactions where light-controlled solubility can be exploited in controlling the product formation. Our attempt to use 5-Pd in Sonogashira coupling remained inconclusive. Not only aqueous Sonogashira couplings are challenging, but also the low solubility of acetylenes and the additional components (CuI) in the reaction mixture did not facilitate the catalytic study.
1. Moreover, the light absorption of several substrates/coupled products might interfere with the irradiation wavelength that decreases (or even eliminates) the possibility of catalyst isomerization. Hence, expanding the substrate scope could lead to individual optimization of conditions (and catalysts) for each starting material. The same holds for expanding the scope of cross-coupling reactions where light-controlled solubility can be exploited in controlling the product formation. Our attempt to use 5-Pd in Sonogashira coupling remained inconclusive. Not only aqueous Sonogashira couplings are challenging, but also the low solubility of acetylenes and the additional components (CuI) in the reaction mixture did not facilitate the catalytic study.
4 Conclusion
In summary, we have designed and synthesized an azobenzene-containing Pd-complex with photocontrollable solubility, which was exploited in the light-enhanced Suzuki cross-coupling reaction. Our design of a photostable Pd complex with switchable solubility could open new ways in the development of photoswitchable organometallic catalysts and catalytic systems with increased complexity. However, care should be taken in choosing the components (solvent, base, starting materials) and conditions (light intensity) of the coupling reaction to avoid the occurrence of unwanted processes (metal loss or dehalogenation) that might influence the outcome or hinder parallel reactions.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Financial support by the Lendület Program of the Hungarian Academy of Sciences (G. L.) and the National Research, Development and Innovation Office, Hungary (NKFIH Grant No. FK 123760 (G. L.) and PD 128504 and K 124544 (T. H.)) is gratefully acknowledged. Rita Dobszay is acknowledged for assistance in crystallography. Dr Pál Szabó and Dr Kornél Szőri are acknowledged for fruitful discussions.Notes and references
- B. M. Neilson and C. W. Bielawski, ACS Catal., 2013, 3, 1874–1885 CrossRef CAS.
- R. Göstl, A. Senf and S. Hecht, Chem. Soc. Rev., 2014, 43, 1982–1996 RSC.
- V. Blanco, D. A. Leigh and V. Marcos, Chem. Soc. Rev., 2015, 44, 5341–5370 RSC.
- M. Vlatković, B. S. L Collins and B. L. Feringa, Chem. –Eur. J., 2016, 22, 17080–17111 CrossRef.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- I. Jablonkai, A. Kunfi, D.-H. Qu and G. London, in Nontraditional Activation Methods in Green and Sustainable Applications, ed. B. Török and C. Schäfer, Elsevier, Amsterdam, 2021, ch. 7 Search PubMed.
- B. L. Feringa, Angew. Chem., Int. Ed., 2017, 56, 11060–11078 CrossRef CAS.
- M. Akita, Organometallics, 2011, 30, 43–51 CrossRef CAS.
- Z. Freixa, Catal. Sci. Technol., 2020, 10, 3122–3139 RSC.
- H. D. Samachetty and N. R. Branda, Pure Appl. Chem., 2006, 78, 2351–2359 CAS.
- D. Sud, R. McDonald and N. R. Branda, Inorg. Chem., 2005, 44, 5960–5962 CrossRef CAS.
- B. M. Neilson and C. W. Bielawski, Organometallics, 2013, 32, 3121–3128 CrossRef CAS.
- A. J. Teator, H. Shao, G. Lu, P. Liu and C. W. Bielawski, Organometallics, 2017, 36, 490–497 CrossRef CAS.
- Z. Xu, Y. Cao, B. O. Patrick and M. O. Wolf, Chem. –Eur. J., 2018, 24, 10315–10319 CrossRef CAS PubMed.
- R. Dorel and B. L. Feringa, Chem. Commun., 2019, 55, 6477–6486 RSC.
- H. Sugimoto, T. Kimura and S. Inoue, J. Am. Chem. Soc., 1999, 121, 2325–2326 CrossRef CAS.
- R. Cacciapaglia, S. Di Stefano and L. Mandolini, J. Am. Chem. Soc., 2003, 125, 2224–2227 CrossRef CAS.
- Z. S. Kean, S. Akbulatov, Y. Tian, R. A. Widenhoefer, R. Boulatov and S. L. Craig, Angew. Chem. Int Ed., 2014, 53, 14508–14511 CrossRef CAS.
- D. Zhao, T. M. Neubauer and B. L. Feringa, Nat. Commun., 2015, 6, 6652 CrossRef CAS.
- N. Priyadarshani, B. Ginovska, J. T. Bays, J. C. Linehan and W. J. Shaw, Dalton Trans., 2015, 44, 14854–14864 RSC.
- T. Arif, C. Cazorla, N. Bogliotti, N. Saleh, F. Blanchard, V. Gandon, R. Métivier, J. Xie, A. Voituriez and A. Marinetti, Catal. Sci. Technol., 2018, 8, 710–715 RSC.
- H. M. D. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC.
- G. S. Hartley and R. J. W. Le Fèvre, J. Chem. Soc., 1939, 531–535 RSC.
- C. Brown, S. K. Rastogi, S. L. Barrett, H. E. Anderson, E. Twichell, S. Gralinski, A. McDonald and W. J. Brittain, J. Photochem. Photobiol. A: Chem., 2017, 336, 140–145 CrossRef CAS.
- D. Schweinfurth, R. Pattacini, S. Strobela and B. Sarkar, Dalton Trans., 2009, 9291–9297 RSC.
- D. Schweinfurth, L. Hettmanczyk, L. Suntrup and B. Sarkar, Z. Anorg. Allg. Chem., 2017, 643, 554–584 CrossRef CAS.
- Q. V. C. van Hilst, N. R. Lagesse, D. Preston and J. D. Crowley, Dalton Trans., 2018, 47, 997–1002 RSC.
- S. Jindabot, K. Teerachanan, P. Thongkam, S. Kiatisevi, T. Khamnaen, P. Phiriyawirut, S. Charoenchaidet, T. Sooksimuang, P. Kongsaeree and P. Sangtrirutnugul, J. Organomet. Chem., 2014, 750, 35–40 CrossRef CAS.
- W. K. C. Lo, G. S. Huff, J. R. Cubanski, A. D. W. Kennedy, C. J. McAdam, D. A. McMorran, K. C. Gordon and J. D. Crowley, Inorg. Chem., 2015, 54, 1572–1587 CrossRef CAS PubMed.
- S. O. Schmidt, H. Naggert, A. Buchholz, H. Brandenburg, A. Bannwarth, W. Plass and F. Tuczek, Eur. J. Inorg. Chem., 2016, 2175–2186 CrossRef CAS.
- R. Lekkala, R. Lekkala, B. Moku, K. P. Rakesh and H.-L. Qin, Eur. J. Org. Chem., 2019, 2769–2806 CrossRef CAS.
- K. Kim and S. H. Hong, Adv. Synth. Catal., 2017, 359, 2345–2351 CrossRef CAS.
- A. Elhage, P. Costa, A. Nasim, A. E. Lanterna and J. C. Scaiano, J. Phys. Chem. A, 2019, 123, 10224–10229 CrossRef CAS PubMed.
- M. Fialkowski, K. J. M. Bishop, R. Klajn, S. K. Smoukov, C. J. Campbell and B. A. Grzybowski, J. Phys. Chem. B, 2006, 110, 2482–2496 CrossRef CAS PubMed.
- C. Biagini, S. D. P. Fielden, D. A. Leigh, F. Schaufelberger, S. Di Stefano and D. Thomas, Angew. Chem., Int. Ed., 2019, 58, 9876–9880 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2050358 and 2050357. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra03838a |
| This journal is © The Royal Society of Chemistry 2021 |