Open Access Article
Open Access ArticleCalcium carbonate modified urea–formaldehyde resin adhesive for strength enhanced medium density fiberboard production
Li Lu a,
Yan Wangb,
Tianhua Lic,
Supeng Wangd,
Shoulu Yange,
Yan Qing
a,
Yan Wangb,
Tianhua Lic,
Supeng Wangd,
Shoulu Yange,
Yan Qing a,
Xingong Lia,
Yiqiang Wua and
Ming Liu
a,
Xingong Lia,
Yiqiang Wua and
Ming Liu *a
*a
aCollege of Materials Science and Engineering, Central South University of Forestry and Technology, Changsha 410000, China. E-mail: liumws@126.com
bCollege of Engineering and Design, Hunan Normal University, Changsha 410000, China
cGuangxi Fenglin Wood Industry Group Co., Ltd, Nanning 530000, China
dShengxiang Group – Shengxiang Industry (Jiangsu) Co., Ltd, Danyang 212300, China
eGuizhou Academy of Forestry, Guiyang 550005, China
First published on 19th July 2021
Abstract
This study investigated the improved properties of calcium carbonate (CaCO3) modified urea–formaldehyde (UF) resin adhesive for medium density fiberboard (MDF) production. The CaCO3 modified UF resins were prepared by adding different proportions of CaCO3 to a low molar ratio UF resin at the initial stage of a typical synthetic process of the resin. The physicochemical properties of the resins were measured. The mechanical and environmental performances of the resin-bonded MDF panels were tested. The results show that the viscosity and free formaldehyde content of UF resins with or without CaCO3 modification were not significantly different. The solid content of the CaCO3 modified UF resin was significantly lower than that of the control group. In addition, the measured gel time of the CaCO3 modified UF resin was 111–149 s, which was longer than that of the control resin (82 s). The gel time was further extended with the increase of the CaCO3 content in the UF resin. The chemical group and crystal structure of UF resins with or without the modification of CaCO3 were not significantly different. The internal bonding (IB) strength of the MDF panels significantly increased from 0.75 MPa to 0.97 MPa when the UF resin was modified with 2% of CaCO3. This study provides scientific support for the preparation of inorganic mineral modified UF resins for strength enhanced wood-based panel manufacturing.
1. Introduction
Urea–formaldehyde resin (UF) has become the most widely used adhesive in the field of wood-based panel industry because of its excellent bonding properties and low cost.1–3 However, the stability of the UF resin is poor. It easily decomposes or degrades due to the attacks of moisture and heat, releasing formaldehyde. This seriously limits the application of UF resin in the field of functional wood-based panels.4,5 Inorganic particles have the advantages of good stability, environmentally friendly nature, abundance in source, and so on. Inorganic–organic hybrid technology is one of the effective ways of material functional modification. By adding inorganic materials into organic materials, the physical properties of organic materials could be improved, including hardness, strength, thermal stability, and chemical stability.6–9 The introduction of inorganic particles or their grafted modified particles into UF resin might form certain chemical bonds between them, which improves the chemical stability of the resin, reduces the consumption of chemical raw materials such as urea and formaldehyde and improves the environmental performance of resin and wood-based panels.UF resin contains hydroxyl, hydroxymethyl, and other active chemical groups. The reported results show that the addition of inorganic mineral particles (mainly composed of silicon dioxide and magnesia) in UF resin generates the silicon oxygen between the mineral particles and the oxygen atom of the magnesium oxygen bond and the hydrogen atom of the methylol group on the structure of the UF resin. This is beneficial to improve the bonding strength of the UF resin.10–12 At the same time, the new bond seals the hydroxymethyl group and improves the durability of the resin binding strength. In addition, the magnesia component of the mineral filler in UF resin could absorb the released acids during the gel process of the UF resin (MgO + 2HCl–MgCl2 + H2O), which improves the durability and the gel process of the resin bonded products.13
However, the enhancement effect of mineral fillers on the properties of the modified UF resin is not significant because of the weak connections between them. The addition of mineral fillers also increases the viscosity of the resin rapidly and produces a large amount of precipitation, which is not conducive to the sizing section of wood-based panels made from agricultural and forestry residues. The modification of the UF resin with nanoparticles further reduces the size of inorganic materials. It is expected to solve the above-mentioned problems of the inorganic filler modified UF resin. This is accounted to the small size effect and more unpaired electrons on the surface of nanoparticles. The content of free formaldehyde in UF resin could be reduced by the addition of nano-silica particles. The introduction of nano-silica particles has a significant effect on the bonding strength of a low molar ratio UF resin. The bonding strength is increased by more than 100%, and the thermal stability is also improved to a certain extent. However, the chemical bonding or cross-linking between them is difficult to form. Silicon dioxide is mainly physically dispersed in the resin, in which delamination and precipitation easily occur.10,14
In order to realize the chemical cross-links between inorganic nanoparticles and UF resin molecules and to prepare an inorganic–organic hybridized resin adhesive, the modification of the UF resin using siloxane grafted nano-SiO2 has been studied. The chemical connections of the resin are characterized using X-ray photoelectron spectroscopy (XPS). The results show that the siloxane grafted nano-SiO2 forms chemical bonds with UF resin molecules. The chemical bonds mainly affect the binding energy and chemical state of oxygen atoms of the resin molecules and finally increases the content of C–O–Si bonds. The study also indicates that only a small amount of added nano-SiO2 improves the bonding strength of UF resin and reduces the content of free formaldehyde.14 The addition of organic montmorillonite in the hydroxymethylation process of UF resin synthesis significantly reduces the content of free formaldehyde. The addition of organic montmorillonite in the condensation stage improves the bonding performance of the adhesive. The introduction of organic montmorillonite destroys the crystalline structure of the UF resin, improves the degree of molecular branching, and finally improves the bonding performance of UF resin.15,16 These studies provide a certain basis and guides the research and development of inorganic–organic hybridized UF resins. By modifying the inorganic nanoparticles with organic componds, there are organic groups on the surface of the particles, chemical cross-links might be formed during the synthetic or curing process of the UF resin.
The properties of the inorganic–organic hybridized resin adhesives are closely related to the nature of the modified particles, which provides a reference for the performance improvement of adhesives. However, the grafting process of inorganic particles is complicated, which is not conducive to industrial utilization. In addition, the grafted inorganic nanoparticles are usually added after the synthesis of the resin and formed chemical cross-links with the resin during the gel process. Under this circumstance, the viscosity of the modified resin is high due to the poor dispersibility of the grafted nanoparticles. The number of chemical connections between the inorganic and organic parts is also limited. To overcome these problems, CaCO3 particles were used to modify the UF resin in this study. The particles were added in the initial stage (hydroxymethylation) of the synthetic process. The resin is synthesized using a typical “alkaline–acid–alkaline” method, which provides conditions for the acid hydrolysis reaction of CaCO3 and allows the chemical cross-linking reactions of CaCO3 with UF resin molecules. Finally, the CaCO3 particles might be chemically bonded to the resin molecules directly without grafting or other chemical modifications.
2. Materials and methods
2.1 Materials
Eucalyptus fiber with a moisture content of 10% was obtained from Guangxi Fenglin Wood Industry Group Co., Ltd; formaldehyde solution (HCHO, 37 ± 0.5%), industrial grade, was purchased from Shangsi Huaping Chemical Co., Ltd; urea (CH4N2O, total nitrogen content ≥46.2%), industrial grade, was purchased from Chongqing Jianfeng Chemical Co., Ltd; NaOH (≥99%), industrial grade, was purchased from Xinjiang Zhongtai Chemical Co., Ltd; formic acid (HCOOH, ≥85%), industrial grade, was purchased from Shanghai Yihua International Trade Co., Ltd; ammonium chloride (NH4Cl, ≥99%), industrial grade, was purchased from Xilong Chemical Co., Ltd; heavy calcium carbonate (CaCO3, 200 mesh), was purchased from Yanxi Mineral Products Processing Plant. All chemicals were used as received without further purification, except for dilution to achieve different desired concentrations.2.2 UF resin preparation
The total molar ratio (F/U) of the UF resin was kept at 1.0. The detailed synthetic procedures of the resin are given below: the formaldehyde solution was added to a three-neck flask, and then CaCO3 was added. The pH value of the formaldehyde solution was adjusted to about 8.0 with 30 wt% NaOH solution. When the temperature of the solution was 40 °C, the first part of urea (50% of the total mass of urea) was added. Then the solution was heated up to 80 °C and kept for 30 min. After the heat preservation, the pH value of the reactant was adjusted to about 5.0 with 15 wt% formic acid solution, and the reaction was continued at 85–87 °C. The pH value of the solution was adjusted to about 6.5 when the viscosity of the solution reached a targeted value. The second part of urea (about 20% of the total mass of urea) was added after the solution temperature was reduced to 80–81 °C. The pH value of the solution was adjusted to 7.0–7.5 with 30 wt% NaOH solution after 20 min. Then the third part of urea (about 30% of the total mass of urea) was added while the reaction was continued at 60–65 °C. When the temperature of the solution was reduced to 40 °C, the pH value of the solution was adjusted to 8.0–8.5 with 30 wt% NaOH solution. Finally, the resin was poured out and packed in a plastic bottle. The synthetic procedures of the control UF resin were the same as the procedures except for the addition of CaCO3. The UF resin in the control group was named UF, and the UF resin modified by 1%, 2%, and 3% of CaCO3 (based on the dry weight of resin) was named 1% CaCO3-UF, 2% CaCO3-UF, and 3% CaCO3-UF, respectively.2.3 Characterization of physical and chemical properties of UF resin
The viscosity of the UF resin was measured by the no. 4 Cup method when the resin temperature was 26 °C (room temperature). The pH value of the resin was measured with a pH test paper. The free formaldehyde content of the resin was measured according to China National Standard GB/T14074.16-1993 “Test method for wood adhesive and its resin, determination of free formaldehyde content”. The solid content and gel time of the resin were measured according to China National Standard GB/T14074-2017 “Test method for adhesives and resins for wood industry”.The sample of 1 ± 0.1 g resin was weighed in an aluminium foil cup. The sample was placed in a constant temperature oven and dried at 120 ± 1 °C for 120 ± 1 min. The solid content of the resin was calculated according to formula (1):
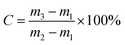 | (1) |
5 ± 0.5 g resin sample was added to a 250 mL iodine flask, followed by the addition of 50 mL distilled water (the same as a blank test), and 8–10 drops of mixed indicator (mixed with two parts of 0.1% methyl red ethanol solution and one part of 0.1% methylene blue ethanol solution). The solution was titrated with hydrochloric acid solution (1 mol L−1) until the solution turned grey-green, and 10 mL ammonium chloride solution (10 wt%) was added and shaken well. 10 mL sodium hydroxide solution (1 mol L−1) was added immediately with a pipette, and the bottle was capped and shaken well enough. The solution was allowed to stand at 20–25 °C for 30 min and then titrated with hydrochloric acid standard solution (1 mol L−1). The solution turned from green to grey-green to purple. The endpoint was grey-green. The content of free formaldehyde in the resin was calculated according to formula (2):
 | (2) |
50 g of the sample was weighed in a small beaker, and 2 mL ammonium chloride solution (25 wt%) was added using a pipette (5 mL) as the hardener. After stirring evenly, 10 g prepared resin was moved into a test tube, and the test tube was put into a beaker with boiling water, and the clock was started at this point. The liquid level of the sample in the test tube is 20 mm lower than the water surface of the boiling water. The metal wire is used as the stirring rod, and the stirring rod is stirred rapidly until the rod cannot be lifted suddenly or the resin suddenly hardens. The clock was stopped at this point, which corresponds to the gel time.
The chemical groups of the UF resin were characterized by a Fourier transform infrared spectroscopy (FTIR), IRTrace-100 (SHIMADZU, Japan). The sample was prepared by a typical potassium bromide (KBr) method. The resolution of the spectrogram was 0.5 cm−1, and the data interval was 4 cm−1. Each spectral curve was derived from the average of 20 scans.
The crystallization characteristics of UF resin were characterized by an X-ray diffractometer (XRD), Ultima IV (RIGAKU Company, Japan). The radiation source was CuKα, and the wavelength was 1.5418 nm. The data were collected at the testing speed of 10° min−1 in the range of 5° to 90° at 40 kV and 40 mA. All sample powders were tested using a non-reflective monocrystalline silicon sample rack.
The thermal stability of the UF resin was tested using a thermogravimetric (TG) analyzer (TGA5500). The sample (about 5 mg) was placed in an alumina crucible with a nitrogen flow rate of 25 mL min−1 and a heating rate of 15 °C min−1 in the temperature range of 30 °C to 790 °C.
2.4 Preparation of UF resin bonded MDF panels
The bonding properties of all the UF resin adhesives were characterized via the properties of the resin bonded MDF panels. The MDF panels were prepared using the procedures below: the amount of added UF resin was maintained at 13% (based on oven dry weight of the wood fibers) for wood fibers. Ammonium chloride (NH4Cl, 1% to solid resin content) was added to the resin as a hardener. The wood fibers were placed in a laboratory drum blender. Then, the UF resin was sprayed evenly to obtain a homogenized mixture. The hot air pipe was connected to the drum, and the resinated fibers were dried till the moisture content reached a value of less than 8%. The specific parameters for the MDF panels preparation are shown in Table 1.| Panel number | Adhesive type | Moisture content of resinated fibers after drying (%) |
|---|---|---|
| 1 | UF | 5.48/7.12 |
| 2 | 1% CaCO3-UF | 7.52/6.88 |
| 3 | 2% CaCO3-UF | 7.93/8.07 |
| 4 | 3% CaCO3-UF | 6.93/6.66 |
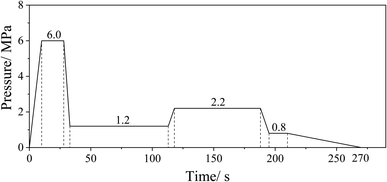
After the drying process, the resinated fibers were manually formed into mats using a wooden frame (340 mm × 340 mm). All the fiber mats were hot-pressed at 195 °C under the same hot-pressing parameters (Fig. 1). Board thickness was controlled at 13.4 mm by stop bars, and the target density was set as 780 kg m−3. Each group of the UF resin was used to prepare two pieces of the MDF panel. The obtained MDF panels were cut to the dimensions of 300 mm × 300 mm and sanded to the thickness of about 12 mm using a band sander before testing.
2.5 Tests of MDF panels
All properties of the MDF panels were measured in accordance with the procedure described in China National Standard GB/T 17657-2013.Ten samples were obtained from each panel, and the dimensions of 50 mm × 50 mm were used to calculate its density. The average value was taken as the density of the panel.
Two samples were obtained from each panel (samples used for density measurement) and placed in an oven at 103 ± 1 °C for 24 h to measure the mass of the samples before and after drying and calculated the moisture content of the samples. The average value was taken as the moisture content of the panel.
Two samples were obtained from each panel (samples used for density measurement) and soaked in water (20 ± 0.1 °C) for 24 h. The central thickness of the samples before and after soaking was measured, and the thickness swelling (TS) of the samples was calculated. The average value was taken as the 24 h TS of the panel.
Five samples were obtained from each panel (samples used for density measurement) to measure its internal bonding (IB) strength. The IB strength tests were conducted using a universal testing machine at room temperature. The loading rate for the IB strength was controlled at 2 mm min−1. The average value was taken as the IB strength of the panel.
Two samples were obtained from each panel with dimensions of 300 mm × 50 mm were used to measure its modulus of rupture (MOR) and modulus of elasticity (MOE). The MOR and MOE tests were measured by a three-point bending test conducted using a universal testing machine at room temperature. The loading rate for the MOR and MOE was controlled at 20 mm min−1. The average value was taken as the MOR and MOE of the panel.
3. Results and discussion
3.1 Effect of CaCO3 modification on physical and chemical properties of UF resins
The basic physical and chemical properties of conventional low molar ratio UF resin and CaCO3 modified UF resins are shown in Table 2. The pH value, viscosity, and free formaldehyde content of all the UF resins were not significantly different. In the synthetic procedure of UF resins, the typical “alkaline–acid–alkaline” method was used to strictly control the pH value, temperature, and target viscosity in each stage of the resin synthesis. Therefore, there was no significant difference in these measured items.| Adhesive type | pH | Viscosity (s) | Solid content (%) | Gel time (s) | Free formaldehyde content (%) |
|---|---|---|---|---|---|
| UF | 7.2 | 15.56 | 56.8 | 82 | 0.24 |
| 1% CaCO3-UF | 7.5 | 16.01 | 51.9 | 111 | 0.26 |
| 2% CaCO3-UF | 7.2 | 15.46 | 51.5 | 123 | 0.26 |
| 3% CaCO3-UF | 7.5 | 16.34 | 51.5 | 149 | 0.24 |
However, the solid content of the CaCO3 modified UF resin was significantly lower than that of the control group. The reason is that the polycondensation process of the UF resin was carried out under acidic conditions. After the introduction of CaCO3 into the reaction system, the added formic acid reacts with CaCO3. The targeted pH value of the solution could be reached after the CaCO3 reacted completely in the system.17 Therefore, in the CaCO3 modified UF resin system, the acid solution consumption, which is used to adjust the pH value of the solution, was tens of times higher than that of the control resin. Hence, the solid contents of the CaCO3 modified UF resins were significantly lower than that of the unmodified UF resin.
The gel times of the CaCO3 modified UF resins were longer than that of the control resin. The gel time could be further prolonged with the increase of CaCO3 content. The prolongation of gel time might be determined by the nature of CaCO3 since it has a strong neutralization or reaction ability to the acid. Although there might be no or only a small amount of CO32− ions in the CaCO3 modified UF resin, the association of calcium ions with hydroxyl groups, hydroxymethyl groups, and other groups in UF resin molecules might still have a strong neutralization ability to acids. Therefore, the gel time of CaCO3 modified UF resin was prolonged.
3.2 Effect of CaCO3 modification on chemical groups of UF resins
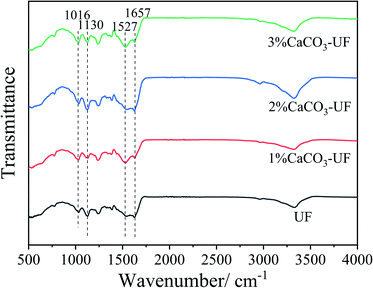
The Fourier transform infrared spectra of the control UF resin, and CaCO3 modified UF resins are shown in Fig. 2. The peaks at 1657 cm−1 and 1527 cm−1 were attributed to the stretching of carbonyl groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and C–N stretching of secondary amines, respectively. The other observed peaks of 1130 cm−1 and 1016 cm−1 were assigned to the C–O–C aliphatic ether and methylene bridge (–NCH2N–).18 The infrared spectrum shows the typical absorption peaks of UF resins. Compared to the reported FTIR spectra of the organic group grafted nano-silica modified UF resin, the chemical group of the UF resin with or without the modification of CaCO3 are not significantly different. There is only a small enhancement effect on the original absorption peak of the resin molecule, such as 1527 cm−1. The results indicate that the CaCO3 modified UF resin maintains the original molecular structure and chemical group of the resin. The introduction of CaCO3 might affect the rheological properties and other chemical properties of the resin through ionic bond complexation, electrostatic interaction, or other chemical bonds.17
O) and C–N stretching of secondary amines, respectively. The other observed peaks of 1130 cm−1 and 1016 cm−1 were assigned to the C–O–C aliphatic ether and methylene bridge (–NCH2N–).18 The infrared spectrum shows the typical absorption peaks of UF resins. Compared to the reported FTIR spectra of the organic group grafted nano-silica modified UF resin, the chemical group of the UF resin with or without the modification of CaCO3 are not significantly different. There is only a small enhancement effect on the original absorption peak of the resin molecule, such as 1527 cm−1. The results indicate that the CaCO3 modified UF resin maintains the original molecular structure and chemical group of the resin. The introduction of CaCO3 might affect the rheological properties and other chemical properties of the resin through ionic bond complexation, electrostatic interaction, or other chemical bonds.17
The absence of new absorption peaks in the infrared spectrum might also be attributed to the low proportion of cross-linking structures formed or the absorption peaks coinciding with the original chemical group. The chemical structure and state of CaCO3 modified UF resin still need to be further analysed and confirmed.
3.3 Effect of CaCO3 modification on crystallization characteristics of UF resins
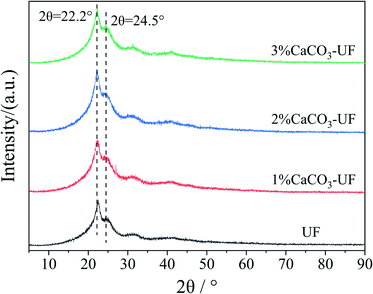
The ratio of the crystalline region of UF resins increases with the decrease in the molar ratio of the UF resins. The existence of a large number of crystalline regions shows that the amorphous structure in the resin is reduced, the content of free active groups is less, and the degree of cross-linking is low, which affects the bonding properties of the resin. Fig. 3 shows the X-ray diffraction (XRD) patterns of UF resins. As shown in the figure, the obvious crystallization characteristic peak can be observed in the UF resin with the molar ratio of 1.0, which is consistent with the reported results of crystallization characteristics of the UF resin.19The crystal structures of CaCO3 modified UF resins were not significantly different from that of the control UF resin. This trend is not changed with the increase in the amount of CaCO3. To quantify the crystalline regions, the crystalline percentages of UF in the control group and the 1%∼3% CaCO3 modified groups were simulated and resulted in 26.86% and 22.22%, 22.71%, 22.12%, respectively. It indicated that the ordered structure of the UF resin is not affected by the introduction of CaCO3. This shows that the cross-linking structure of CaCO3 with UF resin molecules is different from the organic groups grafted inorganic particles that do not directly bind with the resin molecules through the bridge group, but through other forms of non-direct crosslinking with the resin molecules. This association needs to be further demonstrated. However, in terms of macroscopic properties, it is found that CaCO3 modified UF resins have almost no precipitation. Their physical and chemical properties are similar to that of a conventional UF resin, which is significantly different from the UF resin with the addition of inorganic mineral particles in the later stage of resin synthesis.
3.4 Effect of CaCO3 modification on the thermal stability of UF resins
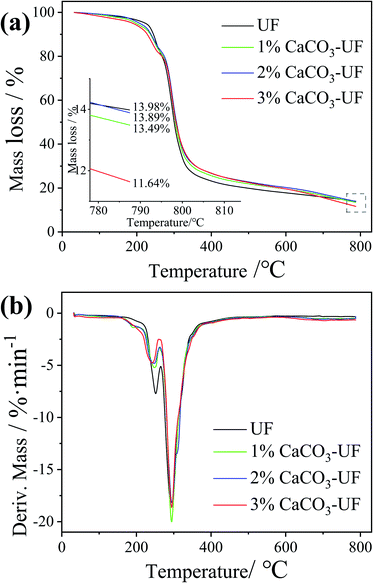
The thermal stability of the UF resin is very important for the hot-pressing process of wood-based panels. The improvement in thermal stability could reduce the pyrolysis of the UF resin in the hot-pressing process of wood-based panels, which might affect the surface quality of the final panels due to the long heating time of the surface. In order to explore the effect of CaCO3 modification on the thermal stability of UF resins, thermogravimetric analysis was carried out, and the results are shown in Fig. 4a. It can be seen from the figure that the CaCO3 modification has no significant effect on the thermal stability of UF resins. The mass loss rate and change trend of UF resins modified by different contents of CaCO3 are basically the same. The introduction of CaCO3 could only slightly reduce the mass loss rate of UF resins.Fig. 4b shows the DTG curves. The mass loss rate of the CaCO3 modified UF resin during thermal decomposition was slightly lower than that of the control UF resin, which is consistent with the result that CaCO3 improves the heat resistance of plastics and other organic compounds.20 This explains that the prolongation of the gel time of the UF resin modified by CaCO3. However, in this study, due to the low CaCO3 content (the highest addition value was only 3% based on the resin solid content), the effect on thermal stability of UF resins was not significant. The preparation of high content of CaCO3 modified UF resin adhesives is expected to further improve their thermal stability.
3.5 Effect of CaCO3 modification on the properties of resin bonded MDF panels
The performance of resin bonded MDF panels is used to further explore the effect of CaCO3 modification on the bonding properties of UF resins. The basic properties of the board are shown in Table 3. The fiberboard density designed in the experiment was 780 kg m−3, but there are slight differences in the fiberboard density due to the difference of solid content and moisture content of the resin, the difference of moisture content of the dried fibers, and the uniformity control in the manual forming process. The density of all the MDF panels was maintained at about 780 kg m−3, which leads to no significant difference in the board density. In addition, the moisture content of the fiberboard was about 5%, which meets the relevant requirements of the national standard.| Panel number | Board density (kg m−3) | The moisture content (%) |
|---|---|---|
| 1 | 782.09 ± 16.99 | 5.0 ± 0.1 |
| 2 | 773.58 ± 10.13 | 5.4 ± 0.2 |
| 3 | 782.12 ± 16.74 | 5.1 ± 0.1 |
| 4 | 783.80 ± 15.71 | 5.3 ± 0.2 |
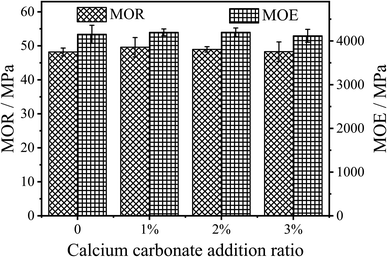
The modulus of rupture (MOR) and modulus of elasticity (MOE) test results of the panels are shown in Fig. 5. It can be seen from the figure that the MOR of the panels prepared with 1% CaCO3 modified UF resin (MOR = 49.59 MPa) is slightly higher than that of the unmodified UF resin (MOR = 48.20 MPa), but there is no significant difference between them. The MOR of the panels did not increase with the increase of CaCO3 content. The MOR of the fiberboard usually increases with the increase in density.21 However, the density and moisture content of the fiberboards prepared in this experiment are basically the same. It can be concluded that CaCO3 modification on UF resin in this study has no significant effect on the MOR of the fiberboards prepared using UF resins.
The change of MOE of the panels is similar to that of MOR. The MOE only increased from 4153 MPa to 4198 Mpa, as shown in Fig. 5, but there is no significant difference between them. This shows that the introduction of CaCO3 in the polycondensation stage of UF resins will not affect the toughness of the panels.
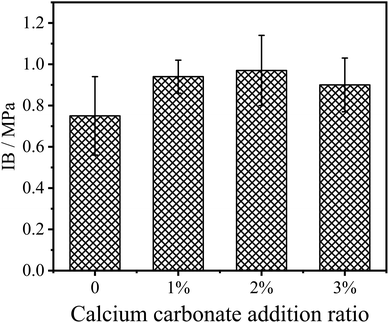
The internal bonding (IB) strength reflects the bonding quality of the fibers inside the fiberboard. Adding inorganic flame retardants (mainly metal oxides, etc.) directly into UF resins will affect the gel time and bonding strength of the resin, thus reducing the IB strength of the fiberboard.22,23 CaCO3 modified UF resin not only has certain flame retardancy, but also prolongs the gel time of the resin. In this study, CaCO3 was used to modify the UF resin, and the IB strength results of the fiberboards are shown in Fig. 6. As can be seen from the figure, with 2% of CaCO3 in UF resin, the IB strength increased from the original 0.75 MPa to 0.97 MPa. However, increasing the CaCO3 amount in the resin shows no significant effect on the IB strength of the panel.
The improvement of IB strength showed that the modified resin cured more fully or CaCO3 improved the cohesion of the modified UF resins via a possible reinforcement of the modified resin in the bond-line under the same hot pressing process of the fiberboard, and the cured modified resin had higher bonding strength. This may be due to the modification of CaCO3 improving the rheological properties of the resin, so that the UF resin can fully flow and disperse in the fiberboard hot pressing process, thus increasing the bonding area and improving the bonding quality. However, the IB strength of the fiberboard does not increase with the increase in CaCO3 content, and different CaCO3 contents have no significant effect on the IB strength of the fiberboard.
Wood materials easily absorb moisture because of their large number of hydrophilic groups. The raw material unit of the fiberboard is wood fiber. Because of its small size and large specific surface area, it easily absorbs moisture, which causes the deformation and cracking of the panel, resulting in the decline of its mechanical properties.24,25 Therefore, the waterproof or moisture-proof properties of the fiberboard are very important for its practical application.
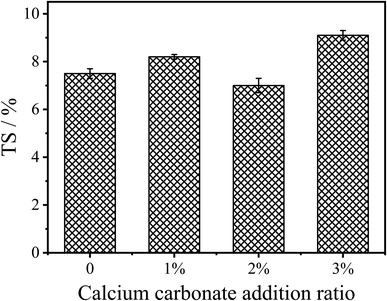
Fig. 7 shows the 24 h thickness swelling (TS) of the fiberboard prepared by the control UF resin and CaCO3 modified UF resins. It can be seen from the figure that CaCO3 modification has no significant effect on the moisture-proof performance of the panels. When the content of CaCO3 was 2%, the 24 h TS of the fiberboard was 7.0%, which is slightly lower than the 7.5% of the control resin. With the continuous increase of the CaCO3 content, the moisture-proof property of the fiberboards prepared by the modified UF resins tends to decline, which may be due to the strong hydrophilicity of inorganic minerals. This is consistent with the changing trend of moisture-proof properties of wood-based panels when inorganic minerals or inorganic nanoparticles are used as fillers of UF resins.23 Therefore, the introduction of CaCO3 during the addition or polycondensation stage of the UF resin synthesis can form chemical bonds through the synthesis environment of CaCO3 with resin and the interaction between resin molecules to maintain the bonding strength and moisture-proof properties of the fiberboard.
4. Conclusions
In this study, the effects of CaCO3 modification on the physical and chemical properties of the UF resin and the physical, mechanical, and moisture-proof properties of prepared fiberboards were investigated. The results show that CaCO3 modification has no significant effect on the viscosity, pH value, and free formaldehyde content on the UF resin. But CaCO3 modification decreased the solid content of the UF resin due to the increase in the amount of acid consumed by pH adjustment in the acidic stage of the UF resin synthetic procedure. In addition, the gel time of the UF resin was prolonged by the modification of CaCO3. The gel time was gradually prolonged with the increase in CaCO3 content. FTIR and XRD patterns show that CaCO3 modification has no significant effect on the chemical groups or the crystalline structure of the UF resin. CaCO3 modification only enhanced the absorption peak of some groups. TG and DTG patterns show that the thermal stabilities of CaCO3 modified UF resins are slightly improved.The properties of fiberboards prepared by 2% CaCO3 modified UF resins show that the IB strength of the panels could be increased from 0.75 MPa to 0.97 MPa, which is about 29% in enhancement. The modification of CaCO3 in UF resin shows no significant improvement of the resin bonded MDF panels on MOR, MOE, and 24 h TS properties.
In summary, the UF resins modified by CaCO3 maintain the excellent properties of the control UF resin. CaCO3 modification could significantly improve the IB strength of MDF panels, while maintaining other physical and mechanical properties. CaCO3 modified UF resin provides support for UF resin modification technology and the manufacture of high-performance fiberboards.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China [32001262]; Outstanding youth project of Hunan Education Department [18B18]; Changsha Science and technology project [kq2004096]; Forestry science and Technology Extension Project [2019:23]; Science and technology innovation project of Shengxiang group - Shengxiang industry (Jiangsu) Co., Ltd. [G.YF-QH-201905001]; the Key Projects of Hunan Science and Technology Plan [2016SK2032]; Guizhou Science and Technology Supporting Plan [2018:2196]; Introduced Talents Start-up Project of Central South University of Forestry and Technology [2018YJ007].References
- H. Khanjanzadeh, R. Behrooz, N. Bahramifar, S. Pinkl and W. Gindl-Altmutter, Carbohydr. Polym., 2019, 206, 11–20 CrossRef CAS PubMed.
- K. C. Akyüz, G. Nemli, M. Baharoğlu and E. Zekoviç, Int. J. Adhes. Adhes., 2010, 30(3), 166–169 CrossRef.
- S. E. V. D. A. Boran, M. U. S. T. A. F. A. Usta and E. S. A. T. Gümüşkaya, Int. J. Adhes. Adhes., 2011, 31(7), 674–678 CrossRef CAS.
- S. I. Tohmura, C. Y. Hse and M. Higuchi, J. Wood Sci., 2000, 46(4), 303–309 CrossRef CAS.
- E. Minopoulou, E. Dessipri, G. D. Chryssikos, V. Gionis, A. Paipetis and C. Panayiotou, Int. J. Adhes. Adhes., 2003, 23(6), 473–484 CrossRef CAS.
- A. Karthik, S. Arunmetha, S. R. Srither, P. Manivasakan and V. Rajendran, J. Sol-Gel Sci. Technol., 2015, 74(2), 460–471 CrossRef CAS.
- A. Afzal, H. M. Siddiqi, N. Iqbal and Z. Ahmad, J. Therm. Anal. Calorim., 2013, 111(1), 247–252 CrossRef CAS.
- H. Yu, Y. Xia, X. Xu, N. Zarshad, M. Wu and H. Ni, J. Appl. Polym. Sci., 2020, 137(41), 49256 CrossRef CAS.
- T. B. Sharmila, J. V. Antony, M. P. Jayakrishnan, P. S. Beegum and E. T. Thachil, Materials & Design, 2016, 90, 66–75 CrossRef.
- E. Roumeli, E. Papadopoulou, E. Pavlidou, G. Vourlias, D. Bikiaris, K. M. Paraskevopoulos and K. Chrissafis, Thermochim. Acta, 2012, 527, 33–39 CrossRef CAS.
- H. R. Taghiyari, R. Majidi, A. Esmailpour, Y. S. Samadi, A. Jahangiri and A. N. Papadopoulos, Polymers, 2020, 12(4), 857 CrossRef CAS.
- A. Esmailpour, H. R. Taghiyari, M. Ghorbanali and G. I. Mantanis, Fire Mater., 2020, 44(6), 759–766 CrossRef CAS.
- D. B. Fan, G. Y. Li, T. F. Qin and F. X. Chu, Polymers, 2014, 6(8), 2221–2231 CrossRef.
- Q. Lin, G. Yang, J. Liu and J. Rao, Front. For. China, 2006, 1(2), 230–237 CrossRef.
- M. A. R. Lubis and B. D. Park, J. Adhes., 2020, 1–20 Search PubMed.
- E. S. Wibowo, M. A. R. Lubis, B. D. Park, J. S. Kim and V. Causin, J. Ind. Eng. Chem., 2020, 87, 78–89 CrossRef CAS.
- T. Ozyhar, T. Depnering, C. Ridgway, M. Welker, J. Schoelkopf, I. Mayer and H. Thoemen, Eur. J. Wood Wood Prod., 2020, 78(1), 75–84 CrossRef CAS.
- S. Samaržija-Jovanović, V. Jovanović, S. Konstantinović, G. Marković and M. Marinović-Cincović, J. Therm. Anal. Calorim., 2011, 104(3), 1159–1166 CrossRef.
- M. Liu, R. V. K. Thirumalai, Y. Wu and H. Wan, RSC Adv., 2017, 7(78), 49536–49541 RSC.
- P. Liu, M. Zhao and J. Guo, J. Macromol. Sci., Part B: Phys., 2006, 45(6), 1135–1140 CrossRef CAS.
- M. K. Hong, M. A. R. Lubis and B. D. Park, Journal of the Korean Wood Science and Technology, 2017, 45(4), 444–455 Search PubMed.
- R. Hashim, O. Sulaiman, R. N. Kumar, P. F. Tamyez, R. J. Murphy and Z. Ali, J. Mater. Process. Technol., 2009, 209(2), 635–640 CrossRef CAS.
- O. Camlibel and M. Akgul, Wood Res., 2020, 65(2), 231–244 CAS.
- H. R. Taghiyari, A. Karimi and P. M. Tahir, J. For. Res., 2015, 26(2), 495–500 CrossRef CAS.
- H. R. Taghiyari, Eur. J. Wood Wood Prod., 2013, 71(3), 353–360 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |