Open Access Article
Open Access ArticleEffect of blending biodiesel produced from waste cooking oil on the properties of residual fuel oil: energy saving and the economic cost
Mahmoud Abd El-Aziz Mohameda,
Mostafa A. A. Mahmoudb and
H. A. El Nagy *b
*b
aMechanical Power Engineering, Faculty of Engineering, Zagazig University, Sharkia, 4419, Egypt
bChemistry Department, Faculty of Science, Suez Canal University, Ismailia, 41522, Egypt. E-mail: hala.elnagy@yahoo.com; Hala_elnagy@science.suez.edu.eg; Tel: +201002375731
First published on 7th October 2021
Abstract
The mazout properties were improved using ecofriendly ways because of its wide range of applications, abundance and low cost. In this study, the effect of biodiesel blending on the properties of mazout was investigated. Biodiesel was prepared from waste cooking oil. The mazout properties such as viscosity and density improved with the increase in volume ratio of biodiesel to mazout. The mazout viscosity decreases with an average value of 12% as the biodiesel is added with a volume ratio of 10%. In contrast, when a 10% volume ratio of the biodiesel is added to mazout, the heating value decreases by 1.5%. Although the calorific value of mazout decreases after the blending process, the blending method is considered a method that saves energy compared to the heating method to reduce the viscosity. The cost of improved mazout depends on the cost of biodiesel production. The more the cost of biodiesel production approaches the cost of mazout, the more expensive the use of the blending method compared to the heating method. Moreover, the blending method is a very effective method to reduce the percentages of harmful compounds such as sulfur, and the compound percentages that occupy volumetric proportions of fuel such as water content.
1. Introduction
Meeting the world's energy needs in a sustainable way is one of the biggest challenges facing humanity.1 Most industries depend on the use of residual heavy fuels due to their high calorific value, low cost and abundance.2 Mazout (also called fuel oil no. 6, residual fuel oil or bunker C) is a residue from crude oil after removing gasoline, fuel oil no. 1 and fuel oil no. 2. Residual fuel oil (fuel oil no. 6) has a composition that is more complex and contains more impurities than the distillate fuels. Limited data are obtainable on its composition. Paraffins, polycyclic aromatic hydrocarbons, asphaltenes and metal-containing derivatives are components of fuel oil no. 6.3The resources of residual heavy oil in the world are more than twice those of the conventional light crude oil.4 The heavy oil of petroleum product contains a high percentage of sulfur, minerals, asphaltenes and carbon residues.5 It is used for industrial purposes, ships, marine vessels, electricity generation, and others. The quality of this product is affected by some properties such as viscosity, flash point, pour point and specific weight. In addition, it is preferable to use less quantity, especially in cities and residential areas, due to the high pollution rates it causes.6
Density, viscosity and calorific value of fuels are the most important criteria that represent the key properties to define and develop the range of heavy fuel economics.7 Usually heavy oil is sold at a lower price than light fuel because it has obstacles and technological costs for preparing it for the burning process. On the other hand, its high viscosity is an obstacle for its use, combustion process, production and transportation.8
Numerous common methods are used to reduce viscosity and to transport heavy fuels from places of production to places of consumption, where burning, for example, in furnaces, ships and in power plants. Common methods used in the transfer process are heating, dilution, emulsification, partial upgrading and annular flow.9
Heating is a common method for improving the flow properties of heavy oils. The viscosity decreases to a noticeable degree with the increase in temperature.10 On the other hand, the process of heating the lines is a difficult and expensive process. This process needs to take into account the expansion of pipelines and the number of pumps for heating stations, as well as the thermal insulation and thermal loss in the transport process and the rate of corrosion that takes place inside the pipes due to heat, and more than that colloidal structure in the case of crude oil transportation. All these considerations represent a fixed economic cost in case of pipelines being prepared to suit the transportation conditions.11
One of the advanced methods used for improving the transportation is the dilution. Heavy fuels are blended with lower-viscosity hydrocarbons such as naphtha and kerosene. According to practical experiments, it has been found that the viscosity decreases with the increasing in volume fraction of the hydrocarbons used for dilution. On the other hand, it is necessary to use pipes with a higher capacity. Making these hydrocarbons available for fear is a problem. Re-separating these thinners requires other solutions, and this requires more preparation to place additional tubes for the additives.12–14
The emulsification method is also used for reducing the viscosity of heavy fuels. The emulsion is composed of 70% crude oil, 30% aqueous phase, and 500–2000 ppm chemical additives. The viscosity of the resulting emulsion was in the range of 50–200 cp under the operating conditions. Subsequently, this method requires re-breaking the emulsion, but this method still poses a problem in completely re-disposing of the water from the fuel.15,16
There are other common methods used for this purpose. One of the technologies used to improve the properties of heavy fuels is the use of blending with biodiesel. Biodiesel is easy to use, biodegradable, non-toxic, has lower greenhouse gas emissions, contains no sulfur and has more effective combustion levels.17–19
The main obstacle in biodiesel production is the cost of raw materials. Raw material source of biodiesel is mostly vegetables oil such as Jatropha, Soybean, Pongamia, Neem, Castor oil etc. that needs large land area to cultivate. The production of biodiesel from low quality feedstock is one of the best methods to diminish the biodiesel cost.20
Waste cooking oil represents a valued source of raw materials due to its waste-nature and availability. It also well fit in the circular economy model, render to interest for developing new sustainable productions.21–23
However, in some heavy fuel applications such as marine fuel, biofuels can be blended with petroleum diesel to form blended biodiesel, which consists of 20 percent by volume biodiesel and 80 percent by volume diesel from oil.24 It was found that when mixing biofuels with heavy marine fuels (20% bulk biofuel), the calorific value was reduced by 1.9%. On the other hand, other characteristics of the heavy marine fuels have improved, such as flash point and viscosity.25 However, private renewable energy projects such as clearing forests to produce biofuels can cause similar or worse environmental damage compared to using fossil fuels.26
Cherng-Yuan Lin investigated the effects of biodiesel blends on residual and distillate marine fuel properties. The fuel characteristics of residual marine fuel increase much more than those of distillate marine fuel when biodiesel is blended in ref. 27. Michael D. Kass et al. studied the properties of blends made up of high viscosity heavy fuel oil and acidic bio-oil. Bio-oil concentrations as low as 5% mass percent were shown to reduce the viscosity of heavy fuel oil considerably.28
From all this it becomes clear that there is a basic strategy for finding alternatives or improving energy sources. First: energy conservation. Second: finding cleaner ways to produce energy. Third: seek to find a lower cost in producing or improving energy.
From the previous introduction, it was found that the viscosity is an important fuel property in the fuel use that must be improved. The fuel properties of heavy residual fuel (mazout) can be adjusted through blending it with a renewable, clean and ecofriendly alternative fuel. The blending of biodiesel with residual heavy fuel is still very limited compared with the widespread biodiesel–diesel blends.
In this study, the improvement of residual fuel oil properties (mazout, API 15.1) using the blending method is discussed. The mazout is blended with different volume ratio of biodiesel produced from waste cooking oil. Moreover, the effectiveness of using this method is judged from the point of view of the energy saving and the cost compared with using heating method to reduce the viscosity.
2. Material and methods
2.1. Materials
The materials used for biodiesel production include waste-cooking oil (WCO) obtained from household and its physicochemical properties were indicated in Table 1, ethanol absolute (99.9%) and sodium hydroxide (used as catalyst). The attained WCO was filtered off for several times to separate it from the solid suspended impurities. Then, it was allowed to settle down for three days to remove further contaminants and filtered once again. Moreover, the WCO sample was heated to remove moisture finally, the WCO sample were dried over anhydrous Na2SO4.| Property | Waste cooking oil (WCO) | Prepared biodiesel |
|---|---|---|
| Density at 15 °C (g cm−3) | 0.920 | 0.880 |
| Viscosity at 40 °C (Cst) | 14 | 12 |
| Pour point (°C) | 9 | −3 |
| Acid value (mg KOH g−1) | 3.1 | 0.215 |
| Sulfur (% wt/wt) | 0.110 | 0.100 |
| Flash point | — | 130 |
| Heating value (kJ kg−1) | — | 38.413 |
| Water content (% v/v) | 0.09 | 0.04 |
Mazout was attained from fuel storage tanks of a local power plant station. Preparation of biodiesel and its test fuels blends with mazout was performed in Suez Canal University, faculty of science, chemistry department laboratories.
2.2. Synthesis of biodiesel procedure
Transesterification is the method used for biodiesel synthesis and the experiment was performed at a laboratory scale. It is a method in which the animal fats or vegetable oils was reacted with alcohol in presence of catalyst to form glycerol and the fatty acid methyl ester (FAME). Ethyl alcohol (20% weight of oil) and sodium hydroxide (1% weight of oil) were mixed using magnetic stirrer. WCO was heated to 60 °C in a separate flask before the ethyl alcohol mixture has been added to it. The reaction was continued for one hour at 60 °C with stirring. Then, the excess alcohol has been distilled off and the reaction product was put into a separating funnel in order to separate biodiesel from glycerin. Finally, the produced biodiesel was washed three times with warm water, separated from water and drying for one hour at 105 °C.2.3. Preparation of blends
Though mixing mazout with biodiesel, six blends were prepared in volumetric percentage (v/v) and named as M100 (100% mazout + 0% biodiesel), M90 (90% mazout + 10% biodiesel), M80 (80% mazout + 20% biodiesel), M70 (70% mazout + 30% biodiesel), M60 (60% mazout + 40% biodiesel) and M50 (50% mazout + 50% biodiesel). Moreover, each blend was further mixed with a magnetic stirrer at room temperature to confirm homogeneity. The prepared blends were kept in glass bottles at ambient temperature.2.4. Fuel properties measurements
Measurements of the physical–chemical properties for the prepared blends were carried out to investigate the effect of adding biodiesel on the mazout properties.Kinematic viscosities of blends were determined using ASTM D 445 standard, kinematic viscosity device, Tamson Tv4000MkIL as shown in Fig. 1(a). The time it takes for a fixed amount of liquid to flow under gravity through the capillary of a calibrated viscometer, under a reproducible driving head, and at a precisely regulated and known temperature is measured.
Sulfur content was measured for the prepared blends according to ASTM D 4294 standard using sulfur X-ray device, Horiba SLFA-6800 as shown in Fig. 1(b). An X-ray tube emits a beam that the sample is placed in. The collected count from the resultant excited characteristic X radiation is compared to counts from previously prepared calibration samples to determine the sulphur concentration in mass percent and/or mg kg−1.
Pour point of blends was determined according to ASTM D 97 standard using pour point device, stanhope-seta 26346 as shown in Fig. 1(c). After preliminary heating, the sample is cooled at a set rate and tested at 3 °C intervals for flow properties. The lowest temperature at which the specimen moves is documented as the pour point.
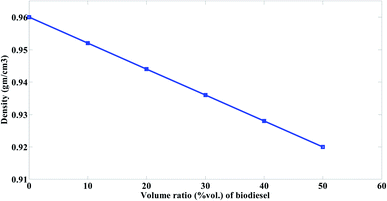
Density was determined using ASTM D 5002 standard by Densitometer A. Kruss Optronic device. An oscillating sample tube is filled with approximately 0.7 ml of oil sample, and the change in oscillating frequency induced by the change in tube mass is utilized in conjunction with calibration data to calculate the density of the sample.
Flash points blends was determined according to ASTM D 93 standard using auto flash point stanhope-seta 35000-04 as shown in Fig. 1(d). The specimen is heated and stirred at set rates in a brass test cup of defined dimensions, filled to the inside mark with test specimen and fitted with a cover of specified dimensions. At regular intervals, an ignition source is delivered into the test cup, interrupting the stirring process, until a flash is detected.
Water content was measured for the prepared blends according to ASTM D 4006 standard using Dean Stark Stanhope-Seta device as shown in Fig. 1(e). The sample is heated in a water immiscible solvent that co-distills with the water in the samples under reflux circumstances. In a trap, condensed solvent and water are continually separated, with the water settling in the graded part and the solvent returning to the distillation flask.
Heating values was determined according to ASTM D 4809 standard. A weighted sample is burned in an oxygen-bomb calorimeter under controlled conditions to estimate the heating value. A temperature-reading instrument is used to measure the temperature increase, allowing the method's precision to be satisfied. The heating value is measured.
3. Results and discussions
The results discuss the effect of temperature on mazout properties (heating method). The heating method was compared to the blending method with the prepared biodiesel and the results are compared with the previous related works. Besides, the energy saving and the cost have been studied to predict the best method to improve the properties of mazout.3.1. The heating method
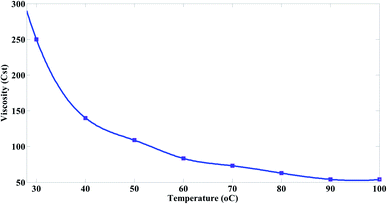
When the temperature of the pure mazout increases from 30 °C to 70 °C, the results were as follow.Fig. 2 shows a reduction in the viscosity of mazout with the increasing in temperature. As the temperature increases from 30 to 70 °C, the viscosity decreases gradually from 250 Cst to 73 Cst. However, when the temperature increases above 70 °C, the viscosity is slightly reduced. With the increasing in temperature, the kinetic and thermal energy increase and the molecules become freer. As a result, the attractive binding energy and the viscosity is reduced.
Typically, the mazout have higher viscosity values due to its complex composition of aromatic hydrocarbons, asphaltenes and paraffins. This leads to high resistance of mazout to flow and transform from one place to another.31 Hence, a great amount of pumping energy or heating is required to reduce the viscosity of the mazout and increase its fluidity so it can easily flow through pipelines for burning process.
3.2. The blending method
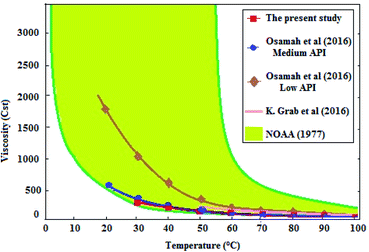
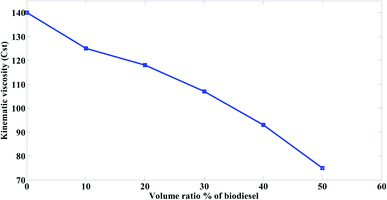
The mazout was blended with the prepared biodiesel at different concentrations (M100, M90, M80, M70, M60 and M50).The results were compared with the results of Cherng-Yuan.27 He used the biodiesel (density (at 15 °C) 860 to 900 kg m−3, kinematic viscosity (at 40 °C) 3.5 to 5 mm2 s−1, and heating value 38 MJ kg−1), to improve the properties of marine fuel (density (at 15 °C) 920 kg m−3, kinematic viscosity (at 40 °C) 10 Cst, and heating value 40 MJ kg−1). The results were as follow.Therefore, when the mazout being blended with the prepared biodiesel, the viscosity of the prepared blend decreased because of the great difference between mazout and the prepared biodiesel in viscosity. Fig. 4 represent much more significant decrease in the kinematic viscosity of mazout–biodiesel blends as the amount of the prepared biodiesel being increased so that much less pumping or heating energy is required.
The viscosity of mazout gradually decreases with an average value of 12% as the prepared biodiesel being added with a volume ratio of 10% in all blends. When the mazout was blended with the prepared biodiesel at a concentration ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (M50 blend), the viscosity of mazout decreased with an average value of 47% as compared with the value of mazout without biodiesel (M100). The change in viscosity because of blending according to the present study is in a good agreement with Cherng-Yuan27 results.
50 (M50 blend), the viscosity of mazout decreased with an average value of 47% as compared with the value of mazout without biodiesel (M100). The change in viscosity because of blending according to the present study is in a good agreement with Cherng-Yuan27 results.
Therefore, biodiesel is combusted much more efficiently than mazout. Hence, biodiesel has lower stoichiometric air to fuel ratio than that of the mazout because lower amount of air will be required for burning. As a result, biodiesel releases much less greenhouse gas emissions and carbon oxides.36
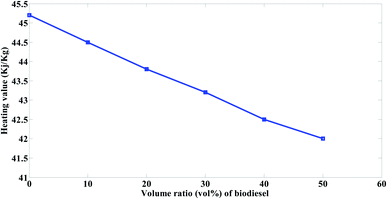
The heating value for the prepared biodiesel is 38.413 kJ kg−1, whereas that of the mazout fuel is 45.2 kJ kg−1. It was observed from the Fig. 6 that the heating values of the prepared blends decrease with the increasing in the volume ratio of the prepared biodiesel at the order of M90 > M80 > M70 > M60 > M50. Moreover, it was observed that the heating value decreased with 1.5% with the addition of 10% volume ratio of the prepared biodiesel (M90). The decrease in heating value because of blending according to the present study is in a good agreement with Cherng-Yuan27 results.
3.3. Energy saving
Although the properties of mazout fuel such as viscosity and density have been improved, its heating value has decreased by 1.5% when adding biodiesel by a volume ratio 10% to it.In order to determine the effective and economical of blending process. The values of energy saving were calculated based on the decrease in the viscosity. The blending method is compared to the heating method of mazout. Then, the energy saving values are calculated when comparing the heating method with the blending method to reduce the viscosity. This required two steps; the first step is to study the change of viscosity with temperature as shown in Fig. 2. It was indicated that the decrease in viscosity, required much greater temperature range for heating. For example, to decrease the viscosity from 250 Cst to 200 Cst, it needs to 5 °C while to decrease the viscosity from 100 Cst to 50 Cst, it needs to nearly 50 °C. The second step is to estimate the amount of energy required to reduce the viscosity corresponding to the volume ratios of biodiesel added in blends as shown in Fig. 7.
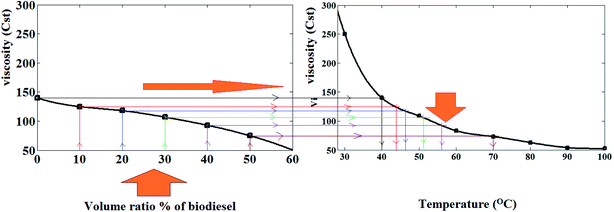 | ||
| Fig. 7 Variation of viscosity with volume ratio [%] of biodiesel blends vs. the effect of temperature on viscosity of mazout. | ||
The amount of energy saving are calculated from eqn (1):
| ΔQ = Cp × ΔT − Δh | (1) |
| Δh = hvmazout − ((1 − α)hvmazout + αhvbiodiesel) | (2) |
The results of the amount of energy saving (kJ kg−1) are summarized in Table 2.
| Blend of volume fraction | The viscosity (Cst) | The corresponding temperature, Tcorres (K) to viscosity | The amount of energy saving (kJ kg−1), Cp × (Tcorres − Treff) − Δh |
|---|---|---|---|
| 0% | 140 | 313 | 0.0 |
| 10% | 125 | 317 | 8−0.678 = 7.322 |
| 20% | 118 | 319 | 12−1.356 = 10.644 |
| 30% | 107 | 325 | 24−2.034 = 21.966 |
| 40% | 93 | 329 | 32−2.712 = 29.288 |
| 50% | 75 | 340 | 54−3.39 = 50.61 |
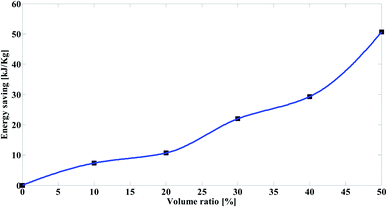
As shown in Fig. 8, when the volume ratios of the prepared biodiesel increase, the values of energy saving increase. Moreover, the amount of energy saving depends more on the equivalent temperature difference (ΔT).
All this indicate that the energy was saved because of adding the prepared biodiesel to mazout through reducing the viscosity of mazout. However, these calculations are not sufficient to judge the use of the blending method in improving the properties of mazout compared to the heating method, as there is a large variation in the price of mazout and the price of the prepared biodiesel.
3.4. The predicted cost of the prepared blends
The effect of production price of biodiesel from waste cooking oil compared to the production of mazout is discussed. Two different prices are studied in this research. According to Mohammadshirazi et al.37 and El-Gharbawy,38 the prices of biodiesel produced from waste cooking oil is 1.2$ and 0.515$, respectively. According to global energy prices estimations, the average price of a liter of mazout is 0.32$.39In this section, the volume of fuel and the amount of energy savings in dollars are evaluated as equivalent volume of mazout. The equivalent volume of mazout is defined as the volume of the fuel that give the amount of energy required to heating mazout for the required viscosity. The equivalent volume of mazout is calculated according to eqn (3):
| The equivalent volume of mazout (liter) = (the amount of energy required for heating/heating value of mazout) × (1/density × 103) | (3) |
The cost of the improved mazout consist of the cost of mazout and biodiesel blended with it. Assuming that the use of fuel depends on the viscosity. In this section, three cases are considered. One of the cases is that of the pure mazout, in this case, the cost depends on the purchase cost of the fuel in addition to the equivalent cost of heating method to reach the required viscosity. The other two cases are considered for the blend method to reach the required viscosity. One of them when the cost of prepared biodiesel is 1.2$, and the other case when the cost of prepared biodiesel is 0.515$ (Table 3).
| Volume ratio% | Equivalent saving volume of mazout (liter) |
|---|---|
| 10 | 0.17 |
| 20 | 0.247 |
| 30 | 0.51 |
| 40 | 0.68 |
| 50 | 1.17 |
The predicted cost of three cases are calculated assuming the same viscosity according to eqn (4) and (5):
The predicted cost by heating method
| The predicted cost = the cost of mazout + the equivalent cost of heating method | (4) |
The predicted cost by blending method
| The predicted cost = α × the cost of biodiesel + (1 − α) × the cost of pure mazout | (5) |
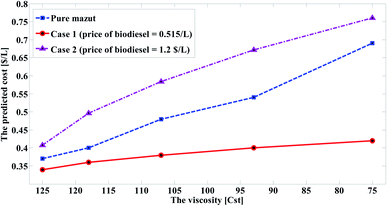
Table 4 summarized the predicted cost of the three cases. As shown in Fig. 9, when the cost of biodiesel production approaches the cost of mazout, the predicted cost of improved mazout becomes less than the cost of heating method. On the contrary, the higher the cost of biodiesel production, the higher the predicted cost of the improved mazout. This indicates that the blending process has less cost than the heating process.
| Blend | Heating | |||||
|---|---|---|---|---|---|---|
| α | μ | Case 1 (price of biodiesel = 1.2$ L−1) | Case 2 (price of biodiesel = 0.515$ L−1) | T | μ | Pure mazout |
| 10% | 125 | 0.408 | 0.34 | 317 | 125 | 0.37 |
| 20% | 118 | 0.496 | 0.36 | 319 | 118 | 0.40 |
| 30% | 107 | 0.584 | 0.38 | 325 | 107 | 0.48 |
| 40% | 93 | 0.672 | 0.40 | 329 | 93 | 0.54 |
| 50% | 75 | 0.76 | 0.42 | 340 | 75 | 0.69 |
Using the biodiesel as a blend fuel to improve the mazout properties depends on the cost of the production of biodiesel. The critical cost of biodiesel production is the cost when the predicted cost from eqn (4) and (5) are equal value. Then, the critical cost of biodiesel can be calculated as eqn (6):
| The critical cost of biodiesel production = the cost of pure mazout + (1/α)the equivalent cost of heating method | (6) |
The capital cost of heating method may be increase because of providing equipment such as thermal insulation materials, heating lines, and annual cost of maintenance of thermal insulation.
The insulation thickness depends on the difference temperature between the maximum temperature allowed on the outside surface of insulation (Tout), considered 40 °C and the operating temperature of the fluid inside pipe (Tin). Formula for steady state heat transfer (Qtransfer) through insulating material wrapped around a pip is as shown in eqn (7):
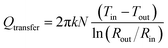 | (7) |
Assume for the same insulating material and dimentions of the pipe contains the heavy oil fuel, the thickness of the insulating material increases as indicated in eqn (8):
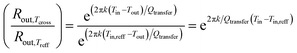 | (8) |
According to eqn (8), the percentage change of the outer radius of insulating material increases with mazout temperature (Tin) for the same insulating material, heat transfer and reference temperature and insulation thickness become as eqn (9):
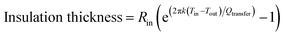 | (9) |
When using the prepared biodiesel, the thickness of insulating material become a value of zero. The predicting cost after considering the value saving of insulating material is as eqn (10):
| The capital cost after considering the value saving of insulating material = the predicting cost from eqn (4) + price of insulation material | (10) |
Price of insulation material depends on the thickness, the length and the type of insulation as eqn (7).
Also the important advantage when using the blend method to improve the properties of mazout. The other properties such as sulfur content, pour point, flash point and water content improved. Further removal of sulfur and water content from mazout translates to additional energy and capital costs and can influence fuel price and availability. Moreover, the blending method is a very effective method to reduce the percentages of harmful compounds such sulfur, and the compounds percentages that occupy volumetric proportions of fuel such water content.
3.5. The effect of blending method on the other properties
The reduction of the heavy fuel sulfur content decreases emissions of sulfur oxides produced in the combustion process.41
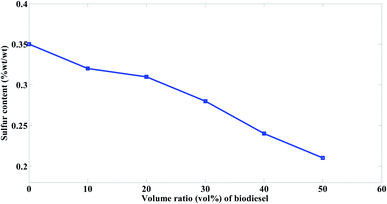
One important property of biodiesel is its low sulfur content as compared to the residual heavy fuels. Therefore, biodiesel is considered as a green fuel, which has low effect on greenhouse gases emissions. The sulfur content of the prepared biodiesel is 0.100% wt/wt while that of mazout is 0.350% wt/wt. Fig. 10 shows reduced sulfur content for mazout with the increased biodiesel volume ratios in the prepared blends at the order of M90 > M80 > M70 > M60 > M50. It was indicated that if 10 vol% biodiesel is added to the mazout, the sulfur content of mazout reduced by 8.5% as shown in Fig. 10.
The decrease of sulfur content because of blending according to the present study is in a good agreement with Cherng-Yuan27 results.
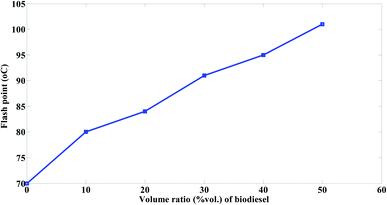
The flash point of biodiesel is usually higher than that of conventional heavy fuel because biodiesel is produced from oil containing fatty acids with longer carbon chains. Catoire and Naudet explained why compounds with longer carbon chain have a higher flash point through using an empirical model for estimation of flash point.42
The flash point for biodiesel is 130 °C, while that for mazout is 70 °C. It was observed that the flash point of mazout increase significantly with the increasing in the biodiesel volume ratios in the order of M90 < M80 < M70 < M60 < M50 as shown in Fig. 11. Furthermore, it points out that the blending of mazout 10 vol% biodiesel increase its flash point noticeably by 14%. This increase in flash point of mazout reduce the problems such as fire hazards that is being caused by the lower flash point of fuel.
The increase of flash point because of blending according to the present study is in a good agreement with Cherng-Yuan27 results.
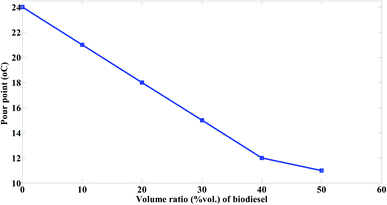
The residual heavy fuels are also rich in asphaltenes, which can lead to several transportation and recovery problems due to increased viscosity. The paraffins may also crystallize or deposit in the storage tanks leading the fuel to be immovable and causing blockages at pipelines.44
The pour point of the prepared biodiesel is −3 °C while that of mazout is 24 °C. The Fig. 12 indicated the effect of adding the prepared biodiesel on the pour point of mazout. The pour point of mazout significantly decrease with the increasing the volume ratio of biodiesel being added to the prepared blends at the order of M90 > M80 > M70 > M60 > M50 so that much less pumping and heating energy is required.
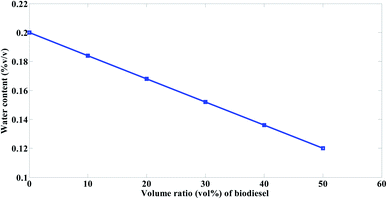
The water content of mazout is 0.2 (% v/v) while that of the prepared biodiesel is 0.04 (% v/v). It was observed from Fig. 13 that the water content of mazout decrease with the increase of the volume ratio of the prepared biodiesel. Fig. 13 also indicates that the water content of mazout decrease by about 8% when 10% of the prepared biodiesel being added.
4. Conclusions
The process of improving fuel properties is not an absolute process, but rather a relative process that depends on the method used to improve the properties. Important criteria must be taken to determine the effectiveness of the method used to improve fuel properties. Environment, energy saving and the cost are three important interrelated criteria that must be taken into account when judging the method used to improve fuel. Using the blending method with biodiesel extracted from the waste cooking oil to improve fuels is more acceptable than heating method to improve mazout properties when it meets these three criteria. This appears more especially when producing fuel from waste cooking oils at lower cost. The mazout properties was improved through blending it with a renewable, clean and ecofriendly alternative fuel produced from waste cooking oil. Addition of the prepared biodiesel on the mazout blends reveals that the viscosity of mazout decrease with an average value of 12% as 10% of the prepared biodiesel being added so that much less pumping and heating energy are required. Heating value of mazout decreased with 1.5% with the addition of 10% volume ratio of the prepared biodiesel. The blending method is considered a method that saves energy compared to the heating method to reduce the viscosity. The blending process also can save the equipments used in heating process such as thermal insulation materials, heating pipes lines, and annual maintenance of the thermal insulation. The blending method is a very effective method to reduce the percentages of harmful compounds such sulfur. Sulfur and water content decreased with 8.5% and 8%, respectively when 10% of the prepared biodiesel being added so that its effect on health and environment, corrosion and microbial problems have being reduced.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work had been reinforced by Suez Canal University (SCU) and Zagazig University.References
- B. R. Conard, Sustainability, 2013, 5, 3368–3381 CrossRef.
- D. Stratiev, Z. Belchev, P. Petkov and K. Kirilov, Oil Gas Eur. Mag., 2008, 34, 199–203 CAS.
- J. G. Speight, in Advances in Clean Hydrocarbon Fuel Processing, ed. M. R. Khan, Woodhead Publishing, 2011, pp. 54–82 Search PubMed.
- R. G. Santos, W. Loh, A. C. Bannwart and O. V. Trevisan, Braz. J. Chem. Eng., 2014, 31, 571–590 CrossRef.
- I. Elsayed, R. Abdallah, A. El-Naggar, M. Mashaly and A. Salem, Nat. Sci., 2012, 10, 72–79 Search PubMed.
- U. Hassan, I. Al-Zubaidi and H. Ibrahim, ChemEngineering, 2018, 2(3) DOI:10.3390/chemengineering2030030.
- F. Bjørnseth, Master's thesis, Institutt for petroleumsteknologi og anvendt geofysikk, 2013.
- A. Hart, J. Pet. Explor. Prod. Technol., 2014, 4, 327–336 CrossRef CAS.
- J. Jing, R. Yin, Y. Yuan, Y. Shi, J. Sun and M. Zhang, ACS Omega, 2020, 5, 9870–9884 CrossRef CAS PubMed.
- A. Saniere, I. Hénaut and J.-F. Argillier, Oil Gas Sci. Technol.-Rev. Inst. Francais Pet. - OIL GAS SCI TECHNOL, 2004, 59, 455–466 CrossRef.
- T. N. Mitusova, N. K. Kondrasheva, M. M. Lobashova, M. A. Ershov, V. A. Rudko and M. A. Titarenko, Chem. Technol. Fuels Oils, 2018, 53, 842–845 CrossRef CAS.
- I. A. Struchkov, P. V. Roschin, V. T. Litvin, V. A. Ol’hovskaya and E. S. Kalinin, J. Pet. Explor. Prod. Technol., 2020, 10, 755–767 CrossRef.
- P. Gateau, I. Hénaut, L. Barré and J.-F. Argillier, Oil Gas Sci. Technol.-Rev. Inst. Francais Pet. - OIL GAS SCI TECHNOL, 2004, 59, 503–509 CrossRef CAS.
- Y.-H. Kuan, F.-H. Wu, G.-B. Chen, H.-T. Lin and T.-H. Lin, Energy, 2020, 201, 117559 CrossRef CAS.
- M. M. Abdulredha, H. Siti Aslina and C. A. Luqman, Arab. J. Chem., 2020, 13, 3403–3428 CrossRef CAS.
- L. Leng, H. Li, X. Yuan, W. Zhou and H. Huang, Energy, 2018, 161, 214–232 CrossRef CAS.
- B. E. do Amaral, D. B. de Rezende and V. M. D. Pasa, Fuel, 2020, 279, 118289 CrossRef.
- S. Subramani, K. Natarajan and G. Lakshmi Narayana Rao, Fuel, 2021, 287, 119438 CrossRef CAS.
- V. K. Viswanathan and P. Thomai, Fuel, 2021, 288, 119611 CrossRef CAS.
- F. Allah, M. Bica and T. Dragos, Proceedings of the 4th International Congress of Automotive and Transport Engineering (AMMA 2018), 2019, pp. 217–224 Search PubMed.
- M. K. Gupta, Practical guide to vegetable oil processing, 2nd edn, Elsevier, Cambridge, MA, 2017 Search PubMed.
- A. Pugazhendhi, A. Alagumalai, T. Mathimani and A. E. Atabani, Fuel, 2020, 273, 117725 CrossRef CAS.
- J. Mercy Nisha Pauline, R. Sivaramakrishnan, A. Pugazhendhi, T. Anbarasan and A. Achary, Fuel, 2021, 285, 119108 CrossRef CAS.
- U. Kesieme, K. Pazouki, A. Murphy and A. Chrysanthou, Sustain. Energy Fuels, 2019, 3, 899–909 RSC.
- F. J. Sánchez-Borrego, P. Álvarez-Mateos and J. F. García-Martín, Processes, 2021, 9, 797 CrossRef.
- A. Gasparatos, P. Stromberg and K. Takeuchi, Anim. Front., 2013, 3, 12–26 CrossRef.
- C.-Y. Lin, Energies, 2013, 6, 4945–4955 CrossRef.
- M. D. Kass, B. L. Armstrong, B. C. Kaul, R. M. Connatser, S. Lewis, J. R. Keiser, J. Jun, G. Warrington and D. Sulejmanovic, Energy Fuels, 2020, 34, 8403–8413 CrossRef CAS.
- R. Thahir, A. Altway, S. R. Juliastuti and Susianto, Energy Rep., 2019, 5, 70–77 CrossRef.
- V. P. Dimri, R. P. Srivastava and N. Vedanti, in Handbook of Geophysical Exploration: Seismic Exploration, ed. V. P. Dimri, R. P. Srivastava and N. Vedanti, Pergamon, 2012, vol. 41, pp. 89–118 Search PubMed.
- B. Tesfa, R. Mishra, F. Gu and N. Powles, Renewable Energy, 2010, 35, 2752–2760 CrossRef CAS.
- O. Alomair, M. Jumaa, A. Alkoriem and M. Hamed, J. Pet. Explor. Prod. Technol., 2015, 6 DOI:10.1007/s13202-015-0184-8.
- K. Grab-Rogalinski and S. Szwaja, IOP Conf. Ser. Mater. Sci. Eng., 2016, 148, 12066 CrossRef.
- H. C. Curl and K. O'Donnell, NOAA technical memorandum ERL MESA; 17, 1977 Search PubMed.
- S. H. Park, K. B. Choi, M. Y. Kim and C. S. Lee, Energy Fuels, 2013, 27, 56–65 CrossRef CAS.
- G. Ayetor, A. Sunnu and J. Parbey, Alex. Eng. J., 2015, 54 DOI:10.1016/j.aej.2015.09.011.
- A. Mohammadshirazi, A. Akram, S. Rafiee and E. Bagheri Kalhor, Renew. Sustain. Energy Rev., 2014, 33, 44–49 CrossRef CAS.
- A. El-Gharbawy, Smart Grid Search PubMed.
- Energy Prices/Monthly Energy Review, U.S. Energy Information Administration, 2021, https://www.eia.gov/totalenergy/data/monthly/pdf/mer.pdf Search PubMed.
- K. Sirviö, S. Niemi, S. Heikkilä and E. Hiltunen, Agron. Res., 2017, 15, 1232–1241 Search PubMed.
- M. Muñoz, F. Moreno, C. Monné, J. Morea and J. Terradillos, Renewable Energy, 2011, 36, 2918–2924 CrossRef.
- L. Catoire, J. Phys. Chem. Ref. Data, 2004, 33 DOI:10.1063/1.1835321.
- S. Farazmand, M. R. Ehsani, M. M. Shadman, S. Ahmadi, S. Veisi and E. Abdi, Pet. Sci. Technol., 2016, 34, 1542–1549 CrossRef CAS.
- J. K. Fink, in Petroleum Engineer's Guide to Oil Field Chemicals and Fluids, ed. J. K. Fink, Gulf Professional Publishing, Boston, 2012, pp. 663–694 Search PubMed.
- T. Tsoutsos, S. Tournaki, Z. Gkouskos, O. Paraíba, F. Giglio, P. Q. García, J. Braga, H. Adrianos and M. Filice, ChemEngineering, 2019, 3(1) DOI:10.3390/chemengineering3010019.
| This journal is © The Royal Society of Chemistry 2021 |