Open Access Article
Open Access ArticleIsolation of HLA-G+ cells using MEM-G/9 antibody-conjugated magnetic nanoparticles for prenatal screening: a reliable, fast and efficient method
Elaheh Emadia,
Abdol-Khalegh Bordbar†
 bc,
Hamid Nadrid,
Ali Shamse,
Asghar Taheri-Kafrani
bc,
Hamid Nadrid,
Ali Shamse,
Asghar Taheri-Kafrani f and
Seyed Mehdi Kalantar†
f and
Seyed Mehdi Kalantar† *ag
*ag
aDepartment of Genetics, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, 8916978477, Iran. E-mail: smkalantar38@gmail.com; smkyzd@gmail.com
bDepartment of Chemistry, University of Isfahan, Isfahan 81746-73441, Iran
cCalifornia Institute for Quantitative Biosciences (QB3), University of California, Berkeley, CA 94720, USA
dDepartment of Medicinal Chemistry, Faculty of Pharmacy and Pharmaceutical Sciences Research Centre, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, 8916978477, Iran
eDepartment of Immunology, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, 8916978477, Iran
fDepartment of Biotechnology, Faculty of Biological Science and Technology, University of Isfahan, Isfahan 81746-73441, Iran
gResearch and Clinical Centre for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, 8916978477, Iran
First published on 20th September 2021
Abstract
The development of an effective and noninvasive early method for obtaining fetal cells is crucial to prenatal screening. Despite proving the presence of fetal cells in the reproductive tract, their use is limited due to their inability to properly isolate them from maternal cells. Magnetic-activated cell sorting (MACS) is a simple technique to separate cells. The present study aimed to develop a MACS-based platform for the isolation of the HLA-G expressing trophoblast cells. For this purpose, first, the triazine functionalized MNPs were synthesized and characterized. Then, MNPs were directly and indirectly conjugated by the MEM-G/9 antibodies targeting HLA-G+ cells. The antibody amount on the surface of the nanoparticles was determined with the Bradford assay. The cell capture efficiency was also investigated. Various characterization methods confirmed the successful nanoparticle synthesis and antibody conjugation. The optimal initial antibody amount for the immobilization was about 20 μg and the optimal time was 3 h. The antibody-nanoparticles by the indirect method had better targeting and capture efficiency than the direct method. The MNPs indirectly conjugated with antibodies are an efficient tool for cell isolation and present considerable potential to be applied in biomedical fields.
1 Introduction
The advanced reproductive age increases the risk of having a newborn with a structural or a chromosomal abnormality.1 A prenatal diagnostic procedure that can provide vital information about the genetic health and other abnormalities of a fetus and poses no considerable risk for the fetus would be invaluable.2 The prenatal diagnosis provides an opportunity for physicians to identify causes and to evaluate corrective interventions and helps the parents by giving them enough time to emotionally and mentally cope with fetal health status and selection of possible clinical options.2,3 Prenatal diagnostic methods are constantly evolving.4 Amniocentesis (at 12 to 14 weeks' gestation) and chorionic villus sampling (CVS) (at 9 to 10 weeks' gestation) are at present the only reliable methods of prenatal diagnosis. Although both these procedures are highly sensitive and accurate, unfortunately, due to their invasiveness, they carry the risk of abortion and fetal structural deformities, even in experienced hands, and are usually performed at stages of pregnancy where clinical options are limited for parents and physicians.5–7 Therefore, in recent decades, attention has been focused on the developing of a noninvasive highly reliable method that would be feasible in the early stages of pregnancy.8,9 Currently, the noninvasive cell-free fetal DNA (cffDNA) method, despite its limitations, such as the low percentage and the fragmented nature of fetal DNA in maternal plasma, the associated problems in their separation and analysis, under the influence of gestational age and maternal weight and available after the first ten weeks of gestation are used for prenatal screening. Due to the limitations of the cffDNA method, an alternative NIPT method is necessary.10–15 Recently, the retrieval of trophoblast cells from the cervix has attracted attention from scientists as a potential source of fetal DNA for prenatal diagnosis.15 In 1971, Shettles first observed the shedding and presence of trophoblast cells in the uterus and cervix.16 The first embryonic lineage that differentiates during fetal development for forming the placenta is trophoblast cells. Trophoblast cells contain two main lineages, villous trophoblast (VT) and extravillous trophoblast (EVT), with different functions. EVT cells differentiate wherein the placenta contacts with the uterine decidua.17,18 Some of them are shed into the reproductive tract from diverse invasive routes and then are trapped in the transcervical mucus and can be retrieved from the endocervical canal in ongoing pregnancies by a cytobrush.15,19 The possibility of capturing the intact fetal EVT cells from the endocervical canal provides a noninvasive alternative for early prenatal diagnosis.10 The number of EVTs in cytobrush-retrieved endocervical samples from pregnancy is approximately 1 EVT cell in 2000 maternal cervical cells.19 The major challenge of using EVTs for the evaluation of prenatal screening is the inability to efficiently isolate them from maternal cells.19 The presence of different antigens (Ags) in trophoblast cells from maternal cervical cells provides the potential to isolate these cells by Ag-based methods.2,20–22 In recent years, attempts at cell isolation by conventional methods of magnetic cell sorting (MACS) and fluorescence-activated cell sorting (FACS) have been reported.23 However, the development of an isolation method with a minimal technical challenge, high separation ability and clinical applicability is still needed.24 Both MACS and FACS isolation methods are dependent on the specific cell surface marker that can be distinguished by magnetic microbead or fluorescent-tagged antibody (Ab).25 Considering FACS is a sophisticated, time-consuming and expensive technique that is not suitable for clinical use. On the other hand, studies show that the MACS method is a powerful, fast and simple strategy for cell isolation and is more cost-effective and time-saving.26 Accordingly, the present study describes a magnetic force-based platform for the isolation of fetal cells.Magnetic nanoparticles (MNPs), due to their special properties including the availability of functional groups for chemical modification, biocompatibility and easy separation from the reaction mixture by use of magnet are a proper immobilization support of bio-macromolecules for magnetic separation.27–30 The smaller nanoparticles (NPs) have the higher surface to volume ratio causing greater binding capacity for ligand and greater separation efficiency.31 Because of the multiple-point attachment, cell separation with large magnetic particles is difficult and they aggregate due to too magnetic and cells get nonspecifically trapped in the aggregates.26 The use of MNPs for separation requires precise physicochemical design and unique targeting.32,33 Iron oxide (Fe3O4) NPs have application potential as a support in magnetic separation owing to their strong superparamagneticity, biocompatibility, low cytotoxicity, simple preparation process, having surface hydroxyl groups for modification and low cost.34 Despite all the advantages, the naked Fe3O4 NPs are unstable and oxidize easily in the air or aqua fluid and their magnetic properties and dispersion reduce.35,36 Therefore, the introduction of an outer shell is very important to maintain the stability of Fe3O4 NPs.37 Fe3O4 NPs are usually coated with various shells such as silica (SiO2), Au, dextran, albumin or polyethylene glycol.35,38 The incorporation of a silica coating is an efficient and appropriate strategy to improve the stability and dispersion of Fe3O4 NPs.39 On the other hand, chemistry of silica is well known and can be conjugated with different functional groups for various biochemical and biomedical purposes.35 In order to isolate rare target cells from a sample, target cell-specific antibody conjugation to the surface of the magnetic NPs is commonly used due to the remarkable binding affinity and specificity between Ab and Ag.40,41 EVT cells can be isolated from the endocervical sample by binding appropriate antibodies to MNPs. Human leukocyte antigen-G (HLA-G) is an EVT-specific Ag and by targeting this specific Ag, EVT cells can be isolated from cervical cells.19,42 The MEM-G/9 antibody shows a strong affinity towards the native form of human HLA-G.43,44 There are five basic methods of Ab immobilization onto NPs: physical adsorption, ionic interaction, and covalent bond, through protein cofactor and by antibody disulfide bond cleavage. Covalent bonding can stably bind Abs onto NPS, which is vital to the immobilization of the Abs in order for them to be used in the isolation process.45,46 The covalent attachment of Abs to the NPs surface generally requires the surface modifications of NPs.47 Thus, the silica-coated magnetite nanoparticles were modified by 3-aminopropyl triethoxysilane (APTES) to introduce the amine groups and then by 2,4,6-trichlorotriazine (TCT) to introduce the chlorine functional group. TCT is an important linker for the immobilization of biomolecules due to its low cost, biocompatibility, and chemoselective reactivity.36 TCT leads to conjugating NPs with antibody amine groups.47 In accordance with existing amine groups in most proteins and their high reactivity, this method does not require chemical manipulation of the antibody structure.48 In the present study, synthesized Fe3O4@SiO2-APTES-TCT nanoparticles were conjugated with MEM-G/9 antibody by direct and indirect methods and then their function in HLA-G+ cells isolation was evaluated. The successful isolation of HLA-G+ cells has provided an opportunity to assess the genetic health of fetus and investigate the placenta function.
2 Experimental
2.1 Materials
All the chemical reagents were commercially purchased and used without further purification. Ferric chloride hexahydrate (FeCl3·6H2O, ≥99%), ferrous chloride tetrahydrote (FeCl2·4H2O, ≥99%), ethanol (96%), ammonium hydroxide (NH4OH, 25%), hydrochloric acid (HCl, 37%), toluene (99.8%), triethylamine (TEA, ≥99.5%), trichlorotriazine (TCT, 99%), acetone (≥99.8%), di-sodium hydrogen phosphate (Na2HPO4, ≥99%), sodium phosphate monobasic (NaH2PO4, ≥99%), sodium chloride (NaCl, ≥99.5%), acetic acid (glacial, 100%), and sodium acetate (≥99%) were provided by Merck company (Germany). 3-Aminopropyl triethoxysilane (APTES, ≥98%), tetraethyl orthosilicate (TEOS, 98%), diisopropylethylamine (DIPEA, ≥98%), bovine serum albumin (BSA, ≥96%) and 1% penicillin–streptomycin were obtained from Sigma Aldrich (Germany). Tetrahydrofuran (THF, >99%) was purchased from Duksan (Korea). Bovine γ-globulin (BGG, 99%) and the Bradford reagent were bought from Bio-Rad (USA). The human breast cancer cell line SK-BR-3 and the human choriocarcinoma cell line JEG-3 were purchased from Pasteur Institute of Iran (Tehran, Iran). Fetal bovine serum (FBS), trypsin–EDTA (0.05%) and RPMI-1640 was obtained from Gibco (UK). The DMEM/F12 medium was acquired from Bio-Idea (Iran). Also, the purified monoclonal MEM-G/9 antibody (Exbio, Czech Republic), the FITC goat anti-mouse (IgG) secondary antibody (ab6785, Abcam, Cambridge, MA), the goat anti-mouse antibody (ab6708, Abcam, Cambridge, MA), the fluorescent dye propidium iodide (PI) (Fluka, 81845, Switzerland), the fluorescent dye 4′,6-diamidino-2-phenylindole (DAPI, Cytocell, UK) and phosphate buffered saline (PBS, pH 7.4, Inoclon) were used in this study. Deionized (DI) water was prepared with ultrapure water system (Easy Pure II, 18.2 MΩ, Barnstead Co).2.2 Synthesis of Fe3O4@SiO2-APTES-TCT
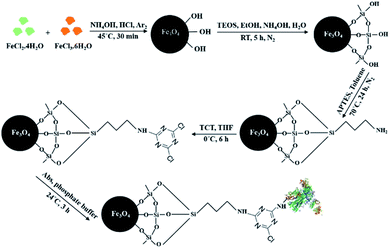
The whole process of synthesis of 1,3,5-triazine functionalized silica-coated iron oxide NPs and the immobilization of MEM-G/9 antibody on MNPs are described here and are shown schematically in Fig. 1.2.3 Immobilization of anti-HLA-G MEM-G/9 Ab onto Fe3O4@SiO2-APTES-TCT NPs
| Immobilization (%) = [(Ci − Cs)/Ci] × 100 | (1) |
2.4 Characterization methods
Fourier infrared spectroscopy (FTIR, PerkinElmer, Spectrum Two, USA), transmission electron microscope (TEM, Philips, CM120, Netherlands), energy dispersive X-ray spectroscopy (EDS, TESCAN, model MIRA III, Czech Republic), zeta potential (Zetasizer Nano-ZS, model Zen3600, Malvern Instrument Ltd, Malvern, UK) and vibrating sample magnetometer (VSM, Quantum Design, USA) were utilized to analyse the certain functional groups, morphology, size, elemental composition, electrical charge and magnetic properties of modified MNPs. Thermogravimetric analysis (TGA) was performed to confirm immobilization of Ab on MNPs using a TA Q600 (USA) from 30 to 600 °C at 10 °C min−1 heating rate in air atmosphere.2.5 Optimization of immobilization time
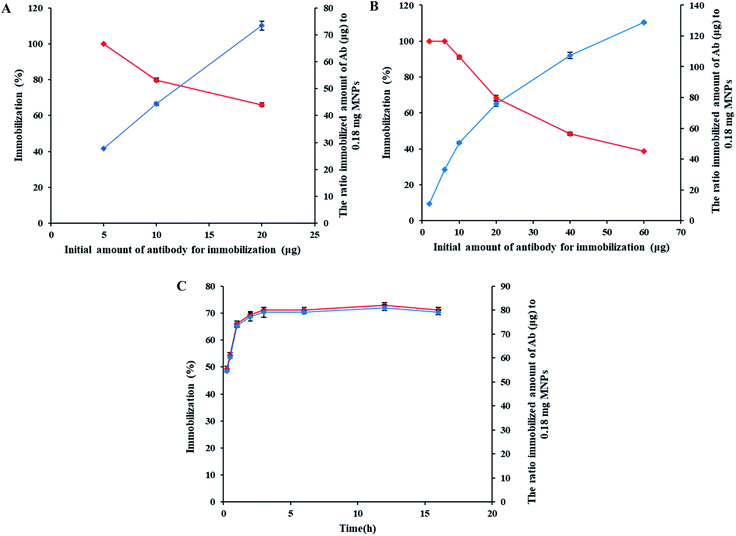
20 μl of goat anti-mouse IgG Ab (1 mg ml−1) was added to 180 μl of the dispersed triazine functionalized MNPs in phosphate buffer. The mixture was shaken at 24 °C for 0.25–16 h. The amount of IgG immobilized on MNPs was quantified by Bradford assay.2.6 Confirmation of immobilization of HLA-G Ab with the immune reactivity
The immobilization of HLA-G Abs onto the modified MNPs was investigated by the following procedure. At first, 20 μg of MEM-G/9 Ab was added to 180 μl of dispersed MNPs (1 mg ml−1), incubated at 24 °C for 3 h and washed with PBS to remove any unconjugated Ab. The MEM-G/9-immobilized MNPs were blocked with 1% BSA for 2 h. Then, goat anti-mouse IgG H&L (FITC), a secondary antibody, was used to recognize the immobilization and the immune reactivity of MEM-G/9 Ab conjugated to the MNPs surface. 200 μl of FITC anti-IgG (50 μg ml−1) was added to the BAS blocked MEM-G/9-MNPs and the resulting mixture was incubated at 24 °C for 2 h. After complete washing with PBS, the collected MNPs were redispersed in 100 μl PBS and immobilized on glass slide by cytospin centrifugation for 5 min, 1500 rpm for examination under a microscope (ESI Fig. 3†).2.7 Cell culture
The human HLA-G-positive JEG-3 trophoblast tumor cells56–58 were cultured in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of Dulbecco's Modified Essential Medium and Ham's F-12 Medium (DMEM/F12 Medium) supplemented with 10% (v/v) inactivated FBS and 1% (v/v) streptomycin/penicillin. SKBR-3 cells with reduced or absent expression of HLA-G gene59–61 were grown in RPMI 1640 medium containing 10% FBS and 1% penicillin/streptomycin.
1 mixture of Dulbecco's Modified Essential Medium and Ham's F-12 Medium (DMEM/F12 Medium) supplemented with 10% (v/v) inactivated FBS and 1% (v/v) streptomycin/penicillin. SKBR-3 cells with reduced or absent expression of HLA-G gene59–61 were grown in RPMI 1640 medium containing 10% FBS and 1% penicillin/streptomycin.
2.8 Quality assessment of the immobilized Ab on the MNP surface by experiment of cell binding
To show that the Ab-MNPs (Ab-conjugated MNPs) are able to target cells when JEG-3 cells have reached the desired number on the coverslip, the culture media was removed and washed gently with PBS. Then, the cells cultured on each coverslip were fixed at room temperature with a mixture of acetate buffer and methanol for 15 min. After washing, cells were blocked with 3% BSA–PBS for 45 min at room temperature. In the case of nanoparticles conjugated directly to MEM-G/9, 50 μl of these nanoparticles were added to the blocked cells. Triazine functionalized MNPs without MEM-G/9 were used as control. The cells were incubated with MNPs (with or without Ab) at 37 °C for 1 h. The coverslip was carefully removed with forceps from the culture plate. It was inverted and placed on a glass slide and examined by light microscope. In the case of nanoparticles conjugated indirectly to MEM-G/9, at first, the blocked cells were incubated with MEM-G/9 (at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 dilution in 1% BSA–PBS) at 37 °C for 1 h. Subsequently, 50 μl of IgG-MNPs were added to the cells and incubated at 37 °C for 1 h. The incubated cells with the antibody diluent alone and no MEM-G/9 Ab were used as control.
50 dilution in 1% BSA–PBS) at 37 °C for 1 h. Subsequently, 50 μl of IgG-MNPs were added to the cells and incubated at 37 °C for 1 h. The incubated cells with the antibody diluent alone and no MEM-G/9 Ab were used as control.
2.9 Showing the ability of Ab-MNPs to capture the JEG-3 cells
About 5 × 105 JEG-3 cells as target cells were poured into a tube and fixed with a mixture of acetate buffer and methanol for 15 min. The cells were centrifuged at 1400 rpm for 7 min and blocked with 3% BSA–PBS for 45 min at room temperature. In the case of nanoparticles conjugated directly to MEM-G/9, the blocked cells were incubated with 50 μl of MEM-G/9-MNPs at 37 °C for 1 h with shaking and were separated by an external magnetic field. After magnetic separation, the number of cells in the supernatant was counted to know the capture efficiency of the Ab-MNPs. The average capture efficiency was obtained according to the results of three experiments.| Capture efficiency (%) = ((initial JEG-3 cells − supernatant JEG-3 cells)/initial JEG-3 cells) × 100 |
In the case of nanoparticles conjugated indirectly to MEM-G/9, first, the cell suspension was incubated with MEM-G/9 at 37 °C for 1 h. Subsequently, 50 μl of IgG-MNPs were added to the cells and incubated at 37 °C for 1 h with shaking. All subsequent steps were identical to those described above.
2.10 Detection and isolation of HLA-G+ cells using Ab-MNPs
To display the capability of Ab-MNPs for HLA-G-positive cells isolation, JEG-3 cells were utilized as HLA-G-positive cells and SK-BR-3 cells were utilized as HLA-G-negative cells. The fixed and blocked JEG-3 cells (about 5 × 105) were stained with DAPI dye (blue) and the fixed and blocked SK-BR-3 cells (about 5 × 105) were stained with Propidium iodide (PI) dye (red). After staining, cells were mixed together. In the case of nanoparticles conjugated directly to MEM-G/9, 50 μl of MEM-G/9-MNPs was added to the mixed cells and incubated for 60 min at 37 °C in the dark. The mixture was vortexed every 15 min. After incubation, the mixture was kept in front of a magnet for 10 min to allow magnetic isolation of the cells + MEM-G/9-MNPs. Both the supernatant and the pellet were carefully collected. The pellet was resuspended in 50 μL PBS. Then, the pellet and the supernatant were immobilized on glass slide and examined with a fluorescence microscopy (BX61, Olympus, Tokyo, Japan) connected to Applied Spectral Imaging (ASI) Acquisition 5.5 software (ASI Inc, Carlsbad, CA). In the case of nanoparticles conjugated indirectly to MEM-G/9, first, the mixed cells were incubated with MEM-G/9 at 37 °C for 1 h. Afterward, 50 μl of IgG-MNPs was added to the cells and incubated at 37 °C for 1 h. All subsequent steps were identical to those described above.3 Results and discussion
3.1 Fe3O4@SiO2-APTES-TCT synthesis
At present, the isolation of target cells from heterogeneous cell populations is important for various biomedical purposes. Methods for isolation and enrichment of cells are evolving. A simple, accurate and inexpensive method that achieves acceptable results in the shortest amount of time possible has been one of the important research goals of scientists in recent years. The magnetic isolation of cells is one of the most appropriate approaches for separating target cells. The magnetic isolation method usually uses MNPs conjugated with antibodies against specific cell surface Ags. Therefore, the synthesis of immunomagnetic nanoparticles using a simple, cost-effective and high-performance method is a critical step for cell isolation. In this study, magnetic nanoparticles were synthesized by the simple and fast co-precipitation method and coated with silica shell for improving their stability. The silica-coated MNPs were then modified through a two-step process with APTES and 2,4,6-trichloro-1,3,5-triazine to create a functional surface with triazine to immobilize the antibody and several techniques were used to characterize them.3.2 Antibody immobilization on MNPs
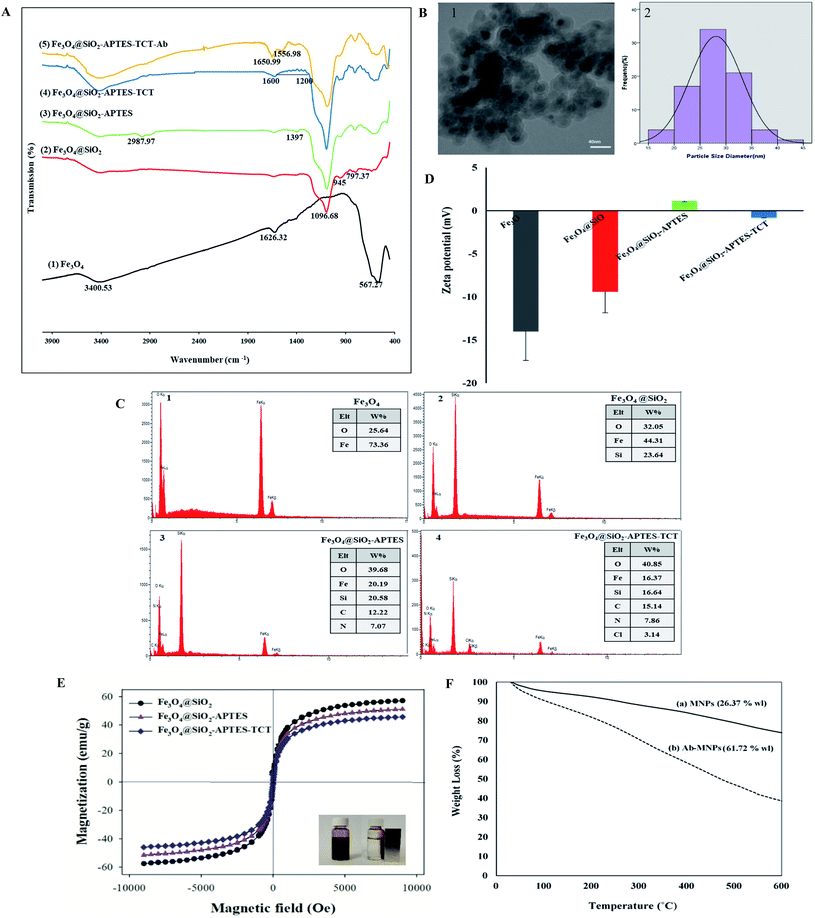
Fe3O4@SiO2 NPs were functionalized with triazine to enable subsequent covalent bond via the amine groups of antibodies. The amount of immobilized antibody was determined according to the linear equation obtained from the BGG standard curve (ESI Fig. 4†).3.3 Characterization of MNPs and Ab-MNPs
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrations which prove the presence of triazine rings onto MNPs.68,69 In Fe3O4@SiO2-APTES-TCT-Ab spectra, two absorption peaks at 1556.98 cm−1 (N–H stretching vibration of antibody amide II) and 1650.99 cm−1 (C
N stretching vibrations which prove the presence of triazine rings onto MNPs.68,69 In Fe3O4@SiO2-APTES-TCT-Ab spectra, two absorption peaks at 1556.98 cm−1 (N–H stretching vibration of antibody amide II) and 1650.99 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration and antibody amide I) confirmed the success of Ab immobilization on modified MNPs.47,70
O stretching vibration and antibody amide I) confirmed the success of Ab immobilization on modified MNPs.47,70
Three magnetization curves showed superparamagnetic behaviour due to the lack of hysteresis.80 As can be seen in Fig. 3E, Fe3O4@SiO2 NPs indicated a slightly higher level of Ms than other MNPs. The decrease of Ms in Fe3O4@SiO2-APTES, and Fe3O4@SiO2-APTES-TCT NPs was due to the modification of MNPs with aminopropyl81 and triazine groups and the increase in the total mass with respect to the magnetic material.55 However, the modifications have had little effect on the magnetization of the MNPs and can be quickly separated from the solution by an external magnet.80
3.4 Effect of immobilization time
Fig. 2C shows the results of the amount of immobilized anti-mouse IgG on MNPs and the immobilization percentage in various reaction times. The amount of immobilized Ab and the immobilization percentage reached their maximum after 3 h and then remained constant, and further elongation of reaction time did not significantly increase the amount and percentage of antibody immobilization. That was probably because there was no free TCT active group left to connect with the Ab amine groups and consequently more immobilization after 3 h.82 Therefore, 3 h was considered as the optimal time for Ab immobilization.3.5 HLA-G Ab immobilization confirmation
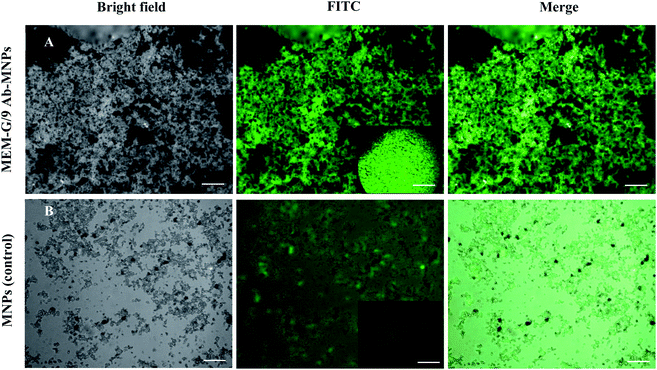
Although, the results of FTIR and TGA confirmed the Ab conjugation with the MNPs. We also confirmed the immobilization of the MEM-G/9 Ab onto MNPs by the immune reactivity. As depicted in Fig. 4A, Ab-MNPs showed a significant green colour. On the other hand, MNPs unconjugated with Ab (control), showed much less colour (Fig. 4B). Fluorescence intensity visualized the conjugation of MEM-G/9 on the surface of the MNPs. In addition, the findings indicated that the FITC-goat anti mouse IgG secondary Ab significantly detected the MEM-G/9 Ab immobilized on the surface of the MNPs and the conjugated Ab maintained its inherent immune reactivity to the secondary Ab.47 Also, the negligible adsorption between FITC-IgG and the MNPs displayed the favored blocking of the MNPs with BSA. In general, the immobilization was successful.3.6 The Ab-MNPs ability to target the HLA-G positive cells
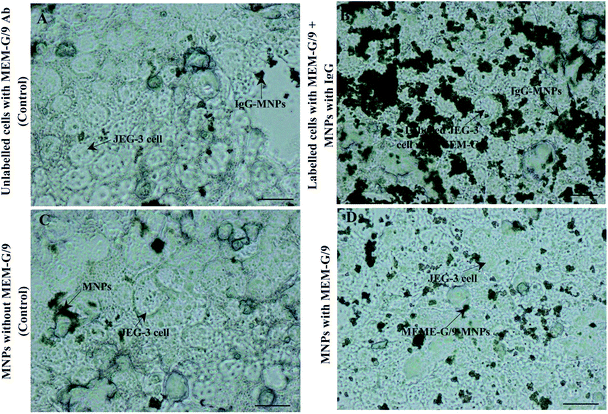
To assess of the Ab-MNPs tendency to HLA-G positive cells, a cell binding experiment was conducted, as described in the methods section. Light microscopy images showed appropriate targeting of MEM-G/9-MNPs to JEG-3 cells (HLA-G-positive) (Fig. 5B and D). Controls also showed very little binding to target cells as expected (Fig. 5A and C). The targeting of MNPs conjugated directly to MEM-G/9 (Fig. 5D) was less than MNPs conjugated indirectly to MEM-G/9 (Fig. 5B). This lower tendency may be due to the loss of biological function of a number of directly immobilized MEM-G/9 Abs. Because Ab immobilization in this study was performed randomly through Ab amine groups, covalent binding may have occurred through some amine groups located in the Ag-binding sites.83 As a result, it can be concluded that the activity of Fab portions of a number of MEM-G/9 antibodies was not maintained after being conjugated to MNPs.3.7 Cell capture efficiency of MEM-G/9-MNPs
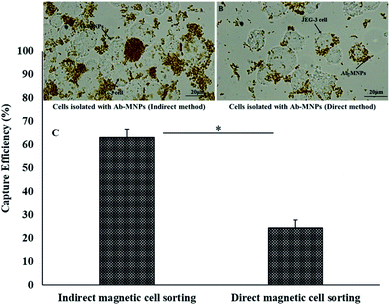
Verification of the targeting ability of Ab-MNPs allowed the investigation of their performance in cell isolation. After incubation and magnetic separation, the capture efficiency of both direct and indirect forms of MEM-G/9-MNPs was determined with microscopy analysis (Fig. 6). The MNPs conjugated directly to MEM-G/9 captured the JEG-3 cells with an efficiency of 24.39 ± 3.41% whereas the MNPs conjugated indirectly to MEM-G/9 captured the JEG-3 cells with an efficiency of 63.07 ± 3.5%. Values are a mean efficiency of three experiments (%) ± standard error of mean (SEM). The t-test analysis showed that the P-value between the capture efficiency of direct and indirect forms of MEM-G/9-MNPs was less than 0.05 (p value = 0.01). The direct conjugation of MEM-G/9 Abs to MNPs may affect their binding affinity to HLA-G Ag and thus the capture efficiency.3.8 Selective isolation of HLA-G+ cells from HLA-G− cells with MEM-G/9-MNPs
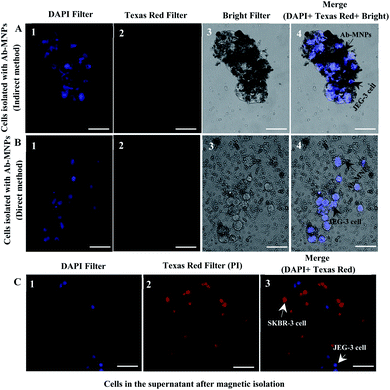
The prepared Ab-MNPs' specificity for HLA-G+ cell separation was determined by incubating a mixture of JEG-3 (HLA-G+) and SKBR-3 (HLA-G−) cells with the Ab-MNPs and then magnetic isolation and microscopic imaging, as mentioned in the methods section. Since the SKBR-3 cells were stained with PI and JEG-3 cells with DAPI, they were visualized with diverse fluorescent dyes under various excitation wavelengths.84 Fluorescence microscopy images after the magnetic isolation from the precipitates showed that blue fluorescent-stained (HLA-G+) cells could be isolated by the Ab-MNPs (Fig. 7A and B), although most of the cells in the supernatant were the red fluorescent-stained (HLA-G−) cells (Fig. 7C). The specificity of the prepared Ab-MNPs to isolate HLA-G+ cells was verified. The MNPs conjugated indirectly to MEM-G/9 had more selective isolation than the MNPs conjugated directly to MEM-G/9. Considering that the limited number of the HLA-G+ cells was not isolated by the MNPs conjugated indirectly to MEM-G/9, the MNPs conjugated indirectly to MEM-G/9 have good target specificity and are the suitable and low-cost tools for detecting and isolating target cells.4 Conclusions
Isolation of fetal cells from maternal cells in endocervical sample remains a problem for prenatal diagnosis. MNPs have been widely used in cell isolation. Several studies have presented the successful covalent immobilization of different biologically active macromolecules (lipase,82 albumin,68 xylanase36 and glucose oxidase37) on triazine-functionalized magnetic NPs. In the present research, similar to the studies mentioned above, triazine-functionalized magnetic NPs were used as a support for covalent immobilization of MEM-G/9 and anti-mouse IgG Abs so that the development of immunomagnetic NPs for the isolation of JEG-3 cells (trophoblastic model system) and a simple and low-cost cell isolation system based on MNPs were exhibited due to the intrinsic Ab–Ag interaction. However, several studies have been performed to isolate fetal cells with immunomagnetic nanoparticles.20,85–92 This study was an attempt to improve isolation efficiency and cost decrease. After confirming the successful Ab-MNPs synthesis by characterization techniques and high immobilization efficiency of Abs by Bradford assay, the Ab-MNPs were used for cell isolation. The most considerable problem was the accumulation of MNP-Ab. The accumulation problem was partly removed by shaking before interaction with target cells and during incubation.The cell capture efficiency of our synthetic MNPs in the direct immunomagnetic method was 24.39% and in the indirect method 63.07%. In the indirect immunomagnetic method, antibody targeting activity was maintained, so their capture efficiency was higher than the direct immunomagnetic method. The results were confirmed by fluorescent microscopy. The improvement in isolation and clinical applicability for prenatal diagnosis could be achieved by further optimization and the elimination of accumulation problem of prepared Ab-MNPs and assessing their reliability for the isolation of EVT cells in actual samples. The MNPs with indirect conjugate strategy also have the potential to be used for other isolation by specific Abs of the target. So, they can be considered as an efficient tool for the isolation of cells and biomolecules.
Conflicts of interest
The authors declare that they have no conflict of interest.Author contributions
E. E: conceptualization, methodology, formal analysis, investigation, data curation, writing – original draft; A. K. B.: conceptualization, methodology, formal analysis, resources, writing – review & editing. H. N.: methodology, investigation, resources; A. Sh.: investigation; A. T. K.: investigation; S. M. K.: conceptualization, resources, project administration, writing – review & editing.Acknowledgements
This research was supported in part by a grant for PhD candidates (project number 4504) from Shahid Sadoughi University of Medical Sciences, Yazd, Iran.References
- J. S. Brandt, M. A. Cruz Ithier, T. Rosen and E. Ashkinadze, Prenatal Diagn., 2019, 39, 81–87 CrossRef PubMed.
- J. M. Bolnick, B. A. Kilburn, S. Bajpayee, N. Reddy, R. Jeelani, B. Crone, N. Simmerman, M. Singh, M. P. Diamond and D. R. Armant, Fertil. Steril., 2014, 102, 135–142 CrossRef PubMed.
- S. Lou, C. P. Nielsen, L. Hvidman, O. B. Petersen and M. B. Risør, BMC Pregnancy Childbirth, 2016, 16, 1–8 CrossRef PubMed.
- K. Wou, J. L. Feinberg, R. J. Wapner and J. L. Simpson, Expert Rev. Mol. Diagn., 2015, 15, 989–998 CrossRef CAS PubMed.
- S. Drewlo and D. R. Armant, Placenta, 2017, 60, S27–S31 CrossRef CAS PubMed.
- A. C. o. Obstetricians and Gynecologists, Obstet. Gynecol., 2007, 110, 1459 CrossRef PubMed.
- M. Sbracia, F. Scarpellini, S. Lalwani, J. Grasso and L. Scarpellini, Ann. N. Y. Acad. Sci., 1994, 731, 170–174 CrossRef CAS PubMed.
- Y. Koumantaki, S. Sifakis, G. Dragatis, I. Matalliotakis, G. Froudarakis, E. Papadopoulou and E. Koumantakis, Prenatal Diagnosis: Published in Affiliation With the, International Society for Prenatal Diagnosis, 2001, 21, 566–570 CrossRef CAS PubMed.
- C. Bussani, R. Cioni, B. Scarselli, F. Barciulli, S. Bucciantini, P. Simi, A. Fogli and G. Scarselli, Prenatal Diagnosis: Published in Affiliation With the, International Society for Prenatal Diagnosis, 2002, 22, 1098–1101 CrossRef PubMed.
- D. R. Armant and M. P. Diamond, US Pat. No. 10344315, U.S. Patent and Trademark Office, Washington, DC, 2019. Search PubMed.
- E. R. Norwitz and B. Levy, Reviews in Obstetrics and Gynecology, 2013, 6, 48 Search PubMed.
- A. Obstetricians, Obstet. Gynecol., 2012, 120, 1532–1534 CrossRef PubMed.
- E. Wang, A. Batey, C. Struble, T. Musci, K. Song and A. Oliphant, Prenatal Diagn., 2013, 33, 662–666 CrossRef CAS PubMed.
- M. E. Norton, N. C. Rose and P. Benn, Obstet. Gynecol., 2013, 121, 847–850 CrossRef PubMed.
- A. N. Imudia, S. Kumar, M. P. Diamond, A. H. DeCherney and D. R. Armant, Fertil. Steril., 2010, 93, 1725–1730 CrossRef PubMed.
- L. B. Shettles, Nature, 1971, 230, 52–53 CrossRef CAS PubMed.
- A. Tarrade, R. L. Kuen, A. Malassiné, V. Tricottet, P. Blain, M. Vidaud and D. Evain-Brion, Lab. Invest., 2001, 81, 1199–1211 CrossRef CAS PubMed.
- G. Moser, S. Drewlo, B. Huppertz and D. R. Armant, Hum. Reprod. Update, 2018, 24, 484–496 CrossRef CAS PubMed.
- A. N. Imudia, Y. Suzuki, B. A. Kilburn, F. D. Yelian, M. P. Diamond, R. Romero and D. R. Armant, Hum. Reprod., 2009, 24, 2086–2092 CrossRef PubMed.
- P. Schueler, D. Yamanishi, J. Pearson, Y. Lee, X. Wu, S. Hashima, M. Madlansacay, C. Cain, E. Collarini and L. Foltz, Placenta, 2001, 22, 702–715 CrossRef CAS PubMed.
- J. N. Bulmer, R. Cioni, C. Bussani, V. Cirigliano, F. Sole, C. Costa, P. Garcia and M. Adinolfi, Prenatal Diagn., 2003, 23, 34–39 CrossRef CAS PubMed.
- M. G. Katz-Jaffe, D. Mantzaris and D. S. Cram, BJOG, 2005, 112, 595–600 CrossRef CAS PubMed.
- A. Grützkau and A. Radbruch, Cytometry, Part A, 2010, 77, 643–647 CrossRef PubMed.
- B. D. Plouffe, S. K. Murthy and L. H. Lewis, Rep. Prog. Phys., 2014, 78, 016601 CrossRef PubMed.
- R. David, M. Groebner and W. M. Franz, Stem Cells, 2005, 23, 477–482 CrossRef CAS PubMed.
- S. Miltenyi, W. Müller, W. Weichel and A. Radbruch, Cytometry, 1990, 11, 231–238 CrossRef CAS PubMed.
- H. Shahrestani, A. Taheri-Kafrani, A. Soozanipour and O. Tavakoli, Biochem. Eng. J., 2016, 109, 51–58 CrossRef CAS.
- H. Guo, Y. Tang, Y. Yu, L. Xue and J.-q. Qian, Int. J. Biol. Macromol., 2016, 87, 537–544 CrossRef CAS PubMed.
- U. Jinendra, J. Kumar, B. Nagabhushana, A. V. Raghu and D. Bilehal, Green Mater., 2019, 7, 137–142 CrossRef.
- K. Kannan, D. Radhika, K. R. Reddy, A. V. Raghu, K. K. Sadasivuni, G. Palani and K. Gurushankar, Nano Express, 2021, 2, 010014 Search PubMed.
- C. T. Yavuz, J. Mayo, W. Y. William, A. Prakash, J. C. Falkner, S. Yean, L. Cong, H. J. Shipley, A. Kan and M. Tomson, Science, 2006, 314, 964–967 CrossRef PubMed.
- S. Trabulo, A. Aires, A. Aicher, C. Heeschen and A. L. Cortajarena, Biochim. Biophys. Acta, 2017, 1861, 1597–1605 CrossRef CAS PubMed.
- U. Jinendra, D. Bilehal, B. Nagabhushana, K. R. Reddy, C. V. Reddy and A. V. Raghu, Mater. Sci. Energy Technol., 2019, 2, 657–666 Search PubMed.
- L. S. Ganapathe, M. A. Mohamed, R. Mohamad Yunus and D. D. Berhanuddin, Magnetochemistry, 2020, 6, 68 CrossRef CAS.
- M. Mostafaei, S. N. Hosseini, M. Khatami, A. Javidanbardan, A. A. Sepahy and E. Asadi, Protein Expression Purif., 2018, 145, 1–6 CrossRef CAS PubMed.
- A. Soozanipour, A. Taheri-Kafrani and A. L. Isfahani, Chem. Eng. J., 2015, 270, 235–243 CrossRef CAS.
- M. Abbasi, R. Amiri, A.-K. Bordbar, E. Ranjbakhsh and A.-R. Khosropour, Appl. Surf. Sci., 2016, 364, 752–757 CrossRef CAS.
- F. Shamsipour, A. H. Zarnani, R. Ghods, M. Chamankhah, F. Forouzesh, S. Vafaei, A. A. Bayat, M. M. Akhondi, M. A. Oghabian and M. Jeddi-Tehrani, Avicenna J. Med. Biotechnol., 2009, 1, 27 CAS.
- X. Hou, C. Zhao, Y. Tian, S. Dou, X. Zhang and J. Zhao, Chem. Res. Chin. Univ., 2016, 32, 889–894 CrossRef CAS.
- Y. Wang and B. Liu, Biosens. Bioelectron., 2009, 24, 3293–3298 CrossRef CAS PubMed.
- S. Taebi, M. Keyhanfar and A. Noorbakhsh, J. Immunol. Methods, 2018, 458, 26–32 CrossRef CAS PubMed.
- Y. Loke, A. King, T. Burrows, L. Gardner, M. Bowen, S. Hiby, S. Howlett, N. Holmes and D. Jacobs, Tissue Antigens, 1997, 50, 135–146 CrossRef CAS PubMed.
- S. Hiby, A. King, A. Sharkey and Y. Loke, Tissue Antigens, 1999, 53, 1–13 CrossRef CAS PubMed.
- I. K. Marcinkowskiego, Isolation of Fetus Cells from Maternal Blood Pre-clinical Study, PhD thesis in Nanobiotechnology, Poznan University of Medical Sciences, 2011 Search PubMed.
- S. R. Makhsin, P. L. Teoh and K. A. Razak, Reviews in Advanced Sciences and Engineering, 2015, 4, 3–21 CrossRef.
- M. H. Jazayeri, H. Amani, A. A. Pourfatollah, H. Pazoki-Toroudi and B. Sedighimoghaddam, Sensing and Bio-Sensing Research, 2016, 9, 17–22 CrossRef.
- M. Poplawska, M. Bystrzejewski, I. P. Grudziński, M. A. Cywińska, J. Ostapko and A. Cieszanowski, Carbon, 2014, 74, 180–194 CrossRef CAS.
- H. Xu, Z. P. Aguilar, L. Yang, M. Kuang, H. Duan, Y. Xiong, H. Wei and A. Wang, Biomaterials, 2011, 32, 9758–9765 CrossRef CAS PubMed.
- A. Schaetz, M. Hager and O. Reiser, Adv. Funct. Mater., 2009, 19, 2109–2115 CrossRef CAS.
- D. Predoi, Dig. J. Nanomater. Biostructures, 2007, 2, 169–173 Search PubMed.
- M. Jonnalagadda, V. B. Prasad and A. V. Raghu, J. Mol. Struct., 2021, 1230, 129875 CrossRef CAS.
- K. Kannan, D. Radhika, A. Nesaraj, K. K. Sadasivuni, K. R. Reddy, D. Kasai and A. V. Raghu, Mater. Sci. Energy Technol., 2020, 3, 853–861 CAS.
- Y. Chi, Q. Yuan, Y. Li, J. Tu, L. Zhao, N. Li and X. Li, J. Colloid Interface Sci., 2012, 383, 96–102 CrossRef CAS PubMed.
- S. Hou, X. Li, H. Wang, M. Wang, Y. Zhang, Y. Chi and Z. Zhao, RSC Adv., 2017, 7, 51993–52000 RSC.
- F. J. Sharahi and A. Shahbazi, Chemosphere, 2017, 189, 291–300 CrossRef PubMed.
- D. Burt, D. Johnston, T. R. de Wit, P. van den Elsen and P. L. Stern, Int. J. Cancer, 1991, 47, 117–122 CrossRef PubMed.
- C. Menier, B. Saez, V. Horejsi, S. Martinozzi, I. Krawice-Radanne, S. Bruel, C. Le Danff, M. Reboul, I. Hilgert and M. Rabreau, Hum. Immunol., 2003, 64, 315–326 CrossRef CAS.
- R. Apps, S. P. Murphy, R. Fernando, L. Gardner, T. Ahad and A. Moffett, Immunology, 2009, 127, 26–39 CrossRef CAS PubMed.
- N. Nazari and S. Farjadian, Iran. J. Immunol., 2016, 13, 178–185 Search PubMed.
- S. Ghoshdastidar, Clinical translation of nanomaterials for early detection of genetic abnormalities in fetus and retinopathy in neonates and adults, PhD thesis, University of Missouri-Columbia, 2017 Search PubMed.
- L. Thiruchelvam-Kyle, S. E. Hoelsbrekken, P. C. Saether, E. G. Bjørnsen, D. Pende, S. Fossum, M. R. Daws and E. Dissen, J. Immunol., 2017, 198, 2556–2567 CrossRef CAS PubMed.
- J. Xu, C. Ju, J. Sheng, F. Wang, Q. Zhang, G. Sun and M. Sun, Bull. Korean Chem. Soc., 2013, 34, 2408–2412 CrossRef CAS.
- M. Yamaura, R. Camilo, L. Sampaio, M. Macedo, M. Nakamura and H. Toma, J. Magn. Magn. Mater., 2004, 279, 210–217 CrossRef CAS.
- J. Zhao, Y. Gui, Y. Liu, G. Wang, H. Zhang, Y. a. Sun and S. Fang, Catal. Lett., 2017, 147, 1127–1132 CrossRef CAS.
- A. H. Gemeay, B. E. Keshta, R. G. El-Sharkawy and A. B. Zaki, Environ. Sci. Pollut. Res., 2019, 1–18 Search PubMed.
- A. Raghu, G. Gadaginamath, N. Mallikarjuna and T. Aminabhavi, J. Appl. Polym. Sci., 2006, 100, 576–583 CrossRef CAS.
- A. Raghu and H. M. Jeong, J. Appl. Polym. Sci., 2008, 107, 3401–3407 CrossRef CAS.
- A. Bordbar, A. Rastegari, R. Amiri, E. Ranjbakhsh, M. Abbasi and A. Khosropour, Biotechnol. Res. Int., 2014, 2014 Search PubMed.
- M. Amirbandeh and A. Taheri-Kafrani, Int. J. Biol. Macromol., 2016, 93, 1183–1191 CrossRef CAS PubMed.
- K. Mu, S. Zhang, T. Ai, J. Jiang, Y. Yao, L. Jiang, Q. Zhou, H. Xiang, Y. Zhu and X. Yang, Mol. Imaging, 2015, 14, 7290 CrossRef PubMed.
- N. A. Frey, S. Peng, K. Cheng and S. Sun, Chem. Soc. Rev., 2009, 38, 2532–2542 RSC.
- W. Liu, L. Nie, F. Li, Z. P. Aguilar, H. Xu, Y. Xiong, F. Fu and H. Xu, Biomater. Sci., 2016, 4, 159–166 RSC.
- Y. Teng, C. Jiang, A. Ruotolo and P. W. Pong, IEEE Trans. Nanotechnol., 2016, 17, 69–77 Search PubMed.
- Y. Kobayashi, H. Inose, T. Nakagawa, K. Gonda, M. Takeda, N. Ohuchi and A. Kasuya, J. Colloid Interface Sci., 2011, 358, 329–333 CrossRef CAS PubMed.
- M. Susewind, A.-M. Schilmann, J. Heim, A. Henkel, T. Link, K. Fischer, D. Strand, U. Kolb, M. N. Tahir and J. Brieger, J. Mater. Chem. B, 2015, 3, 1813–1822 RSC.
- J. H. Min, M.-K. Woo, H. Y. Yoon, J. W. Jang, J. H. Wu, C.-S. Lim and Y. K. Kim, Anal. Biochem., 2014, 447, 114–118 CrossRef CAS PubMed.
- W. Sheng, W. Wei, J. Li, X. Qi, G. Zuo, Q. Chen, X. Pan and W. Dong, Appl. Surf. Sci., 2016, 387, 1116–1124 CrossRef CAS.
- C. Meng, W. Zhikun, L. Qiang, L. Chunling, S. Shuangqing and H. Songqing, J. Hazard. Mater., 2018, 341, 198–206 CrossRef PubMed.
- F. Golmohammadi, M. Hazrati and M. Safari, Microchem. J., 2019, 144, 64–72 CrossRef CAS.
- S. A. A. Noma, A. Ulu, S. Koytepe and B. Ateş, Biocatal. Biotransform., 2020, 38, 392–404 CrossRef CAS.
- M. A. Ghasemzadeh, M. H. Abdollahi-Basir and M. Babaei, Green Chem. Lett. Rev., 2015, 8, 40–49 CrossRef.
- E. Ranjbakhsh, A. Bordbar, M. Abbasi, A. Khosropour and E. Shams, Chem. Eng. J., 2012, 179, 272–276 CrossRef CAS.
- S. Jeong, J. Y. Park, M. G. Cha, H. Chang, Y.-i. Kim, H.-M. Kim, B.-H. Jun, D. S. Lee, Y.-S. Lee and J. M. Jeong, Nanoscale, 2017, 9, 2548–2555 RSC.
- B. S. Cummings and R. G. Schnellmann, Curr. Protoc. Pharmacol., 2004, 25, 12–18 Search PubMed.
- R. Kannan, S. Ghoshdastidar, D. Suresh, D. Schust and A. Upendran, US Pat., 15/150262 Search PubMed.
- U. Mueller, C. Hawes, A. Wright, E. DeBoni, W. Jones, F. Firgaira, A. Morley and D. Turner, Lancet, 1990, 336, 197–200 CrossRef CAS.
- D. Gänshirt-Ahlert, M. Burschyk, H. S. Garritsen, L. Helmer, P. Miny, J. Horst, H. P. Schneider and W. Holzgreve, Am. J. Obstet. Gynecol., 1992, 166, 1350–1355 CrossRef.
- Y. Zheng, N. P. Carter, C. M. Price, S. M. Colman, P. J. Milton, G. A. Hackett, M. F. Greaves and M. A. Ferguson-Smith, J. Med. Genet., 1993, 30, 1051–1056 CrossRef CAS PubMed.
- J. Büsch, P. Huber, E. Pflüger, S. Miltenyi, J. Holtz and A. Radbruch, Prenatal Diagn., 1994, 14, 1129–1140 CrossRef PubMed.
- C. S. Hawes, H. Suskin, B. Kalionis, U. Mueller, G. Casey, J. Hall and Z. Rudzki, Ann. N. Y. Acad. Sci., 1994, 731, 181–185 CrossRef CAS PubMed.
- L. Durrant, K. McDowall, R. Holmes and D. Liu, BJOG, 1996, 103, 219–222 CrossRef CAS PubMed.
- T. H. Lim, A. Tan and V. H. H. Goh, Hum. Genet., 1999, 104, 399–404 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |