Open Access Article
Open Access ArticleNiSe/Ni3Se2 on nickel foam as an ultra-high-rate HER electrocatalyst: common anion heterostructure with built-in electric field and efficient interfacial charge transfer†
Xin Ma,
Jingbo Yang ,
Xiaoqi Xu,
Hangqi Yang and
Chuang Peng
,
Xiaoqi Xu,
Hangqi Yang and
Chuang Peng *
*
School of Resource and Environmental Sciences, Wuhan University, Wuhan 430072, P. R. China. E-mail: Chuang.peng@whu.edu.cn
First published on 25th October 2021
Abstract
One grand challenge in green hydrogen production is to design efficient HER electrocatalysts for high-rate alkaline water electrolysis. Nickel chalcogenide coatings on nickel foam (NF) are promising HER electrocatalysts, but their high-rate performances are yet to be improved. The current work reports a NiSe/Ni3Se2@NF for alkaline HER, which requires an overpotential of only 336 mV to achieve an ultra-high current density of 1250 mA cm−2, outperforming commercial Pt/C. The low onset potential of NiSe/Ni3Se2@NF is attributed to its morphology, and high surface area, as well as multiple active sites and electronic structure modulation because of the heterostructure. While these features are well-known within the current knowledge framework, new understandings are proposed on its superior high-rate performance. The common-anion feature offers abundant interfacial Ni–Se bonding and low resistance for efficient interfacial charge transfer, whereas the heterovalent-Ni-cation in the heterostructure results in a built-in electric field that further enhances the high-rate performance. This work provides new insights on both the mechanistic and methodological aspects of designing high-performance electrocatalysts operating at high current densities.
Introduction
Water electrolysis is a key enabling technology for a sustainable hydrogen economy, because it allows facile conversion of renewable or surplus electricity to hydrogen gas, whilst contributing to the resilience and stability of the electric grid.1,2 Commercial water electrolysis requires effective and robust electrocatalysts for efficient and high-rate hydrogen and oxygen evolution reactions (HER and OER). Alkaline water electrolysis is the most commonly used commercial technology because of its high ionic conductivity and moreover the lower OER overpotential. However, the HER in alkaline medium suffers from a higher energy barrier and poor kinetics compared with acidic solutions. Therefore, the grand challenge in HER electrocatalyst design is to achieve a low overpotential at high current densities in alkaline solutions. Platinum loaded on porous carbon (Pt/C) is the best-performing lab-scale HER electrocatalyst because of its superior catalytic activities.3,4 Nevertheless, the most widely used commercial HER electrocatalyst is porous nickel (Ni) metal, because of its low cost, high robustness and moderate catalytic activities. The performance of Ni metal can be significantly improved by surface treatment or modification. Among others, in situ growth of Ni chalcogenides on Ni metal substrates is an effective and feasible method because of the facile preparation, high activity, conductivity and robustness of the chalcogenide coating.The materials design of the Ni chalcogenide coatings includes morphological, polymorph or crystalline structure, doping, defect and heterostructure. These advanced synthetic strategies drastically improve the HER performances to even compete with the benchmark Pt/C catalyst. Among others, heterostructured Ni chalcogenides allow simultaneous incorporation of high surface area, optimized adsorption energy, enhanced intrinsic catalytic activity, large numbers and multiple active sites.5,6 Recent studies6,7 suggest heterostructured NiSe/Ni3Se2 on Ni foam is a highly promising HER electrocatalyst because of its optimal adsorption free energy for atomic hydrogen, Se on NiSe and Ni on Ni3Se2 dual active sites, and high surface area. Despite their combined merits, these NiSe/Ni3Se2 heterostructures only shows compelling HER catalytic performances at low or moderate current densities (<300 mA cm−2). On the other hand, a recent phenomenon found in heterointerface, i.e., built-in electric field, has been verified to increase the hydrogen yield in photocatalytic HER.8 The presence of a built-in electric field was reported in NiS/Ni3S2 heterostructure,9 which shows platinum-like catalytic behavior as a counter electrode in dye-sensitized solar cell. As a chemical and structural analogue of NiS/Ni3S2, NiSe/Ni3Se2 heterostructure is also expected to yield a built-in electric field that can boost its HER performances at high current densities. Although this hypothesis is highly plausible, it has not verified so far, because there is still a lack of both experimental evidence and design principles for such heterostructure with high-rate HER behaviour. We herein report a common-anion heterovalent-Ni-cation type NiSe/Ni3Se2 heterostructure on Ni foam by one-step hydrothermal synthesis. This rationally designed electrode shows lower overpotential compared with Pt/C at ultra-high current densities up to 1.25 A cm−2. Furthermore, we propose the general rules on optimal heterostructure design for HER, particularly the synergistic effects of intrinsic activity, site exposure and built-in electric field.
Results and discussion
Two single-phase nickel selenide coating on nickel foam, NiSe@NF and Ni3Se2@NF, heterostructured NiSe/Ni3Se2@NF, and dissimilar-anion heterostructured NiSe/Ni3S2@NF were prepared by one-step solvothermal process (details in the Experimental section of the ESI†). SEM images (Fig. 1) show that all the four samples have similar morphologies of uniform 1D nanorod arrays on the nickel substrate, though the sizes, shape and lengths show variations. It is also observed that the nanorods of NiSe/Ni3Se2@NF has a comparatively rougher surface than the other three samples. For all four samples, the nanorods are near-vertically grown on NF, with diameters between 200 and 500 nm and lengths of several micrometers. Electrodes with such nanorod arrays on the surfaces are highly desired because of the increased number of active sites and improved electrolyte accessibility. Moreover, this sub-micrometer-scale architecture commonly generates a hydrophilic and aerophobic surface that leads to enhanced mass transfer during gas evolving reaction.10 The contact angles with water droplet and air bubble (Fig. S1†) indeed suggest that NiSe/Ni3Se2@NF is much more hydrophilic and slightly more aerophobic than bare NF. The powder X-ray diffraction (XRD) patterns (Fig. 2a) indicates single phase NiSe (JCPDS no. 75-0610 and JCPDS no. 29-0935) and Ni3Se2 (JCPDS no. 19-0841) structures of NiSe@NF and Ni3Se2@NF. In contrast, the XRD patterns of the two heterostructures, NiSe/Ni3Se2@NF, and NiSe/Ni3S2@NF, all display two-phase crystalline structures of their individual two constituent phases, confirming successful preparation of the intended heterostructures. Detailed peak assignment of the four samples is shown in Table S1.†11–14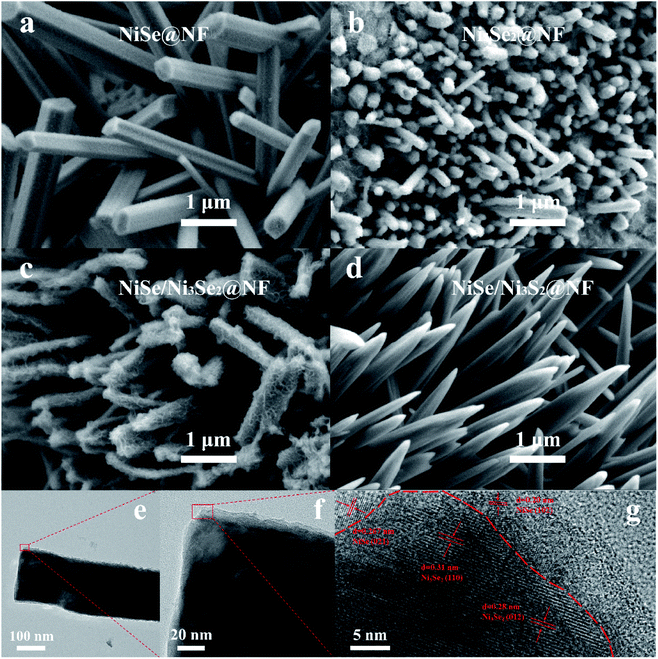 | ||
| Fig. 1 Morphological and structural characterizations. SEM images of NiSe@NF (a) Ni3Se2@NF (b) NiSe/Ni3Se2@NF (c) NiSe/Ni3S2@NF (d) and high-resolution TEM images of NiSe/Ni3Se2@NF (e–g). | ||
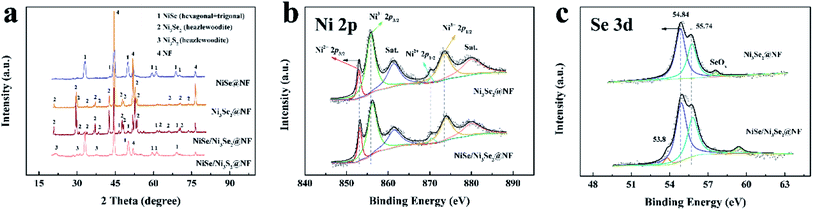 | ||
| Fig. 2 Spectroscopic characterizations. XRD patterns (a) of Ni3Se2@NF, NiSe@NF, NiSe/Ni3Se2@NF, and NiSe/Ni3S2@NF. (b) Ni 2p spectra. (c) XPS fitting of Se 3d peaks of NiSe/Ni3Se2@NF and Ni3Se2@NF. | ||
The high-resolution transmission electron microscopic (HR-TEM) images (Fig. 1e–g) of NiSe/Ni3Se2@NF shows two distinct lattice structures, i.e., the broader lattice fringe spaces of 0.28 and 0.31 nm correspond to the (012) and (110) planes of hexagonal Ni3Se2, while the narrower lattice fringe spaces of 0.20 and 0.267 nm correspond to the (102) and (021) planes of hexagonal NiSe.6,7 The HR-TEM images also reveal that Ni3Se2 is the dominant and skeleton phase with NiSe located at the edge of the nanorods, i.e., Ni3Se2 nanorods wrapped by a thin NiSe layer. The HR-TEM elemental mapping (Fig. S2†) of NiSe/Ni3Se2@NF shows a uniform distribution of nickel and selenide signals. Compared with heterostructures with dissimilar cations or anions, NiSe/Ni3Se2@NF is a unique heterovalent-Ni-cation common-anion type heterostructure that is beneficial for charge transfer across the interface.
The XPS survey spectrum (Fig. S3†) of NiSe/Ni3Se2@NF shows C and O signals, aside from Ni and Se, which is attributed to the contamination/surface oxidation of the samples due to exposure to air. The high-resolution Ni 2p spectrum of NiSe/Ni3Se2@NF (Fig. 2b) reveals spin–orbital Ni2+ 2p3/2 and Ni2+ 2p1/2 peaks at 852.8 and 870.1 eV, while the higher energy peaks at 855.8 and 873.9 eV are assigned to Ni3+ 2p3/2 and Ni3+ 2p1/2, suggesting that the state of Ni in NiSe/Ni3Se2@NF is the mixed phase of Ni2+ and Ni3+.15,16 The peaks at 861.0 eV and 879.6 eV are assigned to the shake-up satellite peaks of Ni3+ 2p3/2 and Ni3+ 2p1/2, respectively. Besides, the high-resolution XPS spectrum of the Se 3d (Fig. 2b) consists of two peaks at binding energy of 54.84 eV and 55.74 eV, corresponding to the Se 3d5/2 and Se 3d3/2 of the metal-rich form hexagonal Ni3Se2 in the heterostructure. The peak at binding energy of 57.6 eV can be ascribed to the bonding structures of SeOx, which is partial oxidation of Se under air atmosphere. The peak at binding energy of 53.8 eV belongs to hexagonal NiSe phase.6,11,17–19 One Ni atom coordinates with 6 Se atoms and other 2 Ni atoms in NiSe; while one Ni in Ni3Se2 is coordinated with 4 Se and 4 Ni atoms. Ni3Se2 possesses more Ni–Ni bonds than NiSe, thereby exhibiting metallic conductivity. The observed lower binding energy of Ni 2p and Se 3d peaks in Ni3Se2 is a result of its metallic feature (Fig. 2c).7
Based on the above morphological and spectroscopic results, the growth and formation mechanisms of different nickel selenide coatings are proposed as follows. At the beginning of the reaction, N2H4·H2O assists the dissolution of Se powder to produce H+ and Se2− (eqn (1)). The resulting H+ reacts with NF, yielding Ni2+ (eqn (2));19,20 the Ni2+ cations and Se2− anions subsequently precipitate to form the NiSe phase (eqn (3)). At a reaction temperature of 140 °C, the reaction terminates at this step, yielding pure NiSe coating on NF, i.e., NiSe@NF. At elevated hydrothermal temperature of 200 °C, Ni2+ can be reduced by hydrazine hydrate to reactive Ni˙ atoms (eqn (4)) upon heating, which further reacts with NiSe to form Ni3Se2 (eqn (5)) under hydrothermal conditions.7,12 At a low dosage of Se powder, pure Ni3Se2 coating on nickel foam is formed, i.e., Ni3Se2@NF. At higher Se dosage, NiSe continues to form on the surface of Ni3Se2, leading to the formation of heterostructured NiSe/Ni3Se2@NF. To sum up, the formation of different Ni selenide coatings on NF is a combined consequence of hydrothermal reaction temperature and Se usage. The XRD, SEM and HR-TEM results agree well with the above proposed reaction mechanisms.
| N2H4·H2O + Se → 4H+ + Se2− + N2↑ + H2O | (1) |
| 2H+ + Ni → Ni2+ + H2 | (2) |
| Ni2+ + Se2− → NiSe | (3) |
| Ni2+ + N2H4·H2O → Ni˙ + N2 + 2H2 + H2O | (4) |
| 2NiSe + Ni˙ → Ni3Se2 | (5) |
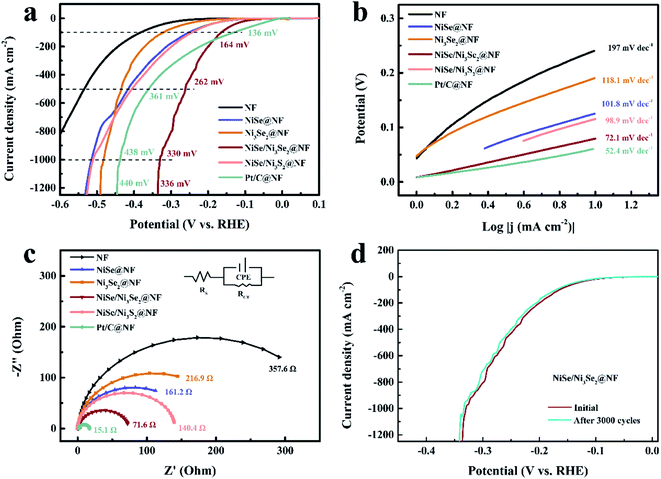
The HER catalytic behaviors of all samples were evaluated and compared with bare NF and Pt/C@NF in a standard three-electrode electrochemical cell using 1.0 M KOH aqueous solution. The linear sweep voltammograms (LSV, Fig. 3a) show that all the samples exhibit HER catalytic behaviors, with more positive onset potentials and higher cathodic currents than bare NF, suggesting that nickel selenides generally have higher HER catalytic activities than nickel metal. Among all the four selenide samples, NiSe/Ni3Se2@NF shows the best electrocatalytic performance as evidenced by its lowest overpotential and highest cathodic current density. Compared with Pt/C@NF, the HER onset potential of NiSe/Ni3Se2@NF is 50 mV more negative. At low current density of 10 mA cm−2, NiSe/Ni3Se2@NF also shows an overpotential that is 61 mV higher than Pt/C (Fig. S4†), but their LSV curves intersect with each other at current density of 180 mA cm−2. At higher current beyond the intersection (>180 mA cm−2), NiSe/Ni3Se2@NF requires lower overpotentials than Pt/C@NF to deliver the same current density. To reach ultra-high current densities of 500, 1000 and 1250 mA cm−2, NiSe/Ni3Se2@NF only requires overpotentials of 262, 330 and 336 mV, respectively, outperforming Pt/C@NF (η500, η1000 and η1250 = 351, 438 and 440 mV) for high-rate HER. Among all the selenide samples, NiSe/Ni3Se2@NF also displays the lowest Tafel slope of 72.1 mV dec−1 (Fig. 4b), indicating that the HER occurs via the Volmer–Heyrovsky mechanism with the Volmer step (water discharge) as the bottleneck. The Vomer-step-determined alkaline HER requires fast water dissociation and appropriate adsorption of Had/OHad.21 In this sense, heterostructured electrocatalysts are generally more prone to meeting the multiple requirements through their multiple active sites and electronic structure modulation.22,23
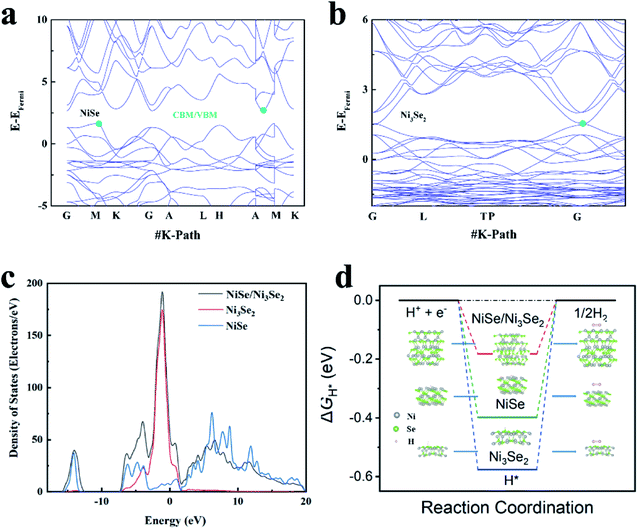 | ||
| Fig. 4 Computed band structures of NiSe (a) and Ni3Se2 (b), computed density of states (DOS) (c) and ΔGH (d) of NiSe, Ni3Se2 and NiSe/Ni3Se2. | ||
The charge transfer and ion diffusion properties of different electrodes were studied by electrochemical impedance spectroscopy (EIS) and electrochemical active surface area (ECSA). The fitted Nyquist plots (Fig. 3c and Table S2†) show that NiSe/Ni3Se2@NF has the lowest charge-transfer resistance (RCT) value of 71.6 Ω among all the selenide samples. The low RCT value and Tafel slope of NiSe/Ni3Se2@NF are both beneficial to its catalytic performances, particularly at high current densities. The ECSA values as derived from the electrochemical double-layer capacitance (Cdl) are plotted in Fig. S5.† NiSe/Ni3Se2@NF displays the highest Cdl value of 25.17 mF cm−2 among all the selenide samples, indicating the highest number of active sites on NiSe/Ni3Se2@NF. The high Cdl value agrees well with the SEM observation (Fig. 2) that the nanorods of NiSe/Ni3Se2@NF have rougher surface than the other samples. The charge density difference plots (Fig. S6†) also reveal electron enrichment at the interface between NiSe and Ni3Se2. This finding suggests the interfacial region can probably provide additional HER active sites, complementing to the two known optimal sites, i.e., Se on NiSe and Ni on Ni3Se2.6,7 As a result of the additional active sites and the higher ECSA, NiSe/Ni3Se2@NF shows a much higher exchange current density (j0, Table S3†) than the other selenide samples.
The stability of NiSe/Ni3Se2@NF was evaluated by the chronoamperometric test, which demonstrates ultra-high durability as evidenced by the nearly overlapped LSV curve after 3000 CV cycles (Fig. 3d). The stability is further verified by the 30 h potentiostatic HER test. At η = 164 mV, a constant current density of 100 mA cm−2 is maintained after 30 h; while at η = 164 mV, NiSe/Ni3Se2@NF shows fairly slow decrease of current density (Fig. S7†). The amount of H2 gas produced by NiSe/Ni3Se2@NF is exactly the same as the theoretical value during galvanostatic HER in 2 h (Fig. S8†), suggesting a nearly 100% faradaic efficiency. The SEM images (Fig. S9†), XRD pattern (Fig. S10†) and the XPS spectra (Fig. S11†) indicate that the NiSe/Ni3Se2@NF electrocatalyst undergo no morphological, structural or chemical change after 3000 CV cycles. The superior stability of NiSe/Ni3Se2@NF is attributed to its intrinsic chemical stability, as well as the robust mechanical properties resulting from the in situ growth of the selenide coating on NF.10,24
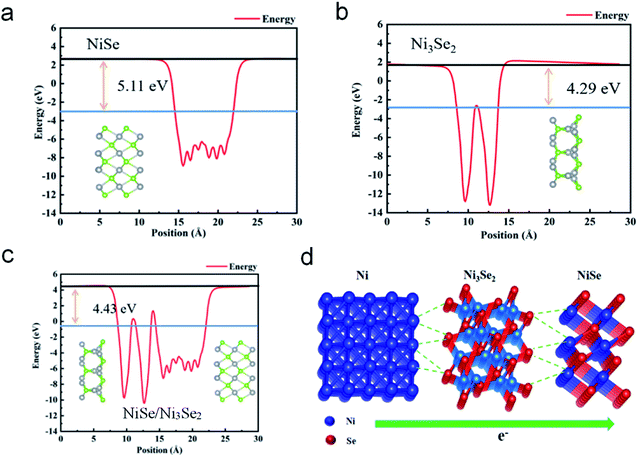
To gain further insight into the heterostructure features, Mott–Schottky (M–S) analysis was performed at an AC frequency of 1 kHz. The M–S diagram (Fig. S12†) exhibits positive slope of n-type semiconductor properties.25,26 A recent work demonstrates that n-type semiconductor electrocatalysts favor cathodic reactions such as HER because of their self-gating under negative electrode potentials.27 All the selenide samples belong to the n-type, so they are promising HER electrocatalysts from this perspective. DFT calculations (structural model shown in Fig. S13†) shows that NiSe and Ni3Se2 possess different band structures (Fig. 4a and b). NiSe shows a clear gap between its valence and conduction band (VB and CB), while the VB and CB of Ni3Se2 shows overlap, suggesting that NiSe is a typical semiconductor but Ni3Se2 exhibits metallic-type conductivity. Therefore, the electric conductivity in NiSe/Ni3Se2@NF follows NF > Ni3Se2 > NiSe. This descending order of conductivity from substrate to the electrode surface is in accordance with the rational design principle of electrocatalyst with rapid charge transfer properties.10 The calculated density of states (DOS, Fig. 4c) suggest that the DOS of NiSe/Ni3Se2 below the Fermi level is mainly contributed by Ni3Se2, while above the Fermi level is jointly contributed by NiSe to a large extent. The DOS results also indicate that the electrical properties of heterojunction, especially the width of valence band, are significantly larger than that of the single component. Therefore, the two components in the heterojunction have strong hybridization. The electronic structure modulation is further verified by the free energy with hydrogen (ΔGH), as illustrated in Fig. 4d. The computed hydrogen adsorption free energy (ΔGH*) of NiSe/Ni3Se2 is the lowest among all the three electrocatalysts. High HER catalytic activity generally requires a ΔGH* value close to 0 eV because it offers both moderate H chemisorption and releasing strength.31 The more negative ΔGH* values of NiSe and Ni3Se2 indicates lower HER catalytic activities, which can be attributed to the formation of NiHads species (Ni + H+ + e− → NiHads) and SeHads species (Se + H+ + e− → SeHads) with strong binding energy during the Volmer reaction in HER processes, respectively.32,33 The near-zero ΔGH* value of the NiSe/Ni3Se2 structure is an indication of its optimal adsorption and dissociation ability for H*, in agreement with the observed higher exchange current density (j0, Table S3†).
Aside from the higher conductivity and electron density, Ni3Se2 has lower work function than NiSe (Fig. 5a and b). The lower work function of Ni3Se2 is in agreement with previous theoretical work,28 which attributes this to the lower oxidation state and lower electronegativity of Ni atoms in Ni3Se2 than NiSe. In the NiSe/Ni3Se2 heterojunction, a built-in electric field (BIEF) is formed which induces electron injection from the lower to higher work function material,29 i.e., electron transfer from Ni3Se2 to NiSe. This BIEF originates from the band alignment in order to achieve a work function equilibrium (Fig. 5c) between NiSe and Ni3Se2. The charge density difference plots (Fig. S6†) also show that the electron density is reduced on Ni3Se2 but increased on NiSe upon contact, confirming the spontaneous electron transfer from Ni3Se2 to NiSe. The resulting BIEF facilitates unidirectional interfacial charge transfer that is in line with the cathodic current flow (Fig. 5d) and hence enhances the HER catalytic activity.30,34 Furthermore, NiSe/Ni3Se2 belongs to a unique type of common-anion heterovalent-common-cation heterojunction, which possesses ample Ni–Se bonds (Fig. 5d) across the interface to reduce the interfacial charge transfer resistance. In fact, NiSe and Ni3Se2 have similar lattice parameters,35 so the lattice mismatch in the NiSe/Ni3Se2 heterostructure is small, also beneficial for interfacial Ni–Se bonds and charge transfer. The dissimilar-anion heterostructured NiSe/Ni3S2@NF exhibits higher overpotentials (Fig. 3a) and higher charge transfer resistance (Fig. 3c) than NiSe/Ni3Se2@NF, confirming that the common-anion feature is favorable for HER electrocatalysis.
Coupled with the experimental results and the theoretical analysis, the superior high-rate HER performance of NiSe/Ni3Se2@NF is rationalized as follows. (1) The nanorod-array morphology and high surface roughness affords high surface area and exposure of catalytic active sites. (2) The multiple active sites and electronic structure modulation in the heterostructure meet the specific requirements of the elementary steps of alkaline HER. (3) The hydrophilic and aerophobic surface originating from the micro-architecture is beneficial for mass transfer at high reaction rates. (4) The common-anion heterostructure presents ample interfacial Ni–Se bonds to facilitates charge transfer across the interface. (5) The different electronegativity of Ni cations results in a built-in electric field that induces spontaneous electron transfer from Ni3Se2 to NiSe. The observed low onset potential of NiSe/Ni3Se2@NF is attributed to standpoint (1) and (2), whereas its superior high-rate HER performance is mainly due to (3), (4) and (5).
Conclusions
In conclusion, we report one-step hydrothermal synthesis of NiSe/Ni3Se2@NF heterostructure through control of reaction temperature and reactant molar ratio. This common-anion heterovalent-common-cation heterostructure shows superior catalytic performances in ultra-high-rate alkaline HER. The morphological features offer high number of active sites and the heterostructure provides multiples catalytic sites and electronic structure modulation. Moreover, the rationally designed NiSe/Ni3Se2@NF results in built-in electric field and efficient interfacial charge transfer, both responsible for its excellent high-rate HER performances.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work received financial support from the Science and Technology Bureau of Shenzhen (Grant No. JCYJ20170306171540744).References
- N. Armaroli and V. Balzani, The hydrogen issue, ChemSusChem, 2011, 4, 21–36 CrossRef CAS PubMed.
- I. Dincer and C. Acar, Smart energy solutions with hydrogen options, Int. J. Hydrogen Energy, 2018, 43, 8579–8599 CrossRef CAS.
- B. Yu, Y. Hu, F. Qi, X. Wang, B. Zheng, K. Liu, W. Zhang, Y. Li and Y. Chen, Nanocrystalline Ni0.85Se as efficient non-noble-metal electrocatalyst for hydrogen evolution reaction, Electrochim. Acta, 2017, 242, 25–30 CrossRef CAS.
- G. Li, J. Yu, J. Jia, L. Yang, L. Zhao, W. Zhou and H. Liu, Cobalt-Cobalt phosphide nanoparticles@nitrogen-phosphorus doped carbon/graphene derived from cobalt ions adsorbed saccharomycete yeasts as an efficient, stable, and large-current-density electrode for hydrogen evolution reactions, Adv. Funct. Mater., 2018, 28, 1801332 CrossRef.
- C. Tang, Z. Pu, Q. Liu, A. M. Asiri, X. Sun, Y. Luo and Y. He, In situ growth of NiSe nanowire film on nickel foam as an electrode for high-performance supercapacitors, ChemElectroChem, 2015, 2, 1903–1907 CrossRef CAS.
- Y. Zhong, B. Chang, Y. Shao, C. Xu, Y. Wu and X. Hao, Regulating phase conversion from Ni3Se2 to NiSe in bifunctional electrocatalyst for overall water splitting enhancement, ChemSusChem, 2019, 12, 2008–2014 CrossRef CAS PubMed.
- H.-B. Wang, Y.-S. Sun, F. Ma, L. Zhou, H.-F. Li, L. Zhang, G.-J. Chen, Y.-K. Xu, Y.-N. Chen, K.-W. Xu and D.-Y. Ma, Se molarity tuned composition and configuration of Ni3Se2/NiSe core-shell nanowire heterostructures for hydrogen evolution reaction, J. Alloys Compd., 2020, 819, 153056 CrossRef CAS.
- X. Wang, X. Wang, J. Huang, S. Li, A. Meng and Z. Li, Interfacial chemical bond and internal electric field modulated Z-scheme Sv-ZnIn2S4/MoSe2 photocatalyst for efficient hydrogen evolution, Nat. Commun., 2021, 12, 4112 CrossRef CAS PubMed.
- Y. Liao, K. Pan, Q. Pan, G. Wang, W. Zhou and H. Fu, In situ synthesis of a NiS/Ni3S2 nanorod composite array on Ni foil as a FTO-free counter electrode for dye-sensitized solar cells, Nanoscale, 2015, 7, 1623–1626 RSC.
- X. Ma, W. Chen, Q. Li, L. Xue and C. Peng, Nitrogen-doped hierarchical heterostructured aerophobic MoSx/Ni3S2 nanowires by one-pot synthesis: system engineering and synergistic effect in electrocatalysis of hydrogen evolution reaction, Energy Environ. Mater., 2020, 1 Search PubMed.
- Y. Tian, Y. Ruan, J. Zhang, Z. Yang, J. Jiang and C. Wang, Controllable growth of NiSe nanorod arrays via one-pot hydrothermal method for high areal-capacitance supercapacitors, Electrochim. Acta, 2017, 250, 327–334 CrossRef CAS.
- X. Zhang, M. Zhen, J. Bai, S. Jin and L. Liu, Efficient NiSe-Ni3Se2/graphene electrocatalyst in dye-sensitized solar cells: the role of hollow hybrid nanostructure, ACS Appl. Mater. Interfaces, 2016, 8, 17187–17193 CrossRef CAS PubMed.
- F. Zhang, Y. Pei, Y. Ge, H. Chu, S. Craig, P. Dong, J. Cao, P. M. Ajayan, M. Ye and J. Shen, Controlled synthesis of eutectic NiSe/Ni3Se2 self-supported on Ni foam: an excellent bifunctional electrocatalyst for overall water splitting, Adv. Mater. Interfaces, 2018, 5, 1701507 CrossRef.
- Z. Zou, X. Wang, J. Huang, Z. Wu and F. Gao, An Fe-doped nickel selenide nanorod/nanosheet hierarchical array for efficient overall water splitting, J. Mater. Chem. A, 2019, 7, 2233–2241 RSC.
- J. Zhang, Y. Li, T. Zhu, Y. Wang, J. Cui, J. Wu, H. Xu, X. Shu, Y. Qin, H. Zheng, P. M. Ajayan, Y. Zhang and Y. Wu, 3D coral-like Ni3S2 on Ni foam as a bifunctional electrocatalyst for overall water splitting, ACS Appl. Mater. Interfaces, 2018, 10, 31330–31339 CrossRef CAS PubMed.
- C. Tang, N. Cheng, Z. Pu, W. Xing and X. Sun, NiSe nanowire film supported on nickel foam: an efficient and stable 3D bifunctional electrode for full water splitting, Angew. Chem., Int. Ed., 2015, 54, 9351–9355 CrossRef CAS PubMed.
- H. Li, S. Chen, H. Lin, X. Xu, H. Yang, L. Song and X. Wang, Nickel diselenide ultrathin nanowires decorated with amorphous nickel oxide nanoparticles for enhanced water splitting electrocatalysis, Small, 2017, 13, 1701487 CrossRef PubMed.
- X. Yu, W. Sun and Y. Chu, One-pot solvothermal synthesis and properties of 1D NiSe and NiSe-Ni3S2 alloyed compound nanorod arrays, New J. Chem., 2014, 38, 70–76 RSC.
- C. Bao, F. Li, J. Wang, P. Sun, N. Huang, Y. Sun, L. Fang, L. Wang and X. Sun, One-pot solvothermal in situ growth of 1D single-crystalline NiSe on Ni foil as efficient and stable transparent conductive oxide free counter electrodes for dye-sensitized solar cells, ACS Appl. Mater. Interfaces, 2016, 8, 32788–32796 CrossRef CAS PubMed.
- L. Zhang, J. C. Yu, M. Mo, L. Wu, Q. Li and K. W. Kwong, A general solution-phase approach to oriented nanostructured films of metal chalcogenides on metal foils: the case of nickel sulfide, J. Am. Chem. Soc., 2004, 126, 8116 CrossRef CAS PubMed.
- L. Zhao, Y. Zhang, Z. Zhao, Q.-H. Zhang, L.-B. Huang, L. Gu, G. Lu, J.-S. Hu and L.-J. Wan, Steering elementary steps towards efficient alkaline hydrogen evolution via size-dependent Ni/NiO nanoscale heterosurfaces, Natl. Sci. Rev., 2020, 7, 27–36 CrossRef CAS.
- Y. Jiang, P. Sun, L. Sharma, B. Mao, R. Kakkar, T. Meng, L. Zheng and M. Cao, Further insights into bifunctional mechanism in alkaline hydrogen evolution for hybridized nanocatalysts and general route toward mechanism-oriented synthesis, Nano Energy, 2021, 81, 105645 CrossRef CAS.
- J. Ge, J. Jin, Y. Cao, M. Jiang, F. Zhang, H. Guo and X. Lei, Heterostructure Ni3S4-MoS2 with interfacial electron redistribution used for enhancing hydrogen evolution, RSC Adv., 2021, 11, 19630–19638 RSC.
- X. Liu, S. Li, B. Akinwolemiwa, D. Hu, T. Wu and C. Peng, Low-crystalline transition metal oxide/hydroxide on MWCNT by Fenton-reaction-inspired green synthesis for lithium ion battery and OER electrocatalysis, Electrochim. Acta, 2021, 387, 138559 CrossRef CAS.
- S. Bolar, S. Shit, J. S. Kumar, N. C. Murmu, R. S. Ganesh, H. Inokawa and T. Kuila, Optimization of active surface area of flower like MoS2 using V-doping towards enhanced hydrogen evolution reaction in acidic and basic medium, Appl. Catal., B, 2019, 254, 432–442 CrossRef CAS.
- D. He, L. Cao, Y. Huang, G. Li, Y. Feng, Y. Zhao, X. Li, D. Li and L. Feng, Tuning the morphologic and electronic structures of self-assembled NiSe/Ni3Se2 heterostructures with vanadium doping toward efficient electrocatalytic hydrogen, Appl. Surf. Sci., 2021, 542, 148598 CrossRef CAS.
- Y. He, et al., Self-gating in semiconductor electrocatalysis, Nat. Mater., 2019, 18, 1098–1104 CrossRef CAS PubMed.
- M. T. Greiner, L. Chai, M. G. Helander, W.-M. Tang and Z.-H. Lu, Transition metal oxide work functions: the influence of cation oxidation state and oxygen vacancies, Adv. Funct. Mater., 2012, 22, 4557–4568 CrossRef CAS.
- Z. Li, Y. Pei, R. Ma, Y. Wang, Y. Zhu, M. Yang and J. Wang, A phosphate semiconductor-induced built-in electric field boosts electron enrichment for electrocatalytic hydrogen evolution in alkaline conditions, J. Mater. Chem. A, 2021, 9, 13109–13114 RSC.
- M. S. Hassan, P. Basera, S. Gahlawat, P. P. Ingole, S. Bhattacharya and S. Sapra, Understanding the efficient electrocatalytic activities of MoSe2-Cu2S nanoheterostructures, J. Mater. Chem. A, 2021, 9, 9837–9848 RSC.
- Z. Pu, J. Zhao, I. S. Amiinu, W. Li, M. Wang, D. He and S. Mu, A universal synthesis strategy for P-rich noble metal diphosphide-based electrocatalysts for the hydrogen evolution reaction, Energy Environ. Sci., 2019, 12, 952–957 RSC.
- Y. Zheng, Y. Jiao, M. Jaroniec and S. Z. Qiao, Advancing the Electrochemistry of the Hydrogen-Evolution Reaction through Combining Experiment and Theory, Angew. Chem., Int. Ed., 2015, 54, 52–65 CrossRef CAS PubMed.
- Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier and H. Dai, Co3O4 Nanocrystals on Graphene as a Synergistic Catalyst for Oxygen Reduction Reaction, Nat. Mater., 2011, 10, 780 CrossRef CAS PubMed.
- Z. Niu, C. Qiu, J. Jiang and L. Ai, Hierarchical CoP-FeP branched heterostructures for highly efficient electrocatalytic water splitting, ACS Sustainable Chem. Eng., 2018, 7, 2335–2342 CrossRef.
- K. Unoki, A. Yoshiasa, G. Kitahara, T. Nishiayama, M. Tokuda, K. Sugiyama and A. Nakatsuka, Crystal structure refinements of stoichiometric Ni3Se2 and NiSe, Acta Crystallogr., Sect. C: Struct. Chem., 2021, 77, 169–175 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra06183f |
| This journal is © The Royal Society of Chemistry 2021 |