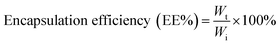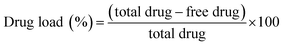Open Access Article
Open Access ArticleSurface-engineered liposomal particles of calcium ascorbate with fenugreek galactomannan enhanced the oral bioavailability of ascorbic acid: a randomized, double-blinded, 3-sequence, crossover study
Ashil Joseph,
Dinesh Kumar,
Abhilash Balakrishnan,
Prasanth Shanmughan,
Balu Maliakel and
Krishnakumar IM *
*
R&D Centre, Akay Natural Ingredients, Ambunad, Malaidamthuruth P. O., Cochin, 683561, India. E-mail: krishnakumar.im@akay-group.com; Fax: +91 484 2680891; Tel: +91 484 2686111
First published on 26th November 2021
Abstract
The antioxidant, anti-inflammatory, immunomodulating, anti-thrombotic, and antiviral effects along with its protective effects against respiratory infections have generated a great interest in vitamin C (vitC) as an attractive functional/nutraceutical ingredient for the management of COVID-19. However, the oral bioavailability and pharmacokinetics of vitC have been shown to be complex and exhibit dose-dependent non-linear kinetics. Though sustained-release forms and liquid liposomal formulations have been developed, only marginal enhancement was observed in bioavailability. Here we report a novel surface-engineered liposomal formulation of calcium ascorbate (CAAS), using fenugreek galactomannan hydrogel in powder form, and its pharmacokinetics following a randomized, double-blinded, single-dose, 3-way crossover study on healthy human volunteers (n = 14). The physicochemical characterization and in vitro release studies revealed the uniform impregnation of CAAS liposomes within the pockets created by the sterically hindered galactomannan network as multilaminar liposomal vesicles with good encapsulation efficiency (>90%) and their stability and sustained-release under gastrointestinal pH conditions. Further human studies demonstrated >7-fold enhancement in the oral bioavailability of ascorbate with a significant improvement in pharmacokinetic properties (Cmax, Tmax, T1/2, and AUC), compared to the unformulated counterpart (UF-CAAS) when supplemented at an equivalent dose of 400 mg of CAAS as tablets and capsules.
Introduction
Vitamins and minerals, collectively known as micronutrients, are essential elements for the survival of organisms owing to their vital role in the maintenance of the body's homeostasis. L-Ascorbic acid (AA), commonly known as vitamin C (vitC), is an important water-soluble antioxidant micronutrient involved in the regulation of a number of gene expressions and is an essential cofactor for various classes of enzymes involved in the biosynthesis of amino acids, neurotransmitters and hormones.1,2 Supplementation of vitC is very often recommended (up to 2000 mg per day) to eliminate its deficiency and hence for efficient cellular processes since a mutation which encodes L-gulono-γ-lactone oxidase does not allow its synthesis in humans.3,4 Large population studies have reported that vitC deficiency is common even in developed countries.5 A significant association has also been demonstrated between vitC deficiency, balanced immune functions and morbidity/mortality.1,5,6 Low levels of plasma vitC (∼20 μM) has also been shown to correlate with the severity of illness among pneumonia, sepsis and COVID-19 patients.7Vitamin C has been shown to degrade quickly depending on the environmental factors such as pH, temperature, light and oxygen.8 Under in vivo conditions, it is mainly found in the reduced or anionic ascorbate (ASC) form which may reversibly be oxidized to dehydroascorbate (DHAA) and further to inactive 2,3-diketogulonic acid.2,9 In humans, body concentration of vitC depends on its intake, intestinal absorption, tissue distribution, renal re-absorption and excretion.10,11 The pharmacokinetics of vitC has been shown to be complex due to a saturable active transport mechanism mediated by sodium-dependent vitC transporters (SVCT) and exhibited dose-dependent non-linear kinetics.11–13 Though regular uptake of 200 to 400 mg dose has been shown to achieve a steady state plasma concentration of 50 to 70 μM, either multiple gram dosage or infusion is suggested to overcome its tight intestinal absorption control and hence to exceed the homeostatic saturation level.11,14 Though 100% absorption was reported for 200 mg single dose, the absorption was reported to reduce to 33% when the dose was increased to 1250 mg.15 Therefore, there exist tremendous interest in food-grade delivery forms that can enhance the bioavailability and hence the pharmacokinetic properties of vitC.
Considering the high water solubility and transporter proteins-mediated absorption, multiple and smaller doses per day was hypothesised to be the best mode of dosage for vitC.11 But, no significant differences in pharmacokinetic variables has been observed between the plain and slow release formulations even after 4 weeks of supplementation.16 Another major approach was based on liposomes. But, supplementation of doses ranging from 4 to 36 g of liposomal vitC was found to offer only marginal enhancement in the oral bioavailability, probably due to its limitations.17–20 Liposomal forms are susceptible to chemical changes (hydrolysis of phospholipid ester bonds and oxidation of unsaturated acyl chains) and exhibit poor stability under stomach and storage conditions.21 The liquid state of liposomes, usage of various chemical stabilizers during its preparation, relatively low drug loading ability and hence the high dosage are the common issues associated with liposomes in addition to opsonisation (strong interaction with the blood proteins leading to rapid elimination from systemic circulation).22
We hypothesised that surface modification of liposomal CAAS using suitable methods and novel food-grade biopolymers would enhance the in vivo stability and hence oral bioavailability of vitC avoiding the use of synthetic excipients and chemicals. Surface modification of liposomes with synthetic hydrophilic polymers such as polyethylene glycol, polyvinyl alcohol, and chemically modified chitosan have already been reported.23,24 Here we report the development of a stable powder formulation of liposomal calcium ascorbate (CAAS) employing a novel surface modification of liposomes with fenugreek (Trigonella foenum gracum) galactomannans (FG) for the first time. The process involved the gel-phase dispersion of a liposomal CAAS into FG-hydrogel network to provide a “capping effect” whereby to stabilize the liposomes from the harsh environmental conditions. Previously, we had reported that FG can form mucoadhesive and amphiphilic gels containing lipophilic molecules as water dispersible powders capable of re-swelling in the gastrointestinal tract to release self-emulsified colloidal particles for better absorption.25–27
Materials and methods
General
All solvents used for analysis were of high performance liquid chromatography (HPLC) grade and those used for the extraction were reagent grade, procured from Merck life sciences Private Limited, Bengaluru, India. MilliQ plus (Millipore India Private Limited, Bengaluru, India) purified water was used for the experiments. The analytical standards of CAAS (CAS no: 5743-28-2 Sigma-Aldrich, Bangalore, India) and ascorbic acid (CAS no: 50-81-7) were procured from Sigma Aldrich, Bangalore, India. Food-grade calcium ascorbate 98% suitable for human supplementation (DSM Nutritional Products Limited, Heanor, UK) and the surface-modified liposomal particles of CAAS with fenugreek galactomannan (FC+) was received from Akay Natural Ingredients, Cochin, India along with a detailed certificate of analysis indicating food safety parameters. Ascorbic acid content was estimated using a HPLC (Shimadzu SPD20AT) coupled with SPD-M20A prominence photodiode array detector (PDA) (Shimadzu India Private Limited, Mumbai, India) and a Phenomenex reverse phase C18 column (150 × 4.6 mm; 3 μm, 100 Å) following a previously reported validated procedure by Robitaille and Hoffer.28Preparation and characterisation
FC+ prepared by a gel-phase dispersion process of lipidated CAAS into FG-hydrogel matrix to form a hybrid-hydrogel (hybrid-FENUMAT™). Briefly, about 4.2 g of CAAS was dissolved in water, mixed with an ethanolic solution of lecithin (2.1 g), and homogenized. The homogeneous solution was then mixed uniformly with fenugreek galactomannan solution and the resulting mass was evaporated to dry powder form.The physicochemical properties including particle size, zeta potential, Fourier-transform infrared spectroscopy (FTIR) and morphology of the formulation FC+ was carried out using high resolution transmission electron microscope (HR-TEM) (JEOL JEM-2100 LaB6, Jeol Co Limited, Japan), field emission scanning electron microscope (FE-SEM) (ZEISS Sigma 500 VP, ZEISS microscopy, Oberkochen, Germany), dynamic light scattering (DLS) (Horiba SZ-100 particle size analyser, Horiba India Private Limited, Bengaluru, India) and FTIR spectra from PerkinElmer Spectrum 400 spectrometer.
where Wt is the total amount of drug (CAAS) in the liposomes suspension and Wi is the total quantity of drug added initially during preparation.
The concentration of drug loaded was determined using a HPLC method. The following equation was used to analyse the efficiency of drug loading
Stability study
Accelerated stability study was performed as per the guidelines of International Conference on Harmonization (ICH) of technical requirements for the registration of pharmaceuticals for human use.30 Briefly, about 10 g sample packets of FC+ were packed in polyethylene kit and kept in air-tight high density polypropylene (HDPE) bottles and incubated at 40 ± 2 °C and 70 ± 5% relative humidity for a period of six months in a stability chamber (Remi, Mumbai, India). Samples were retrieved at 0, 1, 2, 3 and 6 months' intervals and subjected to analysis for various parameters including colour and appearance, ascorbic acid content, moisture content, and microbial parameters (total plate counts, yeast and mould, Coliforms, Escherichia coli and Salmonella).Bioavailability studies
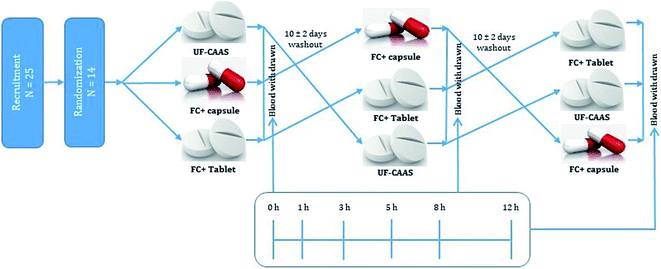
Relative bioavailability of the formulation FC+ with respect to the unformulated CAAS powder with >98% purity (UF-CAAS) was investigated in healthy volunteers.Healthy volunteers (males and females) (n = 14) aged 18 to 65 years having BMI between 18–25 kg m−2 were enrolled for the study. Those who were having a history of gut disorders such as irritable bowel syndrome, ulcerative colitis, gastro-oesophageal reflux disease, duodenal or gastric ulcers, gastritis, kidney stone or those who are using proton pump inhibitors were excluded from the study. Smokers, chronic alcoholics, and those who are under any dietary supplements or medication were also excluded along with others having any symptoms of viral infection. In addition, participants with any condition that in opinion of the investigator does not justify the participation in the study were also excluded.
Selected participants were randomized into one of the three treatment arms consisting of three single dose treatments, with either formulated (tablets and capsules) or unformulated (UF-CAAS) tablets. All the doses were provided after an overnight fasting of 10 ± 1 h as depicted in Fig. 1. The participants were refrained from consuming diets rich in/or supplemented with any form of vitC for five days prior to the study date. A washout period of 10 ± 2 days was provided between supplementation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 minutes at 4 °C. The protein free acid supernatant was immediately frozen and stored at −80 °C for analysis.31
000g for 10 minutes at 4 °C. The protein free acid supernatant was immediately frozen and stored at −80 °C for analysis.31Injection solution of plasma was prepared from thawed samples by dividing them into two parts, each containing 30 μL, in separate Eppendorf tubes and mixing with an equal volume of 5 mmol L−1 tris(2-carboxy ethyl) phosphine hydrochloride (TCEP) at pH 2 in water. The mixture was allowed to react with the sample for about 20 min at room temperature under dark conditions. An equal volume of water was then added and the samples were kept on ice bath and centrifuged for 5 min at 4 °C at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g. The supernatant was stored in cold conditions (4 ± 1 °C) and transferred to auto injection vials for analysis.
000g. The supernatant was stored in cold conditions (4 ± 1 °C) and transferred to auto injection vials for analysis.
Statistical analysis
Statistical analysis was performed using SPSS version 27. All data were expressed as mean ± SD and all analyses were performed using analysis of variance (ANOVA). The differences observed were evaluated using a t-test and P < 0.05 were considered as statistically significant. The pharmacokinetic parameters were deduced from plasma concentration verses time plot using GraphPad Prism 5.0.Results
Food-grade CAAS with not less than 98% purity was used as the vitC supplement in the present study. The formulation (FC+) was a free flowing granular powder with a particle size of 20 to 100 mesh size with a calcium ascorbate content of 40.3% and tapped density 0.8 g mL−1. Technical specification sheet, certificate of analysis and material safety data sheet of the investigational products showed that they are produced under good manufacturing practices (GMP) and possess all necessary documentations and safety data required for human consumption. The properties of both the formulated and unformulated tablets and capsules used in the study are shown in Table 1.| Properties | Tablets (FC+) | Capsules (FC+) | Tablets-unformulated (UF-CAAS) |
|---|---|---|---|
| Weight (mg) | 1000 ± 25 | 500 ± 25 | 1000 ± 25 |
| Calcium ascorbate content (mg) | 397.2 ± 10 | 202.1 ± 5 | 394.5 ± 10 |
| Friability (%) | 0.92 ± 0.2 | — | 1.95 ± 0.4 |
| Thickness (cm) | 0.62 ± 2 | — | 0.65 ± 2 |
| Hardness (kg cm−2) | 5.7 ± 0.3 | — | 4.0 ± 0.5 |
Characterisation
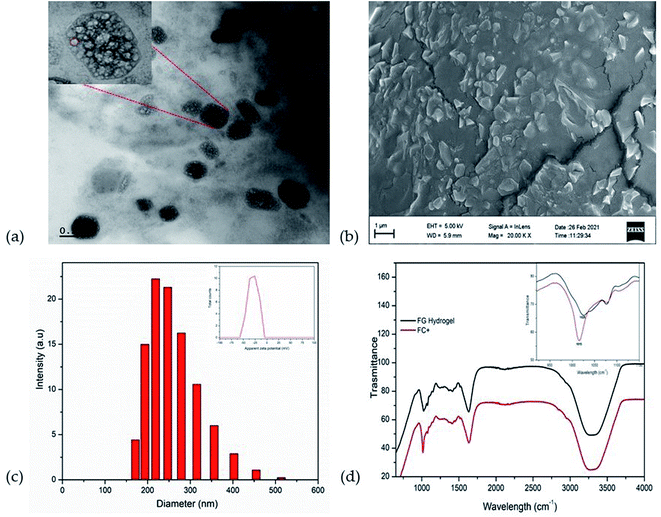
Electron microscopic studies (HR-TEM and FE-SEM) showed that the formulation of CAAS as FC+ is an encapsulated form of liposomal CAAS in the hydrogel matrix of fenugreek galactomannans to form a typical hybrid-hydrogel. The encapsulation was evident from HR-TEM image as non-spherical liposomes with a surrounding membrane structure held in the hydrophobic pockets created by the galactomannan chains in the gel phase (Fig. 2a). The FE-SEM image also showed the uniform impregnation of non-spherical particles of about ∼200 nm diameter, identified as liposomes, in the gel matrix of fibre network (Fig. 2b). The mean particle size of encapsulated liposomal particles was observed as 212.9 ± 5.5 nm with a zeta potential of −29.3 mV (Fig. 2c), while the mean particle size of uncoated liposomes was 89 ± 3.9 nm with a zeta potential of −3.2 mV. CAAS was encapsulated into the liposomes with a high encapsulation efficiency of 95.3 ± 0.55% as determined by HPLC and the drug loading efficiency was found to be 4.65 ± 0.18%.The FTIR spectra of FC+ in comparison with the FG matrix when recorded at in the frequency range 4000 to 800 cm−1 revealed the nature of entrapment of liposomes in the FG hydrogel matrix (Fig. 2d). The C–O stretching vibrations of alcohol group at 1025 cm−1, the C–O–C vibration at 2920 cm−1 and the O–H stretching vibration at 3296 cm−1 were identified as the characteristic frequencies of the carbohydrate moieties in the biopolymer galactomannan.32 In FC+, the peak corresponding to the C–O stretching vibration was found to be shifted from 1025 cm−1 to 1015 cm−1 due to the strong molecular interaction of liposomes with the FG hydrogel network.33
In vitro release studies of FC+ granular powder and the tablet indicated sustained release of ascorbate under both stomach and intestinal pH conditions (Fig. 3). While the granular powder form of FC+ released almost 85% of ascorbate in 4 h, the compression into tablets further delayed the release in such a way that only 25% release was observed in 4 h, at pH 2 and 6.8 respectively.
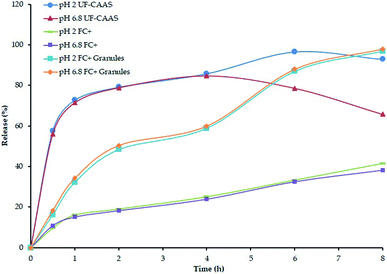 | ||
| Fig. 3 In vitro release of calcium ascorbate from FC+ tablet and granular powder form used for capsules and tablets used in the present study. | ||
Accelerated stability studies revealed no significant changes in the physicochemical properties including colour, appearance, bulk density, moisture content, microbial load and ascorbic acid content. All tested parameters prevailed within ±2% of the initial value, indicating sufficient stability of FC+ for a storage of 2 years when kept in air-tight closed containers under ambient conditions of less than 30 °C in dark, without moisture or direct sunlight (Table 2).
| Parameter | Specification | Initial | 1st month | 2nd month | 3rd month | 6th month |
|---|---|---|---|---|---|---|
| a *NLT denotes ‘not less than’; #each value was presented as an average of three measurements. | ||||||
| Appearance | Free flowing granular powder | Complies | Complies | Complies | Complies | Complies |
| Colour | Off white | Complies | Complies | Complies | Complies | Complies |
| Calcium ascorbate content | NLT* 40% | 40.8% | 39.9% | 39.6% | 40.1% | 38.4% |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Microbiology – USFDA (FDA) | ||||||
| Total plate count# | <10000 cfu g−1 | 100 cfu g−1 | 130 cfu g−1 | 120 cfu g−1 | 130 cfu g−1 | 100 cfu g−1 |
| Yeast & mould# | <200 cfu g−1 | <10 cfu g−1 | <10 cfu g−1 | <10 cfu g−1 | <10 cfu g−1 | <10 cfu g−1 |
| Coliforms# | <3 MPN g−1 | <3 MPN g−1 | <3 MPN g−1 | <3 MPN g−1 | <3 MNP g−1 | <3 MPN g−1 |
| E. coli# | Absent per g | Absent per g | Absent per g | Absent per g | Absent per g | Absent per g |
| Salmonella# | Absent per 25 g | Absent per 25 g | Absent per 25 g | Absent per 25 g | Absent per 25 g | Absent per 25 g |
Bioavailability study
A total of 25 healthy volunteers were screened; out of which 14 (10 males and 4 females) eligible participants were randomized and completed the study as per the double-blinded crossover design depicted in Fig. 1. The demographics and baseline characteristics of the participants is given in Table 3. The validated HPLC-PDA method of Robitaille and Hoffer was adopted for the quantification of ascorbate content in blood plasma.28 The method showed linearity over wide range (50 to 1000 μmol L−1) of vitC concentration with an r2 value of 0.99981 (Fig. 4). Matrix-matched calibration was performed in the present study. Under the specified conditions, ascorbate peak was found at a retention time (Rt) of 3.2 min with a relative standard deviation within the accepted levels of ± 5%. MPA/FEDTA sample preparation method used in the present study showed a recovery of 87.8% in the extraction of vitC from plasma.| Parameter | Male | Female |
|---|---|---|
| a HDL – high-density lipoprotein, LDL – low-density lipoprotein, VLDL – very low density lipoprotein, ALT – alanine aminotransferase, AST – aspartate aminotransferase, ALP – alkaline phosphatase, RBC – red blood cell, BMI – body mass index. | ||
| No. of volunteers | 10 | 4 |
| Age (years) | 31.67 ± 3.73 | 32.33 ± 4.15 |
| Weight (kg) | 65.37 ± 9.65 | 57.3 ± 3.37 |
| BMI | 23.18 ± 2.66 | 24.43 ± 1.20 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Hematology | ||
| Hemoglobin (g dL−1) | 16.28 ± 0.88 | 14.03 ± 1.26 |
| Total leukocyte count (cells per cumm) | 7677.78 ± 1208.07 | 6966.67 ± 585.95 |
| Total RBC count (million per cumm) | 5.49 ± 0.62 | 6.15 ± 0.50 |
| Platelet (lakhs per cumm) | 2.04 ± 0.47 | 2.00 ± 0.63 |
| Lymphocytes (%) | 42.89 ± 5.40 | 38.33 ± 3.51 |
| Eosinophil's (%) | 2.56 ± 0.73 | 3.0 ± 1.00 |
| Neutrophils (%) | 53.2 ± 4.38 | 48.0 ± 2.65 |
| Monocytes (%) | 1.78 ± 0.67 | 1.33 ± 1.15 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Biochemical | ||
| ALT (U L−1) | 38.3 ± 15.76 | 31.3 ± 4.16 |
| AST (U L−1) | 25.3 ± 6.32 | 16.6 ± 6.43 |
| ALP (U L−1) | 59.0 ± 17.2 | 82.3 ± 11.9 |
| Total protein (g dL−1) | 7.56 ± 0.26 | 7.43 ± 0.60 |
| Bilirubin (mg dL−1) | 0.47 ± 0.18 | 0.58 ± 0.14 |
| Albumin (g dL−1) | 4.60 ± 0.43 | 4.56 ± 0.26 |
| Globulin (g dL−1) | 2.74 ± 0.31 | 2.60 ± 0.62 |
| A/G ratio | 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Cholesterol (mg dL−1) | 178.1 ± 21.89 | 178.3 ± 12.1 |
| Triglycerides (mg dL−1) | 114.1 ± 31.56 | 83.3 ± 15.7 |
| HDL (mg dL−1) | 57.6 ± 7.48 | 52.6 ± 4.04 |
| LDL (mg dL−1) | 94.6 ± 7.38 | 16.6 ± 3.14 |
| VLDL (mg dL−1) | 22.8 ± 6.31 | 30.5 ± 2.50 |
| Creatinine (mg dL−1) | 0.79 ± 0.12 | 0.78 ± 0.21 |
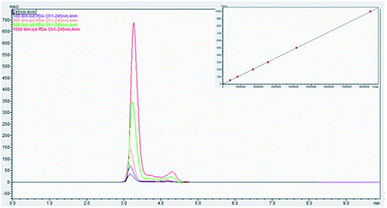 | ||
| Fig. 4 Panel shows HPLC chromatogram for a series of calibration samples and plasma samples. Calibration curve for ascorbate is shown in inset. | ||
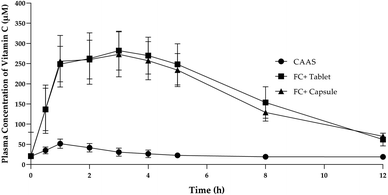
Administration of 1000 mg single dose of FC+ in both capsule and tablet form resulted in significantly (P < 0.05) higher concentration of plasma ascorbate concentration as compared to the equivalent dose of UF-CAAS over 1 to 12 h of post-administration time period (Fig. 5). Each 1000 mg of FC+ was found to contain 400 ± 10 mg of UF-CAAS. The pharmacokinetic properties of the formulations are provided in Table 4. It was observed that the area under curve over 12 h of post-administration time period (AUC0–12 h) for both tablets and capsules of FC+ were 2232 and 2119 respectively, which was about 7.2 and 6.8 times higher than the AUC0–12 h of the corresponding forms of UF-CAAS, indicating ∼7-fold enhancement in the bioavailability of ascorbate from FC+. The maximum observed concentration of ascorbate in plasma (Cmax) was 282.4 and 273 μM for the FC+ tablets and capsules respectively, as compared to 51.7 μM for UF-CAAS tablet. In the case of FC+, maximum plasma concentration of about 230 μM was found to be achieved in 1 h time post-dose and remained in almost 240 ± 20 μM level for more than 5 h (P > 0.05) in both capsules and tablets. Therefore, the time at which the maximum plasma concentration was observed (Tmax) for FC+ can be considered as 1 to 5 h, precisely about 3 h, verses 1 h for Tmax UF-CAAS. The elimination half-life (T1/2) for FC+ tablet was found to be 8.5 h verses 7.6 h for capsules which were significantly higher as compared to UF-CAAS tablets (3.6 h) (Fig. 5 & Table 4). The relative bioavailability (Frel) for the tablet was found to be 716.76 ± 291.01 and that for capsule was 680.47 ± 288.95.
| Sample | Dose (mg) | Cmax (μM) | Tmax (h) | T1/2 (h) | AUC (μM h mL−1) | MRT (h) | Frel |
|---|---|---|---|---|---|---|---|
| UF-CAAS | 1000 ± 25 | 51.7 ± 21 | 1 | 3.6 | 311.4 ± 63.87 | 22.67 ± 2.02 | — |
| FC+ (tablets) | 1000 ± 25 | 282.4 ± 43.86 | 3 | 8.5 | 2232 ± 447.50 | 22.21 ± 1.7 | 716.76 ± 291.01 |
| FC+ (capsules) | 500 ± 25 × 2 | 273 ± 59.88 | 3 | 7.6 | 2119 ± 465.19 | 22.33 ± 2.2 | 680.47 ± 288.95 |
Administration of FC+ did not cause any adverse effects, and none of the participants reported any discomfort or tolerability issues. The haematological and biochemical assessment showed no significant (P > 0.05) differences in the baseline values (Table 3).
Discussion
The present study was aimed at the characterisation and determination of bioavailability and pharmacokinetic properties of a novel, food-grade, and green formulation of CAAS as surface-engineered liposomal microparticles using the galactomannan from fenugreek seeds for the first time. The formulation (FC+) was achieved by a water-based gel-phase dispersion process of lipidated CAAS in FG matrix to form a hybrid-hydrogel where the water-soluble CAAS is first encapsulated in phospholipids and further engulfed within the pockets created by the conformationally restricted highly sterically hindered three-dimensional galactomannan network as shown in Fig. 2 and 6. Electron microscopic images (FE-SEM and HR-TEM) could also reveal the entrapment of liposomal CAAS with a surface coating by galactomannans in an obloid form. The images were in agreement with the earlier reports on the surface modified liposomes.34,35 Particle size analysis and zeta potential measurements could also confirm the surface coating and improved stability of FC+, as evident from the increased mean particle size (212.9 ± 5.5 nm verses 89 ± 3.9 nm) and zeta potential (−29.3 mV verses −3.2 mV) of the formulation as compared to the uncoated liposomes. The non-spherical shape shows the multi-lamellar liposomal structure, probably due to its non-covalent interactions of the vesicles with the galactomannans, as evident from FTIR data. Non-spherical multi-laminar liposomes are of great interest to drug delivery, owing to their controlled and sustained drug release profile, resulting in enhanced bioavailability and efficacy.36 The non-spherical liposomes have longer physiological lifetime when compared to their spherical counterparts. Further, the drug release profile of non-spherical liposomes has been seen to be different from conventional unilaminar spherical liposomes due to the inhomogeneous thickness and larger surface area of the non-spherical particles giving rise to a larger drug flux per unit volume.35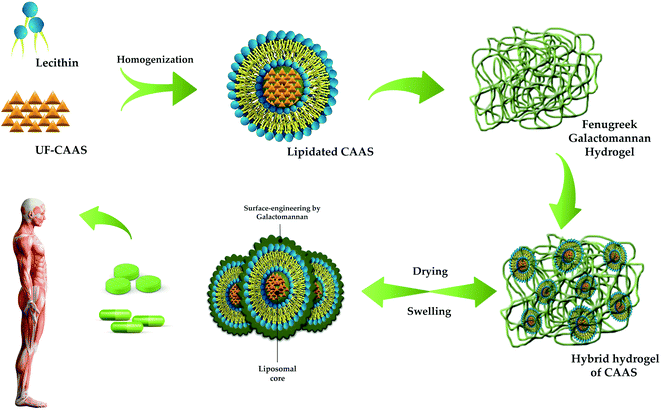 | ||
| Fig. 6 Schematic representation of FC+ formulation using hybrid-FENUMAT technology employed for the surface modification of liposomal CAAS. | ||
The density, particle size and flow ability of FC+ powder was found to be suitable for a wide range of pharma and food delivery forms. It was found to swell extensively under gastrointestinal tract conditions and further to release stable and stabilized liposomal forms of CAAS indicating its behaviour as a reversible hydrogel for drug delivery (Fig. 6). Accelerated stability data demonstrated a shelf life of minimum 2 years if keep under ambient conditions, away from sunlight and moisture. Direct compressibility of FC+ powder and its high drug loading ability (around 40% w/w) further implies the versatility of the present surface modified liposomal hydrogel technology for the delivery of water-soluble and labile bioactive molecules for dietary applications.
Calcium ascorbate is a widely recommended vitC supplement due to its compatibility under gastrointestinal conditions, compared to the ascorbic acid which may results in elevated gastric reflux leading to gastrointestinal disorders.37 Intestinal absorption of ascorbate has been found to be tightly controlled and follow nonlinear pharmacokinetics, with a steady state plasma concentration level attained through a membrane transport mechanism unlike the diffusion-mediated absorption and distribution process of the lipophilic small molecule drugs.11 Table 5 describes the various approaches that have been reported for the oral delivery of vitC to overcome the tight control observed on intestinal absorption and hence to provide pharmacologically relevant plasma concentrations with improved pharmacokinetic properties. Though extended-release forms were initially hypothesised to be better for ascorbate delivery due to its slow elimination by delaying the gastric emptying, no significant enhancement in the bioavailability or plasma concentration of slow release formulations was observed by Viscovich et al.16 A randomised controlled trial on Ester-C® (a formulation of CAAS as a mixture of dehydroascorbate, calcium threonate, and 4-hydroxy-5-methyl-3(2H)-furanone) at a dose of 1000 mg vitC reported no significant change in AUC0–24 h of plasma concentration verses time plot; but showed a significant leukocyte retention of ascorbate.38 In another comparative randomised controlled trial using 1000 mg dose of ascorbic acid, CAAS, Ester-C® and Pureway-C® (a proprietary formulation of vitC-lipid metabolites), both Ester-C® and Pureway-C® showed almost similar enhancement in bioavailability with maximum plasma concentration (Cmax) as: Pureway-C® (2.17 ± 0.19 mg dL−1) > Ester-C® (1.69 ± 0.27 mg dL−1) > ascorbic acid (1.64 ± 0.18 mg dL−1) > CAAS (1.12 ± 0.17 mg dL−1).39 Yet another proprietary form of CAAS as Nutra-C® showed 1.28-fold enhancement in bioavailability and 1.34 times enhancement in Cmax (102.5 μM) followed by a 500 mg single dose when compared with the corresponding dose of UF-CAAS.40 Recent approaches in vitC delivery were based on liposomal technology. Davis et al., has reported about 1.3-fold enhancement in bioavailability when 4.25 g of sodium ascorbate was delivered as liposomes.19 In another study, supplementation of 10 g of liposomal sodium ascorbate was shown to enhance ascorbate bioavailability by 1.79 times with a Cmax of only 300 μM as compared to 180 μM for the equivalent dose of unformulated one.18 Another liposomal formulation of ascorbic acid showed 1.77-fold enhancement in bioavailability when supplemented at a dose of 1000 mg vitC/5 mL.20 Supplementation of very high doses of liposomal vitamin C, as high as 25 and 36 g, was also attempted and observed Cmax in the range of 350–400 μM only.16 The most recent report on liposomal formulation was a powder form with 6-fold enhancement in bioavailability from a single dose of 150 mg of ascorbic acid.40 But, the pharmacokinetic study of this paper faces a serious issue that the reported Cmax of 6.69 mg dL−1 is 2-fold higher than the administered amount of ascorbic acid, warranting further studies on its pharmacokinetic properties.41
| Formulation | Dose | Dose pattern (oral) | Design | Number of subjects (n) | Analysis method | Pharmacokinetic properties | Number of folds of enhancement in bioavailability | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (μM) | Tmax (h) | T1/2 (h) | AUC (μg h mL−1) | ||||||||
| a Abbreviations: DB – double-blind, PC – placebo-controlled, OP – open label, SB – single-blinded, CT – crossover trial, PG – parallel group, RCT – randomized clinical trial, TC – trail controlled, SS – single site, PT – prospective trail, SD – single dose, TT – two-treatment, TS – two-sequences, TP – two-period, TWC – two-way crossover. *HPLC-UV – denote HPLC estimation using ultraviolet spectrophotometric detector; **HPLC-ECD – denotes HPLC estimation using electrochemical detector; ***HPLC-PDA – denotes HPLC estimation using photodiode array detector; #P denotes unformulated ascorbic acid and F represents formulation; $the pharmacokinetics study reported as 2-fold higher Cmax value than the administrated dose. | |||||||||||
| Slow-release ascorbic acid | 250 mg × 2/day 4 weeks | Repeated | SB, RCT, PC, SS | 48 (F19, F19, P10)# | HPLC-UV* | 91.6 | 2.4 | 34.2 | 1010 | 1.33 | 16 |
| Ester-C® (calcium ascorbate) | 500 mg × 2 | Single | DB, PC, CT, SS, RCT | 30 (F15, P15)# | HPLC-UV* | 43.89 | 4.0 | — | 483 | 1.16 | 38 |
| PureWay-C® | 1000 mg | Single | RCT, DB, PT | 10 | HPLC-ECD** | 123.21 | 2 | — | — | — | 39 |
| Nutra-C® (calcium ascorbate) | 500 mg × 1 | Single | SD, OP, RCT, PG | 20 (F10, P10)# | HPLC-UV* | 102.49 | 3.3 | 10.5 | 822 | 1.09 | 40 |
| Liposomal sodium ascorbate | 4 g | Single | PC, RCT | 11 | HPLC-ECD** | 187 | 3 | — | 584 | 1.35 | 19 |
| Liposomal sodium ascorbate | 10 g | Single | — | 20 | HPLC-UV* | 303 | 3 | 6 | 1359 | 1.79 | 18 |
| Liposomal ascorbic acid | 1000 mg/5 mL | Single | OP, RCT, PC, SD, TT, TS, TP, TWC | 24 (P12, F12)# | HPLC-UV* | 296.9 | 3.5 | 12.3 | 3171 | 1.77 | 20 |
| Liposomal sodium ascorbate | 5 g | Single | SB | 2 | HPLC-PDA*** | 230 | 3.5 | — | — | — | 17 |
| Liposomal ascorbic acid -powder | 150 mg | Single | DB, RCT, CT | 8 (F4, P4)# | HPLC-UV* | 379.8 | 3.06 | 3.27 | 3135 | 5.9$ | 41 |
| Hybrid-FENUMAT (FC+) (tablets) | 1000 mg | Single | DB, RCT, CT | 14 | HPLC-UV* | 282.4 | 3 | 8.5 | 2232 | 7.17 | Present study |
| Hybrid-FENUMAT (FC+) (capsules) | 500 × 2 mg | Single | DB, RCT, CT | 14 | HPLC-UV* | 273 | 3 | 7.6 | 2119 | 6.81 | Present study |
It is observed from Table 5 that the pharmacokinetic parameters for FC+ in the present study is significantly higher than the human bioavailability reports on any kind of vitamin C formulation reported so far. FC+ formulation is based on the hypothesis that the protection of vitamin C from its degradation in the gastrointestinal tract and its conversion to mucoadhesive colloidal particles can enhance its absorption and hence the plasma concentration at low dosage. Fenugreek galactomannan-based hybrid-hydrogel technology (hybrid-FENUMAT) provided a novel 100% natural and food-grade powder form for the sustained-release of self-emulsified multi-laminar liposomal particles of CAAS (200 ± 20 nm) encapsulated in mucoadhesive galactomannan networks as evident from the in vitro release, particle size and electron microscopy studies. Enhanced bioavailability (>7-fold) of FC+ with improved pharmacokinetic properties (Cmax, Tmax, T1/2, AUC) and a plasma concentration of around >230 μM for 5 h following the oral administration of a single dose of 1000 mg FC+ containing about 400 mg of CAAS points towards the efficiency of FC+ to deliver stable CAAS particles in the intestine overcoming the acidic stomach conditions. In vitro studies have shown >280 μM L−1 as an optimised dosage for antimicrobial and antiproliferation effects of vitC.17 So, the reported enhancement in ascorbate bioavailability from FC+ is highly significant, since a dosage as high as 2000 mg of CAAS × 3 per day has shown to provide only 250 μM plasma concentration.42
Liposomal formulation of CAAS was achieved with significant encapsulation efficiency of 95.3 ± 0.55%.29 In vitro release studies showed that the powder form of FC+ allowed the sustained release of CAAS by controlling the diffusion process through the galactomannan network, as described by Nguyen et al., for surface modified liposomes.23 The tablets possess relatively more delayed release kinetics as compared to the granular powder form of FC+ used in capsules. However, FC+ provided similar bioavailability and pharmacokinetic properties following the administration of both capsules and tablets. Therefore, it is the in vivo stability, particle size and nature of liposomes released from the fibre matrix, which play critical role in ascorbate pharmacokinetics from FC+ than the extended release profile. It is generally known that the tablets and capsules form can be bioequivalent and provide similar bioavailability.43
Hydrogels are three-dimensional cross-linked polymeric porous structures that absorb and retain huge amount of water.44 The nature of hydrophilic polymer chains and the extent of crosslinking determine the porosity and stability of a hydrogel.45 Swelling in water and shrinkage upon dehydration is a critical property of hydrogel for its drug loading and sustained-release properties through diffusion or environmental stimuli such as pH.44,45 The synthetic hydrogels are widely suggested for the delivery of lipophilic bioactive molecules.46 The novelty of the present study lies in the fact that this accounts the first report on the enhanced bioavailability of water-soluble salts like CAAS using non-chemically altered biopolymer hydrogel derived from a food component source such as fenugreek. It has already been shown that under conditions of optimised swelling, the degree of crosslinking resulting from the interpenetration of sterically hindered galactomannan chains can be tuned to have hydrophobic pockets suitable for the loading of lipophilic molecules and further to convert them as water soluble colloidal particles with enhanced absorption.25 Yet another very important feature is the functionality of fenugreek galactomannan as a clean label (non-genetically modified, allergen-free, vegetarian, non-toxic) soluble dietary fiber with biocompatibility and prebiotic potential.47 Hypoglycaemic, hypolipidemic, hypotriglyceridaemic and gastroprotective effects of fenugreek dietary fibre has already been reported, in addition to its safety and tolerance.48
Conclusion
The present study demonstrated the development of a natural hybrid-hydrogel system for the self-emulsified oral delivery of a water-soluble molecule like CAAS with enhanced bioavailability and pharmacokinetic properties at relatively low dosage of about 400 mg per day of CAAS to provide physiologically significant levels of vitC in the systemic circulation for longer duration as compared to the unformulated counterpart. The methodology used fenugreek galactomannan as a biopolymer matrix for the uniform impregnation of lipidated CAAS and thereby to protect the liposomal forms from its inherent problems of poor in vivo stability and rapid elimination for the first time. Electron microscopy and particle size analysis revealed the surface modification with galactomannan to form the multilaminar vesicles of CAAS engulfed into the galactomannan network. The sustained release of stable nano forms (∼200 nm) was achieved by controlled diffusion process through galactomannan hydrogel network for better absorption. The formulation showed >7-fold enhancement in the bioavailability of vitC, when supplemented as both tablets, and capsules, possessed good stability under accelerated testing conditions. Natural status, biocompatibility, safety, functional benefits, easy availability and water-based process makes hybrid-FENUMAT as a green and food-grade technology for the oral delivery of water-soluble micronutrients.Author contributions
B. M. & K. I. M. – conceptualisation, writing – review & editing, resources, supervision; A. J. – methodology, formal analysis; P. S. – formal analysis, characterisation; D. K. – methodology; A. B. – methodology, software, data curation. All authors have read and agreed to the published version of the manuscript.Data availability
The dataset analysed in the present study are available from the corresponding author on reasonable request.Conflicts of interest
The authors disclose the conflict of interest. Hybrid-FENUMAT is a patented technology of Akay Natural Ingredients, Cochin, India.Acknowledgements
Authors thank Akay Natural Ingredients, Cochin, India, for providing the study samples. Authors are grateful to Dr P. Prathibha, for her support in the preparation of the manuscript.References
- A. C. Carr and S. Maggini, Nutrients, 2017, 9, 1211 CrossRef PubMed , https://pubmed.ncbi.nlm.nih.gov/29099763/.
- S. Englard and S. Seifter, Annu. Rev. Nutr., 1986, 365–406 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/3015170/.
- A. C. Carr and M. C. M. Vissers, Nutr, 2013, 5, 4284–4304 CAS , https://pubmed.ncbi.nlm.nih.gov/24169506/.
- C. S. Tsao, An overview of ascorbic acid chemistry and biochemistry, in, Vitamin C in health and disease, ed. Packer L. and Fuchs J., Marcel Dekker Inc., New York, 1997, pp. 25–58 Search PubMed.
- M. Lindblad, P. Tveden-Nyborg and J. Lykkesfeldt, Nutr, 2013, 5, 2860–2879 CAS , https://pubmed.ncbi.nlm.nih.gov/23892714/.
- S. Rowe and A. C. Carr, Nutr, 2020, 12, 1–20 Search PubMed , /pmc/articles/PMC7400810/.
- P. Holford, A. C. Carr, T. H. Jovic, S. R. Ali, I. S. Whitaker and P. E. Marik, et al., Nutr, 2020, 12(12), 1–17 Search PubMed , https://pubmed.ncbi.nlm.nih.gov/33297491/.
- A. L. Herbig and C. M. G. C. Renard, Food Chem., 2017, 220, 444–451 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/27855924/.
- S. Padayatty and M. Levine, Oral Dis., 2016, 22(6), 463–493, DOI:10.1111/odi.12446.
- A. J. Michels, T. M. Hagen and B. Frei, Annu. Rev. Nutr., 2013, 33, 45–70 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/23642198/.
- J. Lykkesfeldt and P. Tveden-Nyborg, Nutrients, 2019, 11, 2412 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/31601028/.
- I. Savini, A. Rossi, C. Pierro, L. Avigliano and M. V. Catani, Amino Acids, 2008, 34, 347–355 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/17541511/.
- S. Hasselholt, P. Tveden-Nyborg and J. Lykkesfeldt, Br. J. Nutr., 2015, 113(10), 1539–1549 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/25865869/.
- M. Levine, S. J. Padayatty and M. G. Espey, Adv. Nutr., 2011, 2, 78–88 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/22332036/.
- M. Levine, C. Conry-Cantilena, Y. Wang, R. W. Welch, P. W. Washko and K. R. Dhariwal, et al., Proc. Natl. Acad. Sci. U.S.A., 1996, 93(8), 3704–3709 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/8623000/.
- M. Viscovich, J. Lykkesfeldt and H. E. Poulsen, Clin. Nutr., 2004, 23(5), 1043–1050 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/15380894/.
- S. Hickey, H. J. Roberts and N. J. Miller, J. Nutr. Environ. Med., 2008, 17(3), 169–177, DOI:10.1080/13590840802305423.
- M. Łukawski, P. Dałek, T. Borowik, A. Foryś, M. Langner and W. Witkiewicz, et al., J. Liposome Res., 2020, 30(3), 227–234 CrossRef PubMed , https://pubmed.ncbi.nlm.nih.gov/31264495/.
- J. L. Davis, H. L. Paris, J. W. Beals, S. E. Binns, G. R. Giordano and R. L. Scalzo, et al., Nutr. Metab. Insights, 2016, 9, NMI.S39764 CrossRef PubMed , https://pubmed.ncbi.nlm.nih.gov/27375360/.
- S. Gopi and P. Balakrishnan, J. Liposome Res., 2020, 356–364 Search PubMed , https://pubmed.ncbi.nlm.nih.gov/32901526/.
- A. Gabizon, H. Shmeeda and Y. Barenholz, Clin. Pharmacokinet., 2003, 42, 419–436 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/12739982/.
- G. Bozzuto and A. Molinari, Int. J. Nanomed., 2015, 10, 975–999 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/25678787/.
- T. X. Nguyen, L. Huang, M. Gauthier, G. Yang and Q. Wang, Nanomedicine, 2016, 11(9), 1169–1185 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/27074098/.
- H. He, Y. Lu, J. Qi, Q. Zhu, Z. Chen and W. Wu, Acta Pharm. Sin. B, 2019, 9(1), 36–48 CrossRef PubMed.
- M. B. Abhilash, D. Kumar, A. Deepti, A. Nair, V. Greet and V. An-Katrien, et al., J. Funct. Foods, 2021, 79, 104405 CrossRef CAS.
- L. Vijayasteltar, I. J. Jismy, A. Joseph, B. Maliakel, R. Kuttan and I. M. Krishnakumar, Toxicol. Rep., 2017, 4, 382–390 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/28959663/.
- I. M. Krishnakumar, A. Ravi, D. Kumar, R. Kuttan and B. Maliakel, J. Funct. Foods, 2012, 4(1), 348–357 CrossRef.
- L. Robitaille and L. J. Hoffer, Nutr. J., 2016, 15(1), 40 CrossRef PubMed , https://pubmed.ncbi.nlm.nih.gov/27102999/.
- N. Joshi, A. Kaviratna and R. Banerjee, Integr. Biol., 2013, 5(1), 239–248 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/22911380/.
- ICH. ICH Topic Q 1 A (R2) Stability Testing of new Drug Substances and Products Step 5 Note For Guidance On Stability Testing: Stability Testing Of New Drug Substances And Products 2003, http://www.emea.eu.int.
- A. Karlsen, R. Blomhoff and T. E. Gundersen, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2005, 824(1–2), 132–138 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/16046288/.
- Y. L. López-Franco, C. I. Cervantes-Montaño, K. G. Martínez-Robinson, J. Lizardi-Mendoza and L. E. Robles-Ozuna, Food Hydrocolloids, 2013, 30(2), 656–660 CrossRef.
- F. Rashid, S. Hussain and Z. Ahmed, Carbohydr. Polym., 2018, 180, 88–95 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/29103525/.
- U. Baxa, Imaging of liposomes by transmission electron microscopy, in Methods in Molecular Biology, Humana Press Inc., 2018, 73–88, https://pubmed.ncbi.nlm.nih.gov/29039095/ Search PubMed.
- J. Chen, N. E. Clay, N. hyung Park and H. Kong, Chem. Eng. Sci., 2015, 125, 20–24 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/25838583/.
- B. S. Pattni, V. V. Chupin and V. P. Torchilin, Drug Delivery, 2015, 115, 10938–10966 CAS , https://pubmed.ncbi.nlm.nih.gov/26010257/.
- J. K. Lee, S. H. Jung, S. E. Lee, J. H. Han, E. Jo and H. S. Park, et al., Korean J. Physiol. Pharmacol., 2018, 22(1), 35–42 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/29302210/.
- S. H. Mitmesser, Q. Ye, M. Evans and M. Combs, Springerplus, 2016, 5(1), 1161 CrossRef PubMed , https://pubmed.ncbi.nlm.nih.gov/27512620/.
- D. Pancorbo, C. Vazquez and M. A. Fletcher, Med. Sci. Monit., 2008, 14(11), CR547–CR551 CAS.
- K.-M. Choi, K. M. Hoon, H. T. Won, J.-D. Kim, K. duck Park and M.-Y. Kim, et al., Anal. Sci. Technol., 2016, 29(4), 162–169 CrossRef.
- J. Jacob, N. P. Sukumaran and S. Jude, ACS Omega, 2021, 6, 5560–5568 CrossRef CAS PubMed.
- T. K. Nielsen, M. Højgaard, J. T. Andersen, H. E. Poulsen, J. Lykkesfeldt and K. J. Mikines, Basic Clin. Pharmacol. Toxicol., 2015, 116(4), 343–348 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/25220574/.
- S. Kim, J. W. Ko and J. R. Kim, Int. J. Clin. Pharmacol. Ther., 2018, 56(9), 443–450 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/30021691/.
- R. Narayanaswamy and V. P. Torchilin, Molecules, 2019, 24 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/30744011/.
- A. Onaciu, R. A. Munteanu, A. I. Moldovan, C. S. Moldovan and I. Berindan-Neagoe, Pharmaceutics, 2019, 11(9) CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/31450869/.
- S. Grijalvo, J. Mayr, R. Eritja and D. D. Díaz, Biomater. Sci., 2016, 4(4), 555–574 RSC , https://pubmed.ncbi.nlm.nih.gov/26818789/.
- M. Majeed, S. Majeed, K. Nagabhushanam, S. Arumugam, S. Natarajan and K. Beede, et al., Food Sci. Nutr., 2018, 6(3), 666–673 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/29876118/.
- K. Srinivasan, Food Rev. Int., 2006, 22(2), 203–224, DOI:10.1080/87559120600586315.
| This journal is © The Royal Society of Chemistry 2021 |