Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photo-redox catalyzed dehydrazinative acylation of N-heterocycles via Minisci reaction†
Saira Hafeez a and
Aamer Saeed
a and
Aamer Saeed *b
*b
aDepartment of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, China
bDepartment of Chemistry, Quaid-I-Azam University, Islamabad, 45320, Pakistan. E-mail: aamersaeed@yahoo.com; asaeed@qau.edu.pk
First published on 1st December 2021
Abstract
Visible light-induced acylation of heteroaromatic compounds have been achieved using benzoyl hydrazides as an efficient acyl source under mild reaction conditions. The photo-redox catalyzed oxidative cleavage of hydrazides leads to in situ formation of acyl radicals, which subsequently couple with various N-heterocycles to produce acylated products. This synthetic strategy performs the classic Minisci reaction in an eco-friendly and greener way with functional group tolerance and regioselectivity. Control experiments confirm the radical pathway for this transformation.
Introduction
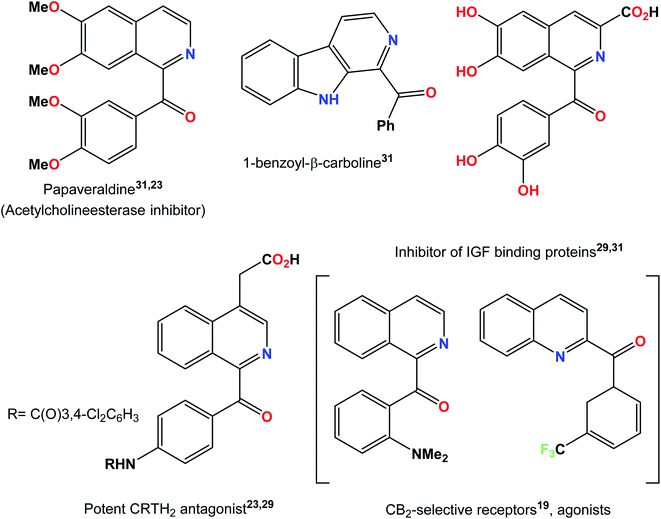
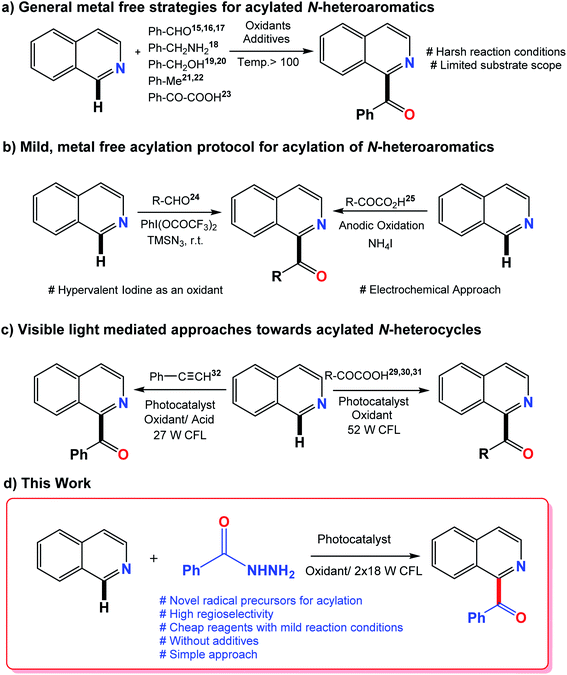
The synthesis of natural products raises the frontiers of synthetic chemistry as it encounters the challenge to construct complex and structurally diverse molecular frameworks.1–5 Natural products that show biological activity frequently serve as vital targets for novel drug design assemblies.6 Efficient and powerful synthetic strategies are required to access these diverse and structurally complex assemblies, which is a multidimensional challenge for synthetic chemists.7 One strategy towards the catalytic activation of organic molecules is visible light photo-redox catalysis.8 Visible light photocatalysis has currently received growing attention from organic chemists because of its extensive applications in organic synthesis as well as its consequences for sustainable chemistry.9N-heterocycles are key structural units for the synthesis of a variety of natural products with widespread application in the field of medicine. Some representative examples are shown in Fig. 1. For example, benzoylated quinolines possess numerous biological activities like antibacterial, antifungal, and anticancer activities.10–12 Different strategies have been designed, of which, Minisci radical C–H functionalization has been considered the most direct and efficient approach to access functionalized N-heterocycles.13,14 Numerous metal-free acylation strategies have been reported via radical transformations including aldehydes,15–17 benzylamines,18 aryl methanol derivatives,19,20 methyl arenes21,22 and α-keto acids23 as coupling counterparts along with oxidants at high reaction temperatures. Some milder protocols from previously reported radical precursors have also been reported by Antonchick and Zhang but with the use of hypervalent iodine as an oxidant and an electrochemical approach, respectively.24,25 A variety of reactions resulting from visible light mediated approaches have been carried out in order to obtain more mild protocols.26–28 In the past few years, the generation of acyl radicals for the acylation of N-heterocycles by the photo-redox strategy have been reported using α-keto acids29–31 and terminal alkynes32 as the acyl radical source. A visible light-induced catalytic system was also developed recently to achieve the acylation of pyridine N-oxides by the decarboxylation of α-oxocarboxylic acids using the organic dye fluorescein dimethylammonium as a new type of photocatalyst.33 The philicity of acetonyl and benzoyl radicals has been investigated by R. H. Verschueren and co-workers recently via experimental as well as computational studies.34
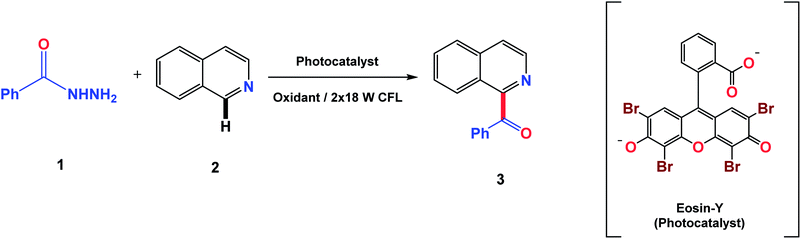
In this context, the production of acyl radicals via the oxidative cleavage of hydrazides introduces an outstanding idea. Acyl hydrazides are a good source of acyl radicals in transition metal catalysis35,36 but their use in the acylation of N-heterocycles via acyl radicals has not been reported yet. In 2017, the oxidative carbamoylation of electron deficient N-heterocycles by hydrazine carboxamide was reported by our group.37 Therefore, in continuation of our interest with N-heterocycles, we envisioned the utility of benzoyl hydrazides as an efficient source of acyl radicals under visible light conditions using eosin-Y as a photocatalyst. Recently, eosin-Y has been reported as a direct hydrogen atom transfer photocatalyst to generate acyl radicals from aldehydes as radical precursors.38 The generation of acyl radicals takes place very easily by oxidative cleavage using oxidants. Traditional Minisci reactions require the use of harsh conditions such as high temperature excess amounts of radical precursors, long reaction times and poor site selectivity. The current strategy eliminates these requirements with the introduction of novel radical precursors for the acylation of heterocycles. Previously reported methods and the current photo-mediated strategy are given in Fig. 2.
Results and discussion
To initiate the study of this transformation, a model reaction between benzoyl hydrazine (1) and isoquinoline (2) was carried out under visible light from 18 W CFL bulbs in the presence of a photocatalyst and an oxidant using DMSO/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature. The benzoylated product was obtained with 49% yield using Ru(bpy)3Cl2·6H2O and K2S2O8 after 12 hours (Table 1 entry 2). The influence of various photocatalysts was then investigated which showed results with moderate to good yields (entries 3–5). The best result is obtained using eosin-Y in combination with K2S2O8 (entry 3). Screening studies of different oxidants showed that K2S2O8 gives the highest yield among all others tested (entries 6–9). Increase or decrease in reaction time results in low yield of the product (entries 10 and 12). The optimal solvent system (DMSO/H2O 4
1) at room temperature. The benzoylated product was obtained with 49% yield using Ru(bpy)3Cl2·6H2O and K2S2O8 after 12 hours (Table 1 entry 2). The influence of various photocatalysts was then investigated which showed results with moderate to good yields (entries 3–5). The best result is obtained using eosin-Y in combination with K2S2O8 (entry 3). Screening studies of different oxidants showed that K2S2O8 gives the highest yield among all others tested (entries 6–9). Increase or decrease in reaction time results in low yield of the product (entries 10 and 12). The optimal solvent system (DMSO/H2O 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was obtained as all other solvents gave poor conversions (entries 12–18).
1) was obtained as all other solvents gave poor conversions (entries 12–18).
| Entry | Photocatalyst (mol%) | Oxidant (mmol) | Solvent (mL) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.6 mmol), 2 (0.2 mmol), oxidant (0.6 mmol), photocatalyst (5 mol%), solvent (2 mL).b Isolated yield.c Reaction at 50 °C in the absence of photocatalyst and light. | |||||
| 1a | — | K2S2O8 | DMSO/H2O | 12 | 11 |
| 2 | Ru(bpy)3Cl2·6H2O | K2S2O8 | DMSO/H2O | 12 | 49 |
| 3 | Eosin-Y | K2S2O8 | DMSO/H2O | 12 | 80 |
| 4 | 9-Fluorenone | K2S2O8 | DMSO/H2O | 12 | 62 |
| 5 | Ir-PC1 | K2S2O8 | DMSO/H2O | 12 | 56 |
| 6 | Eosin-Y | TBHP | DMSO/H2O | 12 | 61 |
| 7 | Eosin-Y | DTBP | DMSO/H2O | 12 | 21 |
| 8 | Eosin-Y | H2O2 | DMSO/H2O | 12 | Trace |
| 9 | Eosin-Y | Na2S2O8 | DMSO/H2O | 12 | 62 |
| 10 | Eosin-Y | K2S2O8 | DMSO/H2O | 10 | 64 |
| 11 | Eosin-Y | K2S2O8 | DMSO/H2O | 15 | 76 |
| 12 | Eosin-Y | K2S2O8 | DMSO | 12 | 56 |
| 13 | Eosin-Y | K2S2O8 | DCE | 12 | 49 |
| 14 | Eosin-Y | K2S2O8 | CH3CN | 12 | 38 |
| 15 | Eosin-Y | K2S2O8 | DMF | 12 | 25 |
| 16 | Eosin-Y | K2S2O8 | CH2Cl2 | 12 | 23 |
| 17 | Eosin-Y | K2S2O8 | MeOH | 12 | 29 |
| 18 | Eosin-Y | K2S2O8 | EtOH | 12 | 35 |
| 19c | — | K2S2O8 | DMSO/H2O | 10 | 56 |
Having the optimized reaction conditions in hand, as indicated in Table 1 entry 3, the scope of this dehydrazinative C–H acylation was then explored with various N-heterocycles. A variety of N-heterocycles, including isoquinoline, quinoline, phenanthridine, dihydroacridine, quinoxaline and phthalazine, underwent dehydrazinative C–H acylation to produce acylated heterocycles with moderate to high yields, as shown in Fig. 3. High regioselectivity was observed in the case of quinazoline (9), quinoxaline (10) and acridine (13) while in the case of phthalazine, a monoacylated compound (7) was detected with 56% yield along with a diacylated product (8) with 21% yield. The benzothiazole moiety may also be expanded using this protocol, furnishing its acylated product (14) with 54% yield. 2-Methylquinoline showed effective coupling with aliphatic hydrazide to give the acylated product (15) with 52% yield under optimized reaction conditions. No acylated product is formed in the case of pyridine and pyrazine under this protocol; however, tert-butyl pyridine furnished the acylated product with 65% yield (16a).
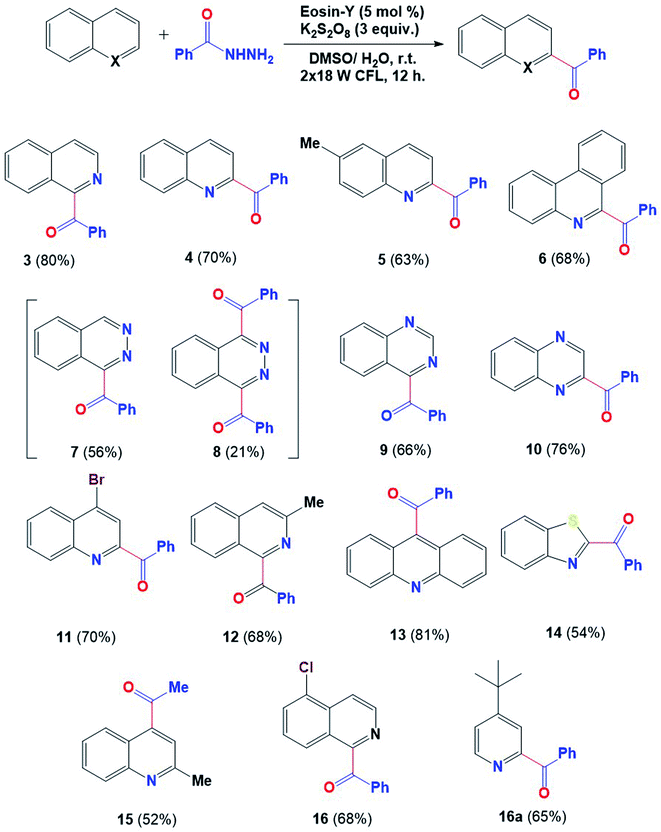 | ||
Fig. 3 Scope of heteroarenes. aReaction conditions: benzoyl hydrazine (0.6 mmol), heteroarene (0.2 mmol), oxidant (0.6 mmol), solvent DMSO/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 2 mL under 36 W (2 CFL of 18 W each). 1) 2 mL under 36 W (2 CFL of 18 W each). | ||
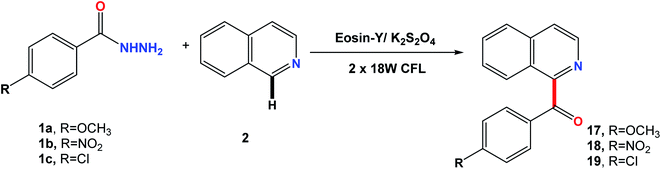
Extending the scope of the reaction with respect to the phenyl ring of 1 substituted with electron donating and withdrawing groups (1a–1c) showed that it worked well and could tolerate various substituents on the ring to afford 17–19. The reaction proceeded with good selectivity and yields, using the optimized conditions mentioned above (Fig. 4).
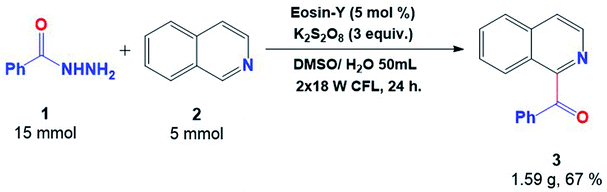
The reaction was performed on a large scale in order to elaborate the utility and applicability of this protocol. The gram-scale reaction proceeded effectively well towards the synthesis of the acylated product (3) with 67% yield, clearly indicating its ability to be useful in the protocol (Fig. 5).
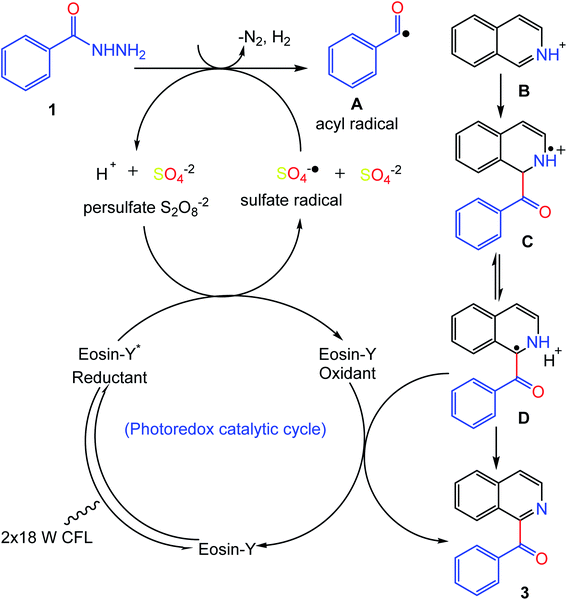
Based on and in comparison with previous literature reports,22,29,30,32 a proposed reaction mechanism for this photocatalytic transformation was developed and is shown in Fig. 6. Possibly, the reaction starts with the excitation of the photocatalyst which undergoes single electron transfer (SET) in the presence of the persulfate salt, generating the sulfate radical anion (SO4−˙) and (eosin-Y oxidant). Meanwhile, benzoyl hydrazine (1) undergoes a removal of nitrogen and hydrogen followed by hydrogen atom transfer (HAT) with the sulfate radical anion (SO4−˙) to produce the acyl radical (A). Consequently, the acyl radical couples with the protonated heteroarene B to give the intermediates C and D. The final product 3 is produced by SET between intermediate D and the (eosin-Y oxidant), thus completing the photo-redox catalytic cycle by regeneration of the photocatalyst.
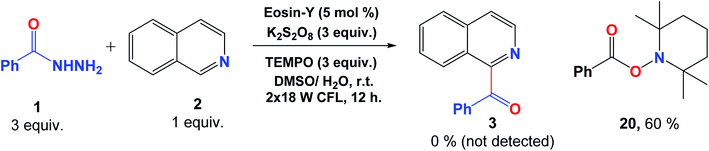
To confirm the proposed mechanism of this photocatalytic transformation, a reaction between benzoyl hydrazine (1) and isoquinoline (2) was conducted in the presence of the radical scavenger TEMPO. The desired acylated product 3 was not observed while the radical capture product 2,2,6,6-tetramethylpiperidino benzoate (20) was detected with 60% yield (Fig. 7). This observation strongly supports the proposal of the formation of radical intermediates in this reaction.
Conclusion
In conclusion, we have developed a visible light-induced acylation of N-heterocycles using benzoyl hydrazides as an efficient acyl source under mild reaction conditions. Photo-redox catalyzed oxidative cleavage of hydrazides leads to in situ formation of acyl radicals, which subsequently couple with various N-heterocycles to produce acylated products. This synthetic strategy performs the classic Minisci reaction in an eco-friendly and greener way with functional group tolerance and regioselectivity. Control experiments confirm the radical pathway for this transformation.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
I am grateful to Chinese Academy of Sciences and the World Academy of Sciences (CAS-TWAS) for their financial support.References
- J. D. Keasling, A. Mendoza and P. S. Baran, Synthesis: A Constructive Debate, Nature, 2012, 492, 188–189 CrossRef CAS PubMed.
- J. Mulzer, Trying to Rationalize Total Synthesis, Nat. Prod. Rep., 2014, 31, 595–603 RSC.
- K. C. Nicolaou and J. S. Chen, The Art of Total Synthesis through Cascade Reactions, Chem. Soc. Rev., 2009, 38, 2993–3009 RSC.
- R. W. Hoffmann, Natural Product Synthesis: Changes over Time, Angew. Chem., Int. Ed., 2013, 52, 123–130 CrossRef CAS PubMed.
- I. S. Young and P. S. Baran, Protecting-Group-Free Synthesis as an Opportunity for Invention, Nat. Chem., 2009, 1, 193–205 CrossRef CAS PubMed.
- A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. Belser and A. von Zelewsky, Coord. Chem. Rev., 1988, 84, 85 CrossRef CAS.
- I. Paterson and E. A. Anderson, The Renaissance of Natural Products as Drug Candidates, Science, 2005, 310, 451–453 CrossRef PubMed.
- C. Grondal, M. Jeanty and D. Enders, Organocatalytic Cascade Reactions as a New Tool in Total Synthesis, Nat. Chem., 2010, 2, 167–178 CrossRef CAS PubMed.
- J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Acc. Chem. Res., 2016, 49(9), 1911–1923 CrossRef CAS PubMed.
- L. Wu, H. Ling, L. Li, L. Jiang and M. He, J. Pharm. Pharmacol., 2007, 59, 695–701 CrossRef CAS PubMed.
- J. Kamei, Pulm. Pharmacol., 1996, 9, 349–356 CrossRef CAS PubMed.
- Y.-F. Zhu, X.-J. Liu, Z.-X. Lu, Q. Xie and N. Ling, J. Med. Chem., 2001, 44, 4001–4010 CrossRef PubMed.
- F. Minisci, R. Bernardi, F. Bertini, R. Galli and M. Perchinummo, Tetrahedron, 1971, 27, 3575–3579 CrossRef CAS.
- F. Fontana, F. Minisci, M. C. Nogueira Barbosa and E. Vismara, J. Org. Chem., 1991, 56, 2866–2869 CrossRef CAS.
- Y. Siddaraju and K. R. Prabhu, Tetrahedron, 2016, 72, 959–967 CrossRef CAS.
- J. Chen, M. Wan, J. Hua, Y. Sun, Z. Lv, W. Li and L. Liu, Org. Biomol. Chem., 2015, 13, 11561–11566 RSC.
- Y. Siddaraju, M. Lamani and K. R. Prabhu, J. Org. Chem., 2014, 79, 3856–3865 CrossRef CAS PubMed.
- R. Sharma, M. Abdullaha and S. B. Bharate, J. Org. Chem., 2017, 82, 9786–9793 CrossRef CAS PubMed.
- M. Adib, R. Pashazadeh, S. Rajai-Daryasarei, R. Kabiri and S. Gohari, Synlett, 2016, 27, 2241–2245 CrossRef CAS.
- H. Jiang, J. Xie, A. Lin, Y. Cheng and C. Zhu, RSC Adv., 2012, 2, 10496–10498 RSC.
- M. Wan, H. Lou and L. Liu, Chem. Commun., 2015, 51, 13953–13956 RSC.
- W. Ali, A. Behera, S. Guin and B. K. Patel, J. Org. Chem., 2015, 80, 5625–5632 CrossRef CAS PubMed.
- N. R. Chaubey and K. N. Singh, Tetrahedron Lett., 2017, 58, 2347–2350 CrossRef CAS.
- K. Matcha and A. P. Antonchick, Angew. Chem., Int. Ed., 2013, 125, 2136–2140 CrossRef.
- J. Q. Chen, R. Chang, Y. L. Wei, J. N. Mo, Z. Y. Wang and P. F. Xu, J. Org. Chem., 2018, 83, 253 CrossRef CAS PubMed.
- J. K. Matsui, D. N. Primmer and G. A. Molander, Chem. Sci., 2017, 8, 3512–3522 RSC.
- J. Liu, Q. Liu, H. Yi, C. Qin, R. Bai, X. Qi, Y. Lan and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 502–506 CrossRef PubMed.
- A. G. Condie, J. C. Gonzalez-Go mez and C. R. J. Stephenson, J. Am. Chem. Soc., 2010, 132, 1464–1465 CrossRef CAS PubMed.
- S. Manna and K. R. Prabhu, J. Org. Chem., 2019, 84, 5067–5077 CrossRef CAS PubMed.
- L. Guillemard, F. Colobert and J. W. Delorda, Adv. Synth. Catal., 2018, 360, 4184–4190 CrossRef CAS.
- M. T. Westwood, C. J. C. Lamb, D. R. Sutherland and A.-L. Lee, Org. Lett., 2019, 21, 7119–7123 CrossRef CAS PubMed.
- S. Sultan, M. A. Rizvi, J. Kumar and B. A. Shah, Chem.–Eur. J., 2018, 24, 10617–10620 CrossRef CAS PubMed.
- C. Hou, S. Sun, Z. Liu, H. Zhang, Y. Liu, Q. An, J. Zhao, J. Ma, Z. Sun and W. Chu, Adv. Synth. Catal., 2021, 363, 2806–2812 CrossRef CAS.
- R. H. Verschueren, J. Schmauck, M. S. Perryman, H. L. Yue, J. Riegger, B. S. Chaput, M. Breugst and M. Klussmann, Chem.–Eur. J., 2019, 25, 9088–9097 CrossRef CAS PubMed.
- J. Tsuji, et al., Tetrahedron, 1980, 36, 1311–1315 CrossRef CAS.
- R. Braslau, M. O. Anderson and F. Rivera, et al., Tetrahedron, 2002, 58, 5513–5523 CrossRef CAS.
- Z. Y. He, C. F. Huang and S. K. Tian, Org. Lett., 2017, 19(18), 4850–4853 CrossRef CAS PubMed.
- H. Ni, Y. Li, X. Shi, Y. Pang, C. Jin and F. Zhao, Tetrahedron Lett., 2021, 68, 152915 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07063k |
| This journal is © The Royal Society of Chemistry 2021 |