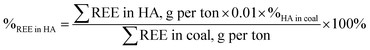Open Access Article
Open Access ArticleConcentrating rare earth elements in brown coal humic acids by mechanochemical treatment
Tatiana Skripkina *a,
Margarita Belokozenko
*a,
Margarita Belokozenko ac,
Svetlana Shatskayaa,
Vera Tikhova
ac,
Svetlana Shatskayaa,
Vera Tikhova b and
Igor Lomovskiy
b and
Igor Lomovskiy a
a
aInstitute of Solid State Chemistry and Mechanochemistry, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, 630128, Russia. E-mail: skripkina.t.s@gmail.com
bN.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry, Siberian Branch of Russian Academy of Science, Russia
cDepartment of Materials Science, Lomonosov Moscow State University, 119991 Moscow, Russia
First published on 8th November 2021
Abstract
Coals are now viewed as a promising source of rare earth elements increasingly often. Rare earth elements (REE) are known to occur both in the organic and mineral components of brown coals. This study aims at investigating the applicability of mechanochemical activation for concentrating rare earth elements (including Sc, Y, La and lanthanides) in different brown coal fractions. Mechanochemical activation of brown coal in the absence of reagents, as well as additives of sodium percarbonate, monosodium phosphate, and sodium chloride, was carried out. Mechanochemical activation does not cause degradation of humic acid–REE complexes contained in pristine coal. The REE concentration process in the samples of mechanochemically activated coal can be attributed both to formation of new oxygen-containing groups in humic acids (HA) and to binding of REEs to oxygen-containing groups already contained in coal due to vigorous solid-phase mechanical mixing. A method for mechanochemical activation of coal, which allows one to transfer up to 93 ± 7% REEs into the organic alkali-soluble fraction – the HA fraction (while HA in the pristine coal contain only 38 ± 3% REE) – has been developed. The estimated total concentration of REEs in pristine coal ash is 8000 ppm. The estimated REE content in the ash of the product (HA fraction) is as high as 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 ppm. Concentrations of Ce, Nd and Y in the ash of the product are 6000 ppm, 4200 ppm and 2500 ppm, respectively.
300 ppm. Concentrations of Ce, Nd and Y in the ash of the product are 6000 ppm, 4200 ppm and 2500 ppm, respectively.
Introduction
Rare earth elements (REEs) are highly demanded in the international market and are used for manufacturing several thousand high-tech products.1,2 Until recently, ores were considered to be the main source of REEs; however, at present, coals (including brown coal) are regarded as a promising source of REEs.2–6Brown coal is viewed as a fuel characterized by low degree of coalification (metamorphism). It is rich in organic matter, including humic acids (HAs), which are involved in the accumulation of REEs.7 Humic acids fraction doesn't contain silicates and is completely soluble in alkaline solutions, so it is assumed that REEs bound with humic acids could be more easily released. Humic acids have a very complex structure as they contain many functional groups of different types, including oxygen-, nitrogen-, and sulfur-bearing ones.8,9 Carboxyl, phenolic, and alcohol groups are considered to be the most important moieties being responsible for metal binding.10,11
Mechanochemical activation of coals has been used for solving various tasks for quite a long time.12–15 Intense mechanical loading causes activation of the organic matter in coal and changes in its physicochemical properties.16,17 Solid-phase treatment in the presence of alkalis is the best-studied method for mechanochemical modification of brown coals. This treatment increases the extraction yield of HAs due to the formation of sodium humate via a neutralization reaction.18 Active sites having excess free energy are formed on the coal surface during mechanochemical activation.19,20 Therefore, molecules of different nature (mainly inorganic salts) can be intensively sorbed on the active sites.21 It is known that the contents of phenolic and carboxyl groups in humic acids increase during brown coal oxidation.22,23 Therefore, it is promising to conduct mechanochemical activation in the presence of an oxidizing agent. REEs are known to occur in both the organic and inorganic components of brown coal.24–26 Furthermore, the inorganic matter of coals includes silicate, aluminosilicate, carbonate and phosphate minerals, and these minerals can contain REEs. In terms of their stability, these complexes are comparable to REE–HA complexes.27 The literature does not describe attempts to bind rare-earth elements by humic acids using mechanochemical treatment, just as there is no data on the possibility of mechanochemical degradation of the humic acids–REE complexes contained in coal.
This study aimed to investigate the applicability of mechanochemical activation for concentrating rare earth elements in various brown coal fractions.
Experimental
Materials and methods
Brown coal mined from the Azeiskoe deposit (Eastern Siberia, Russia; 54°28′05.1′′N, 100°40′16.3′′E) with the total content of REEs of (9.7 ± 0.4) × 102 g per ton, sodium hydroxide (chemically pure grade, Khimmed, Russia), sodium percarbonate 2Na2CO3·3H2O2 (CAS 15630-89-4, percarbonate OJSC, Russia), sodium pyrophosphate (pure for analysis grade, Reachem, Russia), hydrochloric acid (chemically pure grade, Khimmed, Russia), monosodium phosphate (pure for analysis grade, Reachem, Russia), sodium chloride (chemically pure grade, Reachem, Russia), and nitric acid (special purity grade, Khimprom, Russia) were used in this study.Ultrasonic treatment was conducted in an ultrasonic bath (Vilitek, Russia) at a frequency of 40 kHz. A Speedwave four microwave sample preparation system with vertical loading and built-in non-contact temperature and pressure sensors (PerkinElmer, USA) was used for sample decomposition before the elemental analysis. The elemental analysis was conducted on an Agilent 7500a inductively coupled plasma quadrupole mass spectrometer (Agilent Technologies, USA). Water was deionized using a Direct-Q3 UV water purification system (Millipore, Sigma-Aldrich, Supelco, Germany).
The IR spectra of humic acids were recorded on a Vector 22 IR Fourier-transform spectrometer (Bruker, USA) in KBr pellets (150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) in the wavelength range of 400–4000 cm−1. Elemental analysis was carried out using automatic elemental CHNS-analyzer mod. EA3000 (Eurovector Instruments, Italy). The procedure described in ref. 28. The carbon, hydrogen, nitrogen, and oxygen contents were corrected to the ash-free basis.
1 ratio) in the wavelength range of 400–4000 cm−1. Elemental analysis was carried out using automatic elemental CHNS-analyzer mod. EA3000 (Eurovector Instruments, Italy). The procedure described in ref. 28. The carbon, hydrogen, nitrogen, and oxygen contents were corrected to the ash-free basis.
Moisture content in the samples was determined using a WPS 50 SX moisture analyzer (RADWAG, Poland) at 130 °C. Heating was stopped automatically once the content weight had been attained (difference in sample weight being <0.1% for as long as 3 min). Moisture content (18.6%) in the sample was calculated automatically from the final difference in sample weights. Prior to treatment, coal was dried until moisture content in it was 0.7%. Water was removed from coal by drying a weighed sample of coal (100 g) in a drying oven at 95 °C for 24 h. Ash was determined in compliance with the ISO 1171:2010(en) “solid mineral fuels—determination of ash” and was 12.1% in pristine coal.
Mechanochemical activation of coal
Mechanochemical activation of coal was performed using an AGO-2 laboratory-scale planetary-type activator with steel drums and grinding balls equipped with a water cooling system. A 10 g coal sample was placed into the drum to be mechanochemically activated in the absence of reagents (MA). The following agents were used during mechanochemical activation (MCA): Na2CO3·1.5H2O2 (5%), which acted as a solid-phase oxidizing agent;29 NaCl (20%), which was used to shift the equilibrium toward the formation of sodium humate; and Na2HPO4 (20%), which acted as a competitive complexant. The amounts of the reagents added (shown as the percentage of the total weight of the coal-reagents mixture) are given in parentheses. In all the cases, activation took place at the calculated acceleration of the grinding media at the instant of detachment from the grinding chamber walls (20 g); activation time was 5 min, and weight of the mixture sample being activated was 10 g.Extraction of humic acids
Today, there are various methods for extracting HAs from brown coals; however, the conventional procedures are based on dissolving HAs in alkalis, followed by precipitation with a mineral acid.30–32 A weighed sample (∼1 g) of brown coal was placed into a 50 mL polypropylene test tube, and HAs were extracted with 40 mL of 1% NaOH solution in the ultrasonic bath for 15 min. After the extraction, the resulting suspension was centrifuged for 10 min at 6000 rpm. The supernatant (a solution of HAs) was decanted into a separate test tube. For precipitating HAs, 10 wt% HCl was added to the test tube containing the supernatant until pH < 2. At the first extraction stage, 3.0 mL of the acid was added. After the hydrochloric acid had been added, humic acids were separated by centrifugation for 10 min at 6000 rpm. The alkaline extraction procedure was performed twice. The volume of the acid used at the second extraction stage was 3.5 mL.Alkaline extraction was followed by aqueous extraction as the second stage. The fundamental difference was that distilled water was used as an extractant instead of sodium hydroxide. The aqueous extraction procedure was otherwise identical to alkaline extraction, except for the volume of hydrochloric acid being added. The volume of the acid was 1.0 mL at the first stage of aqueous extraction and 0.5 mL, at the second stage.
During the final stage, HAs were washed twice with distilled water supplemented with a small amount of acid (0.1 mL) to prevent the dissolution of HAs in water. These conditions of HA extraction allow one to ensure that concentration of the dissolved salts is <100 mg L−1 as required for performing the inductively coupled plasma mass spectrometry (ICP-MS) analysis.33 The extracted HAs were dried in the drying oven at 95 °C.
Quantification of REEs in humic acids
The contents of REEs in humic acids were determined by ICP-MS. The multi-element calibration standard “Tuning Solution for ICP-MS” (Agilent Technologies, USA) was used for calibrating the ICP-MS. Tuning Solution consisted of nitrates of 5 elements: Li, Y, Ce, Co, Tl in 2% HNO3 with a concentration of each element 10 mg L−1. Different degrees of dilution of the solution under the test was used to minimize the matrix influence. HAs were prepared for the analysis by microwave-assisted decomposition in autoclaves. HAs were decomposed in the presence of nitric acid. Prior to the analysis, a HA sample was ground using an agate mortar until the ultrafine particle size. Samples weighing 0.050 g used for the experiments were placed into autoclaves; 5.00 mL of HNO3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added; and decomposition was performed. After the decomposition, the solution was brought to a volume of 15.0 mL with deionized water. 0.050 mL aliquots of the obtained solution + 5.00 mL of 5 wt% HNO3 were taken, and the measurements were performed.
1) was added; and decomposition was performed. After the decomposition, the solution was brought to a volume of 15.0 mL with deionized water. 0.050 mL aliquots of the obtained solution + 5.00 mL of 5 wt% HNO3 were taken, and the measurements were performed.
The experiments were carried out in at least 4 replicates. Outliers were excluded using Grubbs's test. Confidence intervals were calculated using t-values for P = 0.95.
Results and discussion
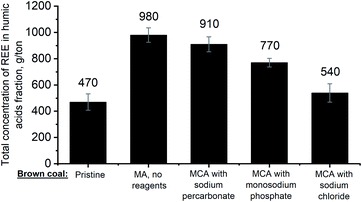
Coal mined from the Azeiskoe deposit is well described in the literature.6,25,34 Besides the high concentration of REE, the coal is characterized by high content of humic acids (77 ± 3%), which bind about 38% REE. Mechanochemical activation increased the extraction yield of humic acids to 82.9 ± 0.1%, 80 ± 1%, and 83 ± 3% in the cases when it was performed in the presence of sodium percarbonate, sodium chloride, and monosodium phosphate, respectively. The increase in the extraction yield of humic acids was most significant (85.5 ± 0.4%) for the sample being mechanochemically activated in the absence of reagents, which can be attributed to the fact that the organic component of coal is partially oxidized by atmospheric oxygen during treatment.Fig. 1 shows the total contents of REEs in the fraction of brown coal humic acids before and after treatment under different conditions.
When an excessive amount of sodium chloride was used as an additive for mechanochemical modification of brown coal, it was expected that the equilibrium would shift toward the formation of sodium humate:
| Hum − COO− + Na+ → Hum − COONa, |
When an excessive amount of monosodium phosphate was used as a reagent for mechanochemical modification of coal, it was expected that REEs would form strong phosphate complexes, and extraction of HAs would yield HAs free of REEs in one fraction and REEs bound into phosphate complexes in the other fraction. The conditional stability constants of the REE–HA complexes (Kc) lie within the range 8.9 < log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc < 16.5 depending on conditions 26. Meanwhile, the Kc for the REE–phosphate complexes belongs to the range of 18.5–25.35
Kc < 16.5 depending on conditions 26. Meanwhile, the Kc for the REE–phosphate complexes belongs to the range of 18.5–25.35
For the samples prepared by mechanochemical activation in the presence of monosodium phosphate, the total content of REEs in HAs was (7.7 ± 0.4) × 102 g per ton, while for the sample prepared from coal treated in the presence of sodium chloride, the total content of REEs was (5.4 ± 0.7) × 102 g per ton. Phosphate ions compete with humic acids and bind to some REEs as evidenced by lower REE content compared to that in other mechanochemically activated samples. The transition of REEs to the humic acid fraction is even less pronounced for coal activated in the presence of an excessive amount of Na+ ions, which indicates that sodium ions protect the acidic functional groups of humic acids during mechanochemical activation and prevent formation of HA–REE complexes. It can be inferred that HA–metal complexes already existing in coal are not ruptured during mechanochemical activation of coal in a planetary ball mill under the conditions used in this study.
Sodium percarbonate was chosen as one of the reagents for mechanochemical activation because this compound has previously proved to be highly efficient during mechanochemical oxidation of humic acids contained in brown coal, which is accompanied by an increase in the number of carboxyl groups.36,37 The enhanced degree of binding of REEs in the fraction of brown coal humic acids after the treatment of coal in the presence of sodium percarbonate can be attributed to the fact that REEs form complexes with the newly formed carboxyl groups.
Mechanochemical activation of coal in the presence of sodium percarbonate increased the total content of REEs in HAs to (9.1 ± 0.6) × 102 g per ton. It is also worth mentioning that the resulting values are comparable to those obtained when treatment was performed in a ball mill without any additional reagents used, where the total content of REEs was (9.8 ± 0.6) × 102 g per ton.
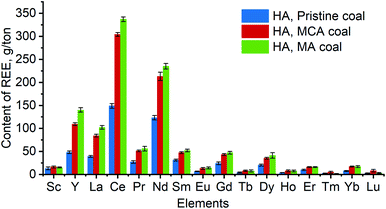
Since the samples of coal mechanochemically activated in the presence of sodium percarbonate in the absence of any additives are the most interesting ones in terms of REE concentration, they were used in further studies: these samples were compared to HAs isolated from the pristine brown coal for which the total REE content was (4.7 ± 0.6) × 102 g per ton (Fig. 1). Fig. 2 shows clearly that mechanochemical activation of brown coal makes REEs concentrate in the HA fraction. Mechanochemical activation of coal without any additional reagents causes even greater degree of REE concentration in HAs.
Table 1 summarizes the data on the percentages of all REEs contained in HAs before and after mechanochemical activation. The percentage of REEs in HAs was calculated using the following formula:
| For pristine coal | For MCA coal | For MA coal |
|---|---|---|
| 38 ± 3 | 80 ± 7 | 93 ± 7 |
The reported findings demonstrate that mechanochemical activation without additions makes REEs concentrate (2.5 ± 0.2)-fold, while mechanochemical activation in the presence of sodium percarbonate causes REE concentration (2.1 ± 0.2)-fold. This fact indicates that it is sufficient to perform mechanochemical activation without using any additional reagents to concentrate REEs in HAs.
Table 2 shows the results of CHN analysis (on an ash-free basis) of the coal samples (pristine and mechanically activated ones, as well as the sample mechanochemically activated in the presence of sodium percarbonate), and the corresponding humic acids.
| Sample | C, %mass | H, %mass | N, %mass | H/C | O/C | N/C |
|---|---|---|---|---|---|---|
| Coal (pristine) | 62.4 ± 0.1 | 4.22 ± 0.03 | 1.10 ± 0.02 | 0.81 ± 0.01 | 0.39 ± 0.00 | 0.02 ± 0.00 |
| Coal (MA) | 60.0 ± 0.2 | 4.1 ± 0.4 | 0.93 ± 0.08 | 0.81 ± 0.08 | 0.44 ± 0.00 | 0.01 ± 0.00 |
| Coal (MCA) | 62.0 ± 0.2 | 4.33 ± 0.08 | 1.16 ± 0.03 | 0.84 ± 0.01 | 0.39 ± 0.01 | 0.02 ± 0.00 |
| HAs (pristine coal) | 58.5 ± 0.1 | 3.65 ± 0.01 | 1.21 ± 0.02 | 0.74 ± 0.00 | 0.47 ± 0.00 | 0.02 ± 0.00 |
| HAs (MA coal) | 56.8 ± 0.3 | 3.64 ± 0.05 | 1.13 ± 0.06 | 0.76 ± 0.01 | 0.51 ± 0.00 | 0.02 ± 0.00 |
| HAs (MCA coal) | 58.0 ± 0.3 | 3.71 ± 0.02 | 1.10 ± 0.01 | 0.76 ± 0.01 | 0.48 ± 0.01 | 0.02 ± 0.00 |
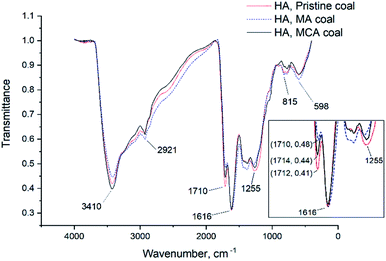
For the MA samples (of coal and HAs), the O/C ratio is higher than that for the pristine samples (of coal and HAs), which indicates that the pristine samples were enriched in oxygen during mechanochemical modification. This can be largely attributed to the increase in the number of carboxyl, phenolic, and hydroxyl groups within the HA structure. In order to test this assumption, we analyzed all the coal and HA samples by IR spectroscopy (Fig. 3 and 4).
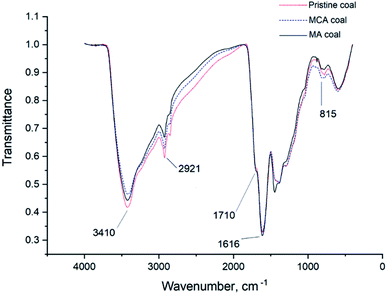 | ||
| Fig. 3 The IR spectra of the pristine coal, coal subjected to mechanochemical activation with Na2CO3·1.5H2O2 (MCA), and mechanochemically activated without using any additional reagents (MA) coal. | ||
One can see that the band at 1710 cm−1 corresponding to carboxyl groups38 in the IR spectra of different HA samples decrease in the following series: HAs in pristine coal → HAs in coal mechanochemically activated in the presence of sodium percarbonate → HAs in coal mechanochemically activated without any reagents added. The reduced number of carboxyl groups can demonstrate that these groups can become bound as a result of mechanochemical activation. Since the total content of REEs for HAs in pristine coal is lower than the total content of REEs for HAs in mechanochemically activated coal, it is fair to assume that carboxyl groups bind mainly to REEs. This process leads to formation of REE complexes with humic acids characterized by high stability constants. The resulting complexes are so stable that they are not degraded during HA extraction from brown coals, so REEs are extracted together with HAs.
The CHN analysis has shown that the MA samples (of both coal and HAs) are rich in oxygen. However, the contents of unbound carboxyl and hydroxyl groups in the IR spectra of these samples are not increased, thus indicating that these groups exist in the bound state. We know that REEs have formed complexes with humic acids both in the MA and MCA coal, but this phenomenon was more pronounced for the MA sample. The reason can be that mechanochemical activation has yielded new oxygen-containing groups that have contributed to better binding of REEs by this sample.
The described method of mechanochemical treatment is easily scalable to machines with a capacity of up to ton per hour.12,39 The product of this treatment, MA brown coal, contains about 86% of humic acids extracted by alkaline extraction. One of the main features of humic acids fraction is that it doesn't contain silicates and it's 100% soluble at pH > 9. REE bound with humic acids could be more easily released during combustion and subsequently incorporated into or adsorbed onto the fly ash.40 The ash content of humic acids extracted from MA brown coal is 5.67%. The estimated total concentration of REEs in pristine coal ash is 8000 g per ton. The estimated REE content in the ash of humic acids in MA coal is as high as 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g per ton. Cerium concentration in this ash is about 6000 g per ton. For comparison, the Clarke value of cerium in coal ash is 130 g per ton. Concentrations of the critical REEs, Nd and Y,41 in terms of the ash of the humic acids extracted from MA coal are 4200 g per ton and 2500 g per ton, respectively; the Clarke values of Nd and Y in coal ash are 67 and 51 g per ton, respectively.42 The REE content in fly ash higher than 1000 g per ton is currently considered a quite high content that is of economic importance.43,44 The organic phase bound form of REE opens up various possibilities for their recovery, in addition to the usual ashing: isolation under controlled heating and combustion,45,46 biosorption5,47,48 and more specific leaching methods.4
300 g per ton. Cerium concentration in this ash is about 6000 g per ton. For comparison, the Clarke value of cerium in coal ash is 130 g per ton. Concentrations of the critical REEs, Nd and Y,41 in terms of the ash of the humic acids extracted from MA coal are 4200 g per ton and 2500 g per ton, respectively; the Clarke values of Nd and Y in coal ash are 67 and 51 g per ton, respectively.42 The REE content in fly ash higher than 1000 g per ton is currently considered a quite high content that is of economic importance.43,44 The organic phase bound form of REE opens up various possibilities for their recovery, in addition to the usual ashing: isolation under controlled heating and combustion,45,46 biosorption5,47,48 and more specific leaching methods.4
Conclusions
It has been demonstrated that REE complexes with HAs contained in pristine coal are not decomposed during mechanochemical activation. Mechanochemical activation makes REE redistribute towards formation of their complexes with HAs. Mechanochemical activation of coal in the absence of any additional reagent allows one to transfer up to 93 ± 7% REEs into the HA fraction, while the HAs in pristine coal contain only 38 ± 3% REEs. In other words, REEs get concentrated in the HA fraction (2.5 ± 0.2)-fold during mechanochemical activation of coal without additional reagents. Mechanochemical activation of coal in the presence of sodium percarbonate results in transfer of 80 ± 7% REEs to the HA fraction; i.e., REEs in HA are concentrated (2.1 ± 0.2)-fold. Therefore, for concentrating REEs in HAs, it is sufficient to conduct mechanochemical activation without using additional reagents.The process of REE concentration in mechanochemically activated coal samples can be attributed both to the formation of new oxygen-containing groups in HAs, which contribute to better binding of REEs by these samples, and to the fact that REEs bind to the already existing oxygen-containing groups due to vigorous mixing.
It has also been shown that during mechanochemical activation of coal in the presence of monosodium phosphate, phosphate ions compete with HAs and also form complexes with REEs as evidenced by the lower REE content in HA fraction compared to that in other mechanochemically activated samples. The transfer of REEs to the HA fraction is even less pronounced for coal subjected to mechanochemical activation in the presence of an excessive amount of sodium chloride. This demonstrates that sodium ions protect the acidic groups in HAs and prevent formation of their complexes with REEs during mechanochemical activation. Therefore, when mechanochemical activation is performed in the presence of monosodium phosphate and sodium chloride, the process of REE concentration in HAs becomes less intense; i.e., REEs contained in the mineral component of coal are transferred to the HA fraction only partially.
A novel promising method for concentrating REEs that allows one to process metal-bearing coals more efficiently has been demonstrated. The fact that humic acids bind REE even from the mineral components of coal during one-step mechanochemical treatment makes it promising to study the possibility of such concentration when processing other metal-bearing coals with humic acids.
Author contributions
Tatiana Skripkina: writing – original draft, review & editing, conceptualization, methodology, investigation. Margarita Belokozenko: writing – original draft, review & editing, formal analysis, investigation. Svetlana Shatskaya: writing – review & editing, formal analysis, investigation. Vera Tikhova: writing – review & editing, investigation. Igor Lomovskiy: writing – review & editing, conceptualization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Mechanochemical treatment of brown coals and chemical analysis (except for the ICP-MS, CHNO, and IR spectrometry) were funded by the Russian Foundation for Basic Research and the Government of the Novosibirsk region (project no. 20-43-543019). ICP-MS, CHNO and IR spectrometry analysis of humic acids and brown coal were funded by the Russian Science Foundation (project no. 21-13-00046). Authors would like to acknowledge the Multi-Access Chemical Research Center SB RAS for spectral and analytical measurements.Notes and references
- G. Charalampides, K. I. Vatalis, B. Apostoplos and B. Ploutarch-Nikolas, Procedia Economics and Finance, 2015, 24, 126–135 CrossRef.
- V. Balaram, Geosci. Front., 2019, 10, 1285–1303 CrossRef CAS.
- S. Chehreh Chelgani, E. Hadavandi and J. C. Hower, Energy Sources, Part A, 2021, 43, 70–79 CrossRef CAS.
- M. Peiravi, F. Dehghani, L. Ackah, A. Baharlouei, J. Godbold, J. Liu, M. Mohanty and T. Ghosh, Mining Metall. Explor., 2021, 38, 1–26 Search PubMed.
- M. Alipanah, D. M. Park, A. Middleton, Z. Dong, H. Hsu-Kim, Y. Jiao and H. Jin, ACS Sustainable Chem. Eng., 2020, 8, 17914–17922 CrossRef CAS.
- S. I. Arbuzov, R. B. Finkelman, S. S. Il'enok, S. G. Maslov, A. M. Mezhibor and M. G. Blokhin, Solid Fuel Chem., 2019, 53, 1–21 CrossRef CAS.
- D. A. Laudal, S. A. Benson, R. S. Addleman and D. Palo, Int. J. Coal Geol., 2018, 191, 112–124 CrossRef CAS.
- I. V. Perminova and K. Hatfield, in Use of Humic Substances to Remediate Polluted Environments: From Theory to Practice, Springer-Verlag, Berlin/Heidelberg, 2005, pp. 3–36 Search PubMed.
- M. Schnitzer, in Soil Organic Matter, 1978, pp. 1–58 Search PubMed.
- E. Tipping, Cation Binding by Humic Substances, Cambridge, 2002 Search PubMed.
- G. R. Aiken, H. Hsu-Kim and J. N. Ryan, Environ. Sci. Technol., 2011, 45, 3196–3201 CrossRef CAS PubMed.
- I. Lomovskiy, A. Bychkov, O. Lomovsky and T. Skripkina, Molecules, 2020, 25, 5345 CrossRef CAS PubMed.
- H. Ji, X. Mi, Q. Tian, C. Liu, J. Yao, S. Ma and G. Zeng, Sci. Total Environ., 2021, 784, 147100 CrossRef CAS PubMed.
- A. P. Burdukov, E. B. Butakov and G. V. Chernova, J. Eng. Thermophys., 2020, 29, 492–502 CrossRef CAS.
- B. Szczęśniak, S. Borysiuk, J. Choma and M. Jaroniec, Mater. Horiz., 2020, 7, 1457–1473 RSC.
- A. G. Proidakov, Solid Fuel Chem., 2009, 43, 9–14 CrossRef.
- S. Mateti, M. Mathesh, Z. Liu, T. Tao, T. Ramireddy, A. M. Glushenkov, W. Yang and Y. I. Chen, Chem. Commun., 2021, 57, 1080–1092 RSC.
- A. V. Savel'eva, A. A. Ivanov, N. V. Yudina, O. I. Lomovsky and D. Dugarzhav, Russ. J. Appl. Chem., 2013, 86, 552–557 CrossRef.
- T. Skripkina, A. Ulihin, A. Bychkov, S. Mamylov, E. Podgorbunskikh, I. Lomovskiy and O. Lomovsky, RSC Adv., 2020, 10, 21108–21114 RSC.
- A. V. Savel'eva, A. A. Ivanov, N. V. Yudina and O. I. Lomovskii, Solid Fuel Chem., 2015, 49, 201–205 CrossRef.
- S. L. Khil'ko, M. I. Rogatko, R. A. Makarova and R. G. Semenova, Colloid J., 2020, 82, 746–757 CrossRef.
- A. G. Proidakov and G. A. Kalabin, Solid Fuel Chem., 2009, 43, 86–93 CrossRef.
- T. Skripkina, A. Bychkov, V. Tikhova, B. Smolyakov and O. Lomovsky, Environ. Technol. Innovation, 2018, 11, 74–82 CrossRef.
- S. Dai, Y. Jiang, C. R. Ward, L. Gu, V. V. Seredin, H. Liu, D. Zhou, X. Wang, Y. Sun, J. Zou and D. Ren, Int. J. Coal Geol., 2012, 98, 10–40 CrossRef CAS.
- S. I. Arbuzov, I. Y. Chekryzhov, R. B. Finkelman, Y. Z. Sun, C. L. Zhao, S. S. Il'enok, M. G. Blokhin and N. V. Zarubina, Int. J. Coal Geol., 2019, 206, 106–120 CrossRef CAS.
- P. Duan, W. Wang, X. Liu, S. Sang, M. Ma and W. Zhang, Int. J. Coal Geol., 2019, 207, 1–11 CrossRef CAS.
- J. E. Sonke and V. J. M. Salters, Geochim. Cosmochim. Acta, 2006, 70, 1495–1506 CrossRef CAS.
- V. P. Fadeeva, V. D. Tikhova and O. N. Nikulicheva, J. Anal. Chem., 2008, 63, 1094–1106 CrossRef CAS.
- T. S. Skripkina, A. L. Bychkov, V. D. Tikhova and O. I. Lomovsky, Solid Fuel Chem., 2018, 52, 356–360 CrossRef CAS.
- N. Q. Arancon, C. A. Edwards, S. Lee and R. Byrne, Eur. J. Soil Biol., 2006, 42, S65–S69 CrossRef CAS.
- F. de Souza and S. R. Bragança, J. Mater. Res. Technol., 2018, 7, 254–260 CrossRef CAS.
- L. Zhou, L. Yuan, B. Zhao, Y. Li and Z. Lin, PLoS One, 2019, 14, e0217469 CrossRef CAS PubMed.
- D. Beauchemin, Anal. Chem., 2008, 80, 4455–4486 CrossRef CAS PubMed.
- S. I. Arbuzov, A. M. Mezhibor, D. A. Spears, S. S. Ilenok, M. V. Shaldybin and E. V. Belaya, Int. J. Coal Geol., 2016, 153, 99–111 CrossRef CAS.
- K. W. Goyne, S. L. Brantley and J. Chorover, Chem. Geol., 2010, 278, 1–14 CrossRef CAS.
- A. McKillop and W. R. Sanderson, Tetrahedron, 1995, 51, 6145–6166 CrossRef CAS.
- L. Doskočil, L. Grasset, D. Válková and M. Pekař, Fuel, 2014, 134, 406–413 CrossRef.
- L. Celi, M. Schnitzer and M. Nègre, Soil Sci., 1997, 162, 189–197 CrossRef CAS.
- E. G. Trofimova, E. M. Podgorbunskikh, T. S. Skripkina, A. L. Bychkov and O. I. Lomovsky, Bulg. Chem. Commun, 2018, 50, 45–48 Search PubMed.
- Z. Wang, S. Dai, J. Zou, D. French and I. T. Graham, Int. J. Coal Geol., 2019, 203, 1–14 CrossRef CAS.
- L. Lefticariu, K. L. Klitzing and A. Kolker, Int. J. Coal Geol., 2020, 217, 103327 CrossRef CAS.
- M. P. Ketris and Y. E. Yudovich, Int. J. Coal Geol., 2009, 78, 135–148 CrossRef CAS.
- W. Zhang, M. Rezaee, A. Bhagavatula, Y. Li, J. Groppo and R. Honaker, Int. J. Coal Prep. Util., 2015, 35, 295–330 CrossRef CAS.
- Ž. Fiket, G. Medunić, M. Furdek Turk and G. Kniewald, Int. J. Coal Geol., 2018, 194, 1–10 CrossRef.
- P. Liu, L. Yang, Q. Wang, B. Wan, Q. Ma, H. Chen and Y. Tang, Int. J. Coal Geol., 2020, 219, 103371 CrossRef CAS.
- J. Widmer, J. Nakano, A. Nakano and J. Bennett, Int. J. Coal Geol., 2021, 235, 103688 CrossRef CAS.
- D. Park, A. Middleton, R. Smith, G. Deblonde, D. Laudal, N. Theaker, H. Hsu-Kim and Y. Jiao, Sep. Purif. Technol., 2020, 241, 116726 CrossRef CAS.
- N. Das and D. Das, J. Rare Earths, 2013, 31, 933–943 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |