Open Access Article
Open Access ArticleDefect induced electrocatalytic hydrogen properties of pentagonal PdX2 (X = S, Se)†
Jingjing Lia,
Dan Liang *a,
Gang Liu
*a,
Gang Liu *a,
Baonan Jiaa,
Jingyu Caoa,
Jinbo Haob and
Pengfei Lu
*a,
Baonan Jiaa,
Jingyu Caoa,
Jinbo Haob and
Pengfei Lu a
a
aState Key Laboratory of Information Photonics and Optical Communications, School of Electronic Engineering, Beijing University of Posts and Telecommunications, Beijing 100876, China. E-mail: liangdan@bupt.edu.cn; liu_g@126.com
bSchool of Science, Xi'an University of Architecture and Technology, Xi'an 710055, Shaanxi, China
First published on 30th November 2021
Abstract
Searching for catalysts of hydrogen evolution reaction (HER) that can replace Pt is critical. Here, we investigated the HER electrocatalytic activity of pentagonal PdS2 (penta-PdS2) and PdSe2 (penta-PdSe2) by first-principles calculations. Three types of vacancies (VS/Se, VPd, DVS/Se) were constructed to activate the inert basal planes of PdS2 and PdSe2. The results show that S/Se and Pd vacancies significantly improve HER performance, and the Gibbs free energy (ΔGH) of systems can be further regulated by vacancy concentration. Particularly, PdS2 with 2.78% VS, 50% VPd and PdSe2 with 12.5% VSe display the optimal ΔGH value and the highest exchange current density. Further analysis of charge transfer and band structures were described that the introduce of vacancies efficiently regulates the electronic properties, resulting in the diminution of bandgap, and accelerates the charge transfer, thereby contributing to an enhanced electron environment for HER process. Our results provide a theoretical guidance for the applications of pentagonal transition-metal dichalcogenides as catalysts of hydrogen evolution reaction.
Introduction
Currently, pollution and the finite nature of fossil fuels make it extremely important to develop sustainable, recyclable and clean energy sources.1–4 Hydrogen energy, prepared by hydrogen evolution reaction (HER), has attracted a lot of attention as an environmentally friendly and sustainable energy source.5–14 Platinum (Pt), with minimal over-potential and slightly negative hydrogen adsorption free energy (ΔGH), is considered to the best catalyst of HER.15,16 However, the scarcity and high price of Pt limit its large-scale use. Therefore, it is urgent to develop abundant and inexpensive catalysts of HER that can replace Pt.17–22Two-dimensional (2D) transition-metal dichalcogenides (TMDs) have attracted wide attention because of rich content, low price, high stability and high catalytic activity.23–32 Many efforts have been made to use TMDs as alternative catalysts of Pt.33–38 Recently, monolayer palladium diselenide (PdSe2) crystals and palladium disulfide (PdS2) with a novel puckered pentagonal structure have been demonstrated experimentally,39–41 and PdS2 has also shown more stable in pentagonal phase than in 1T phase.42 Compared to the hexagonal structure, the puckered pentagonal structure shows some fascinating characteristics. The pentagonal structure exhibits anisotropy due to buckling breaking the symmetry of the lattice and enhances spin coupling.43 Besides, it has a wide adjustable bandgap, ultra high air stability and high electron mobility.44 Our previous work elucidated that the HER catalytic activity of 1T-MX2 (M = Pt, Pd; X = S, Se, Te) with metal, non-metal atom doping and vacancies.45 Lin et al. explored that pentagonal PdSe2 nanosheets show good HER activity and its active sites are located on boundary atoms.46 Liang et al. demonstrated that oxidized 2D PdSe2 can effectively enhance electronic properties and electrocatalytic activity.47 Even with some initial exploration, our knowledge of pentagonal PdX2 (X = S, Se) is far from adequate, especially to enhance its HER activity by defect design. It's worth to reveal the HER catalytic activity of pentagonal structure to extend the potential replacement of Pt.
Herein, on the basis of first-principles calculations, we constructed penta-PdS2, -PdSe2 and introduced three types of vacancies in pristine systems to explore adsorption sites, electrocatalytic performance, vacancy concentration and the origin of improved HER activity. Three types of vacancies include S/Se vacancy (VS/Se), Pd vacancy (VPd) and double S/Se vacancies (DVS/Se). We found that the HER activity can be significantly improved by vacancies, and vacancy concentration efficiently regulates the electrocatalytic performance. The origin of HER activity enhancement was elucidated by electronic properties and charge transfer.
Computational details
The computations were performed by using Vienna ab initio simulation package (VASP) based on density functional theory (DFT).48 The generalized gradient approximation (GGA) of Perdew–Burke–Ernzerhof (PBE) function was adopted to exchange–correction functional energy. The energy cutoff for the plane wave basis was set as 450 eV, and a 4 × 4 × 1 k-point mesh was sampled in Brillouin zones by a Monkhorst–Pack. To avoid the interactions between two layers, we set a 15 Å vacuum space in the z-direction,49 and the effect of spin was also considered. All structures were relaxed until the forces on each atom were less than 0.01 eV Å−1, and 10−5 eV was set to energy convergence criteria for electronic and ionic iterations. The van der Waals (vdW) interaction was considered by using optPBE function.50 Considering that Pd is a rather heavy element, the spin–orbit coupling (SOC) effect was also taken into account in band structure computations. We built a 2 × 2 periodic supercell containing 8 Pd atoms and 16 Se atoms for pristine penta-PdS2 and -PdSe2 to investigate HER catalytic performance. Different vacancy concentrations were considered by removing one S/Se atom from 54 atoms (36 Se), 36 atoms (24 Se), 24 atoms (16 Se), 12 atoms (8 Se), 6 atoms (4 Se) supercells, corresponding to the vacancy concentrations of 2.8%, 4.2%, 6.3%, 12.5% and 25% for VS/Se, respectively. For VPd, the vacancy concentrations of 5.6%, 8.3%, 12.5%, 25% and 50% were constructed by removing one Pd atom from 54 atoms (18 Pd), 36 atoms (24 Pd), 24 atoms (8 Pd), 12 atoms (4 Pd), 6 atoms (2 Pd) supercells, respectively.The hydrogen adsorption energy (ΔEH) is calculated by using the equation:
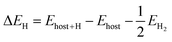 | (1) |
The Gibbs free energy (ΔGH) is a good descriptor of HER electrocatalytic performance, which can be expressed as:
| ΔGH = ΔEH + ΔEZPE − TΔS | (2) |
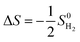 , where
, where 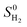 is the entropy of H2 gas molecule.51 Therefore, the ΔGH can be simplified to ΔGH = ΔEH + 0.164 eV.
is the entropy of H2 gas molecule.51 Therefore, the ΔGH can be simplified to ΔGH = ΔEH + 0.164 eV.
Results and discussion
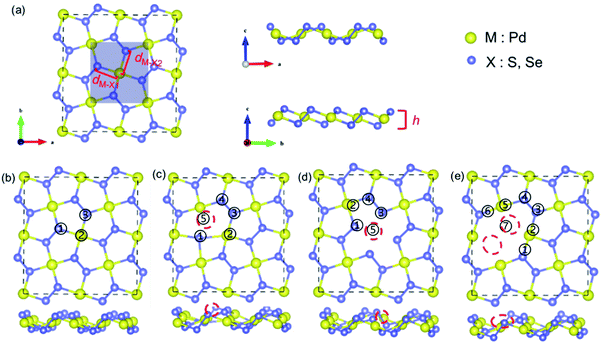
The geometric structure of pentagonal PdX2 (X = S, Se) consists of X–M–X three-atom-thick layers, in which M layer is sandwiched with two X layers, as shown in Fig. 1a. Each M atom binds four X atoms in pentagonal phase, and each unit cell contains two M atoms and four X atoms (highlighted by shaded area in Fig. 1a). The space group is P21/c (no. 14), which exhibits a smaller symmetry compared to the common 2H- and 1T-phase. The lattice parameters that we computed are a = 5.46 Å, b = 5.56 Å for PdS2, and a = 5.71 Å, b = 5.88 Å for PdSe2, agreeing well with previous theoretical studies (a = 5.47 Å, b = 5.57 Å for PdS2, a = 5.74 Å, b = 5.91 Å for PdSe2),52 and experimental values of penta-PdSe2 (a = 5.75 Å, b = 5.87 Å).39 The unique puckering structure allows for the existence of two different metal–nonmetal bonds inside the structure, they are the bond formed by M with X above pucker (M–X1) and the bond formed by M with X below pucker (M–X2), respectively. The bond lengths of M–X1 (dM–X1) and M–X2 (dM–X2) are 2.36 and 2.34 Å in PdS2, 2.49 and 2.47 Å in PdSe2. The difference of bond lengths between M–X1 and M–X2 contributes to the diversity of lattice constants (a, b) along the different orientations in the surface of pentagonal structure. We obtained the vertical puckered distance (h) of the puckering pentagon is 1.29 Å in PdS2 and 1.53 Å in PdSe2, experimentally measured vertical thickness of PdSe2 is 1.6 Å.39For HER on the catalyst under acidic conditions, the first step is the adsorption of H atom via H+ + e− + catalyst → H* − catalyst (Volmer process), where H* denotes H adsorbed to the catalyst. The second step is the release of H2 molecules, which can be described as H* − catalyst + H* − catalyst → H2 + catalyst (Tafel process) or H* − catalyst + H+ + e− → H2 + catalyst (Heyrovsky process). The hydrogen evolution activity of the catalyst can be expressed by the Gibbs free energy (ΔGH). An efficient catalyst means that it has the adsorption and desorption capacity of H and the ability to combine H is neither too strong nor too weak (|ΔGH| ≈ 0). Before exploring the adsorption behavior of H, we firstly determined the most stable adsorption site of H, the calculated adsorption energies (ΔEH) were summarized in Table 1. For pristine PdS2 and PdSe2, three possible adsorption sites were selected, as shown in Fig. 1b, of which site 1 shows the lowest adsorption energy. Considering hydrogen adsorbed at site 1, PdS2 and PdSe2 show ΔGH values of 0.96 eV and 1.03 eV, respectively. The positive and large values of ΔGH indicate that the intrinsic structure does not adsorb H well enough to be catalytically inert. To improve the HER performance, we introduced vacancies in basal plane of PdS2 and PdSe2. The presence of elemental vacancies in materials are generally inevitable. Based on optimized intrinsic structures of PdS2 and PdSe2, we constructed three types of vacancies: a S/Se atom was removed from the surface to form S/Se atom vacancy (VS/Se, Fig. 1c); a Pd atom was removed from the surface to form Pd atom vacancy (VPd, Fig. 1d); two adjacent Se atoms were removed from the surface to form a double Se atom vacancy (DVS/Se, Fig. 1e). Compared with the intrinsic structure, the defective structures display some deformation around the vacancy after optimization, while the deformation is very weak, and the basic structures still maintain.
| Defect type | H-Adsorption sites | ΔEH (eV) | |
|---|---|---|---|
| PdS2 | PdSe2 | ||
| Pristine | 1 | 0.80 | 0.86 |
| 2 | 1.51 | 1.35 | |
| 3 | 0.81 | 1.01 | |
| VS/Se | 1 | −0.32 | 0.01 |
| 2 | — | — | |
| 3 | 0.94 | 0.84 | |
| 4 | 0.62 | 0.97 | |
| 5 | — | — | |
| VPd | 1 | −0.43 | −0.01 |
| 2 | −0.40 | — | |
| 3 | 0.70 | −0.08 | |
| 4 | 0.01 | 0.46 | |
| 5 | −0.52 | 0.72 | |
| DVSe | 1 | 0.20 | 0.50 |
| 2 | — | — | |
| 3 | 0.47 | 0.55 | |
| 4 | 0.37 | 0.97 | |
| 5 | — | — | |
| 6 | — | — | |
| 7 | −0.54 | −0.54 | |
Similarly, we considered the possible H adsorption sites near the vacancy in defective structures. For VS in PdS2 and VSe in PdSe2 (Fig. 1c), site 1 has the lowest adsorption energy (Table 1), and when H is placed at site 2 and 5, H will move to the position of site 1 after optimization. As for VPd in PdS2, site 5 and 1 have the similar and low adsorption energy, and when the initial position of H is site 5, the bond length of the H with Se atom at site 1 is 2.7 Å and with Se atom at site 3 is 3.2 Å after structural relaxation, so we consider site 1 as the most stable H adsorption location for VPd in PdS2. In PdSe2 with VPd, the lowest energy positions are site 3 and site 1, when H is adsorbed at site 3, the H atom will shift to site 1 after structural relaxation, and the bond length formed with the Se atom at site 1 is 2.8 Å, coupled with the fact that the H adsorbed at site 2 will transfer to site 1, which indicates that the site 1 is the most stable adsorption site. For DVS and DVSe, the stable H adsorption sites are only site 1, 3, 4, 7, and site 7 displays the lowest adsorption energy. Summarily, site 1 is the most stable H adsorption site for single-vacancy, site 7 is the most stable H adsorption site for the double-vacancies. Hence, the following discussion is based on site 1 for VS/Se and VPd and site 7 for DVS/Se.
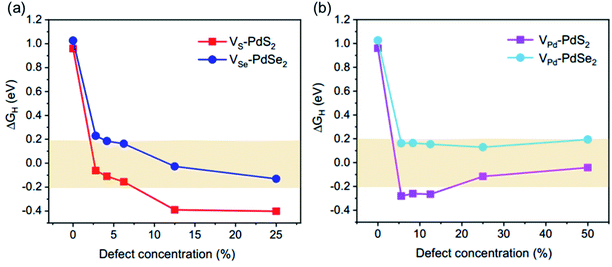
It is obvious that three types of vacancies bring about a drastic decreasing effect in ΔGH, as shown in Fig. 2. We constructed the same vacancy concentration in two systems. For PdS2, the ΔGH of 6.3% VS reduces to −0.16 eV, which indicates an efficient regulation of hydrogen absorption. 12.5% VPd and DVS vacancies more intensely modulate the ΔGH, showing more negative ΔGH values of −0.27 and −0.38 eV, respectively (Fig. 2a). For PdSe2, 6.3% VSe and 12.5% VPd have similar ΔGH values of 0.16 and 0.15 eV (Fig. 2b), DVSe has a largely negative ΔGH value of −0.37 eV and shows the strongest influence on ΔGH. It is noticed that double vacancies give rise to largely negative ΔGH value both in PdS2 and PdSe2. The |ΔGH| value of PdSe2 with metallic vacancy (VPd) is much closer to zero than that of PdS2, displaying a superior HER activity. Considering the approximate |ΔGH| value of systems with VS or VSe, we observe that non-metallic vacancies result in a similar influence on PdS2 and PdSe2.
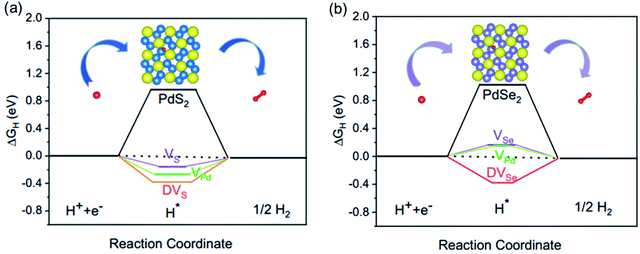 | ||
| Fig. 2 HER free energy diagrams of pristine and defective (a) PdS2 and (b) PdSe2. The illustration represents the adsorption process of H. | ||
We took VS/Se and VPd vacancies to explore the influence of vacancy concentration on ΔGH, 2.8%, 4.2%, 6.3%, 12.5% and 25% vacancy concentrations were constructed for VS/Se, accordingly, 5.6%, 8.3%, 12.5%, 25% and 50% vacancy concentrations for VPd. Considering that the change in supercell size has an effect on the position of the double vacancies, coupled with the existence of interaction between two vacancies, we cannot be sure that it's an isolated effect of the vacancy concentration for the change of ΔGH, so only the single vacancy with different concentrations was adopted in this study. The curves of ΔGH value with different vacancy concentrations were plotted in Fig. 3. The results show that with the increase of VS/Se concentration, ΔGH values display a decrease tendency (Fig. 3a). PdSe2 with VSe exhibits better HER performance than PdS2 with VS. The ΔGH value is more sensitive to low VS/Se concentration, and in the case of high concentration, VS/Se concentration has little effect on the ΔGH. It is also seen that the ΔGH values decrease first and then increase with increasing VPd concentration both in PdS2 and PdSe2 (Fig. 3b). PdSe2 shows good HER activity with VPd concentration in the range between 5.6% and 25%, and PdS2 reaches the optimal ΔGH value (−0.04 eV) at 50% VPd concentration.
In order to intuitively represent the hydrogen evolution activity of the defective structure with different vacancy concentrations, we calculated the exchange current density (i0), which characterized the transfer efficiency of protons from solution to catalyst surface. Under standard conditions (pH = 0 and T = 300 K), if ΔGH < 0, i0 can be calculated by the following formula:
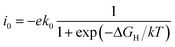 | (3) |
Inversely, if ΔGH > 0, i0 is represented as
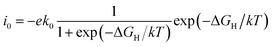 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i0 of −33 and −34 A per site, respectively. The other calculated results were represented by volcano curve plotted in Fig. 4a. Among them, PdS2 with 2.78% VS, 50% VPd and PdSe2 with 12.5% VSe are located at the top of the volcano curve, indicating the optimal ΔGH value and the highest exchange current density (∼10−18 A per site). In particular, PdS2 requires only a small concentration of S vacancy to achieve excellent hydrogen evolution activity. The change of vacancy concentration will lead to the variation of electron environment, and it is important to reveal the inherent charge transfer properties. We calculated the Bader charge of adsorbed H in different vacancy concentrations. As shown in Fig. 4b, there are two cases of charge transfer: when H is adsorbed on PdS2, the electrons are transferred from H to PdS2 in most cases; and when H is adsorbed on PdSe2, the adsorbed H gets electrons from PdSe2. This is because the electronegativity of the atoms connected to H is different, and the electronegativity of S is larger than that of Se, which has an effect on the Bader charge of adsorbed H and thus affects the value of ΔGH. This is also consistent with our previous study.45
i0 of −33 and −34 A per site, respectively. The other calculated results were represented by volcano curve plotted in Fig. 4a. Among them, PdS2 with 2.78% VS, 50% VPd and PdSe2 with 12.5% VSe are located at the top of the volcano curve, indicating the optimal ΔGH value and the highest exchange current density (∼10−18 A per site). In particular, PdS2 requires only a small concentration of S vacancy to achieve excellent hydrogen evolution activity. The change of vacancy concentration will lead to the variation of electron environment, and it is important to reveal the inherent charge transfer properties. We calculated the Bader charge of adsorbed H in different vacancy concentrations. As shown in Fig. 4b, there are two cases of charge transfer: when H is adsorbed on PdS2, the electrons are transferred from H to PdS2 in most cases; and when H is adsorbed on PdSe2, the adsorbed H gets electrons from PdSe2. This is because the electronegativity of the atoms connected to H is different, and the electronegativity of S is larger than that of Se, which has an effect on the Bader charge of adsorbed H and thus affects the value of ΔGH. This is also consistent with our previous study.45
 | ||
| Fig. 4 (a) The exchange current density i0 as a function of ΔGH values. (b) The relationship of ΔGH with Bader charges of adsorbed H in PdS/Se2 with different vacancy concentrations. | ||
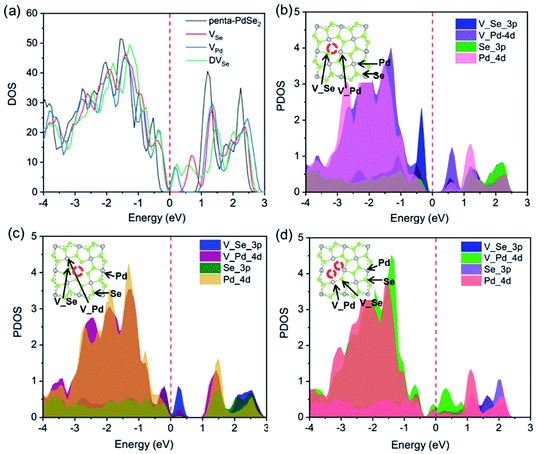
Based on previous investigations, we know that the vacancies give rise to the variation in electronic properties, consequently the improved catalytic performance. As shown in Fig. 5a, the total density of states (TDOS) of defective PdSe2 shows that a large gap state is generated near the Fermi level compared to pristine system, resulting in the bandgap reduction. These new states are attributed to the states resulting from vacancies that are introduced into pristine system. The gap states are beneficial to electron transfer and the conductivity of defective structures and this change may convert the system from being semiconductor to exhibiting metallic properties. We analysed the projected density of states (PDOS) of three defective PdSe2 structures in Fig. 5b–d. It is found that the gap states near the Fermi level are mainly contributed by 4d orbital of Pd and 3p orbital of Se near vacancies. From TDOS and PDOS, it demonstrates that the vacancies effectively modify the electronic structure and increase the occupied states of d and p electrons near the Fermi level, thus affecting the adsorption of H and improving the hydrogen evolution activity.
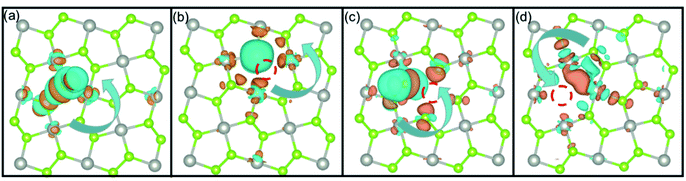
To further explore charge transfer mechanism between defective structures and H, the differential charge transfer density (Δρ(r)) of pristine PdSe2 and three defective structures were calculated. The Δρ(r) is calculated as
| Δρ(r) = ρcat+H(r) − ρcat(r) − ρH(r) | (5) |
The band structures of PdS2 and PdSe2 at different vacancy concentrations were calculated to explore the effect of vacancy concentration on electronic properties. As the SOC has a significant impact on the electronic properties, especially for heavy atoms, we firstly considered the influence of SOC on the band structures of PdS2 and PdSe2, taking 12.5% VS and VSe as an example. Comparing the bands obtained with and without SOC (Fig. S1†), there are splitting bands in the band structures due to the spin–orbit coupling, and the SOC effect slightly alters the bandgap value, a bandgap difference of 0.02–0.03 eV obtained in two cases. While we believe that the SOC effect in PdS2 and PdSe2 is not obvious and especially will not affect the variation trend of electronic structure resulting from the introduction of vacancies, thereby in the subsequent band structure calculations, the SOC is not included.
From the calculated results of band structures (Fig. S2†) and the bandgap values (Table 2) of PdS2 and PdSe2 with different vacancy concentrations, it is found that with the increase of vacancy concentration, the band states would approach to the Fermi level and the position of the valence band maximum (VBM) and the conduction band minimum (CBM) would also change accordingly, thus resulting in the decrease of the bandgap, which leads to the enhancement of the adsorption capacity of H near the vacancy site.
| VS/Se | PdS2 | PdSe2 | VPd | PdS2 | PdSe2 |
|---|---|---|---|---|---|
| 0 | 1.21 | 1.43 | 0 | 1.21 | 1.43 |
| 2.8% | 0.83 | 0.86 | 5.6% | 0.11 | 0.40 |
| 4.2% | 0.71 | 0.74 | 8.3% | — | 0.31 |
| 6.3% | 0.69 | 0.67 | 12.5% | — | 0.31 |
| 12.5% | 0.39 | 0.43 | 25% | — | — |
| 25% | — | — | 50% | — | — |
For PdS2, the intrinsic structure has an indirect bandgap of 1.21 eV, which is consistent with previous studies,53 and after the introduction of S vacancy, the bandgap gradually decreases with the increase of the vacancy concentration, and exhibits metallicity when the VS concentration reaches to 25%. In comparison, PdS2 with VPd displays a smaller bandgap and shows metallic properties as soon as the concentration increases to 8.3%. The intrinsic PdSe2 has an indirect bandgap of 1.43 eV, agreeing well with the experimental value.39 Similarly, the bandgap of PdSe2 with VSe decreases with vacancy concentration and appears metallicity at 25% VSe concentration. For PdSe2 with VPd, it shows metallicity only when the vacancy concentration increases to 25%. It's found that PdS2 with VPd reveals the stronger metallic than PdSe2 under the same VPd concentration, which is also consistent with our calculated HER performance. We noticed that the hydrogen adsorption capacity gradually increases when the bandgap is decreasing, and when the defect concentration reaches a certain value, the bandgap no longer changes and the hydrogen adsorption capacity then changes very little, which is also consistent with our calculated HER activity (Fig. 3). The HER adsorption capacity of VS increases continuously with the defect concentration, while the H adsorption capacity of VPd does not continue to enhance when reaching 5.6% PdS2 and 12.5% PdSe2 and shows a slightly increasing trend. The existence of vacancy efficiently regulates the electronic properties, resulting in the diminution of bandgap, and accelerates the charge transfer, thereby contributing to an enhanced electron environment for HER process.
Conclusions
We explored the HER activity of penta-PdS2 and -PdSe2 by first-principles calculations. The most stable adsorption site of H was determined by comparing the adsorption energy of possible sites. Our results show that the S/Se and Pd vacancies can significantly improve HER performance of PdS2 and PdSe2, and the origin of improvement in HER activity was elucidated through density of states and charge transfer. Moreover, the influence of vacancy concentration on the HER performance was investigated. The ΔGH values display a decrease tendency with the increase of VS/Se concentration, and PdSe2 with VSe exhibits better HER performance than PdS2 with VS. It is also found that the ΔGH values decrease first and then increase with increasing VPd concentration both in PdS2 and PdSe2. PdS2 with 2.78% VS, 50% VPd and PdSe2 with 12.5% VSe are located at the top of the volcano curve, indicating the optimal ΔGH value and the highest exchange current density. Further analysis of Bader charge and band structures were described that the increase of vacancy concentration reduces the bandgap and affects the electron environment. Our results provide a theoretical guidance for electrocatalytic applications of pentagonal transition-metal dichalcogenides.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Open-Foundation of Key Laboratory of Laser Device Technology, China North Industries Group Corporation Limited (No. KLLDT202001), and Fund of State Key Laboratory of IPOC (BUPT), P. R. China (No. IPOC2019ZZ04). We thank for the helpful discussion with Prof. Pengfei Guan and the computational support from the Beijing Computational Science Research Center (CSRC).Notes and references
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed
.
- X. He, S. Luan, L. Wang, R. Wang, P. Du, Y. Xu, H. Yang, Y. Wang, K. Huang and M. Lei, Mater. Lett., 2019, 244, 78–82 CrossRef CAS
.
- B. Hinnemann, P. G. Moses, J. Bonde, K. P. Jørgensen, J. H. Nielsen, S. Horch, I. Chorkendorff and J. K. Nørskov, J. Am. Chem. Soc., 2005, 127, 5308–5309 CrossRef CAS PubMed
.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446–6473 CrossRef CAS PubMed
.
- X. Geng, W. Sun, W. Wu, B. Chen, A. Al-Hilo, M. Benamara, H. Zhu, F. Watanabe, J. Cui and T.-p. Chen, Nat. Commun., 2016, 7, 1–7 Search PubMed
.
- S. Lin, J. C. Liu, W. Z. Li, D. Wang, Y. Huang, C. Jia, Z. W. Li, M. Murtaza, H. Y. Wang, J. N. Song, Z. L. Liu, K. Huang, D. Zu, M. Lei, B. Hong and H. Wu, Nano Lett., 2019, 19, 6853–6861 CrossRef CAS PubMed
.
- W. J. Zou, K. P. Dou, Q. Jiang, J. D. Xiang, C. C. Kaun and H. Tang, RSC Adv., 2019, 9, 39951–39957 RSC
.
- D. Senthilnathan, P. Giunta, V. Vetere, A. Kachmar, P. Maldivi and A. A. Franco, RSC Adv., 2014, 4, 5177–5187 RSC
.
- L. B. Yang, P. Gao, J. H. Lu, W. Guo, Z. Zhuang, Q. Q. Wang, W. J. Li and Z. Y. Feng, RSC Adv., 2020, 10, 20654–20664 RSC
.
- S. C. Chan, Y. L. Cheng, B. K. Chang and C. W. Hong, RSC Adv., 2021, 11, 18500–18508 RSC
.
- M. C. He, F. P. Kong, G. P. Yin, Z. Lv, X. D. Sun, H. Y. Shi and B. Gao, RSC Adv., 2018, 8, 14369–14376 RSC
.
- H. Ogihara, M. Fujii and T. Saji, RSC Adv., 2014, 4, 58660–58663 RSC
.
- Z. Y. Guo, Q. X. Ma, Z. W. Xuan, F. L. Du and Y. Zhong, RSC Adv., 2016, 6, 16730–16735 RSC
.
- L. Wu, X. H. Guo, Y. Xu, Y. F. Xiao, J. W. Qian, Y. F. Xu, Z. Guan, Y. H. He and Y. Zeng, RSC Adv., 2017, 7, 32264–32274 RSC
.
- J. Greeley, I. Stephens, A. Bondarenko, T. Johansson, H. Hansen, T. Jaramillo and J. Rossmeisl, Nat. Chem., 2009, 1, 7 CrossRef PubMed
.
- J. Greeley, T. F. Jaramillo, J. Bonde, I. Chorkendorff and J. K. Nørskov, Nat. Mater., 2006, 5, 909–913 CrossRef CAS PubMed
.
- P. Du and R. Eisenberg, Energy Environ. Sci., 2012, 5, 6012–6021 RSC
.
- E. J. Popczun, J. R. McKone, C. G. Read, A. J. Biacchi, A. M. Wiltrout, N. S. Lewis and R. E. Schaak, J. Am. Chem. Soc., 2013, 135, 9267–9270 CrossRef CAS PubMed
.
- Q. Liu, J. Tian, W. Cui, P. Jiang, N. Cheng, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2014, 53, 6710–6714 CrossRef CAS PubMed
.
- W. Zhong, B. Xiao, Z. Lin, Z. Wang, L. Huang, S. Shen, Q. Zhang and L. Gu, Adv. Mater., 2021, 33, 2007894 CrossRef CAS PubMed
.
- S. Ma, J. Deng, Y. Xu, W. Tao, X. Wang, Z. Lin, Q. Zhang, L. Gu and W. Zhong, J. Energy Chem., 2022, 66, 560–565 CrossRef
.
- D. Wang, D. Zhang, C. Tang, P. Zhou, Z. Wu and B. Fang, Catal. Sci. Technol., 2016, 6, 1952–1956 RSC
.
- J. D. Benck, T. R. Hellstern, J. Kibsgaard, P. Chakthranont and T. F. Jaramillo, ACS Catal., 2014, 4, 3957–3971 CrossRef CAS
.
- A. B. Laursen, S. Kegnæs, S. Dahl and I. Chorkendorff, Energy Environ. Sci., 2012, 5, 5577–5591 RSC
.
- D. Merki and X. Hu, Energy Environ. Sci., 2011, 4, 3878–3888 RSC
.
- P. C. Vesborg, B. Seger and I. Chorkendorff, J. Phys. Chem. Lett., 2015, 6, 951–957 CrossRef CAS PubMed
.
- Q. M. Wang, J. M. Zhang, Z. D. Zhang, Y. N. Hao and K. Bi, Adv. Compos. Hybrid Mater., 2020, 3, 58–65 CrossRef CAS
.
- J. C. Xu, J. Q. Cao, M. H. Guo, S. L. Yang, H. M. Yao, M. Lei, Y. N. Hao and K. Bi, Adv. Compos. Hybrid Mater., 2021, 4, 761–767 CrossRef
.
- P. Lu, J. Sichuan Norm. Univ., Nat. Sci., 2020, 043, 1–20 Search PubMed
.
- J. Mou, Y. Gao, J. Wang, J. Ma and H. Ren, RSC Adv., 2019, 9, 11755–11761 RSC
.
- J. M. Ge, J. X. Jin, Y. M. Cao, M. H. Jiang, F. Z. Zhang, H. L. Guo and X. D. Lei, RSC Adv., 2021, 11, 19630–19638 RSC
.
- L. Song, M. J. Zhao, X. X. Li, Z. P. Zhang and L. T. Qu, RSC Adv., 2016, 6, 70740–70746 RSC
.
- M. Chhetri, U. Gupta, L. Yadgarov, R. Rosentsveig, R. Tenne and C. Rao, Dalton Trans., 2015, 44, 16399–16404 RSC
.
- D. Liang, Y.-W. Zhang, P. Lu and Z. G. Yu, Nanoscale, 2019, 11, 18329–18337 RSC
.
- C. Tsai, F. Abild-Pedersen and J. K. Nørskov, Nano Lett., 2014, 14, 1381–1387 CrossRef CAS PubMed
.
- Y. Xu, L. Wang, X. Liu, S. Zhang, C. Liu, D. Yan, Y. Zeng, Y. Pei, Y. Liu and S. Luo, J. Mater. Chem. A, 2016, 4, 16524–16530 RSC
.
- X. N. Guan, R. Zhang, B. N. Jia, L. Y. Wu, B. Zhou, L. Fan, G. Liu, Y. Wang, P. F. Lu and G. D. Peng, J. Non-Cryst. Solids, 2020, 550, 7 CrossRef
.
- Z. Wang, B. Xiao, Z. Lin, Y. Xu, Y. Lin, F. Meng, Q. Zhang, L. Gu, B. Fang, S. Guo and W. Zhong, Angew. Chem., 2021, 60, 23388–23393 CrossRef CAS PubMed
.
- A. D. Oyedele, S. Yang, L. Liang, A. A. Puretzky, K. Wang, J. Zhang, P. Yu, P. R. Pudasaini, A. W. Ghosh, Z. Liu, C. M. Rouleau, B. G. Sumpter, M. F. Chisholm, W. Zhou, P. D. Rack, D. B. Geohegan and K. Xiao, J. Am. Chem. Soc., 2017, 139, 14090–14097 CrossRef CAS PubMed
.
- A. Guha, R. Sharma, K. R. Sahoo, A. B. Puthirath, N. Shyaga, P. M. Ajayan and T. N. Narayanan, ACS Appl. Energy Mater., 2021, 4, 8715–8720 CrossRef CAS
.
- S. Jiang, C. Zhang, E. Zhao, M. Han, L. Zhu and Y.-Q. Zhao, Appl. Surf. Sci., 2021, 570, 151178 CrossRef CAS
.
- D. Saraf, S. Chakraborty, A. Kshirsagar and R. Ahuja, Nano Energy, 2018, 49, 283–289 CrossRef CAS
.
- R. Quhe, R. Fei, Q. Liu, J. Zheng, H. Li, C. Xu, Z. Ni, Y. Wang, D. Yu, Z. Gao and J. Lu, Sci. Rep., 2012, 2, 1–6 Search PubMed
.
- M. Long, Y. Wang, P. Wang, X. Zhou, H. Xia, C. Luo, S. Huang, G. Zhang, H. Yan, Z. Fan, X. Wu, X. Chen, W. Lu and W. Hu, ACS Nano, 2019, 13, 2511–2519 CAS
.
- G. Liu, J. Li, C. Dong, L. Wu, D. Liang, H. Cao and P. Lu, Int. J. Hydrogen Energy, 2021, 46, 18294–18304 CrossRef CAS
.
- Z. Lin, B. Xiao, Z. Wang, W. Tao, S. Shen, L. Huang, J. Zhang, F. Meng, Q. Zhang, L. Gu and W. Zhong, Adv. Funct. Mater., 2021, 31, 2102321 CrossRef CAS
.
- Q. Liang, Q. Zhang, J. Gou, T. Song, Arramel, H. Chen, M. Yang, S. X. Lim, Q. Wang, R. Zhu, N. Yakolev, S. C. Tan, W. Zhang, K. S. Novoselov and A. T. Wee, ACS Nano, 2020, 14, 5668–5677 CrossRef CAS PubMed
.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS
.
- L. Seixas, A. Carvalho and A. C. Neto, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 155138 CrossRef
.
- S. Grimme, J. Comput. Chem., 2004, 25, 1463–1473 CrossRef CAS PubMed
.
- J. K. Nørskov, T. Bligaard, A. Logadottir, J. Kitchin, J. G. Chen, S. Pandelov and U. Stimming, J. Electrochem. Soc., 2005, 152, J23 CrossRef
.
- W. Xiong, K. Huang and S. Yuan, J. Mater. Chem. C, 2019, 7, 13518–13525 RSC
.
- H. Yang, Y. Li, Z. Yang, X. Shi, Z. Lin, R. Guo, L. Xu, H. Qu and S. Zhang, Vacuum, 2020, 174, 109176 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: The band structures of PdS2 and PdSe2 with SOC and without SOC and under different vacancy concentrations. See DOI: 10.1039/d1ira07466k |
| This journal is © The Royal Society of Chemistry 2021 |