Open Access Article
Open Access ArticleUltrasound assisted synthesis of hybrid quinoline-imidazole derivatives: a green synthetic approach†
Dumitrela Diaconu b,
Dorina Amăriucăi-Mantua,
Violeta Mangalagiubc,
Vasilichia Antocia,
Gheorghita Zbancioc*a and
Ionel I. Mangalagiu
b,
Dorina Amăriucăi-Mantua,
Violeta Mangalagiubc,
Vasilichia Antocia,
Gheorghita Zbancioc*a and
Ionel I. Mangalagiu *ab
*ab
aAlexandru Ioan Cuza University of Iasi, Faculty of Chemistry, 11 Carol I Bd., 700506 Iasi, Romania. E-mail: ionelm@uaic.ro; gheorghita.zbancioc@uaic.ro
bAlexandru Ioan Cuza University of Iasi, Institute of Interdisciplinary Research, Department of Exact and Natural Sciences – CERNESIM Center, 11 Carol I Bd., 700506 Iasi, Romania
cStefan cel Mare University of Suceava, Faculty of Food Engineering, Str. Universitatii 13, Suceava, Romania
First published on 29th November 2021
Abstract
A green, straightforward and efficient study for obtaining hybrid quinoline-imidazole derivatives under ultrasound (US) irradiation as well as under conventional thermal heating (TH) has been presented. The reaction pathway involves only two steps: the N-alkylation of imidazole ring and a Huisgen [3 + 2] dipolar cycloaddition reaction of ylides to dimethyl acetylenedicarboxylate (DMAD). For both types of reactions, a green workup procedure under US irradiation has been presented. Under US irradiation, the N-alkylation of nitrogen atoms from the imidazole nucleus has outstanding benefits in terms of reaction time, energy consumption and yields, and can thereby be considered an environmentally friendly method. Forty new hybrid quinoline-imidazole compounds have been synthesized: 18 salts, 8 dihydro-benzopyrrolo imidazolo quinoline, 9 benzopyrrolo-imidazolo quinoline and 5 dihydro-pyrroloquinoxaline quinoline cycloadducts.
Introduction
Over the past decades, ultrasound (US) assisted reactions have become a modern and successful tool in organic and medicinal chemistry. Compared with conventional thermal heating (TH), the use of US in chemical reactions has some crucial advantages, such as higher yields, better product purity, the possibility to obtain better selectivity and higher reactivity, and reduced reaction times.1–11,31–33,36 In addition, due to milder reaction conditions, less or suppressed side reactions, energy saving nature, and the use of small amounts of solvents, the reactions become eco-friendly.6,9–11,27,28Quinoline and imidazole derivatives are structural scaffolds of huge importance from pharmacological, industrial, and synthetic points of view. As for the pharmacological point of view, they are core scaffolds in medicinal chemistry, having a diverse variety of biological activities such as antiplasmodial and antimalarial, antitubercular, antibacterial, antifungal, anti-HIV, anticancer, anti-inflammatory, antidepressant, analgesic, anti-Alzheimer's, and antihypertensive activities.12–25 The synthesis of quinoline and imidazole derivatives still remains a challenge for the scientific community because conventional synthesis is often inefficient, with low to moderate yields, the formation of by-products, and expensive and harsh reaction conditions (high temperature, long reaction time, large amount of solvents, catalysts, etc.).26
In view of our continued preoccupation with the field of US assisted reactions27–32 and new biological entities with quinoline and imidazole skeletons,16–18,33,34 we decided to perform a thorough study concerning the synthesis of new entities with hybrid quinoline-imidazole cores, both under US irradiation and conventional TH. In addition, we were also interested in developing an environmentally friendly method for the preparation of these hybrids using US technologies. Equally, we were also interested in the anticancer and antimicrobial potential of these compounds.
Results
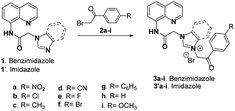
In order to achieve our goal, we decided to use our expertise in cycloimmonium ylides chemistry,35–41 with the Huisgen [3 + 2] dipolar cycloaddition reactions of ylides to dipolarophiles with triple bonds (activated alkynes) being our choice. In this regard, we first obtained the corresponding hybrid quinoline imidazolium salts 3a-i and 3′a-i via N-alkylation with ω-halogeno-acetophenones 2a-i of the acidic nitrogen from benzimidazole 1 and imidazole 1′ (Scheme 1).As we may notice from Table 1, the N-alkylation reactions of the imidazole ring under conventional TH requires long reaction times and the yields are moderate to good (around 50–90%). This is why we decided to modify the workup procedure using US irradiation (ultrasound assisted reactions were carried out using a Sonics VCX-130 reactor with titanium horn, operating in pulse mode, having a nominal power of 130 W and a frequency of 20 kHz). The data from Table 1 reveal that the use of US irradiation in the N-alkylation reactions of the imidazole ring have the advantages of remarkable acceleration of the reaction, substantial decrease of reaction time (from 48–96 hours to 1–2 hours) decrease of consumed energy, and slight increase of yields (by about 5–10%). As a result, this workup procedure could be considered as environmentally friendly.
| Compound | 3a | 3b | 3c | 3d | 3e | 3f | 3g | 3h | 3i | |
| R.t. (h) | CV | 48 | 48 | 48 | 48 | 48 | 96 | 48 | 48 | 48 |
| US | 1.6 | 1.3 | 1.5 | 1.6 | 1 | 1.2 | 1.5 | 1.2 | 1.3 | |
| Compound | 3′a | 3′b | 3′c | 3′d | 3′e | 3′f | 3′g | 3′h | 3′i | |
| R.t. (h) | CV | 48 | 48 | 48 | 48 | 48 | 96 | 96 | 48 | 48 |
| US | 1.6 | 1.3 | 2 | 1.6 | 1.6 | 2.3 | 2.3 | 1.6 | 1.3 | |
| Compound | 3a | 3b | 3c | 3d | 3e | 3f | 3g | 3h | 3i | |
| Yield, % | CV | 90 | 75 | 56 | 87 | 82 | 63 | 74 | 80 | 88 |
| US | 96 | 80 | 63 | 91 | 85 | 65 | 79 | 84 | 90 | |
| Compound | 3′′a | 3′b | 3′c | 3′d | 3′e | 3′f | 3′g | 3′h | 3′′i | |
| Yield, % | CV | 56 | 84 | 78 | 86 | 81 | 51 | 67 | 75 | 83 |
| US | 78 | 86 | 82 | 90 | 83 | 58 | 70 | 84 | 87 | |
As in related cases,27 the efficiency of US irradiation in the N-alkylation reactions could be explained by the cavitation phenomena leading to enhanced mass transfer and better homogenization of the reaction mixture.
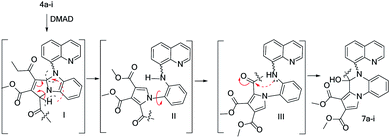
In the second step, benzimidazolium ylides 4a-i and imidazolium ylides 4′a-i (generated in situ from the corresponding imidazolium salts 3a-i and 3′a-i, using triethylamine (Et3N) or 1,2-epoxybutane) were treated with dimethyl acetylenedicarboxylate (DMAD), a symmetrically activated alkyne, and a typical Huisgen [3 + 2] cycloaddition took place (Scheme 3).
In the case of the imidazolium ylides 4′a-i, no matter the conditions we employed for the cycloaddition (US irradiation or conventional TH, with or without catalyst, solvents, etc.), the reactions did not result in any compound, and only decomposition products were obtained (probably because of the instability of the imidazole ring, as we found in a related situation40).
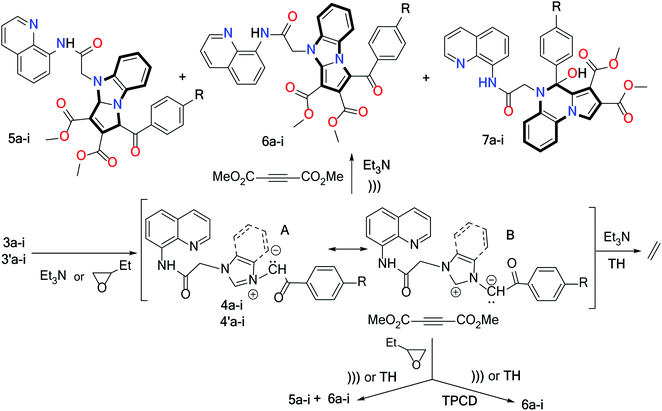
In the case of the benzimidazolium ylides 4a-i, the cycloaddition reactions took place with the formation of the cycloadducts 6a-i and/or 5a-i and/or 7a-i, according to the conditions we employed (Scheme 3 and Tables 2–4). Thus, when the Huisgen [3 + 2] cycloaddition took place using triethylamine as a catalyst, the reactions occurred differently according to the energy source (Table 2). When conventional TH was used, the reactions did not take place, and again only decomposition products were obtained. When US irradiation was used, the reactions took place within a very short period of time (2–4 minutes) with formation of a mixture of the cycloadducts 6a-i and/or 5a-i and/or 7a-i (Table 2).
| Compound | 5a | 5b | 5c | 5d | 5e | 5f | 5g | 5h | 5i | |
| Yield, % | CV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| US | 0 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 14 | |
| Compound | 6a | 6b | 6c | 6d | 6e | 6f | 6g | 6h | 6i | |
| Yield, % | CV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| US | 6 | 6 | 9 | 0 | 5 | 4 | 3 | 2 | 0 | |
| Compound | 7a | 7b | 7c | 7d | 7e | 7f | 7g | 7h | 7i | |
| Yield, % | CV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| US | 17 | 34 | 0 | 12 | 13 | 27 | 0 | 0 | 0 | |
| Compound | 5a | 5b | 5c | 5d | 5e | 5f | 5g | 5h | 5i | |
| Yield, % | CV | 0 | 14 | 15 | 15 | 21 | 13 | 13 | 12 | 18 |
| US | 0 | 24 | 17 | 29 | 25 | 19 | 19 | 19 | 22 | |
| Compound | 6a | 6b | 6c | 6d | 6e | 6f | 6g | 6h | 6i | |
| Yield, % | CV | 32 | 7 | 8 | 10 | 9 | 10 | 14 | 10 | 8 |
| US | 37 | 5 | 6 | 3 | 6 | 10 | 13 | 10 | 8 | |
| Compound | 7a | 7b | 7c | 7d | 7e | 7f | 7g | 7h | 7i | |
| Yield, % | CV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| US | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Compound | 6a | 6b | 6c | 6d | 6e | 6f | 6g | 6h | 6i | |
| Yield, % | CV | 42 | 25 | 21 | 39 | 35 | 31 | 36 | 30 | 37 |
| US | 45 | 29 | 32 | 41 | 42 | 40 | 44 | 34 | 39 | |
Initially, the dihydro-benzopyrrolo-imidazolo quinoline derivatives 5a-i are formed via the Huisgen [3 + 2] cycloaddition of ylides 4a-i (canonical structure B) to DMAD. As in related cases,41–45 the initial cycloaddition is followed by an oxidative dehydrogenation of the dihydro-structure 5, leading to more thermodynamically stable compounds: the aromatized benzopyrrolo-imidazolo quinoline compounds 6a-i. The formation of the dihydro-pyrroloquinoxaline quinoline compounds 7a-i could be explained via the following reaction mechanism (Scheme 2): after the initial Huisgen [3 + 2] cycloadditions which lead to the type I dihydro-benzopyrrolo-imidazolo derivatives, a ring opening of the imidazole cycle occurs simultaneously with a prototropic rearrangement, with the formation of a type II pyrroloaniline derivative. In the next step, rotations of the pyrrole ring around the carbon-nitrogen single bond lead to a favorable conformation of the molecule (type III) which allows cyclization to a quinoxaline cycle (via a nucleophilic attack of the amine electrons to the carbonyl group) with the formation of the dihydro-pyrrolo-quinoxaline quinoline compounds 7a-i. Analogous explications have been furnished by some other authors in related cases.41–45
The formation of cycloadducts 5-7 could be explained via the substituent effect: the electron-repulsive groups favour the formation of the pyrrolo-imidazolo structure of type 5-6, while the electron-withdrawing groups favour the formation of the pyrrolo-quinoxaline structure of type 7.
In view of the above considerations, especially the lack of selectivity and low yields of reactions but also the toxicity of the solvent used (chloroform), we decided to modify the workup protocols by changing the solvents and using catalysts. Our next choice was to use 1,2-epoxybutane as a nontoxic solvent and as a scavenger for hydrobromic acid for the synthesis of the dihydro-benzopyrrolo-imidazolo quinoline compounds 5a-i and the benzopyrrolo-imidazolo quinoline compounds 6a-i (Table 3).
Our first observation is that, under these conditions, the reaction became more selective, with only two products being obtained: the dihydro- and fully aromatized cycloadducts 5a-i and 6a-i. No quinoxaline structures of type 7 were observed. We also noticed that under US irradiation, the global yields are slightly higher (by about 5%) and more importantly, a clear preference for obtaining the dihydro-benzopyrrolo-imidazolo quinoline structure is observed (with about 15% more compared with the aromatized one, in term of yield). We wish to also point out that under US irradiation, the reaction time decreases substantially, from 720–960 minutes under conventional TH to 150–180 minutes under US irradiation (roughly fivefold).
Since we were still not satisfied by the obtained results, we decided to perform the cycloaddition reactions in 1,2-epoxybutane using tetrakis(pyridine)cobalt(II) dichromate (TPCD) as the catalyst. The obtained results are listed in Table 4.
From Table 4 we may notice that, in these conditions, the reactions became selective, and only fully aromatized cycloadducts 6a-i were obtained. We may also notice that under US irradiation, the yields are better, being about 5–10% higher compared with conventional TH. We also wish to point out that under US irradiation, the reaction time decreases dramatically (about twenty times), from 300–480 minutes under conventional TH to 16–20 minutes under US irradiation. Consequently, the consumed energy decreases in the same manner. As a result of all these considerations, we claim that the Huisgen [3 + 2] dipolar cycloaddition reactions of ylides 4a-i with DMAD, in 1,2-epoxybutane and TPCD, are an environmentally friendly workup procedure.
The structures of the compounds were proven by elemental and spectral analyses (IR, 1H-NMR, 13C{1H}-NMR, 2D-COSY, 2D-HMQC, long range 2D-HMBC) and were in accordance with the proposed structures.
Conclusions
In conclusion, a green, straightforward and efficient study for obtaining hybrid quinoline-imidazole derivatives under US irradiation as well as under conventional TH has been presented. The reaction pathway involves only two steps: the N-alkylation of the imidazole skeleton and a Huisgen [3 + 2] dipolar cycloaddition reaction of the ylides to DMAD. The use of US irradiation in the N-alkylation reactions of the imidazole ring has the advantages of remarkable acceleration of the reaction, substantial decrease of reaction time (from 48–96 hours to 1–2 hours), decrease of consumed energy, and slight increase of yields (no more than 10%). As a consequence, this workup method could be considered environmentally friendly. The Huisgen [3 + 2] dipolar cycloaddition reaction of benzimidazolium ylides to DMAD generates three types of hybrid quinoline-imidazole cycloadducts and according to the source of energy and solvent used, the reactions pathway could be conducted selectively. Thus, if the fully aromatized benzopyrrolo-imidazolo quinoline cycloadducts are to be obtained, the TPCD catalyst and 1,2-epoxybutane, have to be used both under US irradiation and conventional TH. When dihydro-pyrrolo-quinoxaline quinoline compounds are the final goal, the triethylamine catalyst and chloroform as the solvent are compulsory to be used under US irradiation. If the dihydro-benzopyrrolo-imidazolo quinoline derivatives are intended as major products, 1,2-epoxybutane and US irradiation have to be used. Taking into account the above mentioned points, alongside an obvious decrease in reaction time and energy consumption, and the fact that in the cycloaddition reactions we have used a nontoxic solvent (1,2-epoxybutane), the 1,3 dipolar cycloaddition reactions under US irradiation can be considered an environmentally friendly workup procedure. Forty new hybrid quinoline-imidazole compounds belonging to four different classes have been synthesized: 18 salts, as well as 8 dihydro-benzopyrrolo-imidazolo quinoline, 9 benzopyrrolo-imidazolo quinoline and 5 dihydro-pyrroloquinoxaline quinoline cycloadducts.Author contributions
The manuscript was written with contributions from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to the Romanian Ministry of Research and Innovation, CNCS-UEFISCDI, project number PN-III-P4-ID-PCE-2020-0371, for financial support. The authors are also thankful to the project POC/448/1/1 Research Center with Integrated Techniques for Atmospheric Aerosol Investigation in Romania-RECENT AIR (grant agreement MySMIS no. 127324) for infrastructure used and the POSCCE-O 2.2.1, SMIS-CSNR 13984-901, No. 257/28.09.2010 Project, CERNESIM, for NMR experiments.Notes and references
- J. M. Léveque, G. Cravotto, F. Delattre and P. Cintas, Organic Sonochemistry, Challenges and Perspectives for the 21st Century, Springer Nature Switzerland AG, Cham, 2018 Search PubMed.
- C. Dong, K. Sanjay and M. Ackmez, H and book on Applications of Ultrasound: Sonochemistry for Sustainability, CRC Press, Taylor & Francis Group, Boca Raton, 2012 Search PubMed.
- T. J. Mason, and D. Peters, Practical Sonochemistry: Power Ultrasound Uses and Applications, Woodhead Publishing Ellis Horwood, Chichester, Cambridge, 2nd edn, 2002 Search PubMed.
- J. L. Luche, Synthetic Organic Sonochemistry, Plenum Press, New York, 1998 Search PubMed.
- G. Cravotto and P. Cintas, Chem. Soc. Rev., 2006, 35, 180–196 RSC.
- T. J. Mason, Ultrason. Sonochem., 2007, 14(4), 476–483 CrossRef CAS PubMed.
- G. Cravotto and P. Cintas, Chem.–Eur. J., 2007, 13, 1902–1909 CrossRef CAS PubMed.
- M. Fujita, J. M. Lévêque, N. Komatsu and T. Kimura, Ultrason. Sonochem., 2015, 27, 247–251 CrossRef CAS PubMed.
- P. Cintas, Ultrason. Sonochem., 2016, 28, 257–258 CrossRef CAS PubMed.
- M. Lupacchini, A. Mascitti, G. Giachi, L. Tonucci, N. d'Alessandro, J. Martinez and E. Colacino, Tetrahedron, 2017, 73(6), 609–653 CrossRef CAS.
- G. Chatel, Ultrason. Sonochem., 2018, 40, 117–122 CrossRef CAS PubMed.
- Y. Q. Hu, C. Gao, S. Zhang, L. Xu, Z. Xu, L. S. Feng, X. Wu and F. Zhao, Eur. J. Med. Chem., 2017, 139, 22–47 CrossRef CAS PubMed.
- P. N. Kalaria, S. C. Karad and D. K. A. Raval, Eur. J. Med. Chem., 2018, 158, 917–936 CrossRef CAS PubMed.
- J. Zhang, S. Wang, Y. Ba and Z. Xu, Eur. J. Med. Chem., 2019, 174, 1–8 CrossRef CAS PubMed.
- Y. L. Fan, X. H. Jin, Z. P. Huang, H. F. Yu, Z. G. Zeng, T. Gao and L. S. Feng, Eur. J. Med. Chem., 2018, 150, 347–365 CrossRef CAS PubMed.
- V. Antoci, D. Cucu, G. Zbancioc, C. Moldoveanu, V. Mangalagiu, D. Amariucai-Mantu, A. Aricu and I. I. Mangalagiu, Future Med. Chem., 2020, 12(3), 207–222 CrossRef CAS PubMed.
- C. N. Lungu, B. I. Bratanovici, M. M. Grigore, V. Antoci and I. I. Mangalagiu, Curr. Med. Chem., 2020, 27(1), 154–169 CrossRef CAS PubMed.
- D. Mantu, V. Antoci, C. Moldoveanu, G. Zbancioc and I. I. Mangalagiu, J. Enzyme Inhib. Med. Chem., 2016, 31(supp. 2), 96–103 CrossRef CAS PubMed.
- F. Gao, X. Zhang, T. Wang and J. Xiao, Eur. J. Med. Chem., 2019, 165, 59–79 CrossRef CAS PubMed.
- N. M. Raghavendra, D. Pingili, S. Kadasi, A. Mettu and S. V. U. M. Prasad, Eur. J. Med. Chem., 2018, 143, 1277–1300 CrossRef CAS PubMed.
- Y. Fang, H. Zhou, Q. Gu and J. Xu, Eur. J. Med. Chem., 2019, 167, 133–145 CrossRef CAS PubMed.
- Y. X. Xu, H. Wang, X. K. Li, S. N. Dong, W. W. Liu, Q. Gong, T. D. Wang, Y. Tang, J. Zhu, J. Li, H. Y. Zhang and F. Mao, Eur. J. Med. Chem., 2018, 143, 33–47 CrossRef CAS PubMed.
- S. Mukherjee and M. Pal, Drug Discovery Today, 2013, 18(7–8), 389–398 CrossRef CAS PubMed.
- M. Gaba, S. Singh and C. Mohan, Eur. J. Med. Chem., 2014, 76, 494–505 CrossRef CAS PubMed.
- A. D. Brown, S. K. Bagal, P. Blackwell, D. C. Blakemore, B. Brown, P. J. Bungay, M. Corless, J. Crawforth, D. Fengas, D. R. Fenwick, V. Gray, M. Kemp, W. Klute, L. Malet-Sanz, D. Miller, Y. Murata, C. E. Payne, S. Skerratt, E. B. Stevens and J. S. Warmus, Bioorg. Med. Chem., 2019, 27(1), 230–239 CrossRef CAS PubMed.
- A. R. Katritzky and A. F. Pozharskii, Handbook of Heterocyclic Chemistry, Pergamon, Amsterdam, 2nd edn, 2000 Search PubMed.
- G. Zbancioc, I. I. Mangalagiu and C. Moldoveanu, Ultrason. Sonochem., 2015, 23, 376–384 CrossRef CAS PubMed.
- G. Zbancioc, A. M. Zbancioc and I. I. Mangalagiu, Ultrason. Sonochem., 2014, 21, 802–811 CrossRef CAS PubMed.
- V. Bejan, D. Mantu and I. I. Mangalagiu, Ultrason. Sonochem., 2012, 19(5), 999–1002 CrossRef CAS PubMed.
- D. Mantu, M. C. Luca, C. Moldoveanu, G. Zbancioc and I. I. Mangalagiu, Eur. J. Med. Chem., 2010, 45(11), 5164–5168 CrossRef CAS PubMed.
- V. Bejan, C. Moldoveanu and I. I. Mangalagiu, Ultrason. Sonochem., 2009, 16(3), 312–315 CrossRef CAS PubMed.
- D. Mantu, C. Moldoveanu, A. Nicolescu, C. Deleanu and I. I. Mangalagiu, Ultrason. Sonochem., 2009, 16(4), 452–454 CrossRef CAS PubMed.
- M. C. C. Luca, V. V. Tura and I. I. Mangalagiu, Med. Hypotheses, 2010, 75(6), 627–629 CrossRef CAS PubMed.
- A. M. Zbancioc, G. Zbancioc, C. Tanase, A. Miron, C. Ursu and I. I. Mangalagiu, Lett. Drug Des. Discovery, 2010, 7(9), 644–649 CrossRef CAS.
- I. I. Mangalagiu, I. Druta, M. Constantinescu, I. Humelnicu and M. Petrovanu, Tetrahedron, 1996, 52(26), 8853–8862 CrossRef CAS.
- I. I. Mangalagiu, G. Mangalagiu, C. Deleanu, G. Drochioiu and M. Petrovanu, Tetrahedron, 2003, 59(1), 111–114 CrossRef CAS.
- M. Caprosu, G. Zbancioc, C. Moldoveanu and I. I. Mangalagiu, Collect. Czech. Chem. Commun., 2004, 69(2), 426–434 CrossRef CAS.
- G. Zbancioc, T. Huhn, U. Groth, C. Deleanu and I. I. Mangalagiu, Tetrahedron, 2010, 66(24), 4298–4306 CrossRef CAS.
- R. Tucaliuc, V. Cotea, M. Niculaua, C. Tuchilus, D. Mantu and I. I. Mangalagiu, Eur. J. Med. Chem., 2013, 67, 367–372 CrossRef CAS PubMed.
- D. Cucu, V. Mangalagiu, D. Amariucai-Mantu, V. Antoci and I. I. Mangalagiu, Studia UBB Chemia, 2019, LXIV, 59–66 CrossRef.
- C. Moldoveanu, G. Zbancioc, D. Mantu, D. Maftei and I. I. Mangalagiu, PLoS One, 2016, 11(5), e0156129 CrossRef PubMed.
- E. Georgescu, A. Nicolescu, F. Georgescu, F. Teodorescu, S. Shova, A. T. Marinoiu, F. Dumitrascu and C. Deleanu, Tetrahedron, 2016, 72(19), 2507–2520 CrossRef CAS.
- E. Georgescu, A. Nicolescu, F. Georgescu, S. Shova, F. Teodorescu, A. M. Macsim and C. Deleanu, Synthesis, 2015, 47(11), 1643–1655 CrossRef CAS.
- E. Georgescu, A. Nicolescu, F. Georgescu, F. Teodorescu, D. Marinescu, A. M. Macsim and C. Deleanu, Beilstein J. Org. Chem., 2014, 10, 2377–2387 CrossRef PubMed.
- A. Nicolescu, C. Deleanu, E. Georgescu, F. Georgescu, A. M. Iurascu, S. Shova and P. Filip, Tetrahedron Lett., 2013, 54(11), 1486–1488 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07484a |
| This journal is © The Royal Society of Chemistry 2021 |