Reactive behavior of isoquinoline alkaloid in a green reduction process assisted by ionic liquids and solvent-free techniques†
Jie
Tang
,
Sara
Toufouki
,
Alula
Yohannes
and
Shun
Yao
 *
*
School of Chemical Engineering, Sichuan University, Chengdu 610065, P. R. China. E-mail: Cusack@scu.edu.cn; Fax: +86 028 85405221; Tel: +86 028 85405221
First published on 17th November 2020
Abstract
The green reduction of berberine (BBR) to tetrahydroberberine (THB) and dihydroberberine (DHB) was realized via ionic liquid (IL)-assisted and solvent-free synthetic technologies; and the reactive behaviour and mechanisms under related conditions were investigated in detail. In particular, a reaction in a tablet was realized through a pressing method for the first time. The performances of ten Brønsted acidic ILs in three series together with four solid-phase reactive technologies were comprehensively compared.
Green reaction chemistry is an eternally hot topic in the field of seeking a cleaner synthetic route while the expected reaction results can still be obtained satisfactorily. The optimal synthesis of a molecule should be assessed through environmentally relevant metrics;1 and the key is to replace harmful reagents with green solvents or non-solvents. Water, supercritical fluids, ionic liquids (ILs) and solvent-free technologies are generally under consideration by researchers. Among these, ILs and molten salts below 200 °C are widely applied in many chemical reactions either as solvents or as catalysts.2 Solvent-free synthesis (SFS) has also attracted a lot of attention in the field of organic synthesis, which is commonly realized by grinding, microwave radiation or ultrasonication.3 A pressurized method has not yet been reported for solid-phase reactions, but its feasibility is worth exploring according to theoretical analysis and its similarity to existing methods like extrusion.4 The emergence of various green techniques provides a broad space for the structural modification of natural products. On the whole, the performance of the above methods is still unknown because of the lack of sufficient exploration in current reduction studies for natural compounds.
It is well known that natural products with pharmacological activities are important sources of medical intermediates, and exploring green synthetic pathways can make the fullest use of these compounds. Herein, berberine was selected as a representative among these compounds for this study, and it is a well-known isoquinoline alkaloid. Its two most important hydrogenated derivatives, tetrahydroberberine and dihydroberberine (THB and DHB) have been reported to possess strong neuroprotective and anti-inflammatory activities.5 Traditionally, THB and DHB are synthesized from borohydride reagents in alcoholic/pyridine solution. On the one hand, volatile organic solvents are harmful to the environment and human beings; on the other hand, alcoholysis of borohydride reagents is inescapable and lipid-soluble products are not easy to precipitate from the above systems. Besides, the simultaneous synthesis of THB and DHB in one reaction has not yet been reported. According to the current literature, there is a lack of reports focusing on the control of reduction extent and selective reduction, and how the different reaction systems would affect the product composition also attracted our interest. Therefore, new methodologies are urgently needed for similar reactions and meanwhile the mechanism of controlled synthesis for THB and DHB should be further clarified. As is known to all, berberine hydrochloride (BH) is primarily extracted by ethanol or acidic solvents; hence it is possible that acidic ILs have an affinity for BH molecules, which would promote its dissolution and improve reduction efficiency during the reaction. Moreover, the catalytic activities of Brønsted acidic ILs have already been proved in many reactions. Here the reduction of berberine to THB and DHB with Brønsted acidic ILs or solvent-free technologies or their combination is an important endeavour, and related reactive rules and possible mechanisms were explored as follows.
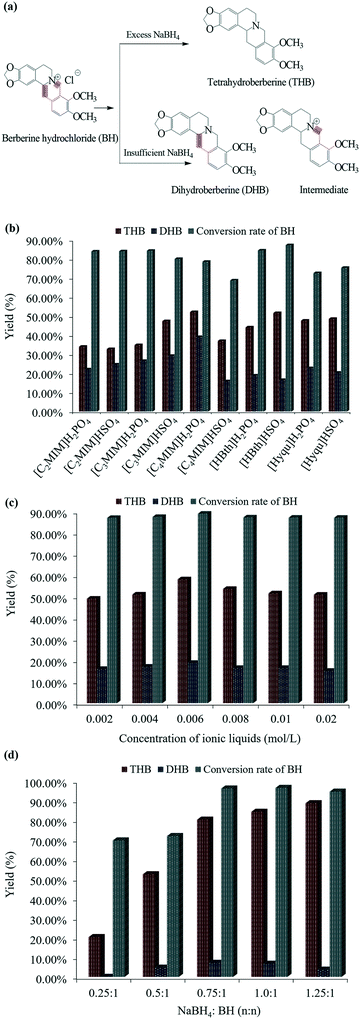
Theoretically, 1 mol of BH could be reduced to THB with 1 mol of NaBH4. When nNaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nBH < 1
nBH < 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the amount of NaBH4 would be relatively insufficient, and the two double bonds of BH would be selectively reduced (as shown in Fig. 1a). After initial screening, 10 ILs in imidazolium, benzothiazolium and quinolinium series were compared for the reduction process: [C2MIM]H2PO4, [C2MIM]HSO4, [C3MIM]H2PO4, [C3MIM]HSO4, [C4MIM]H2PO4, [C4MIM]HSO4, [HBth]H2PO4 [HBth]HSO4, [Hyqu]H2PO4 and [Hyqu]HSO4. All of them were prepared within one or two steps and checked by liquid chromatography according to reported methods. And these ILs were stable up to 200 °C or higher, as reported.6 Based on the IL-assisted reactive process described in ESI,† it was found for both the yields of THB and DHB that the catalytic performance of imidazole-based ILs with H2PO4− as the anion was better with a longer alkyl chain length of the cation (see Fig. 1b), while for ILs with an anion of HSO4−, the catalytic performance increased first and then decreased with an increase in the side chain on the imidazolium ring according to their yields. This trend accorded with the findings for the effect of chain length on reactive results in previous studies.7 The changes in viscosity, hydrophobicity and steric hindrance resulting from the extended alkyl chain can affect the catalytic activity of ILs. For benzothiazole-based ILs, [HBth]H2PO4 was more advantageous to the formation of DHB than [HBth]HSO4 whereas the latter obtained a higher yield of THB. And the conversion rate of BH is the highest among the 10 ILs. This is because the acidity of [HBth]HSO4 is relatively stronger. And according to a previous study,8 the acidity of the ionic liquids has a decisive effect on the reaction rate; the higher the acidity, the stronger the catalytic activity of the ILs (for the pH of 1–5% aqueous solution, [HBth]HSO4: 1.33–0.68, [HBth]H2PO4: 1.58–0.89, H2SO4: 1.32–0.65). Also, the structure of benzothiazole makes it more compatible with the reaction system. Although the acidity of sulfuric acid is close to that of [HBth]HSO4, it will violently react with sodium borohydride as a strong oxidant and release a lot of heat and hydrogen. Similarly, for 8-hydroxyquinoline-based ILs, the yield of DHB obtained by H2PO4− was higher than that of HSO4−, and the yield of THB obtained by H2PO4− was lower than that by HSO4−. Overall, the selectivity of imidazole-based ILs was the lowest among the three and [HBth]HSO4 was found to be superior to other ILs. Before it was applied for reduction, [HBth]HSO4 had only exhibited excellent performance in an esterification reaction as a catalyst, which was more closely related to its strong acidity. In addition, this ionic liquid had the advantage of automatic phase separation from the products after reaction.
1, the amount of NaBH4 would be relatively insufficient, and the two double bonds of BH would be selectively reduced (as shown in Fig. 1a). After initial screening, 10 ILs in imidazolium, benzothiazolium and quinolinium series were compared for the reduction process: [C2MIM]H2PO4, [C2MIM]HSO4, [C3MIM]H2PO4, [C3MIM]HSO4, [C4MIM]H2PO4, [C4MIM]HSO4, [HBth]H2PO4 [HBth]HSO4, [Hyqu]H2PO4 and [Hyqu]HSO4. All of them were prepared within one or two steps and checked by liquid chromatography according to reported methods. And these ILs were stable up to 200 °C or higher, as reported.6 Based on the IL-assisted reactive process described in ESI,† it was found for both the yields of THB and DHB that the catalytic performance of imidazole-based ILs with H2PO4− as the anion was better with a longer alkyl chain length of the cation (see Fig. 1b), while for ILs with an anion of HSO4−, the catalytic performance increased first and then decreased with an increase in the side chain on the imidazolium ring according to their yields. This trend accorded with the findings for the effect of chain length on reactive results in previous studies.7 The changes in viscosity, hydrophobicity and steric hindrance resulting from the extended alkyl chain can affect the catalytic activity of ILs. For benzothiazole-based ILs, [HBth]H2PO4 was more advantageous to the formation of DHB than [HBth]HSO4 whereas the latter obtained a higher yield of THB. And the conversion rate of BH is the highest among the 10 ILs. This is because the acidity of [HBth]HSO4 is relatively stronger. And according to a previous study,8 the acidity of the ionic liquids has a decisive effect on the reaction rate; the higher the acidity, the stronger the catalytic activity of the ILs (for the pH of 1–5% aqueous solution, [HBth]HSO4: 1.33–0.68, [HBth]H2PO4: 1.58–0.89, H2SO4: 1.32–0.65). Also, the structure of benzothiazole makes it more compatible with the reaction system. Although the acidity of sulfuric acid is close to that of [HBth]HSO4, it will violently react with sodium borohydride as a strong oxidant and release a lot of heat and hydrogen. Similarly, for 8-hydroxyquinoline-based ILs, the yield of DHB obtained by H2PO4− was higher than that of HSO4−, and the yield of THB obtained by H2PO4− was lower than that by HSO4−. Overall, the selectivity of imidazole-based ILs was the lowest among the three and [HBth]HSO4 was found to be superior to other ILs. Before it was applied for reduction, [HBth]HSO4 had only exhibited excellent performance in an esterification reaction as a catalyst, which was more closely related to its strong acidity. In addition, this ionic liquid had the advantage of automatic phase separation from the products after reaction.
Obviously, the existence of [HBth]HSO4 at a certain concentration was beneficial for improving the yields of THB and DHB (see Fig. 1c). With a further increase in the concentration, the yields of THB and DHB were both negatively affected. This was because an excessively high concentration of ILs would increase the viscosity of the reaction system, which was not conducive to chemical kinetics or mass transfer. Hence the appropriate concentration of ionic liquid in the whole system should be controlled at around 0.006 mol L−1. Moreover, Fig. 1d indicates that the yield of THB increased with the dosage of sodium borohydride when the IL was determined, while the yield of DHB increased at first and then decreased over the observed range, which could be attributed to the conversion of DHB to THB. It is worth mentioning that the reaction was the nucleophilic addition to BH by a hydride ion. Each sodium borohydride molecule in the solution can ionize four protons. However, when the molar ratio of NaBH4 to BH was less than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, THB was still the dominant product in the solution system, which indicated that the carbon–nitrogen double bonds of BH were not preferentially broken. This phenomenon should account for the spatial effects and electronic effects; chloridion was close to the C–N double bond owing to electrostatic interaction, which augmented the steric resistance of the C
1, THB was still the dominant product in the solution system, which indicated that the carbon–nitrogen double bonds of BH were not preferentially broken. This phenomenon should account for the spatial effects and electronic effects; chloridion was close to the C–N double bond owing to electrostatic interaction, which augmented the steric resistance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. Another possibility combined with the following contents was that it depends on the reaction system, where the preferentially broken bond varied in different conditions. In summary, the order of influence of these factors on both the yield and the selectivity of THB and DHB was basically determined as dosage of sodium borohydride > type of ILs > concentration of ILs.
N bond. Another possibility combined with the following contents was that it depends on the reaction system, where the preferentially broken bond varied in different conditions. In summary, the order of influence of these factors on both the yield and the selectivity of THB and DHB was basically determined as dosage of sodium borohydride > type of ILs > concentration of ILs.
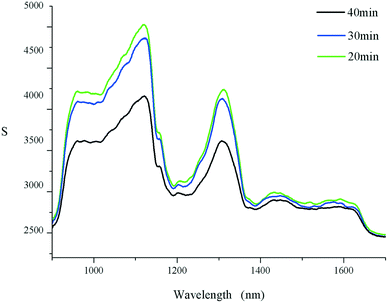
Next, SFS was realized under grinding, microwaves, ultrasound and pressurization. As displayed in Fig. 2a, the yields of THB and DHB reached their highest after 2 h of grinding (60 rpm, 80 mesh). Meanwhile, the difference value of the yields was also the highest, which indicated that grinding for 2 h was beneficial for preparing THB. But excessive grinding time in air was not conducive to such a kind of reduction. What can be concluded from Fig. 2b1 is that when the microwave power exceeded 350 W, the yield of DHB surpassed that of THB, even though the molar ratio of NaBH4 to BH was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A finding opposite to that in the solution environment indicated that higher microwave power was more favourable to the formation of DHB. It might be that the polarity of THB was greater than that of DHB, and thus THB had a stronger ability to absorb microwaves. Another possible mechanism was that the energy barrier of THB formation from DHB could not easily be reached under this condition. In addition, the effect of radiation time on the reaction is shown in Fig. 2b2. Generally, the preferable time was 15 min for both THB and DHB under 500 W. The prolongation of microwave time was not beneficial to the formation of THB or DHB. This was contributed by the thermal effects caused by longer microwave radiation; a higher temperature was not beneficial for this exothermic reaction. Moreover, the violent collision between molecules also aggravated the instability of the products. As for the ultrasound-assisted method, it is particularly powerful because of high frequency and cavitation.9 Here Fig. 2c1 indicates that the maximum THB yield was obtained at 350 W, which was similar to microwave radiation. In addition, ultrasonic power had little effect on the yield of DHB, as reflected in the small fluctuation in the yield. The yield of THB reached its highest after 10 min of ultrasound, but the ultrasonic time had almost no influence on the yield of DHB (see Fig. 2c2). This indicated that ultrasound had definite selectivity for the generation of THB. Finally, compared with the reaction duration (3–4 h) in previous methods,10 it has been shortened to 40 min, which proved the improved efficiency by ILs.
1. A finding opposite to that in the solution environment indicated that higher microwave power was more favourable to the formation of DHB. It might be that the polarity of THB was greater than that of DHB, and thus THB had a stronger ability to absorb microwaves. Another possible mechanism was that the energy barrier of THB formation from DHB could not easily be reached under this condition. In addition, the effect of radiation time on the reaction is shown in Fig. 2b2. Generally, the preferable time was 15 min for both THB and DHB under 500 W. The prolongation of microwave time was not beneficial to the formation of THB or DHB. This was contributed by the thermal effects caused by longer microwave radiation; a higher temperature was not beneficial for this exothermic reaction. Moreover, the violent collision between molecules also aggravated the instability of the products. As for the ultrasound-assisted method, it is particularly powerful because of high frequency and cavitation.9 Here Fig. 2c1 indicates that the maximum THB yield was obtained at 350 W, which was similar to microwave radiation. In addition, ultrasonic power had little effect on the yield of DHB, as reflected in the small fluctuation in the yield. The yield of THB reached its highest after 10 min of ultrasound, but the ultrasonic time had almost no influence on the yield of DHB (see Fig. 2c2). This indicated that ultrasound had definite selectivity for the generation of THB. Finally, compared with the reaction duration (3–4 h) in previous methods,10 it has been shortened to 40 min, which proved the improved efficiency by ILs.
 | ||
| Fig. 2 Effects of (a) grinding time, (b1) microwave power, (b2) microwave time, (c1) ultrasonic power, (c2) ultrasonic time, (d1) pressure and (d2) pressure time on the yields of THB and DHB. | ||
As a newly developed non-solvent method using a laboratory table press, the pressure and time were crucial and easily realized by a common laboratory tablet press for the first time. In the process of axial mechanical compression, the whole system would go through the following three stages: (1) compression, (2) compaction and (3) consolidation. The compression force, shear force and friction force exerted on the solid particles make them continuously broken. Constantly, the gap between particles was reduced and the contact area was increased, and meanwhile the coating layer on the product surface was continuously destroyed, and thus more “fresh” surface could be exposed to reagents so that the reaction was promoted. As shown in Fig. 2d1, the yield of THB reached its highest at 20 MPa, while pressure had little effect on the yield of DHB when it was less than 25 MPa. A further increase in pressure was adverse to the formation of both THB and DHB. Furthermore, the duration of the stress at 20 MPa was explored (see Fig. 2d2), where the yield of THB decreased with a prolongation in pressure time, while the yield of DHB increased at first and then tended to remain unchanged. In terms of mechanism, increasing pressure caused an increase in active molecules per unit volume and in effective collision probability. As a result, this reactive method is more convenient for scaling up in a linear way than other solvent-free techniques. To the best of our knowledge, the above findings have not been reported in solid-phase synthesis before.
Combined with the above conclusions, ultrasound had definite selectivity for the generation of THB. In detail, the order of yield of THB was pressurization > microwave radiation > grinding > ultrasonication. The highest yield of DHB was in the order of microwave radiation > pressurization > grinding > ultrasonication. The yield of DHB was inversely higher than that of THB with microwave radiation, indicating that a certain intensity of microwave had selectivity for the formation of DHB. And the difference in value of yields of THB and DHB was pressurization > grinding > ultrasonication > microwave radiation. The greater the difference in value, the more favourable the approach was for THB and vice versa. In terms of the above evaluation indexes, pressurization was first. The newly developed pressurization method was really unique among various assisted methods. Moreover, microwave radiation reached higher yields of both THB and DHB than the ultrasonic method at the same power within the same duration. By comparison, pressurization was beneficial to THB, while microwave radiation was selective for DHB. As shown in Fig. 3, after pressurization for 10 min at 20 MPa, a colour change of the tablet was apparent, which could reflect the reaction process; the intersecting surface of the products after pressurization was not as smooth as the reactants, and it became rougher because of the formation of water in the process, which could also be felt in the button surface of the mould.
So far, the above solvent-free methods had shown obvious facilitation for reduction without the use of ILs, and meanwhile ILs had performed well in the solution environment for the same reaction. Considering the universal use of microwave and ultrasound and their different effects on this reaction, what the reaction results would be under the two methods when combined with ILs attracted our attention. Here [HBth]HSO4 was selected as an ideal representative and applied in the reduction process assisted by microwave or ultrasound; it has a higher melting point and is in the solid state at room temperature. It is very suitable for mixing with other solid reactants and participating in the reduction. As shown in Fig. 4a, the yields of both THB and DHB obtained by combining [HBth]HSO4 with microwaves were lower than those without them. This could be attributed to the attenuation of microwave radiation caused by the lattice structure.11 For ILs in the solid state at room temperature, the lattices can be viewed as compositions of anions and cations. Resonance interaction between lattices and strong ions absorbed external energy and thereby weakened the microwave radiation. Obviously, ILs were not advantageous to microwave-assisted synthesis in this reaction. When the reaction was implemented under ultrasound coupled with ILs, the results were completely different. As shown in Fig. 4b, the yields of THB and DHB both increased in the presence of ILs. This implies that ILs play different roles in various reaction systems with exclusive mechanisms, and that the addition of ILs is not always favourable.
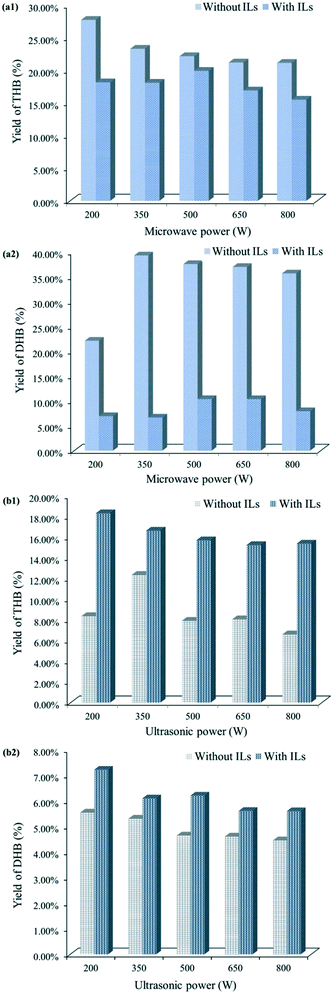 | ||
| Fig. 4 Yields of (a1) THB and (a2) DHB under microwave radiation with [HBth]HSO4 or without [HBth]HSO4; yields of THB (b1) and DHB (b2) under ultrasound with [HBth]HSO4 or without [HBth]HSO4. | ||
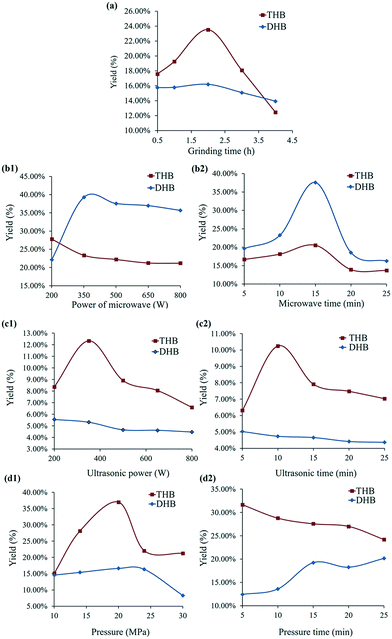
Finally, investigation of the reduction dynamics was carried out in solution, microwave, pressed tablet and ultrasound conditions. From the correlation coefficient (R2) shown in Table 1, the reaction was more in accordance with the pseudo-second-order kinetics in both solvent and solvent-free environments. It can be suggested that the rate-limiting step is related to chemical interactions between molecules rather than particle diffusion.12 For this reaction, although the diffusion patterns were very different in various reaction systems, there should be little difference in chemical interactions; hence it was proved that solvent-free methods were a good alternative to conditional methods. For tracking the reaction process, near infrared spectroscopy (NIR) and powder X-ray diffraction (PXRD) in ESI† are recommended for a solution reaction system and a solid-phase reaction system, respectively. The change in peak intensity around 1120 and 1320 nm in NIR (Fig. 5) was closely related to the chemical composition in the reactive system during the reduction process; PXRD patterns can also obviously show the characteristic peaks of THB and DHB, and the whole profile reflected their combination to varying degrees at different time points (see Fig. 6).
| Modes | Pseudo-first-order model | Pseudo-second-order model | |||||
|---|---|---|---|---|---|---|---|
| C e (me) | K 1 (1/min) | R 2 | C e 2 (me2) | K 2 (me1/2) | R 2 | ||
| 1 | T | 0.0448 | 0.0021 | 0.8942 | 0.0020 | −4.3098 | 0.9892 |
| D | 0.0448 | 0.0016 | 0.9105 | 0.0020 | 0.4278 | 0.9912 | |
| 2 | T | 0.4564 | −0.0034 | 0.8948 | 0.2083 | −0.1351 | 0.9331 |
| D | 0.4537 | −0.0041 | −0.2906 | 0.2058 | −0.1769 | 0.9961 | |
| 3 | T | 0.4564 | −0.0069 | 0.9227 | 0.2083 | −0.3672 | 0.9886 |
| D | 0.4537 | 0.0068 | 0.7750 | 0.2058 | 0.1479 | 0.9943 | |
| 4 | T | 0.4564 | −0.0004 | −0.3047 | 0.2083 | −0.1525 | 0.9578 |
| D | 0.4537 | −0.0004 | 0.9301 | 0.2058 | −0.1175 | 0.9987 | |
In conclusion, hydrogenation of BH in IL aqueous solution and solvent-free conditions was implemented in this study. The results indicated that the highest yield of THB was obtained in a solution environment, and it took less time than the 4 h traditional reduction time for BH. It was also proved that 0.006 mol L−1 [HBth]HSO4 could achieve the best selectivity among the tested ILs, and THB was the dominant product in a solution environment, even though NaBH4 was relatively insufficient. By comparison, solvent-free technologies achieve a relatively high yield within a shorter time. Meanwhile, the highest yield of DHB was reached in solvent-free conditions within 15 min. According to the IL performance in solution and non-solvent reactions, an appropriate concentration of IL was favourable to the reduction in solvent. Meanwhile, from a comparison of solvent-free methods with or without IL, the addition of IL to different non-solvent conditions could lead to different reactive behaviours. An optimal strategy could be selected by researchers according to their expected goals and different technical merits, and the reaction in a pressed tablet is truly an easy and effective method among these.
Experimental section
Synthesis of ILs
All the ILs used in this work were synthesized according to previous methods.13–15 The synthetic route, 1H NMR, elemental analysis and melting points of the ILs are given in ESI.†Reduction of berberine hydrochloride in IL aqueous solution
0.5 g of berberine hydrochloride was accurately weighed and dissolved in 25 mL of a 0.006 mol L−1 ionic liquid aqueous solution. 0.05 g of sodium borohydride was pre-dissolved in 1 mL of water and then added in batches. After stirring for 40 minutes at 50 °C, the reaction was transferred to and cooled in an ice bath for 15 min to precipitate the products. After filtration, the residue, i.e. the crude product, was collected and dried for 24 h. The filtrate containing ionic liquids was back-extracted by ethyl acetate and recycled for reuse.Reduction of berberine hydrochloride under solvent-free conditions without ILs
Four solvent-free synthesis methods were grinding, microwave radiation, ultrasonication and pressurization. Grinding was conducted by milling the reactants in a mortar with a diameter of 1 decimetre. 0.5 g of BH and an equivalent amount of sodium borohydride (0.05 g, pre-dissolved in 1 mL of water) were added together into the mortar. After grinding at room temperature for 0.5–4 h, the crude product was sifted through a mesh size of 80 and dried for 24 h. Microwave radiation and ultrasonication were both carried out in an ultrasound microwave integrated synthesizer. 0.5 g of BH and 0.05 g of sodium borohydride (pre-dissolved in 1 mL of water) were well-mixed and reacted at a power of 200, 350, 500, 650 and 800 W for 5–25 min, respectively. Finally, the pressing method was implemented with a laboratory table press under different pressures varying from 10 MPa to 30 MPa. Identical to the microwave radiation and ultrasonication methods, the reaction lasted for 5–25 min with an internal of 5 min.Reduction of berberine hydrochloride under solvent-free conditions with IL
A combination of solvent-free technologies and ionic liquid was realized with the most commonly used methods, including microwave radiation and ultrasonication. For a convenient comparison, the procedure and conditions were exactly the same as those of the aforementioned solvent-free technologies without ILs, except for the addition of 0.035 g of [HBth]HSO4 in the reactive system.Quantitative analysis of reduced products
Quantitative analysis of THB and DHB was realized via HPLC based on previous research.16 The optimal conditions were as follows. A C18 column was applied as the mobile phase and acetonitrile-0.1% triethylamine (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, V/V) at a flow rate of 1.0 mL min−1 with a 20 μL injection volume was the liquid phase. The temperature was set at 40 °C. THB was detected at 230 nm and DHB was detected at 374 nm based on UV full wavelength scanning. The samples and mobile phases were filtered through a 0.45 μm filter membrane before HPLC injection. An external standard method was applied for quantitative analysis of the crude product. Individual analyses of each sample were repeated six times to evaluate their repeatability.
35, V/V) at a flow rate of 1.0 mL min−1 with a 20 μL injection volume was the liquid phase. The temperature was set at 40 °C. THB was detected at 230 nm and DHB was detected at 374 nm based on UV full wavelength scanning. The samples and mobile phases were filtered through a 0.45 μm filter membrane before HPLC injection. An external standard method was applied for quantitative analysis of the crude product. Individual analyses of each sample were repeated six times to evaluate their repeatability.
Process analysis for solution and non-solvent reduction
The details and results are shown in ESI.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The research is supported by the National Natural Science Foundation of China (No. 81673316).Notes and references
- J. Li and M. D. Eastgate, React. Chem. Eng., 2019, 4, 1595–1607 RSC.
- (a) S. K. Kabra, M. Huuhtanen, R. L. Keiski and G. D. Yadav, React. Chem. Eng., 2016, 1, 330–339 RSC; (b) A. G. Amir, D. E. T. James and P. H. Jason, ACS Sustainable Chem. Eng., 2020, 8, 2462–2471 CrossRef; (c) Y. Zhang and C. Pozo-Gonzalo, Chem. Commun., 2018, 54, 3800–3810 RSC.
- (a) N. Kumar, K. Naveen, A. Bhatia, S. Muthaiah, V. Siruguri and A. K. Paul, React. Chem. Eng., 2020, 5, 1264–1271 RSC.
- D. E. Crawford, C. K. G. Miskimmin and A. B. Albadarin, Green Chem., 2017, 19, 1507–1518 RSC.
- C. A. B. Rodrigues, I. Neto, P. Rijo and C. A. M. Afanso, J. Chem. Educ., 2018, 95, 492–495 CrossRef CAS.
- (a) S. Hua, L. I. Sheng-Qing and C. Hao, et al. , J. Chem. Res., 2008, 19, 32–36 Search PubMed; (b) H. Ye, J. Huang and J. J. Xu, et al. , J. Power Sources, 2008, 178, 651–660 CrossRef CAS.
- J. Sun, H. Li, H. Song, Q. Wu, Y. Zhao and Q. Jiao, RSC Adv., 2015, 106, 87200–87205 RSC.
- H. F. Zhang, D. M. Liu, T. T. Kang, Y. Wang, X. Zhang and X. Zhu, Chin. J. Org. Chem., 2016, 36, 1104–1110 CrossRef CAS.
- D. Roy, S. James and D. Crawford, Chem. Commun., 2019, 55, 5463–5466 RSC.
- (a) J. M. Gao, W. T. Liu, M. L. Li, H. W. Liu, X. C. Zhang and Z. X. Li, J. Mol. Struct., 2008, 892, 466–469 CrossRef CAS; (b) S. Pingali, J. P. Donahue and F. Payton-Stewart, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 262–265 CrossRef CAS; (c) N. Turner, J. Y. Li, A. Gosby, S. W. C. To, Z. Cheng, H. Miyoshi, M. M. Taketo, G. J. Cooney, E. W. Kraegen, D. E. James, L. H. Hu, J. Li and J. M. Ye, Diabetes, 2008, 57, 1414–1418 CrossRef CAS; (d) L. Tan, Y. Wang, G. Ai, C. Luo, H. Chen, C. Li, H. Zeng, J. Xie, J. Chen and Z. Su, Int. Immunopharmacol., 2019, 75, 105802 CrossRef CAS.
- Y. Huang, X. Yuan, M. Chen, W. L. Song, J. Chen, Q. Fan, L. Tang and D. Fang, Carbon, 2019, 114, 449–456 CrossRef.
- K. Yimyam, A. Wongrueng, P. Rakruam and S. Nitayavardhana, Eng. J., 2017, 21, 11–23 CrossRef CAS.
- G. Y. Zhao, T. Jiang, H. X. Gao, B. X. Han, J. Huang and D. H. Sun, Green Chem., 2004, 6, 75–77 RSC.
- X. Li, Y. Q. Gu, Y. Yang, H. Song and S. Yao, Adv. Mater. Res., 2012, 396, 1969–1974 Search PubMed.
- X. Z. Dai, T. Yao, D. Tang, H. Song, L. C. Peng and S. Yao, J. Mol. Liq., 2016, 219, 923–929 CrossRef CAS.
- Z. Zhai, Y. Shi, X. M. Wu and X. P. Luo, Anal. Bioanal. Chem., 2006, 384, 939–945 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0re00362j |
| This journal is © The Royal Society of Chemistry 2021 |