Open Access Article
Open Access ArticleA chiroptical approach for the absolute stereochemical determination of P-stereogenic centers†
Debarshi
Chakraborty
a,
Hadi
Gholami‡
a,
Aritra
Sarkar
a,
Leo A.
Joyce§
 b,
James E.
Jackson
b,
James E.
Jackson
 a and
Babak
Borhan
a and
Babak
Borhan
 *a
*a
aMichigan State University, Department of Chemistry, East Lansing, MI 48824, USA. E-mail: babak@chemistry.msu.edu
bDepartment of Process Research and Development, Merck and Co, Inc., Rahway, NJ 07065, USA
First published on 7th December 2020
Abstract
A simple chiroptical solution for the absolute stereochemical determination for asymmetric phosphorus V stereocenters is presented. Strong coordination of the phosphorus oxide with the Zn-metallo center of the racemic host Zn-MAPOL 2 leads to an induced axial chirality of the host, yielding a strong ECCD signal. A mnemonic is proposed to correlate the asymmetry of the guest molecule with the observed ECCD signal.
Introduction
Optically active compounds containing P-stereogenic centers are of great importance and have been widely applied as chiral ligands, organocatalysts, and versatile synthons.1 Furthermore, a number of biologically active molecules, agrochemicals, and pharmaceuticals possess P-chirogenic centers.1a With the progress in asymmetric synthesis, access to these compounds has become more routine.2 Despite these recent advancements, there is a dearth of straightforward methods for the rapid stereochemical determination of these P-stereogenic centers.Current methods rely on X-ray crystallography, optical rotation, 2D NMR spectroscopy, and chemical derivatization to known compounds.3 These methods are generally slow and not a simple solution for most cases. Well diffracting crystals are required for X-ray analysis, a slow and laborious process that depends on the propensity of the molecule to form suitable crystals. Homochiral ortho-palladated complexes have been employed as a “reporter complex” to establish the stereochemistry of P-chiral compounds using 2D NMR spectroscopy.3a,3g These complexes bind with chiral phosphines, leading to diastereomeric forms that generate unique 1H NMR signals. Although this methodology has enjoyed some success, it is limited to symmetrically substituted bis or polydentate phosphines. Binding of non-symmetric bisphosphines leads to the formation of regio-isomers that complicates absolute stereochemical determination. Chiral mono-phosphines pose a more difficult problem due to their high rotameric lability that leads to ambiguous determination of absolute stereochemistry. Mislow and coworkers used ‘intersystem matching’ to correlate the Cotton effects seen for chiral sulfoxides to assign the absolute stereochemistry of analogous phosphine oxides.4 Nonetheless, the absolute stereochemistry of the sulfoxides is needed for this method. Zygo et al.5 utilized VCD to solve this problem for a single substrate, although, it has yet to become a routine method for this class of compounds. The difficulty often stems from the inability to predict an accurate set of solution phase conformers, due to the flexibility of the molecules and complexity of their structures.
With our prior experience in using porphyrin-based hosts as reporters of chirality, we set our goal to develop a simple, rapid, direct (derivatization free), and micro-scale methodology to assign the chirality of P-stereogenic compounds. We envisioned utilizing the principles of Exciton Coupled Circular Dichroism (ECCD) to develop a procedure that addresses this long-standing problem. This method enables the non-empiricalassignment of helicity for two or more interacting chromophores via coupling of their electric transition dipole moments.6 The porphyrin tweezer methodology, a host-guest interacting system, has been used successfully for the absolute stereochemical determination of various functionalities.7 Porphyrin tweezers are achiral, but highly chromophoric host systems that are able to adopt a specific helicity upon binding chiral guest molecules.
Results and discussion
The helicity of the porphyrin tweezer (P or M), derived from the ECCD signal, leads to the assignment of stereochemistry of the bound chiral molecule. A major limitation in using the tweezer strategy is the need for bidentate coordination with the guest molecule to induce helicity within the host/guest complex. Mono-phosphine oxides lack the presence of a secondary coordination site and also do not possess a suitable functional group to append the required secondary binding element. Recently, our group has developed a new host system MAPOL 1,8 having a rigid bis-phenol backbone which brings two porphyrins in closer proximity compared to a traditional tweezer, thus creating a smaller cavity. MAPOL 1, adopts a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of its P and M stereoisomers at room temperature (Fig. 1). This equilibrium is shifted in one direction through its interaction with a chiral guest molecule. Using this concept, MAPOL 1 has been successfully implemented as a reporter of chirality for chiral monoamines.8 Translation of point to axial chirality through hydrogen bonding interaction between the phenolic hydrogen atoms in the host with the chiral amine guest was the key to its success.
1 mixture of its P and M stereoisomers at room temperature (Fig. 1). This equilibrium is shifted in one direction through its interaction with a chiral guest molecule. Using this concept, MAPOL 1 has been successfully implemented as a reporter of chirality for chiral monoamines.8 Translation of point to axial chirality through hydrogen bonding interaction between the phenolic hydrogen atoms in the host with the chiral amine guest was the key to its success.
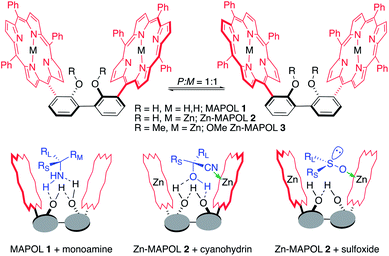 | ||
| Fig. 1 MAPOL and its analogs, used in the absolute stereochemical determination of amines, cyanohydrins, and sulfoxides. | ||
On the other hand, Zn-MAPOL 2, the zincated version of host MAPOL 1 bears two orthogonal binding elements i.e. hydrogen bonding with phenolic functionality and complexation with the zinc metal center (Fig. 1). Although for cyanohydrin both hydrogen bonding and ligand coordination to Zn metallo-center was necessary,9 chiral sulfoxides required coordination of the sulfoxide oxygen atom to the Zn metal to induce helicity of Zn-MAPOL 2 (Fig. 1).10 Intrigued by the results observed with sulfoxides, we realized that a similar approach might provide an effective chiroptical solution for the absolute stereochemical assignment of P-chiral phosphorus oxides. Coordination between the Zn2+ and the oxygen atom should yield a tightly bound host–guest complex, leading to the induction of axial chirality in the host molecule. Moreover, with recent advances in conversion of phosphine oxides to phosphines with stereochemical fidelity,2g–i,11 a routine method for the absolute stereochemical determination of phosphine oxides remains a critically important, but yet an unmet need.
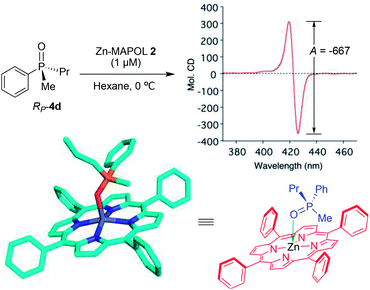
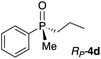
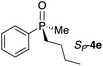
First, we turned our attention to UV-vis measurements to explore the binding of phosphine oxides to Zn-MAPOL 2. Addition of phosphine oxide RP-4d to Zn-MAPOL 2 led to a redshift from 413 nm to 423 nm, indicating the binding event between the host and the guest molecules (Fig. S1†). Titration of Zn-MAPOL 2 with different equivalents of RP-4d provided a binding isotherm revealing a Kassoc of ∼2.7 × 105 M−1 in hexane (Fig. S2†), while Job plot analysis indicated the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex (Fig. S3†).
1 complex (Fig. S3†).
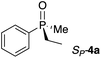
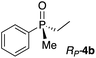
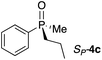
Gratifyingly, a strong negative ECCD signal (A = −667) was observed when only 5 equivalents of compound RP-4d was added to a solution of Zn-MAPOL 2 (1 μM) in hexane at 0 °C (Fig. 2). This low detection limit is the consequence of the high binding affinity for phosphine oxides with Zn-MAPOL 2. As anticipated, a positive ECCD signal was observed for SP-4c, the enantiomer of RP-4d (Table 1). The strength of the ECCD signal was sensitive to the temperature of measurement. As expected, the ECCD amplitude decreased with increasing temperature (Fig. S24†). Nevertheless, a significant signal is observed even at 45 °C, also indicative of the strong binding between the host and guest molecule.
| Entry | Predicted sign | λ nm, Δε | A/Acorra |
|---|---|---|---|
| a A corresponds to the amplitude for the ECCD spectrum, while Acorr is the amplitude after correction for the enantiopurity. b 5 equiv. of phosphine oxide. c 20 equiv. of phosphine oxide. | |||
|
Positive | 426, +127 | +223b/+328 |
| 419, −96 | |||
|
Negative | 426, −126 | −233b/−315 |
| 419, +107 | |||
|
Positive | 426, +279 | +497b/+637 |
| 419, −218 | |||
|
Negative | 426, −358 | −667b/−725 |
| 419, +309 | |||
|
Positive | 426, +308 | +561b/+813 |
| 419, −253 | |||
|
Negative | 427, −163 | −316c/−445 |
| 419, +153 | |||
|
Negative | 426, −561 | −1023b |
| 419, +462 | |||
|
Positive | 426, +287 | +501b/+590 |
| 419, −214 | |||
|
Negative | 425, −434 | −785b/−872 |
| 418, +351 | |||
With these preliminary results in hand, a variety of molecules possessing an asymmetric phosphorus oxide moiety with different alkyl and aryl substituents were synthesized according to reported procedures.2a,12 Complexation of these P-stereogenic compounds with Zn-MAPOL 2 resulted in strong ECCD signals centered around the Soret band of the host system (Table 1). In general, all SP phosphorus oxides produce positive ECCD spectra while RP phosphorus oxides yield negative ECCD spectra. Binding of RP-4d with methoxy Zn-MAPOL 3 produced a complex spectrum, (Fig. S31†) suggesting the presence of the hydroxyl groups in host 2 is necessary to yield a consistent ECCD signal. This observation can be explained based on the fact that intramolecular hydrogen bonding between the hydroxyl groups at 2,2′ positions of the host system helps in rigidifying the favored CD active conformer.
In order to derive a binding model for the host–guest system, numerous attempts to obtain a single crystal of any of the host–guest complexes were unfruitful. Nonetheless, the crystal structure of the phosphine oxide RP-4d bound to the Zn-tetraphenylporphyrin(Zn-TPP) as a mimic of the porphyrin ring in Zn-MAPOL 2 was obtained (Fig. 2). The crystal structure confirmed the coordination between the oxygen atom in phosphine oxide and the zinc metal of the metal porphyrin. It also revealed that upon coordinating with the zinc center, the chiral phosphine oxide RP-4d projects its smallest substituent (the methyl group) towards the porphyrin ring (Zn–O–P bond angle is 138°), thus projecting the medium and larger group away (Fig. 2). As depicted in Fig. 3, coordination of the oxygen lone pair with the Lewis acidic Zn center orients the guest molecule RP-4d in between the two porphyrin rings of Zn-MAPOL 2. Minimization of steric interactions leads to Zn-MAPOL 2 adopting a specific helicity. While the small methyl group is projected towards the bound porphyrin, the other two substituents are pointed towards the unbound porphyrin ring. An energetically minimized conformer would be achieved by placing the larger phenyl group (based on A strain values13) in a less sterically hindered space, thus favoring the M-(RP) complex. As such, the M helicity is energetically favored, leading to a negative ECCD signal. The latter speculation agrees with the observed ECCD signal for the complex of RP-4d with Zn-MAPOL 2 (Fig. 2). The predicted signs of all phosphine oxides 4a–4f, based on their A strain values, are in full agreement with the experimentally observed values (Table 1). The Zn-MAPOL 2 host system is capable of discerning small differences with ease. For example, steric differentiation of methyl and ethyl, or phenyl and o-anisyl, leads to large ECCD signals, although their sizes are not significantly different (Table 1). A note regarding substituents that do not have a reported A strain value; in such cases, we rely on prediction of size based on the relative size of the substituent, favoring the decision on factors that affect A strain values the most. These factors are the nature of the first atom at the point of attachment (degree of substitution), and its associated bond length.13
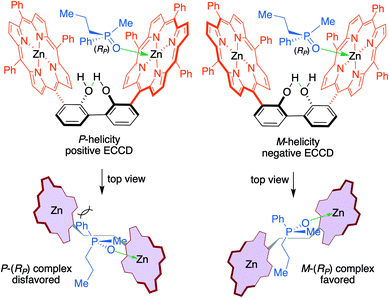 | ||
| Fig. 3 Putative binding of RP-4d to Zn-MAPOL 2, illustrating the competing diastereomeric forms [P-(RP) and M-(RP)]. Considering the binding paradigm in Fig. 2, with the small methyl group directed towards the porphyrin ring bound to the phosphine oxide, the projection of the medium (propyl) and the large (phenyl) groups towards the second porphyrin leads to the prediction for the preponderance of the M-helicity (relieving strain associated with the large phenyl group). As observed experimentally, this arrangement would predict a negative ECCD spectrum. | ||
The successful application of Zn-MAPOL 2 to determine the absolute configuration of simple phosphorus oxides prompted the investigation of more complex substrates to examine the generality and applicability of this methodology. Compound RP-4g and its epimer SP-4h (epimeric at the P center only) bear multiple stereocenters. Nonetheless, they produced opposite ECCD signals when complexed with Zn-MAPOL 2, thus highlighting the sensitivity of the system to the asymmetry at the P-chiral center (Table 1). The same binding model as described above (Fig. 3) also predicts the absolute stereochemistry of these two epimers based on the size of the substituents in the order of Ph > OMen > Me (as described above, OMen is perceived to be smaller than Ph although its A strain value is not known). Expanding the substrate scope with compound RP-4i, which has a hydrogen atom in place of the Me group in RP-4g (Ph > OMen > H), produced the anticipated ECCD signal (Table 1).
Next, the system was challenged with molecules that not only contain multiple stereocenters, but also have coordinating functionalities, which have the potential to bind with the zincated porphyrin. This would demonstrate the practical use of Zn-MAPOL 2 with a pharmaceutical API with a complex structure. Hence, we decided to analyze sofosbuvir 5, a commercial antiviral agent, widely used for the treatment of hepatitis C virus (HCV).2l The absolute stereochemistry of 5 was determined previously by X-ray crystallography.14 In addition to the phosphoramidate functionality, sofosbuvir 5 contains hydroxyl, ester, fluoride, and imide moieties, which could bind to the zincated porphyrin. Nonetheless, we posited that the strong binding affinity for phosphorus oxide complexation with the zincated porphyrin (∼270![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1) should prevent interference from other groups. As a point of comparison, to date we have found amines and sulfoxides as the strongest binders of Zn-TPP, with binding affinities of ∼11
000 M−1) should prevent interference from other groups. As a point of comparison, to date we have found amines and sulfoxides as the strongest binders of Zn-TPP, with binding affinities of ∼11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–15
000–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1.7b,10 Complexation of Sofosbuvir 5 with Zn-MAPOL 2 in hexane resulted in a strong positive ECCD signal (Fig. S13†). Unexpectedly, epi-sofosbuvir (epi-5), epimeric only at the P-stereocenter, also resulted in a positive ECCD signal (Fig. S14†), albeit with lower amplitude. We proposed that although various functional groups in sofosbuvir 5, such as the ester, imide, fluoride, or hydroxyl, would have a substantially reduced binding affinity in comparison to the phosphoramidate, the presence of the second metalloporphyrin does not preclude the potential dual binding of other functional groups. In fact, the initial strong binding of the phosphoramidate would entropically favor the binding of another functional group with the second porphyrin.
000 M−1.7b,10 Complexation of Sofosbuvir 5 with Zn-MAPOL 2 in hexane resulted in a strong positive ECCD signal (Fig. S13†). Unexpectedly, epi-sofosbuvir (epi-5), epimeric only at the P-stereocenter, also resulted in a positive ECCD signal (Fig. S14†), albeit with lower amplitude. We proposed that although various functional groups in sofosbuvir 5, such as the ester, imide, fluoride, or hydroxyl, would have a substantially reduced binding affinity in comparison to the phosphoramidate, the presence of the second metalloporphyrin does not preclude the potential dual binding of other functional groups. In fact, the initial strong binding of the phosphoramidate would entropically favor the binding of another functional group with the second porphyrin.
We surmised that the use of the non-interacting solvent, hexane, increases the availability for binding of a second functionality. It is noteworthy that the metalloporphyrin strategy (i.e., the porphyrin tweezer methodology or the Zn-MAPOL system) for absolute stereochemical determination of most functional groups fails in polar solvents, presumably because of the attenuated binding affinities, either as a result of solvation of the functional group, and/or competing solvent coordination with the metalloporphyrin. Nonetheless, the high binding affinity of the phosphoramidate moiety with the zincated porphyrin suggests a solution to remove secondary interactions that originate from less efficient binding functionalities. The use of a more polar solvent would not only solvate the interfering groups, but also, would decrease their binding affinity with the zincated porphyrin. This would eliminate the secondary interaction that interferes with desired sole stereodifferentiation based on the asymmetry of the P chiral center.
After a quick survey, chlorinated solvents, such as dichloromethane, chloroform, and chlorobenzene provided the best balance, where sofosbuvir 5, maintained its positive ECCD signal when bound to Zn-MAPOL 2, while complexation of epi-5 resulted in a negative ECCD signal (Fig. 4). The binding model follows the mnemonic proposed above (Fig. 3), in which the smallest group (phenoxyl) on the P-stereogenic center points towards the bound porphyrin ring, while the medium (amino ester) and large (uracil) substituents on the phosphorus atom project towards the unbound porphyrin ring (note that the assignment of size here is based on our perceived notion of volume, since A strain values are not available for complex substituents as those found in 5). This leads to the prediction of a positive ECCD signal for 5, as observed experimentally. Expectedly, a counterclockwise twist would be favored in the binding of epi-5 with Zn-MAPOL 2, yielding a negative ECCD signal. Indeed, the measured ECCD signals are in full agreement with the proposed binding scenario (Fig. 4).
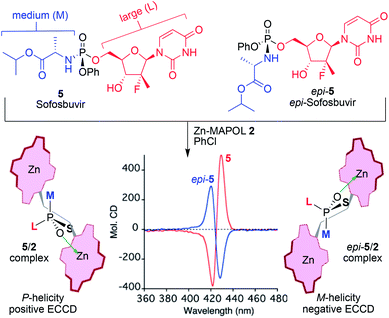 | ||
| Fig. 4 Sofosbuvir and epi-sofosbuvir bound to Zn-MAPOL 2 lead to positive and negative ECCD, respectively. The sign of the ECCD spectra depends solely on the absolute stereochemistry of the P-chiral center. The helicity of 2 is correlated to the absolute stereochemistry of the phosphorus atom by applying the mnemonic described in Fig. 3, considering the large (L), medium (M), and small (OPh, S) as depicted in the structure of sofosbuvir 5. | ||
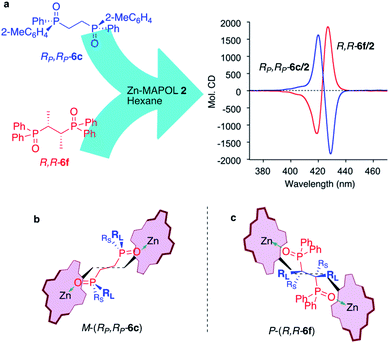
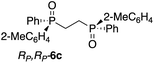
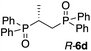
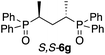
The use of Zn-MAPOL 2 as a ‘reporter of chirality’ was extended to the chiroptical sensing of P-chiral bis-phosphine oxides, molecules that are important in asymmetric catalysis.1a The bidentate coordination of bis-phosphine oxides with Zn-MAPOL 2 was anticipated, putatively leading to even a stronger complex as compared to the binding of mono-phosphorous oxides. The investigations were initiated with the complexation of RP,RP-6c with Zn-MAPOL 2, which led to the generation of a strong ECCD signal (A = −3460, Fig. 5a). Due to strong binding affinities, only 1 equivalent of the substrate (∼0.5 μg of sample, 1 nmol, 1 μM) is necessary to obtain ECCDs with large amplitudes for all of the bis-phosphine oxides examined. Compound SP,SP-6a, easily obtained from the oxidation of DIPAMP,15 also generates the anticipated ECCD signal (Table 2), illustrating the practical applicability of the methodology for the absolute stereochemical determination of chiral bis-phosphines that are widely used in transition metal catalysis. As expected, its enantiomer RP,RP-6b yields a negative ECCD signal of similar intensity.
| Entry | Predicted sign | λ nm, Δε | A/Acorra |
|---|---|---|---|
| a A corresponds to the amplitude for the ECCD spectrum, measured with the addition of 1 equiv. of the bis-phosphine oxide to a 1 μM Zn-MAPOL 2 solution. | |||
|
Positive | 427, +416 | +612 |
| 418, −196 | |||
|
Negative | 427, −426 | −654 |
| 418, +228 | |||
|
Negative | 429, −1838 | −3460 |
| 420, +1622 | |||
|
Positive | 429, +332 | +586 |
| 421, −254 | |||
|
Negative | 427, −1646 | −2770 |
| 419, +1124 | |||
|
Positive | 427, +1862 | +3110 |
| 419, −1248 | |||
|
Negative | 427, −1350 | −2154 |
| 418, +804 | |||
Fig. 5b depicts the proposed mnemonic for stereodifferentiation that leads to the observed ECCD spectra. We assume each phosphorus center dictates the orientation of the attached porphyrin independently, leading to a preferred helicity of the host system. As such, each porphyrin binds the phosphorus center in a manner to minimize interactions with the larger of the two substituents (2-Me-C6H4vs. Ph, RP,RP-6c). Note that in this mnemonic the chain connecting the two phosphorus centers is not part of the stereodifferentiation. Also important is the fact that the two substituents on the phosphorus center are oriented away from the biphenol linker in order to reduce steric interactions. RP,RP-6c represented in Fig. 5b, leads to a counter-clockwise rotation of the two porphyrin rings with respect to each other, thus predicting a negative ECCD (M-helicity), in accordance to the experimentally observed signal.
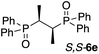
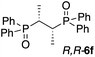
Thus far, all of the examples have demonstrated ‘chiral sensing’ of molecules with an asymmetric phosphorus atom. Nonetheless, an important cohort of the bidentate phosphorus ligands are not asymmetric at the P-center, but rather the adjacent carbon atom. An example is Chiraphos,16 which upon routine oxidation (H2O2)11c yields the bis-phosphine oxide R,R-6f. Illustrated in Fig. 5a, complexation of 1 equivalent of R,R-6f with Zn-MAPOL 2 yields a positive ECCD signal (A = +3110). Table 2 lists a number of these molecules (6d–6g) complexed with Zn-MAPOL 2, where consistently strong ECCD signals were observed. Fig. 5c depicts the proposed binding model that yields the predicted helicity of the complex. Similar to the model described above, the phenyl substituents on the phosphorus atom are placed in the most sterically unencumbered space, while RL and RS (the large and small α-carbon substituents, respectively) dictate the helicity by avoiding interactions with the biphenol linker (the small group points towards the linker). In the mnemonic developed for R,R-6f (Fig. 5c), this leads to a clockwise (P-helical) orientation of the porphyrins, matching the experimentally observed positive ECCD signal.
Conclusions
In conclusion, we have developed a simple chiroptical based methodology for the direct assignment of absolute stereochemistry of chiral phosphorus compounds without the need for derivatization. The protocol is suitable for the stereochemical determination of phosphorus stereocenters in mono-phosphine oxides, phosphinates, phosphoramidates, as well as bis-phosphine oxides, chiral at either the P-center or the α-carbon. High binding affinities enable the detection of stereochemistry for P-stereogenic center of molecules with multiple functionalities that could also bind with the zincated porphyrin. Simple mnemonics for each class of molecules correlates the observed ECCD signal with the stereochemistry.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We thank Dr Richard Staples (Michigan State University) for solving the crystal structure of RP-4d bound to Zn-TPP. We are grateful to the NSF (CHE-1213759) for the support of this research.Notes and references
- (a) M. Dutartre, J. Bayardon and S. Juge, Chem. Soc. Rev., 2016, 45, 5771–5794 RSC; (b) A. Grabulosa, J. Granell and G. Muller, Coord. Chem. Rev., 2007, 251, 25–90 CrossRef CAS; (c) C. J. A. Warner, A. T. Reeder and S. Jones, Tetrahedron: Asymmetry, 2016, 27, 136–141 CrossRef CAS; (d) J. Loup, V. Muller, D. Ghorai and L. Ackermann, Angew. Chem., Int. Ed., 2019, 58, 1749–1753 CrossRef CAS PubMed.
- (a) H. Adams, R. C. Collins, S. Jones and C. J. A. Warner, Org. Lett., 2011, 13, 6576–6579 CrossRef CAS PubMed; (b) R. Beaud, R. J. Phipps and M. J. Gaunt, J. Am. Chem. Soc., 2016, 138, 13183–13186 CrossRef CAS PubMed; (c) E. Bergin, C. T. O'Connor, S. B. Robinson, E. M. McGarrigle, C. P. O'Mahony and D. G. Gilheany, J. Am. Chem. Soc., 2007, 129, 9566–9567 CrossRef CAS PubMed; (d) Z. X. S. Han, N. Goyal, M. A. Herbage, J. D. Sieber, B. Qu, Y. B. Xu, Z. B. Li, J. T. Reeves, J. N. Desrosiers, S. L. Ma, N. Grinberg, H. Lee, H. P. R. Mangunuru, Y. D. Zhang, D. Krishnamurthy, B. Z. Lu, J. H. J. Song, G. J. Wang and C. H. Senanayake, J. Am. Chem. Soc., 2013, 135, 2474–2477 CrossRef CAS PubMed; (e) Z. J. Huang, X. Huang, B. S. Li, C. L. Mou, S. Yang, B. A. Song and Y. R. Chi, J. Am. Chem. Soc., 2016, 138, 7524–7527 CrossRef CAS PubMed; (f) Y. S. Jang, L. Wozniak, J. Pedroni and N. Cramer, Angew. Chem., Int. Ed. Engl., 2018, 57, 12901–12905 CrossRef CAS PubMed; (g) Q. Dai, W. B. Li, Z. M. Lo and J. L. Zhang, J. Am. Chem. Soc., 2019, 141, 20556–20564 CrossRef CAS PubMed; (h) B. M. Trost, S. M. Spohr, A. B. Rolka and C. A. Kalnmals, J. Am. Chem. Soc., 2019, 141, 14098–14103 CrossRef CAS PubMed; (i) D. Xu, N. Rivas-Bascon, N. M. Padial, K. W. Knouse, B. Zheng, J. C. Vantourout, M. A. Schmidt, M. D. Eastgate and P. S. Baran, J. Am. Chem. Soc., 2020, 142, 5785–5792 CrossRef CAS PubMed; (j) G. R. Genov, J. L. Douthwaite, A. S. K. Lahdenpera, D. C. Gibson and R. J. Phipps, Science, 2020, 367, 1246–1251 CrossRef CAS PubMed; (k) K. W. Knouse, J. N. deGruyter, M. A. Schmidt, B. Zheng, J. C. Vantourout, C. Kingston, S. E. Mercer, I. M. McDonald, R. E. Olson, Y. Zhu, C. Hang, J. Zhu, C. Yuan, Q. Wang, P. Park, M. D. Eastgate and P. S. Baran, Science, 2018, 361, 1234–1238 CrossRef CAS PubMed; (l) D. A. DiRocco, Y. N. Ji, E. C. Sherer, A. Klapars, M. Reibarkh, J. Dropinski, R. Mathew, P. Maligres, A. M. Hyde, J. Limanto, A. Brunskill, R. T. Ruck, L. C. Campeau and I. W. Davies, Science, 2017, 356, 426–429 CrossRef CAS PubMed.
- (a) Q. Z. Jiang, H. Ruegger and L. M. Venanzi, J. Organomet. Chem., 1995, 488, 233–240 CrossRef CAS; (b) I. L. Karle, J. M. Karle, W. Egan, G. Zon and J. A. Brandt, J. Am. Chem. Soc., 1977, 99, 4803–4807 CrossRef CAS PubMed; (c) O. Korpium and K. Mislow, J. Am. Chem. Soc., 1967, 89, 4784–4786 CrossRef; (d) O. Korpiun, R. A. Lewis, J. Chickos and K. Mislow, J. Am. Chem. Soc., 1968, 90, 4842–4846 CrossRef CAS; (e) R. A. Lewis, O. Korpiun and K. Mislow, J. Am. Chem. Soc., 1968, 90, 4847–4853 CrossRef CAS; (f) R. A. Lewis and K. Mislow, J. Am. Chem. Soc., 1969, 91, 7009–7012 CrossRef CAS; (g) V. V. Dunina, L. G. Kuz'mina, M. Y. Rubina, Y. K. Grishin, Y. A. Veits and E. I. Kazakova, Tetrahedron: Asymmetry, 1999, 10, 1483–1497 CrossRef CAS.
- F. D. Saeva, D. R. Rayner and K. Mislow, J. Am. Chem. Soc., 1968, 90, 4176–4178 CrossRef CAS.
- F. Wang, Y. Wang, P. L. Polavarapu, T. Y. Li, J. Drabowicz, K. M. Pietrusiewicz and K. Zygo, J. Org. Chem., 2002, 67, 6539–6541 CrossRef CAS PubMed.
- (a) C. Wolf and K. W. Bentley, Chem. Soc. Rev., 2013, 42, 5408–5424 RSC; (b) L. You, D. J. Zha and E. V. Anslyn, Chem. Rev., 2015, 115, 7840–7892 CrossRef CAS PubMed; (c) H. Lu and N. Kobayashi, Chem. Rev., 2016, 116, 6184–6261 CrossRef CAS PubMed; (d) D. Pasini and A. Nitti, Chirality, 2016, 28, 116–123 CrossRef CAS PubMed; (e) N. Berova, P. L. Polavarapu, K. Nakanishi and R. W. Woody, Applications in Stereochemical Analysis of Synthetic Compounds, Natural Products, and Biomolecules, Wiley, Hoboken, NJ, 2012 Search PubMed.
- (a) X. Li and B. Borhan, J. Am. Chem. Soc., 2008, 130, 16126–16127 CrossRef CAS PubMed; (b) X. Li, M. Tanasova, C. Vasileiou and B. Borhan, J. Am. Chem. Soc., 2008, 130, 1885–1893 CrossRef CAS PubMed; (c) X. Y. Li, C. E. Burrell, R. J. Staples and B. Borhan, J. Am. Chem. Soc., 2012, 134, 9026–9029 CrossRef CAS PubMed; (d) M. Tanasova, M. Anyika and B. Borhan, Angew. Chem., Int. Ed., 2015, 54, 4274–4278 CrossRef CAS PubMed; (e) M. Tanasova, C. Vasileiou, O. O. Olumolade and B. Borhan, Chirality, 2009, 21, 374–382 CrossRef CAS PubMed; (f) X. Huang, B. Borhan, B. H. Rickman, K. Nakanishi and N. Berova, Chem.–Eur. J., 2000, 6, 216–224 CrossRef CAS PubMed.
- M. Anyika, H. Gholami, K. D. Ashtekar, R. Acho and B. Borhan, J. Am. Chem. Soc., 2014, 136, 550–553 CrossRef CAS.
- H. Gholami, M. Anyika, J. Zhang, C. Vasileiou and B. Borhan, Chem.–Eur. J., 2016, 22, 9235–9239 CrossRef CAS PubMed.
- H. Gholami, J. Zhang, M. Anyika and B. Borhan, Org. Lett., 2017, 19, 1722–1725 CrossRef CAS PubMed.
- (a) T. Imamoto, S. Kikuchi, T. Miura and Y. Wada, Org. Lett., 2001, 3, 87–90 CrossRef CAS PubMed; (b) K. V. Rajendran and D. G. Gilheany, Chem. Commun., 2012, 48, 817–819 RSC; (c) H. C. Wu, J. Q. Yu and J. B. Spencer, Org. Lett., 2004, 6, 4675–4678 CrossRef CAS PubMed.
- (a) I. Fernandez, N. Khiar, A. Roca, A. Benabra, A. Alcudia, J. L. Espartero and F. Alcudia, Tetrahedron Lett., 1999, 40, 2029–2032 CrossRef CAS; (b) Q. Xu, C. Q. Zhao and L. B. Han, J. Am. Chem. Soc., 2008, 130, 12648–12655 CrossRef CAS.
- E. L. Eliel, S. H. Wilen and L. N. Mander, Stereochemistry of organic compounds, Wiley, New York, 1994 Search PubMed.
- M. J. Sofia, D. Bao, W. Chang, J. Du, D. Nagarathnam, S. Rachakonda, P. G. Reddy, B. S. Ross, P. Wang, H. R. Zhang, S. Bansal, C. Espiritu, M. Keilman, A. M. Lam, H. M. Steuer, C. Niu, M. J. Otto and P. A. Furman, J. Med. Chem., 2010, 53, 7202–7218 CrossRef CAS PubMed.
- B. D. Vineyard, W. S. Knowles, M. J. Sabacky, G. L. Bachman and D. J. Weinkauff, J. Am. Chem. Soc., 1977, 99, 5946–5952 CrossRef CAS.
- (a) A. S. C. Chan, J. J. Pluth and J. Halpern, J. Am. Chem. Soc., 1980, 102, 5952–5954 CrossRef CAS; (b) M. Yamaguchi, T. Shima, T. Yamagishi and M. Hida, Tetrahedron: Asymmetry, 1991, 2, 663–666 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2005419. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc02940h |
| ‡ Present address: Janssen Pharmaceutical Research & Development, LLC, 3210 Merryfield Row, San Diego, CA 92121, USA. |
| § Present address: Arrowhead Pharmaceuticals, 502 South Rosa Rd, Madison, WI 53719, USA. |
| This journal is © The Royal Society of Chemistry 2021 |