Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cd-driven surface reconstruction and photodynamics in gold nanoclusters†
Xu
Liu‡
 a,
Guo
Yao‡
b,
Xinglian
Cheng‡
a,
Jiayu
Xu
a,
Xiao
Cai
a,
Weigang
Hu
a,
Wen Wu
Xu
a,
Guo
Yao‡
b,
Xinglian
Cheng‡
a,
Jiayu
Xu
a,
Xiao
Cai
a,
Weigang
Hu
a,
Wen Wu
Xu
 *c,
Chunfeng
Zhang
b and
Yan
Zhu
*c,
Chunfeng
Zhang
b and
Yan
Zhu
 *a
*a
aSchool of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, China. E-mail: zhuyan@nju.edu.cn
bSchool of Physics, Nanjing University, Nanjing 210093, China
cSchool of Physical Science and Technology, Ningbo University, Ningbo 315211, China. E-mail: xuwenwu@nbu.edu.cn
First published on 5th January 2021
Abstract
With atomically precise gold nanoclusters acting as a starting unit, substituting one or more gold atoms of the nanocluster with other metals has become an effective strategy to create metal synergy for improving catalytic performances and other properties. However, so far detailed insight into how to design the gold-based nanoclusters to optimize the synergy is still lacking, as atomic-level exchange between the surface-gold (or core-gold) and the incoming heteroatoms is quite challenging without changing other parts. Here we report a Cd-driven reconstruction of Au44(DMBT)28 (DMBT = 3,5-dimethylbenzenethiol), in which four Au2(DMBT)3 staples are precisely replaced by two Au5Cd2(DMBT)12 staples to form Au38Cd4(DMBT)30 with the face-centered cubic inner core retained. With the dual modifications of the surface and electronic structure, the Au38Cd4(DMBT)30 nanocluster exhibits distinct excitonic behaviors and superior photocatalytic performances compared to the parent Au44(DMBT)28 nanocluster.
Introduction
Metal synergy is of paramount importance as the rationale to modulate the intrinsic properties of metal nanoparticles.1,2 However, the precise synergistic interaction in an intermetallic nanoparticle has so far been elusive, due to the challenges in determining the atomic-level arrangement of the metal heteroatoms in the nanoparticle. Atomically precise metal nanoparticles (often called nanoclusters) lead to unprecedented opportunities in signalling clear directions to exploit the cooperativity between the two metal elements within a single nanocluster.3 Thiolate-protected gold nanoclusters, Aun(SR)m, where n is the number of gold atoms and m is the number of thiolate ligands, SR, have gained momentum over the past few years as an exciting area and have opened up new horizons in precise tailoring of the composition and structure to control the physicochemical properties.4–12 The Aun(SR)m nanoclusters are typically configured with an inner gold core (or kernel) and various surface motifs, in which the motifs containing both gold and thiolate resemble staples. Both the gold core and the surface motifs can contribute to the physicochemical properties such as the optical and electronic properties, as well as catalysis.13–18 It has been recognized that substituting one or more gold atoms in either the core or the motifs with other metals can tune the overall performances of the parent nanoclusters.19–26 Therefore, it has become possible to access the previously inaccessible metal synergy in the bimetallic nanoclusters with atomic-precision.Among the gold-based bimetal nanoclusters, cadmium-containing bimetal clusters provide synergistic strategies to adjust the electronic structures and further modulate the physicochemical properties in the clusters, since Cd has one more valence electron than Au.21,26,27 Cd introduction usually causes surface reconstruction of gold nanoclusters. For example, Au19Cd2(SR)16 was obtained through the substitution of two neighboring surface Au atoms with one Cd with the cuboctahedral Au13 unchanged.26 Au19Cd3(SR)18 was formed by retaining the icosahedral Au13 core but only changing the surface of Au25(SR)18.27 However, the surface reconstruction strategy remains challenging and no examples of bimetal clusters formed without breaking the face-centered cubic (fcc) core of the parent gold clusters have been documented, which might thus impede gaining a higher understanding of how to tailor the surface structure of gold-based nanoclusters and accordingly optimize their synergy.
Herein, we report our success in synthesizing a Au38Cd4(DMBT)30 (DMBT = 3,5-dimethylbenzenethiol) nanocluster that is obtained from the surface reconstruction of Au44(DMBT)28 induced by Cd2+. The fcc Au26 kernel originating from Au44(DMBT)28, which is assembled from Au4 tetrahedra, is retained in the Au38Cd4(DMBT)30. The Au38Cd4(DMBT)30 nanocluster exhibits distinctly different excited-state dynamics from Au44(DMBT)28. More interestingly, the Au38Cd4(DMBT)30 nanocluster as a photocatalyst shows better visible light-driven catalytic activity than the parent Au44(DMBT)28 catalyst.
Results and discussion
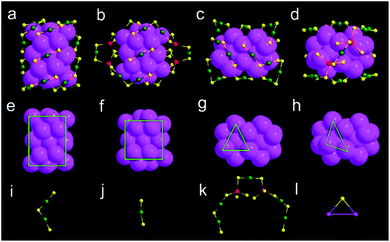
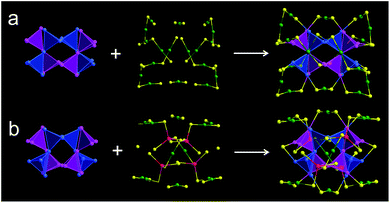
X-ray crystallography analysis shows that the parent Au44(DMBT)28 nanocluster is composed of an Au26 kernel, six Au2(SR)3 and two Au(SR)2 staples (Fig. 1a, c and Table S1†). The formula of Au44(DMBT)28 is further confirmed by electrospray ionization mass spectroscopy (ESI-MS, Fig. S1a†). The structural framework of Au44(DMBT)28 is identical to that of the reported Au44(TBBT)28 (TBBT = 4-tert-butylbenzenethiol) (Fig. S2†),28 both of which can be assembled into the layered structures (Fig. S3–S5†). Notably, a significant difference is observed in the layer's interior, where all the molecules of Au44(TBBT)28 in the layer (marked with the same color, Fig. S3†) are packed along the same direction, while Au44(DMBT)28 molecules are arranged in different directions (Fig. S5†). Such a difference may be ascribed to the different steric hindrance between TBBT and DMBT. The UV-vis-NIR spectra of the two Au44(SR)28 nanoclusters show only small deviations. As shown in Fig. S6,† the prominent peak at 380 nm for Au44(TBBT)28 is slightly red-shifted to 388 nm for Au44(DMBT)28, and the broad peaks at 650 and 725 nm become apparent when TBBT is replaced by DMBT.With Au44(DMBT)28 as a starting unit, a Cd-doped nanocluster was further synthesized via an ion-exchange strategy. From ESI-MS data (Fig. S1b†), the prominent peak at 6025.43 m/z with a +2 charge is assigned to Au38Cd4(DMBT)30 (theoretical value: 6025.48 m/z), which is further confirmed by the excellent match between experimental and calculated isotopic patterns (inset of Fig. S1b†). Single crystallography analysis reveals that Au38Cd4(DMBT)30 contains a 26-Au-atom kernel, two Au5Cd2(SR)12 staples, two Au(SR)2 staples and two bridging SR ligands, as shown in Fig. 1b, d, and Table S2.† Note that the retained kernel of Au38Cd4(DMBT)30 experiences a slight distortion from “slender” to “stocky” in comparison with that of the parent Au44(DMBT)28 (Fig. 1e–h). Further analysis shows that the Au26 kernel in Au38Cd4(DMBT)30 can be viewed as the assembly of tetrahedral Au4 units in a double-helical mode, as well as that in Au44(DMBT)28 (Fig. 2). Furthermore, the two nanoclusters have almost identical distances between neighboring Au4 units, which is clearly manifested in the similar Au–Au bond lengths according to the different positions of the Au atoms (Fig. S7†). Therefore, Au38Cd4(DMBT)30 can be viewed as the gentle surface reconstruction without breaking the double-helical Au26 kernel based on the parent Au44(DMBT)28. In addition, Au38Cd4(DMBT)30 is also patterned along different directions in the layer structure (Fig. S8†).
To gain an in-depth insight into the Cd-induced surface reconstruction mechanism, density functional theory (DFT) calculations were performed. Starting from the Au44(SR)28 cluster, as presented in Fig. S9,† four Au2(SR)3− protecting motifs of Au44(SR)28 are substituted by six Cd(SR)2, resulting in the formation of an unstable intermediate, Au36Cd6(SR)284+, due to the very high substitution energy (24.80 eV, step 1). After this, a surface isomerization of Au36Cd6(SR)284+ is considered via the reorganization of the motifs (step 2). In this step, the Cd atom in Cd(SR)2 binds with a neighboring S atom in the SR[Au(SR)]2− motif, which is accompanied by the breaking of a Au–S bond and the formation of a naked Au atom. The S atom in Cd(SR)2 binds with this naked Au atom to form a new Au–S bond. Therefore, the structure in which the three-coordinated μ3-Cd atoms bind with two SR− and one Au(SR)2− can be obtained during the structural isomerization of Au36Cd6(SR)284+. In addition, the Au36Cd6(SR)284+ becomes more stable after isomerization via lowering the energy by 3.37 eV. Then, two S atoms of the SR[Au(SR)]− motif further bind with two μ3-Cd atoms to form two four-coordinated μ4-Cd atoms, resulting in the formation of a more stable intermediate structure, Au38Cd6(SR)322+, with a formation energy of −18.64 eV (step 3). In step 4, the SR− motif binds with the Cd atom of Cd(SR)2 to form Cd(SR)3−, in which the Cd(SR)2 is quickly separated from Cd(SR)3− leaving a bridging SR− motif on the surface of the Au core. Finally, the stable Au38Cd4(SR)30 is formed with a formation energy of −12.50 eV. The proposed conversion process from Au44(SR)28 to Au38Cd4(SR)30 includes two key steps: (i) the substitution of SR[Au(SR)]2− by Cd(SR)2 and (ii) the structural isomerization of surface ligands.
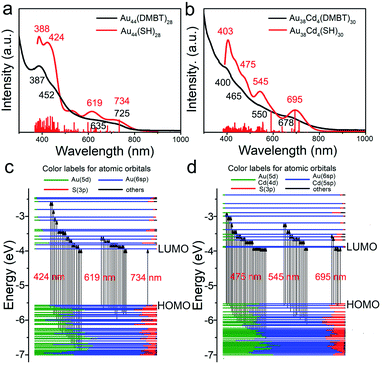
To investigate the electronic structure changes induced by Cd-atom surface modification, the optical adsorption spectra of the Au44(DMBT)28 and Au38Cd4(DMBT)30 nanoclusters were measured. The absorption peaks of Au38Cd4(DMBT)30 are mainly centered at 400, 465, 550 and 678 nm (Fig. 3b), which differ from those observed in the parent nanocluster (387, 452, 635 and 725 nm; Fig. 3a). These optical features can be well reproduced by theoretical calculations (Fig. 3a, b and S10†). The Kohn–Sham (KS) molecular orbital (MO) energy levels and atomic orbital components in each KS MO of Au44(SR)28 and Au38Cd4(SR)30 suggest that the absorption peaks mainly involve the Au(sp) → Au(sp) transitions (Fig. 3c and d). In particular, for Au44(SR)28, the first absorption peak centered at 734 nm originates from the highest occupied molecular orbital → the lowest unoccupied molecular orbital (HOMO → LUMO) transition, while for Au38Cd4(SR)30, the first absorption peak centered at 695 nm originates from the HOMO → LUMO, HOMO → LUMO+1, HOMO → LUMO+4, HOMO−1 → LUMO, HOMO−1 → LUMO+1, and HOMO−1 → LUMO+5 transitions. The more complex orbital transitions in Au38Cd4(SR)30 than in Au44(SR)28 can be attributed to the dopant Cd. This behaviour can also be observed for other absorption peaks.
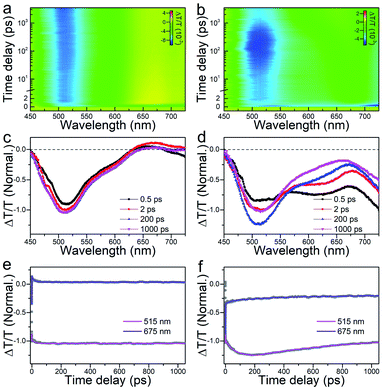
Moreover, femtosecond and nanosecond carrier dynamics of the two nanoclusters were measured via time-resolved transient absorption (TA) spectroscopy to decipher their potential energy-related applications. The femtosecond-resolved TA spectra of the Au44(DMBT)28 and Au38Cd4(DMBT)30 nanoclusters are provided in Fig. 4a and b. Similarly, both Au44(DMBT)28 and Au38Cd4(DMBT)30 nanoclusters showed broad excited state absorption (ESA) signals overlapped with ground state bleaching (GSB) peaks near 675 nm. We selectively extracted the TA spectra at different delay times, combined with the dynamic traces probed at 515 and 675 nm to study the transient evolution and the relaxation dynamics (Fig. 4c–f). A 0.6 ps process at the early stage, which is attributed to the ultrafast internal conversion from higher excited states to lower excited states,29 was observed in the two nanoclusters (Fig. S11 and Table S3†). It is worth noting that the major divergence between the two nanocluster systems emerged after a delay of 2 ps. For Au44(DMBT)28, the TA spectra remained nearly unchanged after 2 ps (Fig. 4c), which is consistent with the flat decay kinetic traces shown in Fig. 4e. A 19 ps process obtained by exponential fitting was ascribed to the structural relaxation caused by conformational changes after pumping.29–31 For Au38Cd4(DMBT)30, interestingly, an obvious spectral transformation was observed and the lifetime of this component was determined to be 57 ps (Table S3†), which differs from the 19 ps structural relaxation observed in Au44(DMBT)28 and might be related to the charge transfer states between the ligand and the metal core of Au38Cd4(DMBT)30,32–35 which can be manifested by the overall Hirshfeld charge of the Au26 core in Au38Cd4(SH)30 (0.46) and in Au44(SH)28 (0.56). Of note, deduced from nanosecond-resolved TA analysis, as shown in Fig. S12,† the Au38Cd4(DMBT)30 nanocluster exhibits a faster carrier recombination process with a lifetime of 253 ns than the Au44(DMBT)28 nanocluster (389 ns lifetime), readily supporting the putative synergy in the Au38Cd4(DMBT)30 nanocluster being invoked in underpinning the excited-state dynamics.
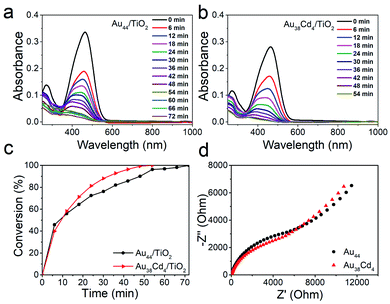
The distinguishable electronic and optical properties of the two nanoclusters would apparently impact their catalytic properties. Thus, visible light-driven degradation of methyl orange was selected to explore the photocatalysis of the two nanoclusters. From Fig. 5a and b, within 50 min, methyl orange can be completely degraded on the Au38Cd4(DMBT)30 catalyst under visible light illumination, while on the Au44(DMBT)28 catalyst it was completed in 70 min. The plots of methyl orange degradation on the catalysts versus reaction time further indicate the better photocatalytic performance of the Au38Cd4(DMBT)30 catalyst (Fig. 5c). Electrochemical impedance spectroscopy was performed to investigate the interfacial transfer of electrons. In Fig. 5d, the semicircular diameter of Au38Cd4(DMBT)30 was smaller than that of Au44(DMBT)28, which implies faster electron-transfer in the Au38Cd4(DMBT)30 system. The photocatalysis difference in the two cluster catalysts is suggested to arise from their different equilibria established between the carrier recombination and the electron transfer influenced by metal synergy.
Conclusions
In summary, we have developed a Cd-driven surface reconstruction strategy for synthesizing a new Au38Cd4(DMBT)30 bimetallic nanocluster with the fcc Au26 core retained from the parent Au44(DMBT)28 nanocluster. The two nanoclusters that exhibit elegant patterns of Au4 tetrahedra show distinct differences in the electronic structures, optical properties, and photocatalytic performances. Beyond the Cd-mediated surface reconstruction case, we anticipate that this heteroatom-doping mechanism will find applications in using gold and other metals in a series of challenging gold-based nanocluster formations and tuning of their intrinsic properties.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (21773109, 91845104 and 11974195), China Postdoctoral Science Foundation (2019M650106, 2020T130286), and Programs for high-level entrepreneurial and innovative talents introduction of Jiangsu Province (individual and group program).Notes and references
- A. C. Deacy, A. F. R. Kilpatrick, A. Regoutz and C. K. Williams, Nat. Chem., 2020, 12, 372–380 CrossRef CAS.
- N. Zou, X. Zhou, G. Chen, N. M. Andoy, W. Jung, G. Liu and P. Chen, Nat. Chem., 2018, 10, 607–614 CrossRef CAS.
- Y. Sun, W. Pei, M. Xie, S. Xu, S. Zhou, J. Zhao, K. Xiao and Y. Zhu, Chem. Sci., 2020, 11, 2240–2247 Search PubMed.
- P. D. Jadzinsky, G. Calero, C. J. Ackerson, D. A. Bushnell and R. D. Kornberg, Science, 2007, 318, 430–433 CrossRef CAS.
- C. Zeng, Y. Chen, K. Kirschbaum, K. J. Lambright and R. Jin, Science, 2016, 354, 1580–1584 CrossRef CAS.
- J. Yan, B. K. Teo and N. Zheng, Acc. Chem. Res., 2018, 51, 3084–3093 CrossRef CAS.
- Y. Negishi, K. Nobusada and T. Tsukuda, J. Am. Chem. Soc., 2005, 127, 5261–5270 CrossRef CAS.
- Y. Song, F. Fu, J. Zhang, J. Chai, X. Kang, P. Li, S. Li, H. Zhou and M. Zhu, Angew. Chem., Int. Ed., 2015, 127, 8550–8554 CrossRef.
- Z. Wu, Y. Du, J. Liu, Q. Yao, T. Chen, Y. Cao, H. Zhang and J. Xie, Angew. Chem., Int. Ed., 2019, 58, 8139–8144 CrossRef CAS.
- A. Baksi, P. Chakraborty, S. Bhat, G. Natarajan and T. Pradeep, Chem. Commun., 2016, 52, 8397–8400 RSC.
- C. Zeng, Y. Chen, C. Liu, K. Nobusada, N. L. Rosi and R. Jin, Sci. Adv., 2015, 1, e1500425 CrossRef.
- L. Shi, L. Zhu, J. Guo, L. Zhang, Y. Shi, Y. Zhang, K. Hou, Y. Zheng, Y. Zhu, J. Lv, S. Liu and Z. Tang, Angew. Chem., Int. Ed., 2017, 56, 15397–15401 CrossRef CAS.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS.
- M. A. Tofanelli, K. Salorinne, T. W. Ni, S. Malola, B. Newell, B. Phillips, H. Häkkinen and C. J. Ackerson, Chem. Sci., 2016, 7, 1882–1890 RSC.
- X. Cai, G. Saranya, K. Shen, M. Chen, R. Si, W. Ding and Y. Zhu, Angew. Chem., Int. Ed., 2019, 58, 9964–9968 CrossRef CAS.
- K. Kwak, W. Choi, Q. Tang, M. Kim, Y. Lee, D. E. Jiang and D. Lee, Nat. Commun., 2017, 8, 14723 CrossRef.
- S. Li, H. Chen, X. Liu, H. Liu, J. Ma and Y. Zhu, Chem. Sci., 2020, 11, 8000–8004 RSC.
- X. Wan, J. Wang, Z. Nan and Q. Wang, Sci. Adv., 2017, 3, e1701823 CrossRef.
- S. Takano, S. Hasegawa, M. Suyama and T. Tsukuda, Acc. Chem. Res., 2018, 51, 3074–3083 CrossRef CAS.
- Q. Li, T. Luo, M. G. Taylor, S. Wang, X. Zhu, Y. Song, G. Mpourmpakis, N. L. Rosi and R. Jin, Sci. Adv., 2017, 3, e1603193 CrossRef.
- S. Zhuang, D. Chen, L. Liao, Y. Zhao, N. Xia, W. Zhang, C. Wang, J. Yang and Z. Wu, Angew. Chem., Int. Ed., 2020, 59, 3073–3077 CrossRef CAS.
- W. Fei, S. Antonello, T. Dainese, A. Dolmella, M. Lahtinen, K. Rissanen, A. Venzo and F. Maran, J. Am. Chem. Soc., 2019, 141, 16033–16045 CrossRef CAS.
- H. Qian, D. E. Jiang, G. Li, C. Gayathri, A. Das, R. R. Gil and R. Jin, J. Am. Chem. Soc., 2012, 134, 16159–16162 CrossRef CAS.
- X. Cai, W. Hu, S. Xu, D. Yang, M. Chen, M. Shu, R. Si, W. Ding and Y. Zhu, J. Am. Chem. Soc., 2020, 142, 4141–4153 CrossRef CAS.
- M. Suyama, S. Takano, T. Nakamura and T. Tsukuda, J. Am. Chem. Soc., 2019, 141, 14048–14051 CrossRef CAS.
- Q. Li, K. L. Lambright, M. G. Taylor, K. Kirschbaum, T. Luo, J. Zhao, G. Mpourmpakis, S. Mokashi-Punekar, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2017, 139, 17779–17782 CrossRef CAS.
- C. Yao, C. Xu, I. Park, M. Zhao, Z. Zhu, J. Li, X. Hai, H. Fang, Y. Zhang, G. Macam, J. Teng, L. Li, Q. Xu, F. Chuang, J. Lu, C. Su, J. Li and J. Lu, Angew. Chem., Int. Ed., 2020, 59, 8270–8276 CrossRef CAS.
- C. Zeng, Y. Chen, K. Iida, K. Nobusada, K. Kirschbaum, K. J. Lambright and R. Jin, J. Am. Chem. Soc., 2016, 138, 3950–3953 CrossRef CAS.
- M. Zhou, C. Zeng, M. Y. Sfeir, M. Cotlet, K. Iida, K. Nobusada and R. Jin, J. Phys. Chem. Lett., 2017, 8, 4023–4030 CrossRef CAS.
- M. Zhou, S. Tian, C. Zeng, M. Y. Sfeir, Z. Wu and R. Jin, J. Phys. Chem. C, 2017, 121, 10686–10693 CrossRef CAS.
- M. Zhou, T. Higaki, Y. Li, C. Zeng, Q. Li, M. Y. Sfeir and R. Jin, J. Am. Chem. Soc., 2019, 141, 19754–19764 CrossRef CAS.
- Z. Wu and R. Jin, Nano Lett., 2010, 10, 2568–2573 CrossRef CAS.
- S. A. Miller, C. A. Fields-Zinna, R. W. Murray and A. M. Moran, J. Phys. Chem. Lett., 2010, 1, 1383–1387 CrossRef CAS.
- K. L. D. M. Weerawardene and C. M. Aikens, J. Am. Chem. Soc., 2016, 138, 11202–11210 CrossRef CAS.
- X. Kang, S. Wang, Y. Song, S. Jin, G. Sun, H. Yu and M. Zhu, Angew. Chem., Int. Ed., 2016, 55, 3611–3614 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2022214 and 2022215. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc05163b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |