Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Discovery of a size-record breaking green-emissive fluorophore: small, smaller, HINA†
Rui
Kang
 a,
Laura
Talamini
b,
Elisa
D'Este
c,
Bianca Martins
Estevão
b,
Luisa
De Cola
a,
Laura
Talamini
b,
Elisa
D'Este
c,
Bianca Martins
Estevão
b,
Luisa
De Cola
 *ab,
Wim
Klopper
*ab,
Wim
Klopper
 *ad and
Frank
Biedermann
*ad and
Frank
Biedermann
 *a
*a
aInstitute of Nanotechnology (INT), Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz Platz 1, 76344 Eggenstein-Leopoldshafen, Germany. E-mail: frank.biedermann@kit.edu; willem.klopper@kit.edu
bInstitut de Science et d'Ingénierie Supramoléculaires (ISIS), Université de Strasbourg, CNRS, 8Rue Gaspard Monge, 67083 Strasbourg, France. E-mail: decola@unistra.fr
cOptical Microscopy Facility, Max Plank Institute for Medical Research, Jahnstraße 29, D-69120 Heidelberg, Germany
dInstitute of Physical Chemistry (IPC), Karlsruhe Institute of Technology (KIT), Fritz-Haber-Weg 6, 76131 Karlsruhe, Germany
First published on 25th November 2020
Abstract
Astonishingly, 3-hydroxyisonicotinealdehyde (HINA) is despite its small size a green-emitting push–pull fluorophore in water (QY of 15%) and shows ratiometric emission response to biological relevant pH differences (pKa2 ∼ 7.1). Moreover, HINA is the first small-molecule fluorophore reported that possesses three distinctly emissive protonation states. This fluorophore can be used in combination with metal complexes for fluorescent-based cysteine detection in aqueous media, and is readily taken up by cells. The theoretical description of HINA's photophysics remains challenging, even when computing Franck–Condon profiles via coupled-cluster calculations, making HINA an interesting model for future method development.
Introduction
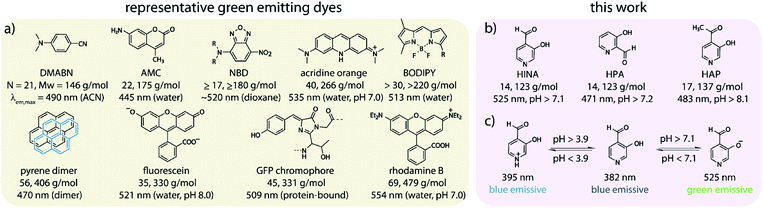
Fluorescence is both fundamentally fascinating and practically relevant. Countless examples of fluorescent compounds have been described and hundreds of dyes have found commercial use.1–4 Decade-long debates were spurred about the origin of the dual fluorescence of DMABN, a small, weakly emissive push–pull system (Scheme 1a).5–7 For comparison, the GFP chromophore is, discounting the surrounding protein pocket, one of the smallest green-emitting fluorophores of biological origin.8 However, synthetic protein-free GFP analogues suffer from emission quenching by protic solvents.8–10 Structurally related polymethine or squarylium push–pull dyes consist of at least two methylene-bridged aromatic moieties.11–15 Nitrobenzoxadiazole-derivatives (NBDs) are typical push–pull fluorophores that display good quantum yields (QY) of ∼10% in hydrophobic environments but are not emissive in water.16–18 Thus, rhodamines, fluoresceins, coumarins, BODIPYs (Scheme 1a) and other polyaromatic dyes are commonly utilized as water-soluble green-emitting dyes.11–13,19,20 However, motivated by the need for smaller, non-disturbing fluorescent labels,16,21 the search for compact fluorophores that are applicable to aqueous media continues.13,22 Furthermore, luminescent compounds that possess a rich photophysical behaviour also provide valuable test cases for theoretical electron-dynamics method development.23–25We have stumbled over the unexpected emission of 3-hydroxyisonicotinealdehyde (HINA, see Scheme 1b) in aqueous media. Surprisingly, while the absorbance properties of HINA have been known for decades,26–28 its unique fluorescent properties were completely overlooked.29 HINA consists of only 14 atoms (123 g mol−1); for comparison, the similar-sized fluorophores naphthalene and azulene (both 18 atoms, 128 g mol−1) are blue emitting, λem,max = 327 and 373 nm in methanol, respectively.30–32 Typical green-emitting dyes are much larger in terms of atom number, molecular weight and size than HINA, see Scheme 1a.
Results
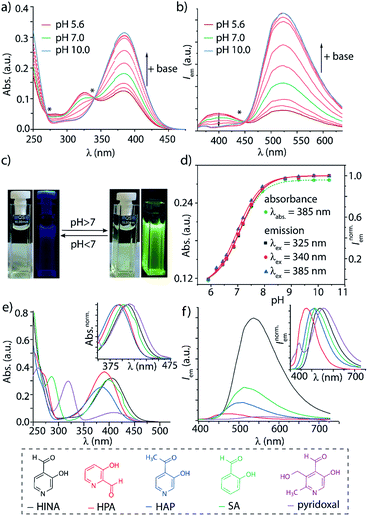
Systematic investigations by absorbance and emission spectroscopy were carried out to elucidate the photophysical behaviour of HINA (Fig. 1). At 3.8 < pH < 7.0, HINA occurs in its neutral form and is blue emissive (λem,max = 382 nm) with a respectable QY of 7% (Table 1). Upon addition of NaOH, a new absorbance centered at 385 nm arose while the band at 325 nm decreased (Fig. 1a). Simultaneously, the blue emission of neutral HINA vanished and an even stronger green emission (λem,max = 525 nm) with a QY of 15% appeared;33 the corresponding colour change can be seen by the naked eye (Fig. 1b and c). The pKa value for deprotonation of HINA was obtained by absorbance-based titration, pKa2 = 7.05 ± 0.01, and agreed with the value from pH-meter recordings, see Fig. 1d and S10.† Coincidentally, this pKa value lies perfectly in the biologically relevant range. Interestingly, very similar pKa2 values (∼7) were obtained by fluorescence-based titrations regardless of the excitation wavelength, i.e. by exciting the neutral form of HINA, the anion or both at an isosbestic point. This indicates that deprotonation of the neutral form of HINA is not a significant process during the lifetime of its excited state34 despite its largely negative value estimate of −5.5, which was obtained by a Förster cycle analysis (see the ESI†).35,36 Unlike many other hydroxarenes such as phenols and naphthols,34,37 HINA does not function as a photoacid. HINA becomes N-protonated below pH 3.9, where it remains fluorescent, albeit with a low QY of 0.9%. The emission band of cationic HINA is centred at 395 nm, and thus surprisingly bathochromically shifted compared to neutral HINA (Fig. S1† and Table 1). Conversely, the absorbance spectra showed the expected hypsochromic shift upon addition of acid. The emission lifetimes of the cationic, neutral and anionic forms of HINA identified them as fluorophores (Table 1). Indeed, HINA is a fascinating emissive dye that occurs in three distinct emissive forms. We are unaware of any other small fluorophore with this property.
value estimate of −5.5, which was obtained by a Förster cycle analysis (see the ESI†).35,36 Unlike many other hydroxarenes such as phenols and naphthols,34,37 HINA does not function as a photoacid. HINA becomes N-protonated below pH 3.9, where it remains fluorescent, albeit with a low QY of 0.9%. The emission band of cationic HINA is centred at 395 nm, and thus surprisingly bathochromically shifted compared to neutral HINA (Fig. S1† and Table 1). Conversely, the absorbance spectra showed the expected hypsochromic shift upon addition of acid. The emission lifetimes of the cationic, neutral and anionic forms of HINA identified them as fluorophores (Table 1). Indeed, HINA is a fascinating emissive dye that occurs in three distinct emissive forms. We are unaware of any other small fluorophore with this property.
| Cationic form at pH < pKa1 | Neutral form at pKa1 < pH < pKa2 | Anionic form at pH > pKa2 | pKa1, pKa2 in H2Oh | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
λ
abs (nm) & [log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε]a ε]a |
λ em (nm) & QYc | τ (ns) |
λ
abs (nm) & [log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε]a ε]a |
λ em (nm) & [QY]f | τ (ns) |
λ
abs (nm) & [log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε]a ε]a |
λ em (nm) & [QY]f | τ (ns) | ||
| a Absorbance peak maximum and logarithmic extinction coefficient. b Emission peak maximum, λex = 285 nm. c Emission quantum yield, λex = 300 nm. d Emission lifetime, λex = 373 nm. e Emission peak maximum, λex = 330 nm. f Emission quantum yield, λex = 370 nm. g Emission peak maximum, λex = 380 nm. h From pH titration experiments. Estimated errors ± 0.1 from different methods, see the ESI. i Very weak emission. | ||||||||||
| HINA | 286 [3.71] | 395 [0.9%] | 0.2 | 325 [3.32] | 382 [7.0%] | 0.9 | 385 [3.74] | 525 [15.0%] | 1.0 | 3.9, 7.1 |
| HPA | 285 [3.84] | 395 [0.4%] | 0.2 | 316 [3.63] | 376 [1.2%] | 0.4 | 369 [3.87] | 484 [1.4%] | 0.2 | 3.2, 7.2 |
| HAP | 318 [3.64] | n.a.i | — | 329 [3.53] | 410 [0.1%] | 0.2 | 365 [3.72] | 484 [0.8%] | 0.9 | 2.6, 8.1 |
Strongly solvent-dependent absorbance and emission spectra of HINA were observed. For instance, the characteristic ∼340 nm absorbance maxima of neutral HINA and a weak blue emission was found in anhydrous acetonitrile (ACN). In contrast, a long-wavelength absorbance band and green emission appeared when water was added to ACN (Fig. S13†). Indeed, the emission of HINA is centred at ∼400 nm in all anhydrous organic solvents tested (DMSO, MeOH, ethylene glycol, Fig. S14–S18†), but shifts to the 450–600 nm range upon addition of water. Generally, the emission quantum yield of HINA increases upon deprotonation and is higher in polar protic than in polar aprotic solvents, e.g. reaching up to 24% in basified methanol (Table S8†).
In aqueous environments, the aldehyde moiety of HINA equilibrates with its hydrate structure, as confirmed by UV/Vis38 and NMR experiments (ESI, Fig. S42–S46†). At first, we wondered if one of the hydrated forms of HINA is the green-emitting species. However, fluorescence in the 450 to 700 nm wavelength region was also detected when adding anhydrous base or anion-stabilising urea to a solution of HINA in organic solvents (see Fig. S16–S18†), confuting hydrate-contributions. Unlike pyrenes (Scheme 1a) or other aggregation-induced emitting (AIE) systems,3,24,39 HINA emission is not caused by aggregate formation (Fig. S27 and S28†).
The photophysical properties of 3-hydroxypicolinealdehyde (HPA, an isomer of HINA), of 3-hydroxy-4-acetylpyridine (HAP, a ketone analogue of HINA), of salicylaldehyde (SA, a phenyl analogue of HINA) and of pyridoxal (a biological hydroxy-formyl-pyridine) were evaluated to obtain insights into the photophysical mechanism, see Fig. 1e, f and Table 1. HPA showed spectroscopic features that were similar to that of HINA, but its absorbance and emission bands for both the neutral and anionic forms are hypsochromically shifted. Moreover, HPA is a much weaker emitter than HINA. The ketone HAP is also poor emissive in its neutral form and as an anion. HAP requires a higher pH for deprotonation than HINA or HPA. Notably, HAP does not form hydrates in water as was confirmed by 1H NMR experiments (Fig. S55†), providing additional evidence that these are not the blue and green-emitting species. Like for HINA, absorbance and emission-based titrations of HPA and HAP yielded essentially overlaying pH–property plots (Fig. S6–S9†). Again, effective deprotonation of the neutral form of the hydroxy-pyridines apparently does not occur during the lifetime of the excited state despite the strong acidity of the excited state 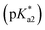 of the fluorophores that was estimated by Förster's method (see ESI†).
of the fluorophores that was estimated by Förster's method (see ESI†).
7-Azaindole (7AI, 15 atoms) and SA (15 atoms) are to the best of our knowledge the smallest green-emitting fluorophores that have been reported so far, but they require the use of an unphysiological basic pH of >13.5 and >8.4, respectively.40–42 SA is structurally closely related to HINA (14 atoms) but shows despite its more electron-rich phenyl ring somewhat counterintuitively a hypochromic shifted absorbance and emission spectra (Fig. 1e and f). Compared to HINA, SA is an inferior fluorophore because its neutral form is not fluorescent and its anionic form is seven times weaker emissive (QY = 2% at pH 10.0). Pyridoxal is both significantly larger in size and much less emissive than the other formyl-hydroxy-pyridines.
The character of the emitting electronic states has been investigated by computing the Franck–Condon profiles through coupled-cluster (CC) calculations with the def2-TZVPPD basis set, see Table S20.† For the anionic form of HINA, the predicted absorption and emissions bands, and the Stokes shift are in good agreement with the experimental findings (Fig. S72 and Tables S20–S22†). The n → π* transition displayed an almost zero oscillator strength, whereas that of the π → π* transition is four orders of magnitude larger, resulting in computed RGB values (Table S22†) that agreed well with the visually observed green emission. Also, the relative emission wavelength maxima trends between HINA, HPA and SA were correctly predicted (Table S20†). Nevertheless, the theoretical description of HINA and its analogues is not simple, despite the small size of these fluorophores. In fact, electronic states which are very close in energy were present and it was found that vibronic effects23,24,43 are crucial for the description of the photophysical properties.44
Very large discrepancies with the experiments were encountered when computing the emission transitions for the neutral forms of HINA and its analogues. Indeed, an excited-state intramolecular proton-transfer process (ESIPT)23,45–47 likely occurs for these substances (Fig. S73†). An intramolecular ESIPT may also explain the large discrepancies seen between the expected pH-dependent deprotonation of the excited state based on calculated 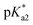 values, and the “ground-state like” protonation status observed in fluorescence experiments. Thus, explicit theoretical treatment of the electron dynamics is required for computing the photophysical properties of (neutral) HINA, which is beyond the scope of this work. HINA and its analogues represent challenging test cases for advanced theoretical studies and method developments of the nuclear and electronic dynamics, because they combine fascinating photophysical properties with advantages for carrying out the computations (small number of atoms, electrons, and conformers).
values, and the “ground-state like” protonation status observed in fluorescence experiments. Thus, explicit theoretical treatment of the electron dynamics is required for computing the photophysical properties of (neutral) HINA, which is beyond the scope of this work. HINA and its analogues represent challenging test cases for advanced theoretical studies and method developments of the nuclear and electronic dynamics, because they combine fascinating photophysical properties with advantages for carrying out the computations (small number of atoms, electrons, and conformers).
The large observed Stokes shifts, e.g. 6700, 4600 and 6900 cm−1 for protonated, neutral and deprotonated HINA characterize it as a push–pull dye, e.g. ∼5000 cm−1 for NBDs and ∼8400 cm−1 for recently reported benzoxazole-thiophenes48 and stilbenes.17 This raises the question if more compact but emissive push–pull fluorophores are even conceivable because single aryl ring-based HINA, HPA and SA already feature some of the smallest but most powerful donor and acceptor moieties, i.e. –O− with a donor resonance const. R+ of −2.04, and –CHO with an acceptor resonance const. R− of 0.70.49 Note that similarly-sized cyanophenols (R− = 0.49 for –CN) are not emissive.
Unlike most other push–pull fluorophores, HINA shows a good emission quantum yield in water, which makes it attractive for applications. For instance, it may be used as ratiometric, fluorescent pH indicator dye because its protonated, neutral and deprotonated forms display characteristic emission profiles. Complementary to many other green emitting dyes that interact with hydrophobic species, HINA's high hydrophilicity diminishes its binding to β-cyclodextrin and cucurbit[7]uril as macrocyclic hosts, to serum albumin as a carrier protein, and to polymethacrylate- and polystyrene surfaces.
HINA can also function as a fluorescent indicator in supramolecular sensing assays: we observed that HINA coordinates to Pt- and Pd-complexes, resulting in a full fluorescence switch off (see Fig. 2). From those complexes, the HINA ligand can be readily displaced by a stronger ligand, e.g. thiols, yielding in an emission switch-on response in aqueous and organic media. In fact, micromolar concentrations of L-cysteine (L-Cys) can be rapidly (∼10 min) detected through a fluorescence-based indicator-displacement assay in that way (Fig. 2b). Analogously, HINA can be displaced from a Pd-complex by pyridine (Fig. S65†). We believe that HINA-capped metal–organic building blocks could be reacted on demand with stronger ligands to form metal–organic cages or -frameworks,50–52 thereby providing an in situ fluorescence response for monitoring reactions in real time. Noteworthy, HINA also forms covalent conjugates with L-Cys in aqueous media (Fig. S66–S68†), via the formation of a 1,3-thiazolidine ring, which is reminiscent to other aldehyde moiety-containing cysteine-reactive probes.53–55 However, like for most reported CHO-based probes, a large excess of L-Cys and long reaction times are needed, making it less attractive for the sensing applications than the HINA-based indicator displacement assay introduced above.
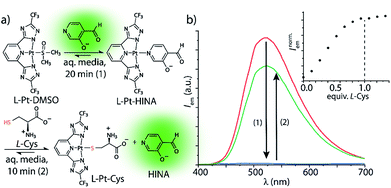 | ||
| Fig. 2 (a) HINA can be reacted with a Pt(II)-precursor to form a non-emissive L-Pt-HINA complex with a binding constant of Ka ∼ 106 M−1, see ESI.† Upon addition of L-Cys, the HINA ligand is displaced and becomes emissive. (b) Emission spectra of HINA (50 μM, red line) upon addition of L-Pt-DMSO (50 μM, blue line), and subsequent addition of L-Cys (50 μM, green line). The inset shows a titration of L-Pt-HINA (50 μM) with L-Cys, 0 to 73 μM (λem = 533 nm). All the spectra were obtained in 25 mM NaHCO3 buffer (pH 9.5) containing 0.9 mM CTAB. | ||
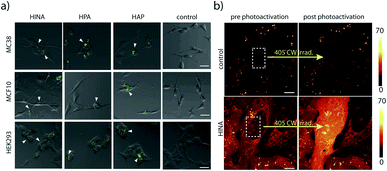
HINA, HPA and HAP readily permeate through biological membranes19,56 as was confirmed for different cancerous and non-cancerous cell lines, see the ESI.† Preliminary results indicate that they localize in the perinuclear region of the cells (Fig. 3a) and the cell toxicity level of the dyes is low (Fig. S69†). Interestingly, a rapid fluorescence photoactivation of HINA-treated cells was observed within seconds during the fluorescence imaging experiments (Fig. 3b and Videos in the ESI†). A similar behaviour was seen in control experiments upon irradiation of HINA solutions with a strong light source, and in the presence of hydrogen peroxide (Fig. S71†).
Conclusions
In conclusion, 3-hydroxyisonicotinealdehyde (HINA) has been identified as the smallest known green fluorescent dye, and may be reaching the fundamental size-limit. Uniquely, HINA occurs in three different protonation states that are each distinctly fluorescent. The QY of HINA in water is surprisingly high, despite being a push–pull chromophore. HINA's commercial availability, and its favourable photophysical properties (large Stokes shift, pH-dependent ratiometric emission properties that are switching in biorelevant pH 7 regime) will enable future applications, ranging from its use as a fluorescent dye to its function as an indicator in supramolecular assays. The synthesis and investigation of additional hydroxyl-functional pyridine-aldehydes and -ketones will lead to the discovery of novel green-emissive labels with improved photophysical properties. These dyes will like HINA provide excellent test cases to evaluate theoretical predictions of emission spectra using highly advanced computational methods capable of considering both vibronic effects and excited-state intramolecular proton-transfer process (ESIPT).Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. K. acknowledges the China Scholarship Council (No. 201806870028). B. M. E. thanks the funding from São Paulo Research Foundation (FAPESP). F. B. thanks the Emmy Noether program of the Deutsche Forschungsgemeinschaft (DFG) for funding. F. B. and W. K. thank the SPP 1807 of the DFG for funding.Notes and references
- L. D. Lavis, Annu. Rev. Biochem., 2017, 86, 825–843 CrossRef CAS.
- J. Liu, Y. Q. Sun, H. Zhang, H. Shi, Y. Shi and W. Guo, ACS Appl. Mater. Interfaces, 2016, 8, 22953–22962 CrossRef CAS.
- Y. Hong, J. W. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- F. Pina, M. J. Melo, C. A. Laia, A. J. Parola and J. C. Lima, Chem. Soc. Rev., 2012, 41, 869–908 RSC.
- A. Demeter, S. Druzhinin, M. George, E. Haselbach, J. L. Roulin and K. A. Zachariasse, Chem. Phys. Lett., 2000, 323, 351–360 CrossRef CAS.
- M. Sayed, F. Biedermann, V. D. Uzunova, K. I. Assaf, A. C. Bhasikuttan, H. Pal, W. M. Nau and J. Mohanty, Chem.–Eur. J., 2015, 21, 691–696 CrossRef CAS.
- J. Catalán, Phys. Chem. Chem. Phys., 2013, 15, 8811–8820 RSC.
- J. Dong, K. M. Solntsev and L. M. Tolbert, J. Am. Chem. Soc., 2006, 128, 12038–12039 CrossRef CAS.
- C. Y. Lin, M. G. Romei, L. M. Oltrogge, I. I. Mathews and S. G. Boxer, J. Am. Chem. Soc., 2019, 141, 15250–15265 CrossRef CAS.
- H. Deng, C. Yu, D. Yan and X. Zhu, J. Phys. Chem. B, 2020, 124, 871–880 CrossRef CAS.
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS.
- X. Li, X. Gao, W. Shi and H. Ma, Chem. Rev., 2014, 114, 590–659 CrossRef CAS.
- R. N. Dsouza, U. Pischel and W. M. Nau, Chem. Rev., 2011, 111, 7941–7980 CrossRef CAS.
- A. S. Tatikolov, J. Photochem. Photobiol., C, 2012, 13, 55–90 CrossRef CAS.
- B. Tang, Y. Xing, P. Li, N. Zhang, F. Yu and G. Yang, J. Am. Chem. Soc., 2007, 129, 11666–11667 CrossRef CAS.
- S. Benson, A. Fernandez, N. D. Barth, F. de Moliner, M. H. Horrocks, C. S. Herrington, J. L. Abad, A. Delgado, L. Kelly, Z. Chang, Y. Feng, M. Nishiura, Y. Hori, K. Kikuchi and M. Vendrell, Angew. Chem., Int. Ed., 2019, 58, 6911–6915 CrossRef CAS.
- X. Liu, Q. Qiao, W. Tian, W. Liu, J. Chen, M. J. Lang and Z. Xu, J. Am. Chem. Soc., 2016, 138, 6960–6963 CrossRef CAS.
- S. Saha and A. Samanta, J. Phys. Chem. A, 1998, 102, 7903–7912 CrossRef CAS.
- L. Wang, M. Tran, E. D'Este, J. Roberti, B. Koch, L. Xue and K. Johnsson, Nat. Chem., 2020, 12, 165–172 CrossRef CAS.
- K. Zamojc, W. Wiczk, B. Zaborowski, D. Jacewicz and L. Chmurzynski, Spectrochim. Acta, Part A, 2015, 136, 1875–1880 CrossRef CAS.
- C. P. Toseland, J. Chem. Biol., 2013, 6, 85–95 CrossRef.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer Science & Business Media, 2013 Search PubMed.
- S. Mai and L. Gonzalez, Angew. Chem., Int. Ed., 2020, 59, 16832–16846 CrossRef CAS.
- Y. Tu, J. Liu, H. Zhang, Q. Peng, J. W. Y. Lam and B. Z. Tang, Angew. Chem., Int. Ed., 2019, 58, 14911–14914 CrossRef CAS.
- J. Chmela, J. F. Greisch, M. E. Harding, W. Klopper, M. M. Kappes and D. Schooss, J. Phys. Chem. A, 2018, 122, 2461–2467 CrossRef CAS.
- K. Nakamoto and A. Martell, J. Am. Chem. Soc., 1959, 81, 5863–5869 CrossRef CAS.
- C. M. Harris, R. J. Johnson and D. E. Metzler, Biochim. Biophys. Acta, 1976, 421, 181–194 CrossRef CAS.
- D. Sanz, A. Perona, R. M. Claramunt and J. Elguero, Tetrahedron, 2005, 61, 145–154 CrossRef CAS.
- The purity of the HINA specimen was confirmed by TLC, HPLC and NMR (ESI†).
- R. Weinberger and L. J. C. Love, Spectrochim. Acta, Part A, 1984, 40, 49–55 CrossRef.
- T. Yamaguchi, Y. Kimura and N. Hirota, J. Chem. Phys., 2000, 113, 2772–2783 CrossRef CAS.
- M. Lamberto, C. Pagba, P. Piotrowiak and E. Galoppini, Tetrahedron Lett., 2005, 46, 4895–4899 CrossRef CAS.
- Isosbestic points at 270 nm and 341 nm and an isosemissive point at 450 nm ruled out chemical decomposition of HINA.
- L. M. Tolbert and K. M. Solntsev, Acc. Chem. Res., 2002, 35, 19–27 CrossRef CAS.
- B. Szczepanik, J. Mol. Struct., 2015, 1099, 209–214 CrossRef CAS.
- J. Zhou, H. Liu, B. Jin, X. Liu, H. Fu and D. Shangguan, J. Mater. Chem. C, 2013, 1, 4427–4436 RSC.
- E. L. Wehry and L. B. Rogers, J. Am. Chem. Soc., 1965, 87, 4234–4238 CrossRef CAS.
- E. G. Sander and W. P. Jencks, J. Am. Chem. Soc., 1968, 90, 6154–6162 CrossRef CAS.
- N. Meher, S. Panda, S. Kumar and P. K. Iyer, Chem. Sci., 2018, 9, 3978–3985 RSC.
- F. M. F. Santos, Z. Dominguez, J. P. L. Fernandes, C. Parente Carvalho, D. Collado, E. Perez-Inestrosa, M. V. Pinto, A. Fernandes, J. F. Arteaga, U. Pischel and P. M. P. Gois, Chem.–Eur. J., 2020, 26, 14064–14069 CrossRef CAS.
- J. E. Wampler and J. E. Churchich, J. Biol. Chem., 1969, 244, 1477–1480 CAS.
- Y. S. Wu, H. C. Huang, J. Y. Shen, H. W. Tseng, J. W. Ho, Y. H. Chen and P. T. Chou, J. Phys. Chem. B, 2015, 119, 2302–2309 CrossRef CAS.
- A. Baiardi, J. Bloino and V. Barone, J. Chem. Theory Comput., 2013, 9, 4097–4115 CrossRef CAS.
- Computed emission wavelength maxima from non-vibronic DFT and CC-based methods largely deviated from experimental values, even after consideration of solvation effects through COSMO.
- J. Zhao, S. Ji, Y. Chen, H. Guo and P. Yang, Phys. Chem. Chem. Phys., 2012, 14, 8803–8817 RSC.
- C. C. Hsieh, C. M. Jiang and P. T. Chou, Acc. Chem. Res., 2010, 43, 1364–1374 CrossRef CAS.
- N. Basilio, C. A. Laia and F. Pina, J. Phys. Chem. B, 2015, 119, 2749–2757 CrossRef CAS.
- Z. Gao, Y. C. Hao, M. L. Zheng and Y. Chen, RSC Adv., 2017, 7, 7604–7609 RSC.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- D. Samanta, J. Gemen, Z. Chu, Y. Diskin-Posner, L. J. W. Shimon and R. Klajn, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 9379–9384 CrossRef CAS.
- S. Pullen and G. H. Clever, Acc. Chem. Res., 2018, 51, 3052–3064 CrossRef CAS.
- A. J. McConnell, C. S. Wood, P. P. Neelakandan and J. R. Nitschke, Chem. Rev., 2015, 115, 7729–7793 CrossRef CAS.
- H. Peng, W. Chen, Y. Cheng, L. Hakuna, R. Strongin and B. Wang, Sensors, 2012, 12, 15907–15946 CrossRef CAS.
- Y. Zhou and J. Yoon, Chem. Soc. Rev., 2012, 41, 52–67 RSC.
- W. Wang, O. Rusin, X. Xu, K. K. Kim, J. O. Escobedo, S. O. Fakayode, K. A. Fletcher, M. Lowry, C. M. Schowalter, C. M. Lawrence, F. R. Fronczek, I. M. Warner and R. M. Strongin, J. Am. Chem. Soc., 2005, 127, 15949–15958 CrossRef CAS.
- F. Biedermann, G. Ghale, A. Hennig and W. M. Nau, Commun. Biol., 2020, 3, 383 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc05557c |
| This journal is © The Royal Society of Chemistry 2021 |