 Open Access Article
Open Access ArticleMetalloid gold clusters – past, current and future aspects
Sebastian
Kenzler
and
Andreas
Schnepf
 *
*
Institute of Inorganic Chemistry, Universität Tübingen, Auf der Morgenstelle 18, D-72076 Tübingen, Germany. E-mail: andreas.schnepf@uni-tuebingen.de; Fax: +49-7071-28-2436; Tel: +49-7071-29-76635
First published on 4th February 2021
Abstract
Gold chemistry and the synthesis of colloidal gold have always caught the attention of scientists. While Faraday was investigating the physical properties of colloidal gold in 1857 without probably knowing anything about the exact structure of the molecules, 150 years later the working group of Kornberg synthesized the first structurally characterized multi-shell metalloid gold cluster with more than 100 Au atoms, Au102(SR)44. After this ground-breaking result, many smaller and bigger metalloid gold clusters have been discovered to gain a better understanding of the formation process and the physical properties. In this review, first of all, a general overview of past investigations is given, leading to metalloid gold clusters with staple motifs in the ligand shell, highlighting structural differences in the cores of these clusters. Afterwards, the influence of the synthetic procedure on the outcome of the reactions is discussed, focusing on recent results from our group. Thereby, newly found structural motifs are taken into account and compared to the existing ones. Finally, a short outlook on possible subsequent reactions of these metalloid gold clusters is given.
Human curiosity is one of the impelling factors that promote innovation and understanding of the world around us. One example for this is the research and development in the area of the element gold and thus, more precisely, gold chemistry. Gold, with its properties and value, has stirred the fantasies of mankind and influenced the fate of cultures for centuries.1–12 One of the first scientific studies of the modern era on the properties of gold was performed by Faraday in 1857. In this work, Faraday described the interaction of light with different metals like gold, platinum, palladium or silver in solution and thin layers.13
Scientific research continued, and insights into the structure of elemental gold, the synthesis of different gold compounds, the properties of gold and much more grew steadily.14–24 In 1951, the working group of Turkevich published their ‘study of the nucleation and growth processes in the synthesis of colloidal gold’.25 This work gave an overview of different synthetic methods to obtain colloidal gold of various morphologies and sizes with a relatively narrow size distribution and with different reducing agents and protecting groups. Many working groups used their results as a foundation for their own work26–29 and citrate stabilized gold nanoparticles are still synthesized via a similar procedure.30–32 In 1969, the group of Malatesta presented the first, via X-ray diffraction, structurally characterized metalloid gold cluster, Au11(PPh3)7(SCN)3 (Fig. 1A). This result was possible due to some groundwork on this topic.33,34 After this success, Malatesta, Naldini and others continued their research, leading to a variety of new gold clusters.35–40 In 1981, the group of Schmid presented the first example of a multi-shell metalloid gold cluster, Au55Cl8(PPh3)12 (Fig. 1B).41,42 By means of different analytical methods and geometrical considerations they suggested that the arrangement of the gold atoms within the metalloid Au55 cluster can be described as a cut-out of the fcc packing of elemental gold, but structural characterization via X-ray diffraction has not been possible, even up to this day.
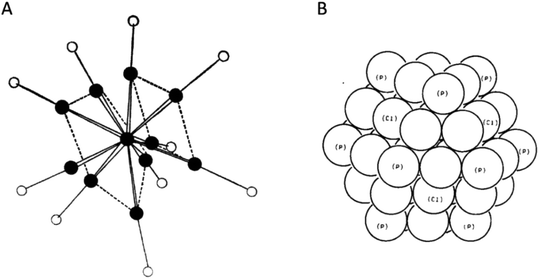 | ||
| Fig. 1 (A) Schematic structure of Au11(PPh3)7(SCN)3 as shown by Malatesta et al.34 The black circles show the position of the Au atoms and the white circles show the position of the PPh3 or SCN groups (adapted from ref. 34 with permission from The Royal Society of Chemistry). (B) Model of the cuboctahedral gold core of Au55Cl8(PPh3)12 as proposed by Schmid et al.41 All spheres represent Au atoms. In the brackets the proposed positions of the protecting atoms, namely P and Cl, are shown (reproduced from ref. 41 with permission from WILEY-VCH, Copyright © 1981, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim). | ||
Metalloid clusters of the general formula MnRm (n > m, M = metals like Al, Au, etc., R: organic substituents like Si(SiMe3)3 or ligands like PPh3), which are actually often called “metal clusters” or “metal nanoclusters” or “monolayer protected metal clusters”,43 are thereby ideal model compounds to understand the chemistry of the dissolution and deposition of metals from molecular precursors and the properties of the intermediary formed particles. At the same time research in colloidal gold chemistry continued and in 1994 and 1995 Brust et al. developed methods to synthesize gold nanoparticles with a narrow size distribution in the size ranges of 1 to 3 nm and 2 to 7 nm.44,45 In these methods thiols are used to stabilize the particles and the reduction is conducted in a two-phase system.46 This work was the continuation of the already rich chemistry of colloidal gold and led to numerous publications and discussions.47–60
Despite the successes in the field of gold nanoparticles protected by thiols, for a very long time it was not possible to obtain any information on the exact structure of these gold nanoparticles as a pure compound in the form of single crystals for X-ray diffraction was not available. This changed in 2007 when the working group of Kornberg published the ground-breaking result of the first structurally characterized multi-shell metalloid gold cluster with more than 100 Au atoms: Au102(p-MBA)44 (p-MBA = para-mercaptobenzoic acid; Fig. 2A).61
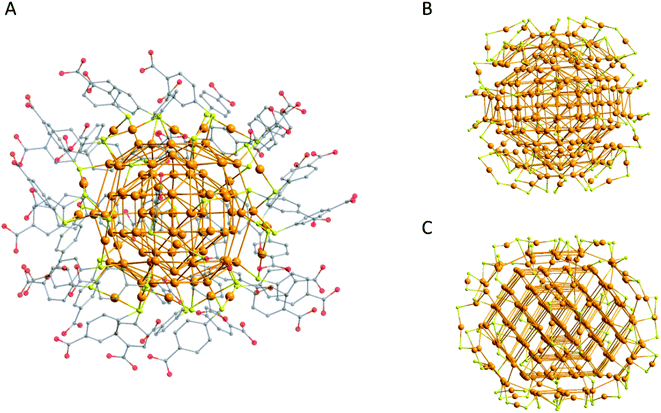 | ||
| Fig. 2 Molecular structures of (A) Au102(p-MBA)44,61 (B) Au246(SR)80 (ref. 63) and (C) Au279(SR)84.64 For (A) the hydrogen atoms are omitted for clarity and the substituents are shown with 50% transparency. For (B) and (C) only the Au and S atoms are shown. Au gold; S yellow; C grey; O red. | ||
This result was possible due to a modified Brust method and adaptations of the work-up procedure. After this first result numerous publications of structurally characterized smaller and bigger metalloid gold clusters appeared, mostly applying aryl based thiols as ligands, whereas Au246(S-Ph-Me)80 (Fig. 2B) and Au279(S-Ph-tBu)84 (Fig. 2C) are the biggest examples up to now.62–64
On looking at the arrangement of the Au atoms in the different metalloid gold clusters a variety of structures can be found.65 Thereby, the Au atoms within the cluster can be divided into different groups: those that form the cluster core and others integrated into the protecting shell. The arrangement of the Au atoms in the core can resemble the fcc packing of elemental gold as it is the case for Au92(SR)44, Au44(SR)28 and others.64,66–75 Furthermore, a bcc like arrangement has been found in Au38S2(SR)20,76 as well as hcp like arrangements as can be found in Au18(SR)14 and Au30(SR)18 or within the recently described Au191(SR)66.77–80 Nevertheless, even decahedral structures are realized which are not restricted to only small Au clusters like Au25(SR)18 and further examples,81–87 but are also found in the big multi-shell metalloid clusters like Au130(SR)50, Au133(SR)52, etc. (Fig. 3).61,63,88–91
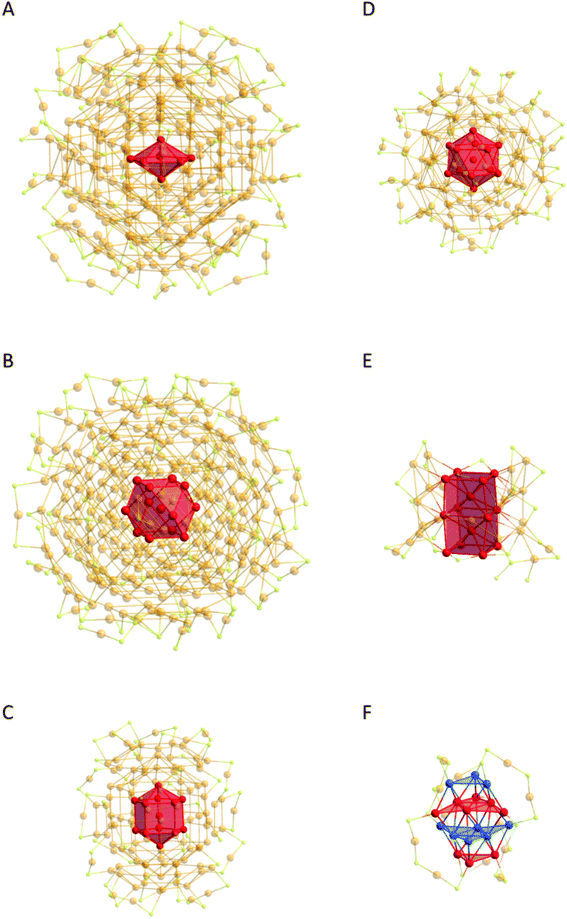 | ||
| Fig. 3 Core motifs of metalloid gold clusters shown as polyhedra. (A) Au246(SR)80 (ref. 63) with the pentagonal bipyramid, (B) the Au279(SR)84 (ref. 64) fcc structure with the central cuboctahedron, (C) Au130(SR)50 (ref. 88) with the Ino decahedron, (D) Au133(SR)52 (ref. 89) with the icosahedron, (E) the Au38S2(SR)20 (ref. 76) central bcc structure and (F) Au30(SR)18 (ref. 79) central Au atoms form a hcp structure. All substituents are omitted for clarity, and outer Au and S atoms have 60% transparency. Au gold, red, blue; S yellow. | ||
Consequently, within metalloid gold clusters a variety of different structural motifs can be realized, depending most likely on the number of bonding electrons within the cluster and the arrangement of the stabilizing ligand shell. As metalloid clusters can be seen as molecular intermediates on the way to the bulk phase of elemental gold with an fcc lattice, the structural diversity of the large multi-shell metalloid gold clusters with more than 100 Au atoms is unexpected. However, at a certain size a structural transition to the fcc packing of elemental gold should take place. In this context another important question arises, that is, at what point (size) does a metalloid cluster show metallic behaviour? Is there a certain size or number of Au atoms that triggers some kind of electronic or structural transition from non-bulk to bulk properties? A lot of research has been conducted in this field and different sizes and numbers of Au atoms have been identified where such a transition seems to happen.80,82,92–95 Hence, not only the number of Au atoms but also the arrangement are important factors and thus a combination of the size of the metalloid cluster (number of gold atoms) and the arrangement of the Au atoms within the cluster core seems to affect the properties and thus, depending on the combination, different cluster sizes will be found where an electronic or structural transition from molecular non-bulk properties to bulk properties will take place. Thereby in recent years the appearance of a plasmon resonance was used as a criterion for the electronic transition, e.g. a sharp transition was found between Au246(SR)80 and Au279(SR)84 both exhibiting thiol ligands.95 Thereby the transition might also be influenced by the ligand used as indicated by a recent result from the multishell cluster [Au110(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C6H4–CF3)48]2−.96
C–C6H4–CF3)48]2−.96
Beside the different “families” of arrangements of the Au atoms in the cluster core (fcc, decahedral, etc.) the protecting shell of thiol protected metalloid gold clusters is more defined, showing so-called staple motifs of the form –SL–(Au–SL–)n (n = 1–4).97,98 Thereby, depending on the size of the cluster, bigger and smaller staple motifs are realized, whereby bigger clusters like Au279(SR)84 or Au144(SR)60 tend to have shorter staple motifs while smaller clusters like Au30(SR)18 or Au18(SR)14 exhibit longer staple motifs.64,77–79,91 This was explained by the fact that smaller clusters show an increasing surface curvature which has to be accommodated by longer staple motifs.97
After the detection of the staple motif many calculations were performed focusing on the question of why the staple motif seemed to be the only stable shell motif for thiolated metalloid gold clusters and if there are other possible motifs. However, this question could not be answered that easily. Already in 2006 the working group of Häkkinen came to the conclusion that Au–S ring motifs are stable and that there is a possibility to find such structural motifs in metalloid gold clusters.99 Two years later the working group of Jiang proposed that the staple motif is energetically preferred over the ring motif.100 Further quantum chemical calculations showed that the stabilizing motifs in the shell of metalloid gold clusters may differ a lot more.101–103 Nevertheless, the only experimentally found shell motif in metalloid gold clusters, stabilized by sulphur containing substituents were for many years the staple motifs and thus it seems as if this structural motif is essential to obtain a stable and isolable metalloid gold cluster. However, ten years after the first structurally characterized multishell metalloid gold cluster exhibiting sulphur containing ligands (Au102(p-MBA)44) it became obvious that even other structural motifs can be realized in the stabilizing ligand shell. Hence, in 2017 and 2018 we isolated two metalloid gold clusters, namely Au108S24(PPh3)16 (Au108) and Au70S20(PPh3)12 (Au70), which did not show the typical Au–S staple motif but the novel (AuS)4 ring motif (Fig. 4A).104,105 This (AuS)4 ring motif is thereby new in the area of metalloid gold clusters but it is well known within the field of Au(I) chemistry, for example, within the molecular clusters Au4(STsi)4 (Tsi = C(SiMe3)3)106 (Fig. 4B) and others,107–109 or the cube-shaped cluster compound [Ph4As]4[Au12S8] (Fig. 4C).110 Consequently, the isolation and structural characterization of Au108 and Au70 directly show that also other structural motifs can be realized within the ligand shell of a stable metalloid gold cluster exhibiting sulphur substituents and there are definitely more motifs to be found in future investigations.
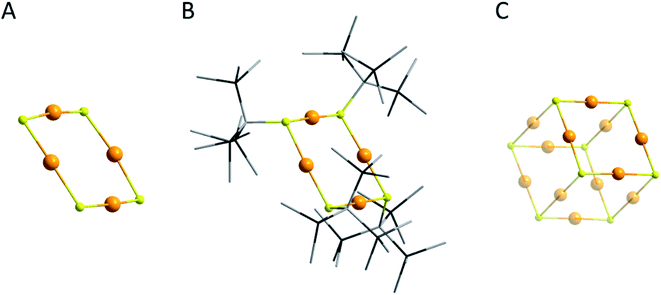 | ||
| Fig. 4 Molecular structures of (A) the (AuS)4 ring motif found in Au108 and Au70,104,105 (B) Au4(STsi)4 (ref. 107) and (C) [Au12S8]4−.110 The H atoms are omitted for clarity. Au gold; S yellow; Si black; C grey. | ||
Beside the structural differences with respect to the other so far discussed metalloid clusters exhibiting sulphur ligands, Au108 and Au70 are both synthesized via a comparable synthetic route. Actually, most of the metalloid gold clusters protected by sulphur-containing ligands are synthesized from HAuCl4 mixed with an aryl based thiol like p-MBA, S-Ph-Me, etc. and reduced with NaBH4.61,63–66,85,89,90 Thereby, in most cases the primary Au precursor, obtained by mixing HAuCl4 with the thiol and which is afterwards reduced with NaBH4, is unknown.61,63,65,66,69,79,85,111 Hence, the formation mechanism of a metalloid gold cluster from molecular precursors is still less understood. To shed light on this area it is useful to know as much as possible and thus starting from defined molecular precursors is a useful step in this direction.
In the case of Au108 the starting materials are Ph3PAuCl and HSTsi, where HSTsi acts as a sulphur atom donating agent during the reaction.105,112 The reaction procedure thereby shows only slight differences in the two-phase synthesis described by Brust et al., where NaBH4 is also used as the reducing agent.44 After the work-up procedure the remaining brown solid is extracted using thf and the thf-extract is stored at 40 °C. This resembles the thermal etching process which is nowadays commonly used for the synthesis of metalloid gold clusters.63,64,66,85,90 After about four weeks black crystals of Au108 had formed.
For Au70, however, Ph3PAuSTsi is used as the Au(I) precursor, which is easily obtained by the reaction of Ph3PAuCl with LiSTsi.104,129 Additionally, a different reducing agent, Li(sec-Bu)3BH (L-selectride), is used because the reduction with NaBH4 failed, showing that the reducing agent expectedly has a strong influence on the reaction.113 As L-selectride and Ph3PAuSTsi are well soluble in organic solvents, a one-phase reaction without the need of an aqueous solution is applied. This cancelled out any possible interferences coming from the aqueous phase like the concentration of the decomposing NaBH4 or the pH-value of the aqueous phase.114–117 The work-up procedure was the same as before and the remaining black solid was extracted in toluene and stored at 40 °C again. After about twelve weeks black crystals of Au70 had formed. Hence, in both cases the heating and etching (altering) times are quite long with respect to the synthesis of other metalloid gold clusters where etching times between several hours and a few days are normal and where normally higher temperatures between 60 and 85 °C are applied.63,64,66,85,90
Comparable to metalloid gold clusters exhibiting staple motifs, both clusters (Au108 and Au70) can be described by a core–shell description (Fig. 5). Thereby Au108 consists of a Au44 core, a Au48S24 shell and additional 16 Ph3PAu groups and is highly symmetric, which is underlined by the fact that the cluster crystallizes in a cubic crystal system (Fig. 5A). Au70 consists of a Au22 core, a Au36S20 shell and additional 12 Ph3PAu groups (Fig. 5B). In contrast to Au108, Au70 shows a strong distortion of the core and the shell (vide infra) and thereby has a lower symmetry and crystallizes in a trigonal crystal system. This shows once more that metalloid gold clusters are structurally quite flexible to obtain a stable compound. The structural motif of the Au22 core can also be found in Au44 and Au44 resembles a cut-out of the solid state phase of elemental Au, namely the fcc structure (Fig. 5C).
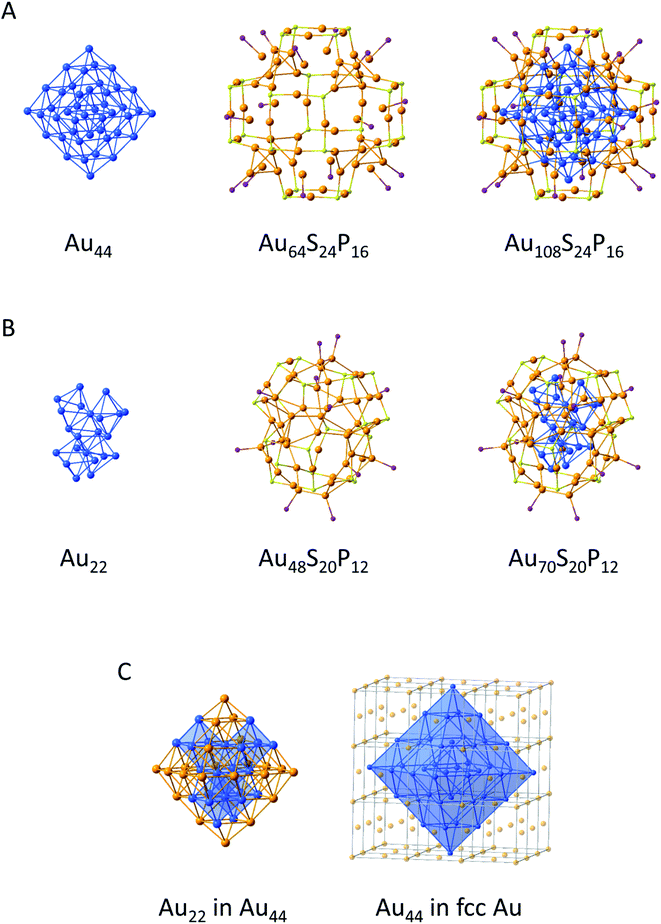 | ||
| Fig. 5 Build-up of the (A) Au108,105 (B) Au70 (ref. 104) and (C) Au22 core from Au70 marked inside the Au44 core from Au108 and Au44 core marked inside the solid state structure of elemental gold.113 Ph groups are omitted for clarity. Au gold, blue; S yellow; P violet. | ||
So both metalloid gold clusters, Au108 and Au70, have a metallic structure in their cores and a molecular structure in their shells. The fcc structure is also found in Au92(SR)44, Au279(SR)84 and others.64,66 Metalloid gold clusters with hollow polyhedra in the centre are also known. The centre of Au108 and Au70 consists of an octahedron while the centres of Au144(SR)60 and Au246(SR)80 are made up of a hollow icosahedron and a pentagonal bipyramidal Au7 unit, respectively.63,91,104,105 Despite the arrangement of the Au atoms in the cluster core the Au–Au distance with respect to the one in elemental gold is another important factor. Hence, to further analyse and compare the structures of different metalloid gold clusters the Au–Au distances of the core as well as the Au–Au distance from the core to the shell motifs of all bigger metalloid gold clusters from 70 to 279 Au atoms are compared in Table 1.
| Cluster | Au–Au core | Au–Au core to shell | Crystalline yield |
|---|---|---|---|
| Au279(SR)84 (ref. 64) | 287.0 ± 4.0 | 284.8 ± 7.9 | * |
| Au246(SR)80 (ref. 63) | 287.2 ± 4.7 | 290.5 ± 16.4 | * |
| Au191(SR)66 (ref. 80) | 287.1 ± 3.9 | 284.5 ± 6.0 | 0.5% |
| Au146(SR)57 (ref. 119) | 282.8 ± 4.8 | 277.7 ± 6.4 | * |
| Au144(SR)60 (ref. 91) | 287.6 ± 5.7 | 291.8 ± 15.9 | 16.0% |
| Au133(SR)52 (ref. 89) | 289.4 ± 8.1 | 288.3 ± 21.5 | **90% |
| Au130(SR)50 (ref. 88) | 287.4 ± 5.5 | 287.0 ± 9.0 | * |
| Au108S24(Ph3P)16 (ref. 105 and 113) | 284.8 ± 9.0 | 291.4 ± 18.5 | 4.8% |
| Au103S2(SR)41 (ref. 90) | 287.7 ± 5.5 | 286.6 ± 10.5 | **40% |
| Au102(SR)44 (ref. 61) | 287.9 ± 6.2 | 286.1 ± 10.4 | * |
| Au92(SR)44 (ref. 66) | 290.2 ± 2.8 | 288.2 ± 11.2 | * |
| Au70S20(Ph3P)12 (ref. 104 and 113) | 287.5 ± 13.6 | 293.4 ± 17.7 | 12.0% |
Interestingly, the average Au–Au distances in the cores are all very similar to the distance of Au in the solid state (288.4 pm).118 The only exception in this case is Au146(SR)57, where significantly smaller Au–Au distances are realized. The standard deviations are relatively small and only show larger values in a few cases like Au70S20(Ph3P)12. Therefore, the differences in the Au–Au distances in the cluster core might be seen as a criterion for how the core and shell structure fit together. Hence, if the core structure does not fit well under the boundary conditions given by the shell or vice versa, the arrangement must be adjusted, leading to distortions and different Au–Au distances and thus to larger standard deviations.
When looking at the Au–Au distances between the core and the shell motifs the average Au–Au distances are spread over a wider range and also larger standard deviations are realized. This indicates that the shell of the metalloid gold clusters shows a wide variety of positions for the Au atoms and the alignment.
Taking a look at the space filling model of Au108 and Au70 as shown in Fig. 6 it is obvious that the Au core in both metalloid clusters is completely covered by the Ph3P groups except for the (AuS)4 ring motifs. Consequently, further build–up reactions to larger aggregates of metalloid gold clusters via linker molecules are possible. Another possibility may be the exchange of the phosphine groups to alter the solubility or the structural arrangement. However, due to the long work-up procedure, the low crystalline yield of about 5% and the unfavourable solubility, both clusters are only soluble in 1,3-dimethyl-2-imidazolidinone (DMI), and no follow-up reactions have been conducted with the two compounds to date. Nevertheless, the isolation and structural characterization of Au108 and Au70 directly show that within metalloid gold clusters a variety of structural motifs can be realized whereby the staple or the (AuS)4 ring-motif are definitely not the only motifs realizable for thiol stabilized metalloid clusters. The situation gets even more complex if other stabilizing ligands are also considered as discussed in the following.
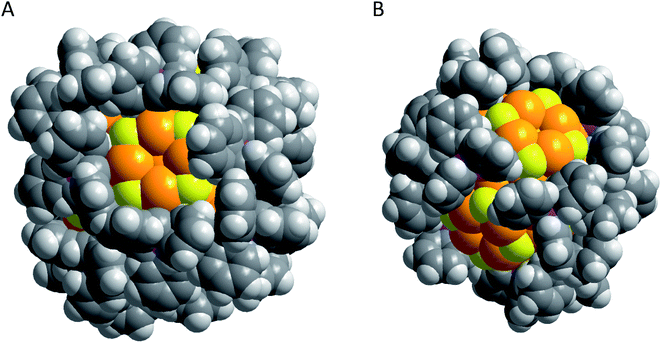 | ||
| Fig. 6 Space filling models of (A) Au108 (ref. 105) and (B) Au70.104 Au gold; S yellow; P violet; C dark grey; H grey. | ||
Until now the above mentioned results have already led to a number of discoveries. (i) Metalloid gold clusters show a wide flexibility and variability concerning their structural arrangement. (ii) The addition or removal of even single Au atoms can significantly change the structure. (iii) The change of the substituents can likewise change the structure remarkably. (iv) The reaction is influenced by the solvent, the gold precursors and the reducing agent. (v) Different structural motifs in the ligand shell, staple or (AuS)4 ring motifs, are realized depending on the ligand source applied during the synthesis.
As there are a variety of parameters present that govern the reaction system it is important to change only one parameter to gain a better control and/or understanding of the reaction parameters for the formation of metalloid gold clusters. Thereby one can for example change the amount of used educts, the solvent in which the reaction is conducted or the functional groups like the phosphines or thiols. In the 1980s Malatesta and the successors of his kind of gold chemistry used different compounds of the form R3PAuX (R = Ph, Ph2Me, PhMe2, X = Cl, SCN) to synthesize small gold clusters.37–40 During these investigations phosphines with aromatic substituents are always used and when solely Ph3PAuCl is reduced with NaBH4 in most cases the metalloid gold cluster Au11(Ph3P)7Cl3 is obtained. One remarkable exception is the metalloid gold cluster [Au39(PPh3)14Cl6]Cl2.120
Hence, compounds like Et3PAuCl, nPr3PAuCl or nBu3PAuCl had not been applied for the synthesis of gold cluster compounds at that time and thus we decided to check if these Au(I) compounds could also be used for the synthesis of metalloid gold clusters. To our surprise, in all cases novel isostructural metalloid gold clusters, namely Au32(PR3)12Cl8 (R = Et, nPr, nBu), are obtained.121 This result again shows that small changes in the reaction system can lead to a complete change of the outcome of the reaction thereby showing that the class of the donor ligand also has an important impact. In this case alkyl phosphines direct the reaction system to the formation of metalloid gold clusters with 32 Au atoms while aryl phosphines mostly lead to smaller clusters like Au11(PPh3)7Cl3.42 However, as the yield of the metalloid clusters is often low to moderate many other clusters might be present in the reaction solution and only the least soluble crystallizes out of the complex reaction system. Consequently, a direct correlation between the change of a parameter and the change on the outcome of the reaction is mostly difficult.
Structurally, the 32 gold atoms within the Au32 clusters are arranged in a shell like manner where the central 12 Au atoms form an empty icosahedron while the next 20 Au atoms are arranged in the form of a pentagonal dodecahedron (Fig. 7). All outer Au atoms bear a ligand whereby the 12 R3P groups are arranged in an icosahedral way and the 8 Cl atoms form a cube. The arrangement of the Au12 icosahedron in the Au20 pentagonal dodecahedron (in the following depicted as M12@M′20) has been described a few times for other elements. Yang et al. showed that different metalloid silver clusters of the form Ag44(SL)30 exhibit the Ag12@Ag20 core motif and the bimetallic clusters of the form Au12Ag32(SL)30 include the motif Au12@Ag20 as well.122 In 2017, the working group of Zhu presented the metalloid silver cluster Ag50(dppm)6(SL)30 (dppm = bis(diphenylphosphino)-methane) which also showed the Ag12@Ag20 motif.123 There are also examples of such motifs from clusters that do not consist of noble metals as it has been described by the working groups of Eichhorn and Fässler.124–126 All of these results have in common the fact that the core and the shell are not perfectly symmetrical but show a slight distortion.
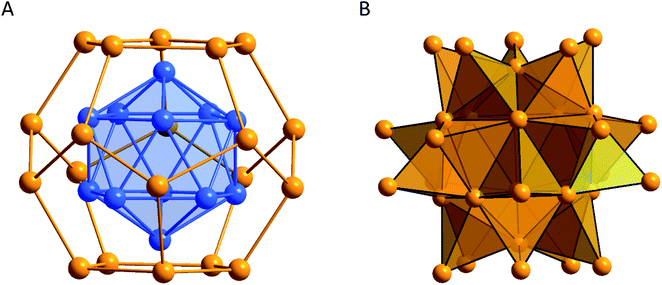 | ||
| Fig. 7 Comparison of the different possible ways to describe the arrangement of the gold atoms within the metalloid Au32(PR3)12Cl8 clusters.121 (A) Shell-like structure and (B) composition of 20 tetrahedral Au4 units. Au gold, blue. | ||
In the case of Au32(PR3)12Cl8 it was shown by quantum chemical calculations that the distortion can be traced back to a Jahn–Teller effect.121 The M12@M′20 description is optically striking (Fig. 7A). However, the Au–Au distances within the Au20 pentagonal dodecahedron are very long (312.8 ± 13.8 pm) and thus in the area of aurophilic interactions.127 If only short Au–Au distances are taken into account it is possible to fully construct the cluster out of 20 tetrahedral Au4 units where either a phosphine or a Cl atom is attached to the outer vertex of the tetrahedron (Fig. 7B). The Au4 tetrahedron is thereby a structural motif that is frequently observed within all kinds of gold clusters and which is also a central structural motif within the fcc packing of elemental gold (Fig. 8).
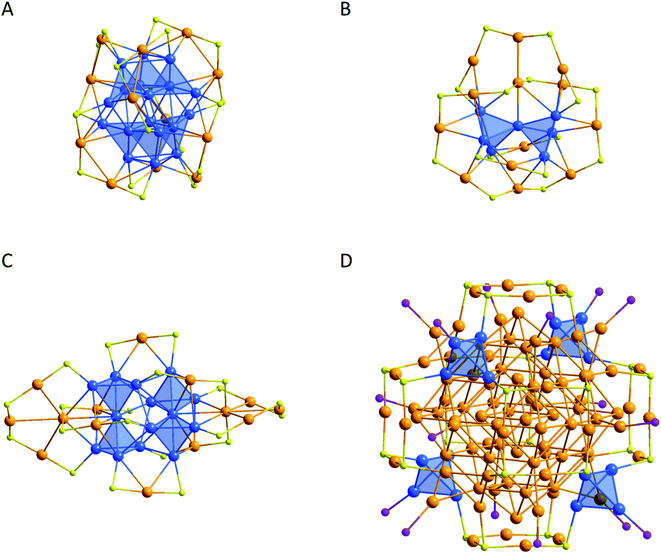 | ||
| Fig. 8 Molecular structure with highlighted Au4 tetrahedra of (A) Au30(SR)18,79 (B) Au20(SR)16,131 (C) Au28(SR)20 (ref. 132) and (D) Au108.105 The substituents are omitted for clarity. Au gold, blue; S yellow; P violet. | ||
A cluster with a single Au4 tetrahedron, Au4(μ-I)2(PPh3)4, was isolated and characterized by Demartin et al. in 1981.128 It was also possible to synthesize a few gold clusters with six Au atoms that form two edge-fused tetrahedra.38,129,130 The Au4 tetrahedron is also often found as a core motif in small metalloid gold clusters like Au30(SR)18, Au20(SR)16 or Au28(SR)20 (Fig. 8A–C).79,131,132 In the case of Au108 the Au4 tetrahedral motif is also observed in the cluster shell (Fig. 8D). Cheng and coworkers also performed quantum chemical calculations on Au70 and showed that Au70 might be described via a superatom133 network model exhibiting 2e-Au4 superatoms.134 In much broader studies Zhou et al. and Cheng et al. showed the important impacts of the Au4 motif onto the structures of metalloid Au clusters.135,136
Another example of a metalloid Au cluster with Au4 tetrahedra is [Au39(PPh3)14Cl6]Cl2 which is made up of 14 Ph3PAu4 units, 6 ClAu5 units and a central Au atom (Fig. 9A).120 Additionally, the working group of Strähle was able to synthesize Au16(AsPh3)8Cl6 which is made up of a Au12 icosahedron with a central Au atom, which makes it possible to describe the icosahedron as a combination of 20 Au4 units, and an outer Au4 tetrahedron with two AsPh3 groups and one Cl atom (Fig. 9B).137
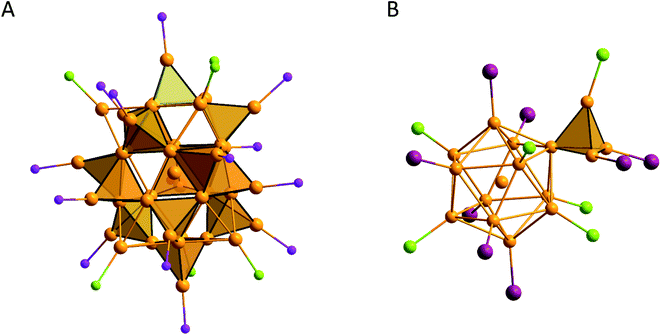 | ||
| Fig. 9 (A) [Au39(PPh3)14Cl6]Cl2 (ref. 120) with highlighted PAu4 groups and (B) Au16(AsPh3)8Cl6 (ref. 137) with highlighted exterior Au4 tetrahedron. Ph groups are omitted for clarity. Au gold; Cl green; P violet; As purple. | ||
Hence, in case of the Au32 clusters again the ever-occurring Au4 motif is present, showing that this motif is another essential motif in a large variety of metalloid gold clusters. However, the difference this time is that the Au32 clusters not only consist of single Au4 motifs but are completely constructed out of this motif. Summarizing these findings, the depiction of Au12@Au20 is easier to comprehend while the display via Au4 tetrahedra reflects the connectivity more accurately (Fig. 7B).
The UV/VIS spectra for the Au32(PR3)12Cl8 compounds look nearly the same, showing that the ligands seem to have a minor impact on the electronic structure of the cluster. Nevertheless, the solubility of these clusters differs remarkably. Au32(PEt3)12Cl8 can only be dissolved in CH2Cl2 while Au32(PnPr3)12Cl8 is additionally soluble in toluene, thf and Et2O and Au32(PnBu3)12Cl8 is also soluble in pentane.121 Consequently, the solubility is directed by the ligands of these isostructural Au32 clusters.
As the reactions with alkyl phosphines PR3 (R = Et, nPr, nBu) lead to Au32 clusters in good to moderate yield one could easily think that alkyl phosphines are the key to obtaining such Au32 clusters. However, the reality looks different and, as in most cases, more difficult. Yuan et al. were able to synthesize and characterize [Au32(PPh3)8(dpa)6]2+ (Hdpa = 2,2′-dipyridylamine) which has an isostructural Au12@Au20 core as found in Au32(PR3)12Cl8.138 In this case other ligands are used and the aryl phosphine Ph3P takes the position of the Cl atoms of Au32(PR3)12Cl8, showing once again the utter importance of the used ligands, but also showing that the Au32 core can be stabilized by ligands beside alkyl phosphines.
The situation gets even more complicated when taking a closer look at the system using PEt3 as the stabilizing phosphine. Hence, during the synthesis of Au32(PEt3)12Cl8 even a slight change of the work-up procedure leads to another and even larger metalloid gold cluster, Au54(PEt3)20Cl12. In this case the reduction reaction was conducted similarly and a simple change of the solvent in which the remaining black solid was extracted gives access to another metalloid cluster.139 Structurally, Au54(PEt3)20Cl12 looks like the fusion of two Au32(PEt3)12Cl8 cluster fragments with one pentagonal Au5 unit missing and thus shows similar structural properties. Hence, the cluster can either be described via a shell-like structure or via 20 edge-sharing and 20 face-sharing Au4 tetrahedra (Fig. 10).
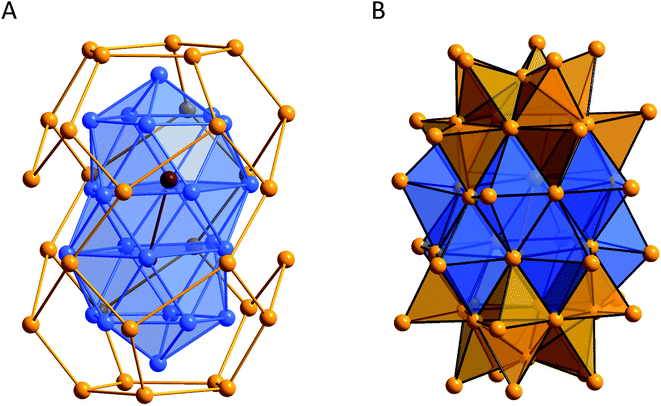 | ||
| Fig. 10 Comparison of the different possible ways to describe the arrangement of the gold atoms within Au54(PEt3)20Cl12.139 (A) shell-like structure and (B) composition of 20 edge-sharing (gold) and 20 face-sharing (blue) tetrahedral Au4 units. Au gold, blue, brown. | ||
Consequently, even in the case of Au54(PEt3)20Cl12 the structural motif of Au4 tetrahedra is striking and this time even face sharing bitetrahedral Au5 units are realized. As in both clusters (Au32(PEt3)12Cl8 and Au54(PEt3)20Cl12) similar structural motifs are present and as Au54(PEt3)20Cl12 can be described as a fusion of two Au32(PEt3)12Cl8 fragments, a common precursor might be present in solution. Depending on the work-up procedure the system then evolves in one or the other direction and there might be more directions possible, giving a slight insight into the reaction system during the formation of a large metalloid gold cluster from smaller units. The results additionally show that the primary obtained reaction mixture must not be a static system but can be still highly dynamic and, depending on the work-up procedures, can lead to quite different products. Hence, beside the concentration, the reagents and solvents used during the reduction of molecular precursors to metalloid clusters, and also the work-up procedure after the reaction is finished, can change the isolated product. This view is supported by 31P-NMR investigations, where the metalloid clusters show distinctly different signals: Au32(PEt3)12Cl8 (41.97 ppm); Au54(PEt3)20Cl12 (96.93 ppm). However, within the primary reaction solution, as well as in the primary obtained extracts from the crude reaction product, no signals of the clusters are found, indicating that primarily another precursor is present within the extracts which finally reacts to the isolated metalloid clusters during the work-up procedure. As we are actually just beginning to collect and maybe understand the influence of the different parameters that govern the reaction system a planned synthesis is still far away but will be the ultimate goal for this kind of synthesis.
In the case of the different Au32(PR3)12Cl8 clusters, further investigations are more easily performed with respect to Au108 and Au70 as the Au32 clusters are easily and relatively quickly synthesized and, depending on the phosphine used, the solubility can be controlled. Thereby, it could be shown lately that the crystalline order leads to additional optical transitions and an enhancement of the charge carrier transport by two orders of magnitude with respect to a polycrystalline thin film.140 Hence, the single crystalline state is not only of central importance for the structure solution but also influences the properties of the metalloid cluster compounds, an aspect that was also observed for the metalloid gallium cluster [Ga84[N(SiMe3)2]20]4−,141,142 showing that the synthesis and characterization of metalloid clusters open the door for future novel applications.
In the case of the Au32(PR3)12Cl8 clusters subsequent reactions and thus alterations of the properties might be possible due to the well accessible Cl atoms and the possible exchange of the phosphine donors. Hence, simple salt metathesis reactions might be used to change the Cl atoms with other substituents like Tsi, Me, N(SiMe3)2 or other groups. Initial investigations indicate that the cluster stays intact after the substitution although up to now it was not possible to isolate and fully characterize any product of such substitution reactions. Nevertheless, such alterations are important for future investigations to better understand the influence of the substituent on the physical and chemical properties of a metalloid cluster. Additionally, such alterations open the door for a variety of applications of metalloid gold clusters as, for example, the solubility might be fine-tuned or specific linkers might be bound to the cluster.
Beside these possible applications, the isolation of two structurally comparable clusters out of the same reaction system might give access to the reaction process. Hence, even though research has been conducted for a very long time and countless insights have been gained, one has to admit that a lot of topics like the specific synthesis of a metalloid gold cluster with a favoured composition, the influence of the ligands and substituents, the role of the solvent, the used reduction reagents and the topic if a cluster shows a more metal-like or molecule-like behaviour cannot be addressed satisfactorily. Consequently, still a lot of future research is needed. Also the comparison with metalloid clusters of other elements could help to improve our understanding, especially with respect to the metallic state as there are strong similarities between metals irrespective of precious, bare or main group metal. Hence, similarities should be present within the metalloid clusters as well. As our knowledge in this area is steadily increasing but still in its infancy there is still a lot of work to be done until we understand the underlying principles in this area to gain at the end a thorough understanding about the relation of metalloid clusters and the respective metal. This might lead us to the point where we might understand the formation and dissolution of metals on an atomic scale, fundamental processes used for centuries but still less understood with respect to the intermediary formed metalloid cluster compounds.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Tanja Kunz, Lars Preiβing, Florian Fetzer and Dr Claudio Schrenk for helpful discussions and the DFG for financial support.References
- T. Higham, J. Chapman, V. Slavchev, B. Gaydarska, N. Honch, Y. Yordanov and B. Dimitrova, Antiquity, 2007, 81, 640–654 CrossRef.
- D. Klemm, R. Klemm and A. Murr, J. Afr. Earth Sci., 2001, 33, 643–659 CrossRef CAS.
- E. C. Bunker, Artibus Asiae, 1993, 53, 27–50 CrossRef.
- Z. Huaizhi and N. Yuantao, Gold Bull., 2001, 34, 24–29 CrossRef.
- W. B. Sedgwick, Greece Rome, 1936, 5, 148–154 CrossRef.
- J. C. Edmondson, J. Roman Stud., 1989, 79, 84–102 CrossRef.
- D. M. Pendergast, Am. Antiq., 1962, 27, 520–545 CrossRef CAS.
- A. M. Watson, Econ. Hist. Rev., 1967, 20, 1–34 Search PubMed.
- G. B. Kauffman, Gold Bull., 1985, 18, 31–44 CrossRef CAS.
- G. B. Kauffman, Gold Bull., 1985, 18, 69–78 CrossRef CAS.
- G. B. Kauffman, Gold Bull., 1985, 18, 109–119 CrossRef CAS.
- R. W. Paul, Miss. Valley Hist. Rev., 1960, 47, 34–50 CrossRef.
- M. Faraday, Philos. Trans. R. Soc. London, 1857, 147, 145–181 CrossRef.
- L. Vanino and L. Seeman, Ber. Dtsch. Chem. Ges., 1899, 32, 1968–1972 CrossRef CAS.
- M. L. Malvano, Atti Accad. Naz. Lincei, Rc. Sed. Solen., 1908, 17, 847–857 Search PubMed.
- V. Lenher, J. Am. Chem. Soc., 1913, 35, 546–552 CrossRef CAS.
- F. Haber, Angew. Chem., 1927, 40, 303–314 CrossRef CAS.
- C. S. Gibson, Nature, 1933, 131, 130 CrossRef CAS.
- C. W. Denko and A. K. Anderson, J. Am. Chem. Soc., 1945, 67, 2241 CrossRef CAS.
- K. Huang, Proc. Phys. Soc., 1948, 60, 161–175 CrossRef CAS.
- E. B. Sandell, Anal. Chem., 1948, 20, 253–256 CrossRef CAS.
- G.-M. Schwab and S. Pesmatjoglo, J. Phys. Chem., 1948, 52, 1046–1053 CrossRef CAS.
- G. Wilkinson, Phys. Rev., 1949, 75, 1019–1029 CrossRef CAS.
- S. D. Stookey, J. Am. Ceram. Soc., 1949, 32, 246–249 CrossRef CAS.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55–75 RSC.
- R. H. Doremus, J. Chem. Phys., 1964, 40, 2389–2396 CrossRef.
- N. Gane, Proc. R. Soc., 1970, 317, 367–391 CAS.
- N. Uyeda, M. Nishino and E. Suito, J. Colloid Interface Sci., 1973, 43, 264–276 CrossRef CAS.
- M. P. A. Viegers and J. M. Trooster, Phys. Rev. B: Solid State, 1977, 15, 72–83 CrossRef CAS.
- G. Frens, Kolloid Z. Z. Polym., 1972, 250, 736–741 CrossRef CAS.
- G. Frens, Nat. Phys. Sci., 1973, 241, 20–22 CrossRef CAS.
- J. Kimling, M. Maier, B. Okenve, V. Kotaidis, H. Ballot and A. Plech, J. Phys. Chem. B, 2006, 110, 15700–15707 CrossRef CAS.
- L. Malatesta, L. Naldini, G. Simonetta and F. Cariati, Chem. Commun., 1965, 212–213 RSC.
- M. McPartlin, R. Mason and L. Malatesta, Chem. Commun., 1969, 334 RSC.
- F. Cariati and L. Naldini, Inorg. Chim. Acta, 1971, 5, 172–174 CrossRef CAS.
- F. Cariati and L. Naldini, J. Chem. Soc., Dalton Trans., 1972, 2286–2287 RSC.
- L. Malatesta, Gold Bull., 1975, 8, 48–52 CrossRef CAS.
- J. W. A. van der Velden, J. J. Bour, W. P. Bosman and J. H. Noordik, Inorg. Chem., 1983, 22, 1913–1918 CrossRef CAS.
- C. E. Briant, K. P. Hall and D. M. P. Mingos, J. Organomet. Chem., 1983, 254, C18–C20 CrossRef CAS.
- K. P. Hall and D. M. P. Mingos, Prog. Inorg. Chem., 1984, 32, 237–325 CAS.
- G. Schmid, R. Pfeil, R. Boese, F. Bandermann, S. Meyer, G. H. M. Calis and J. W. A. van der Welden, Chem. Ber., 1981, 114, 3634–3642 CrossRef CAS.
- Also a variety of PPh3 stabilized cationic clusters have been described as gas-phase species like [Au20(PPh3)8]2+; H.-F. Zhang, M. Stender, R. Zhang, C. Wang, J. Li and L.-S. Wang, J. Phys. Chem. B, 2004, 108, 12259–12263 CrossRef CAS.
- The term ‘metalloid cluster’ was introduced in 1999 by Schnöckel and co-workers to differentiate the different kinds of metal clusters of Cotton's widespread definition of ‘metal clusters’ from 1964. Thereby, a metalloid cluster exhibits more metal-metal than metal-ligand contacts, as well as so-called ‘naked’ metal atoms showing metal-metal contacts only. The general formula for metalloid clusters is MnLm (n > m, M = metal, L = ligand, substituent). This definition directly highlights the correlation of these clusters to the bulk phase of the corresponding metal. Although this term was mainly used for metalloid clusters of the main group metals, it already included all metals like Au, Ag, Pt etc. The term ‘metal cluster’ or the later introduced term ‘metal nanocluster’ do not include this information depth. For further reading see: A. Purath, R. Köppe and H. Schnöckel, Angew. Chem., Int. Ed., 1999, 38, 2926–2928 ( Angew. Chem. , 1999 , 111 , 3114–3116 ) CrossRef CAS; A. Schnepf and H. Schnöckel, Angew. Chem. Int. Ed., 2002, 41, 3532–3554 ( Angew. Chem. , 2002 , 114 , 3682–3704 ) CrossRef; A. Schnepf in Clusters – Contemporary Insight in Structure and Bonding (Hrsg.: J. S. Dehnen), Structure and Bonding, Springer, Berlin, 2017, vol. 174, pp. 135–200 Search PubMed.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801–802 RSC.
- M. Brust, J. Fink, D. Bethell, D. J. Schiffrin and C. Kiely, J. Chem. Soc. Chem. Commun., 1995, 1655–1656 RSC.
- D. Bethell, M. Brust, D. J. Schiffrin and C. Kiely, J. Electroanal. Chem., 1996, 409, 137–143 CrossRef.
- R. H. Terrill, T. A. Postlethwaite, C.-h. Chen, C.-D. Poon, A. Terzis, A. Chen, J. E. Hutchison, M. R. Clark, G. Wignall, J. D. Londono, R. Superfine, M. Falvo, C. S. Johnson Jr, E. T. Samulski and R. W. Murray, J. Am. Chem. Soc., 1995, 117, 12537–12548 CrossRef CAS.
- D. V. Leff, P. C. Ohara, J. R. Heath and W. M. Gelbart, J. Phys. Chem., 1995, 99, 7036–7041 CrossRef CAS.
- D. V. Leff, L. Brandt and J. R. Heath, Langmuir, 1996, 12, 4723–4730 CrossRef CAS.
- R. P. Andres, J. D. Bielefeld, J. I. Henderson, D. B. Janes, V. R. Kolagunta, C. P. Kubiak, W. J. Mahoney and R. G. Osifchin, Science, 1996, 273, 1690–1693 CrossRef CAS.
- M. M. Alvarez, J. T. Khoury, T. G. Schaaff, M. N. Shafigullin, I. Vezmar and L. Whetten, J. Phys. Chem. B, 1997, 101, 3706–3712 CrossRef CAS.
- T. G. Schaaff, M. N. Shafigullin, J. T. Khoury, I. Vezmar, R. L. Whetten, W. G. Cullen, P. N. First, C. Gutiérrez-Wing, J. Ascensio and M. J. Jose-Yacamán, J. Phys. Chem. B, 1997, 101, 7885–7891 CrossRef CAS.
- A. C. Templeton, M. J. Hostetler, C. T. Kraft and R. W. Murray, J. Am. Chem. Soc., 1998, 120, 1906–1911 CrossRef CAS.
- C. J. Kiely, J. Fink, M. Brust, D. Bethell and D. J. Schiffrin, Nature, 1998, 396, 444–446 CrossRef CAS.
- W. W. Weare, S. M. Reed, M. G. Warner and J. E. Hutchison, J. Am. Chem. Soc., 2000, 122, 12890–12891 CrossRef CAS.
- P. Jiang, J. F. Bertone and V. L. Colvin, Science, 2001, 291, 453–457 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Langmuir, 2001, 17, 6782–6786 CrossRef CAS.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957–1962 CrossRef CAS.
- H. Fan, K. Yang, D. M. Boye, T. Sigmon, K. J. Malloy, H. Xu, G. P. López and C. J. Brinker, Science, 2004, 304, 567–571 CrossRef CAS.
- Y. Negishi, K. Nobusada and T. Tsukuda, J. Am. Chem. Soc., 2005, 127, 5261–5270 CrossRef CAS.
- P. D. Jadzinsky, G. Calero, C. J. Ackerson, D. A. Bushnell and R. D. Kornberg, Science, 2007, 318, 430–433 CrossRef CAS.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS.
- C. Zeng, Y. Chen, K. Kirschbaum, K. J. Lambright and R. Jin, Science, 2016, 4, eaat7259 Search PubMed.
- N. A. Sakthivel, S. Theivendran, V. Ganeshraj, A. G. Oliver and A. Dass, J. Am. Chem. Soc., 2017, 139, 15450–15459 CrossRef CAS.
- L. Liao, S. Zhuang, P. Wang, Y. Xu, N. Yan, H. Dong, C. Wang, Y. Zhao, N. Xia, J. Li, H. Deng, Y. Pei, S.-K. Tian and Z. Wu, Angew. Chem., Int. Ed., 2017, 56, 12644–12648 ( Angew. Chem. , 129 , 12818–12822 ) CrossRef CAS.
- C. Zeng, C. Liu, Y. Chen, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2016, 138, 8710–8713 CrossRef CAS.
- C. Zeng, Y. Chen, K. Iida, K. Nobusada, K. Kirschbaum, K. J. Lambright and R. Jin, J. Am. Chem. Soc., 2016, 138, 3950–3953 CrossRef CAS.
- S. Chen, L. Xiong, S. Wang, Z. Ma, S. Jin, H. Sheng, Y. Pei and M. Zhu, J. Am. Chem. Soc., 2016, 138, 10754–10757 CrossRef CAS.
- A. Das, T. Li, K. Nobusada, C. Zeng, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2013, 135, 18264–18267 CrossRef CAS.
- C. Zeng, T. Li, A. Das, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2013, 135, 10011–10013 CrossRef CAS.
- D. Crasto, S. Malola, G. Brosofsky, A. Dass and H. Häkkinen, J. Am. Chem. Soc., 2014, 136, 5000–5005 CrossRef CAS.
- H. Dong, L. Liao, S. Zhuang, C. Yao, J. Chen, S. Tian, M. Zhu, X. Liu, L. Li and Z. Wu, Nanoscale, 2017, 9, 3742–3746 RSC.
- C. Zeng, H. Qian, T. Li, G. Li, N. L. Rosi, B. Yoon, R. N. Barnett, R. L. Whetten, U. Landman and R. Jin, Angew. Chem., Int. Ed., 2012, 51, 13114–13118 ( Angew. Chem. , 124 , 13291–13295 ) CrossRef CAS.
- C. Zeng, Y. Chen, C. Liu, K. Nobusada, N. L. Rosi and R. Jin, Sci. Adv., 2015, 1, e1500425 CrossRef.
- Z. Gan, J. Chen, J. Wang, C. Wang, M.-B. Li, C. Yao, S. Zhuang, A. Xu, L. Li and Z. Wu, Nat. Commun., 2017, 8, 14739 CrossRef CAS.
- C. Liu, T. Li, G. Li, K. Nobusada, C. Zeng, G. Pang, N. L. Rosi and R. Jin, Angew. Chem., Int. Ed., 2015, 54, 9826–9829 ( Angew. Chem. , 127 , 9964–9967 ) CrossRef CAS.
- A. Das, C. Liu, H. Y. Byun, K. Nobusada, S. Zhao, N. Rosi and R. Jin, Angew. Chem., Int. Ed., 2015, 54, 3140–3144 ( Angew. Chem. , 127 , 3183–3187 ) CrossRef CAS.
- S. Chen, S. Wang, J. Zhong, Y. Song, J. Zhang, H. Sheng, Y. Pei and M. Zhu, Angew. Chem., Int. Ed., 2015, 54, 3145–3149 ( Angew. Chem. , 127 , 3188–3192 ) CrossRef CAS.
- T. Higaki, C. Liu, C. Zeng, R. Jin, Y. Chen, N. L. Rosi and R. Jin, Angew. Chem., Int. Ed., 2016, 55, 6694–6697 ( Angew. Chem. , 128 , 6806–6809 ) CrossRef CAS.
- N. A. Sakthivel, M. Shabaninezhad, L. Sementa, B. Yoon, M. Stener, R. L. Whetten, G. Ramakrishna, A. Fortunelli, U. Landman and A. Dass, J. Am. Chem. Soc., 2020, 142, 15799–15814 CrossRef CAS.
- M. W. Heaven, A. Dass, P. S. White, K. M. Holt and R. W. Murray, J. Am. Chem. Soc., 2008, 130, 3754–3755 CrossRef CAS.
- M. Zhu, C. M. Aikens, F. J. Hollander, G. C. Schatz and R. Jin, J. Am. Chem. Soc., 2008, 130, 5883–5885 CrossRef CAS.
- M. Zhu, W. T. Eckenhoff, T. Pintauer and R. Jin, J. Phys. Chem. C, 2008, 112, 14221–14224 CrossRef CAS.
- T. Dainese, S. Antonello, J. A. Gascón, F. Pan, N. V. Perera, M. Ruzzi, A. Venzo, A. Zoleo, K. Rissanen and F. Maran, ACS Nano, 2014, 8, 3904–3912 CrossRef CAS.
- H. Qian, W. T. Eckenhoff, Y. Zhu, T. Pintauer and R. Jin, J. Am. Chem. Soc., 2010, 132, 8280–8281 CrossRef CAS.
- Y. Zhao, S. Zhuang, L. Liao, C. Wang, N. Xia, Z. Gan, W. Gu, J. Li, H. Deng and Z. Wu, J. Am. Chem. Soc., 2020, 142, 973–977 CrossRef CAS.
- S. Zhuang, L. Liao, J. Yuan, C. Wang, Y. Zhao, N. Xia, Z. Gan, W. Gu, J. Li, H. Deng, J. Yang and Z. Wu, Angew. Chem., Int. Ed., 2018, 57, 15450–15454 ( Angew. Chem. , 130 , 15676–15680 ) CrossRef CAS.
- Y. Chen, C. Zeng, C. Liu, K. Kirschbaum, C. Gayathri, R. R. Gil, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2015, 137, 10076–10079 CrossRef CAS.
- C. Zeng, Y. Chen, K. Kirschbaum, K. Appavoo, M. Y. Sfeir and R. Jin, Sci. Adv., 2015, 1, e1500045 CrossRef.
- T. Higaki, C. Liu, M. Zhou, T.-Y. Luo, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2017, 139, 9994–10001 CrossRef CAS.
- N. Yan, N. Xia, L. Liao, M. Zhu, F. Jin, R. Jin and Z. Wu, Sci. Adv., 2018, 4, eaat7259 CrossRef CAS.
- M. M. Alvarez, J. T. Khoury, T. G. Schaaff, M. N. Shafigullin, I. Vezmar and R. L. Whetten, J. Phys. Chem. B, 1997, 101, 3706–3712 CrossRef CAS.
- S. H. Yau, O. Varnavski and T. Goodson III, Acc. Chem. Res., 2013, 46, 1506–1516 CrossRef CAS.
- Y. Negishi, T. Nakazaki, S. Malola, S. Takano, Y. Niihori, W. Kurashige, S. Yamazoe, T. Tsukuda and H. Häkkinen, J. Am. Chem. Soc., 2015, 137, 1206–1212 CrossRef CAS.
- T. Higaki, M. Zhou, K. J. Lambright, K. Kirschbaum, M. Y. Sfeir and R. Jin, J. Am. Chem. Soc., 2018, 140, 5691–5695 CrossRef CAS.
- J.-Q. Wang, S. Shi, R.-L. He, S.-F. Yuan, G.-Y. Yang, G.-J. Liang and Q.-M. Wang, J. Am. Chem. Soc., 2020, 142, 18086–18092 CrossRef CAS.
- C. Zeng, Pure Appl. Chem., 2018, 90, 1409–1427 CAS.
- C.-G. Ning, X.-G. Xiong, Y.-L. Wang, J. Li and L.-S. Wang, Phys. Chem. Chem. Phys., 2012, 14, 9323–9329 RSC.
- H. Häkkinen, M. Walter and H. Grönbeck, J. Phys. Chem. B, 2006, 110, 9927–9931 CrossRef.
- D.-e. Jiang, M. L. Tiago, W. Luo and S. Dai, J. Am. Chem. Soc., 2008, 130, 2777–2779 CrossRef CAS.
- D.-e. Jiang, M. Walter and S. Dai, Chem.–Eur. J., 2010, 16, 4999–5003 CrossRef CAS.
- Y. Pei, N. Shao, H. Li, D.-e. Jiang and X. C. Zeng, ACS Nano, 2011, 5, 1441–1449 CrossRef CAS.
- G. Hu, R. Jin and D.-e. Jiang, Nanoscale, 2016, 8, 20103–20110 RSC.
- S. Kenzler, C. Schrenk, A. R. Frojd, H. Häkkinen, A. Z. Clayborne and A. Schnepf, Chem. Commun., 2018, 54, 248–251 RSC.
- S. Kenzler, C. Schrenk and A. Schnepf, Angew. Chem., Int. Ed., 2017, 56, 393–363 ( Angew. Chem. , 129 , 402–406 ) CrossRef CAS.
- C. Eaborn and J. D. Smith, J. Chem. Soc., Dalton Trans., 2001, 1541–1552 RSC.
- P. J. Bonasia, D. E. Gindelberger and J. Arnold, Inorg. Chem., 1993, 32, 5126–5131 CrossRef CAS.
- Y. Takino, K. Tsuge, A. Igashira-Kamiyama, T. Kawamoto and T. Konno, Chem.–Asian J., 2011, 6, 2931–2935 CrossRef CAS.
- W. Wojnowski, B. Becker, J. Saßmannshausen, E.-M. Peters, K. Peters and H. G. von Schnering, Z. Anorg. Allg. Chem., 1994, 620, 1417–1421 CrossRef CAS.
- G. Marbach and J. Strähle, Angew. Chem., Int. Ed., 1984, 23, 715–716 ( Angew. Chem. , 96 , 695–696 ) CrossRef.
- L. Liao, S. Zhuang, C. Yao, N. Yan, J. Chen, C. Wang, N. Xia, X. Liu, M.-B. Li, L. Li, X. Bao and Z. Wu, J. Am. Chem. Soc., 2016, 138, 10425–10428 CrossRef CAS.
- T. Kunz, C. Schrenk and A. Schnepf, Chem.–Eur. J., 2019, 25, 7210–7217 CrossRef CAS.
- S. I. J. Kenzler, Synthese und Charakterisierung metalloider Goldcluster, PhD-thesis, Cuvillier, Göttingen, 2020, pp. 79–98.
- D. L. van Hyning and C. F. Zukoski, Langmuir, 1998, 14, 7034–7046 CrossRef CAS.
- A. R. Tao, S. Habas and P. Yang, Small, 2008, 4, 310–325 CrossRef CAS.
- D. Wang and Y. Li, Adv. Mater., 2011, 23, 1044–1060 CrossRef CAS.
- T. S. Rodrigues, M. Zhao, T.-H. Yang, K. D. Gilroy, A. G. M. da Silva, P. H. C. Camargo and Y. Xia, Chem.–Eur. J., 2018, 24, 16944–16963 CrossRef CAS.
- A. F. Holleman and E. Wiberg, Lehrbuch der Anorganischen Chemie, 103. Aufl., de Gruyter, Berlin, 2016, pp. 1686–1744 Search PubMed.
- S. Vergara, D. A. Lukes, M. W. Martynowycz, U. Santiago, G. Plascencia-Villa, S. C. Weiss, M. J. de la Cruz, D. M. Black, M. M. Alvarez, X. López-Lozano, C. O. Barnes, G. Lin, H.-C. Weissker, R. L. Whetten, T. Gonen, M. J. Yacaman and G. Calero, J. Phys. Chem. Lett., 2017, 8, 5523–5530 CrossRef CAS.
- B. K. Teo, X. Shi and H. Zhang, J. Am. Chem. Soc., 1992, 114, 2743–2745 CrossRef CAS.
- S. Kenzler, F. Fetzer, C. Schrenk, N. Pollard, A. R. Frojd, A. Z. Clayborne and A. Schnepf, Angew. Chem., Int. Ed., 2019, 58, 5902–5905 ( Angew. Chem. , 131 , 5962–5966 ) CrossRef CAS.
- H. Yang, Y. Wang, H. Huang, L. Gell, L. Lehtovaara, S. Malola, H. Häkkinen and N. Zheng, Nat. Commun., 2013, 4, 3422 Search PubMed.
- W. Du, S. Jin, L. Xiong, M. Chen, J. Zhang, X. Zou, Y. Pei, S. Wang and M. Zhu, J. Am. Chem. Soc., 2017, 139, 1618–1624 CrossRef CAS.
- M. J. Moses, J. C. Fettinger and B. W. Eichhorn, Science, 2003, 300, 778–780 CrossRef CAS.
- Y. Wang, M. Moses-DeBusk, L. Stevens, J. Hu, P. Zavalij, K. Bowen, B. I. Dunlap, E. R. Glaser and B. Eichhorn, J. Am. Chem. Soc., 2017, 139, 619–622 CrossRef.
- S. Stegmaier and T. F. Fässler, J. Am. Chem. Soc., 2011, 133, 19758–19768 CrossRef CAS.
- H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2008, 37, 1931–1951 RSC.
- F. Demartin, M. Manassero, L. Naldini, R. Ruggeri and M. Sansoni, J. Chem. Soc., Chem. Commun., 1981, 222–223 RSC.
- C. E. Briant, K. P. Hall, D. M. P. Mingos and A. C. Wheeler, J. Chem. Soc., Dalton Trans., 1986, 687–692 RSC.
- S. Kenzler, M. Kotsch and A. Schnepf, Eur. J. Inorg. Chem., 2018, 3840–3848 CrossRef CAS.
- C. Zeng, C. Liu, Y. Chen, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2014, 136, 11922–11925 CrossRef CAS.
- N. Xia, J. Yuan, L. Liao, W. Zhang, J. Li, H. Deng, J. Yang and Z. Wu, J. Am. Chem. Soc., 2020, 142, 12140–12145 CrossRef CAS.
- For further reading concerning the concept of superatoms in cluster chemistry see: Z. Luo and A. W. Castleman, Acc. Chem. Res., 2014, 47, 2931–2940 CrossRef CAS; A. C. Reber and S. V. Khanna, Acc. Chem. Res., 2017, 50, 255–263 CrossRef.
- Z. Tian, Y. Xu and L. Cheng, Nanomaterials, 2019, 9, 1132 CrossRef CAS.
- M. Zhou, T. Higaki, G. Hu, M. Y. Sfeir, Y. Chen, D. E. Jiang and R. Jin, Science, 2019, 364, 279–282 CrossRef CAS.
- L. Cheng, Y. Yuan, X. Zhang and J. Yang, Angew. Chem., Int. Ed., 2013, 52, 9035–9039 ( Angew. Chem. , 2013 , 125 , 9205–9209 ) CrossRef CAS.
- M. Richter and J. Strähle, Z. Anorg. Allg. Chem., 2001, 627, 918–920 CrossRef CAS.
- S.-F. Yuan, C.-Q. Xu, J. Li and Q.-M. Wang, Angew. Chem., Int. Ed., 2019, 58, 5906–5909 ( Angew. Chem. , 131 , 5967–5970 ) CrossRef CAS.
- S. Kenzler, C. Schrenk and A. Schnepf, Dalton Trans., 2020, 49, 10765–10771 RSC.
- F. Fetzer, A. Maier, M. Hodas, O. Geladari, K. Braun, A. J. Meixner, F. Schreiber, A. Schnepf and M. Scheele, Nat. Commun., 2020, 11, 6188 CrossRef CAS.
- O. N. Bakharev, D. Bono, H. B. Brom, A. Schnepf, H. Schnöckel and L. J. de Jongh, Phys. Rev. Lett., 2006, 96, 117002 CrossRef CAS.
- D. Bono, A. Schnepf, J. Hartig, H. Schnöckel, G. J. Nieuwenhuys, A. Amato and L. J. de Jongh, Phys. Rev. Lett., 2006, 97, 077601 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |
