Open Access Article
Open Access ArticlePressure-driven, solvation-directed planar chirality switching of cyclophano-pillar[5]arenes (molecular universal joints)†
Jiabin
Yao
 a,
Hiroaki
Mizuno
b,
Chao
Xiao
a,
Hiroaki
Mizuno
b,
Chao
Xiao
 a,
Wanhua
Wu
a,
Wanhua
Wu
 a,
Yoshihisa
Inoue
a,
Yoshihisa
Inoue
 *c,
Cheng
Yang
*c,
Cheng
Yang
 *a and
Gaku
Fukuhara
*a and
Gaku
Fukuhara
 *bd
*bd
aKey Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, State Key Laboratory of Biotherapy, Healthy Food Evaluation Research Center, Sichuan University, Chengdu 610064, China. E-mail: yangchengyc@scu.edu.cn
bDepartment of Chemistry, Tokyo Institute of Technology, 2-12-1 Ookayama, Meguro-ku, Tokyo 152-8551, Japan. E-mail: gaku@chem.titech.ac.jp
cDepartment of Applied Chemistry, Osaka University, Suita 565-0871, Japan. E-mail: inoue@chem.eng.osaka-u.ac.jp
dJST, PRESTO, 4-1-8 Honcho, Kawaguchi, Saitama 332-0012, Japan
First published on 2nd February 2021
Abstract
Planar chiral cyclophanopillar[5]arenes with a fused oligo(oxyethylene) or polymethylene subring (MUJs), existing as an equilibrium mixture of subring-included (in) and -excluded (out) conformers, respond to hydrostatic pressure to exhibit dynamic chiroptical property changes, leading to an unprecedented pressure-driven chirality inversion and the largest ever-reported leap of anisotropy (g) factor for the MUJ with a dodecamethylene subring. The pressure susceptivity of MUJs, assessed by the change in g per unit pressure, is a critical function of the size and nature of the subring incorporated and the solvent employed. Mechanistic elucidations reveal that the in–out equilibrium, as the origin of the MUJ's chiroptical property changes, is on a delicate balance of the competitive inclusion of subrings versus solvent molecules as well as the solvation of the excluded subring. The present results further encourage our use of pressure as a unique tool for dynamically manipulating various supramolecular devices/machines.
Introduction
Stimulus-responsive artificial molecular machines attract much research interest because of their ability to alter the structure and function through the molecular-level motion triggered by external factors.1–3 A wide range of external stimuli, such as light,4–6 temperature,7,8 redox,9–13 pH,14 and chemical additives,15–19 have hitherto been exploited to achieve diverse stimulus-responsive supramolecular architectures and devices with the aid of deep insights into the kinetic and thermodynamic driving forces involved.Hydrostatic pressure is another fundamental and ubiquitous stimulus that is orthogonal to the other stimuli yet substantially influences various biological, chemical, and physical phenomena.20–24 Indeed, hydrostatic pressure is known to cause the conformational changes of biomacromolecules, such as DNA,25 RNA,26 and proteins,27 and also affect the molecular recognition events.28–32 We have revealed that pressure can manipulate the supramolecular complexation and chiral photoreaction behaviors.24,33–37 Despite these intriguing findings, the pressure effects on artificial supramolecular devices and molecular machines still remain an unchartered realm.
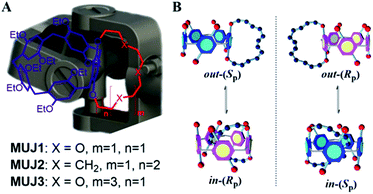
As a relatively new class of synthetic macrocyclic hosts, pillar[n]arenes (P[n]s) have been the target of intensive studies in the past decade.38–47 In particular, planar chirality is one of the most intriguing aspects of P[n] chemistry.48–50 In a recent study, we synthesized a series of inherently chiral cyclophano-P[5] and -P[6] termed molecular universal joints (MUJs) and established their absolute configurations by comparing the experimental versus theoretical CD spectra.51–54 The P[5]-based MUJ1–MUJ3 (Scheme 1, left) employed in the present study incorporate a tetra(oxyethylene)-, dodecamethylene-, and hexa(oxyethylene)-bridged 1,4-hydroquinone unit, respectively, as a fused subring. The subring introduced lowers the symmetry and makes the conformation before/after chiral inversion not enantiomeric anymore. As illustrated in Scheme 1 (right panel), the subring-included “in” and subring-excluded “out” conformers of the MUJ are interconvertible through tumbling of the 1,4-bridged hydroquinone unit, which however occurs only between the in-(Rp) and out-(Sp) and between the in-(Sp) and out-(Rp) isomers without any racemization.
We now report an unprecedented supramolecular chirality inversion of MUJs, which is driven by hydrostatic pressure and facilitated or suppressed by solvation. We further elucidate its origin and operating mechanisms, the results of which allow us to develop a concept of using pressure as a powerful, otherwise-inaccessible, orthogonal-to-the-other-stimuli, yet widely applicable tool for manipulating supramolecular structures, properties, and functions and on/off-switching supramolecular devices and machines.
Results and discussion
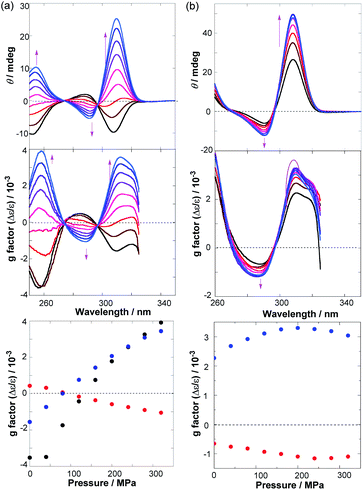
Racemic MUJ1–MUJ3 prepared as reported51 were optically resolved by preparative chiral HPLC (Fig. S1–S3†). The second-eluted in-(Rp)/out-(Sp)-enantiomers of the MUJs in 95–99% enantiomeric excess were used throughout the work. Using a high pressure vessel equipped with birefringence-free diamond windows for spectral measurements, we examined the effects of hydrostatic pressure on the UV-vis and circular dichroism (CD) spectra of in-(Rp)/out-(Sp)-MUJs in hexane (n-H), methylcyclohexane (MCH), carbon tetrachloride (CTC), tetrahydrofuran (THF), ethyl acetate (EA), chloroform (CHL), dichloromethane (DCM), and acetonitrile (AN) at pressures from atmospheric 0.1 MPa to 320 or 160 (for CTC) MPa.Fig. 1 shows the two extreme CD spectral behaviors observed for in-(Rp)/out-(Sp)-MUJ2 in (a) AN and (b) EA upon gradual increase of pressure up to 320 MPa, while Fig. 2 summarizes the pressure-induced variation ranges of the anisotropy (g) factors of in-(Rp)/out-(Sp)-MUJ1, MUJ2, and MUJ3 in all the examined solvents (see Fig. S4–S72 and Table S1† for the original data); note that g = Δε/ε, where Δε denotes the molar CD and ε the molar extinction coefficient. By releasing the applied pressure, the original spectra were immediately recovered without any hysteresis, indicating that the pressure-induced changes observed are fully reversible and no chemical transformation or conformational lock is involved.
The g factor of in-(Rp)/out-(Sp)-MUJ2 was a vital function of both pressure and solvent, exhibiting a dramatic sign-inversion in AN (Fig. 1a) in the middle of the applied pressure range, but no such behavior in EA (Fig. 1b). Thus, in AN, the g factors at 258 and 310 nm (g258 and g310) are substantially enhanced (with a sign inversion) from −0.0035 to +0.0039 and from −0.0016 to +0.0034, respectively, by increasing the pressure from 0.1 to 320 MPa. The net change in g amounts to 0.0074 at 254 nm and to 0.0050 at 310 nm, which are the largest ever reported,24,30 and are expected to grow further at yet higher pressures. In contrast, the g310 factor in EA merely shows a modest initial increase followed by a slow decrease to give a much smaller net change of 0.0007.
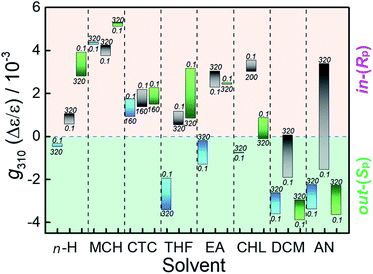
As can be seen from Fig. 2, the pressure-dependent CD spectral behaviors of the three MUJs are distinctly different from each other in all the solvents examined (except CTC), but may be placed somewhere in between the two extreme cases mentioned above (Fig. 1a and b). One of the most intriguing (and unexpected) findings is the pressure-driven sign inversion of the g factor observed for MUJ2 in DCM and AN. The same phenomenon is seen also for MUJ3 in CHL and is anticipated to occur at much higher pressures for MUJ1 in CTC, EA, DCM, and AN, for MUJ2 in CTC, THF, and CHL, and for MUJ3 in n-H, CTC, THF, DCM, and AN with a smaller pressure-dependence in most cases (Fig. 2).
In an attempt to elucidate the origin of the dynamic pressure-dependence (including the sign inversion) of the g factor observed for MUJ2 in DCM and AN, we calculated the van der Waals volumes (VvdW) of the out- and in-conformers of MUJs geometry-optimized in vacuo by the DFT method at the B3LYP/6-31G(d) level. It turned out that the VvdW obtained (Table S2†) is slightly larger for the out- than for the in-conformer, but the difference is mere 0.26–0.36 Å3, which is rather negligible if compared with the whole volumes of the MUJs (944–1030 Å3). This result reveals that the difference in VvdW between the naked in- and out-conformers is not a main driving force of the pressure-driven chirality switching of MUJs.
Judging from the very disparate behaviors of g exhibited in different solvents (Fig. 2), we deduce that the pivotal role is played by solvent in determining the in/out conformation of MUJs. Before closely examining the pressure effects, we analyze the original conformation of MUJ2 at 0.1 MPa in all the solvents examined. Our recent study51 has shown that the sign of the g factor is directly related to the in/out conformation of MUJ2 and hence serves as a reliable tool for assessing the absolute configuration of the MUJ, i.e., in-(Rp) or out-(Sp). This criterion and the g factor monitored at 310 nm under the atmospheric pressure (g3100.1 MPa) listed in Table 1 indicate that, at 0.1 MPa, MUJ2 favors the in-(Rp)-conformation in n-H, MCH, CTC, THF, EA, and CHL, but the out-(Sp)-conformation in DCM and AN.
| Solvent | E T | g 310 0.1 MPa (×103)b | Δg/ΔPc (TPa−1) | ||||
|---|---|---|---|---|---|---|---|
| MUJ1 | MUJ2 | MUJ3 | MUJ1 | MUJ2 | MUJ3 | ||
| a Reichardt's empirical solvent polarity parameter. b Positive (negative) g3100.1 MPa factor indicates preference for the in (out) conformer at atmospheric pressure. c Δg/ΔP = (g310Pmax − g310Pmin)/(Pmax − Pmin); the pressure range applied (ΔP): 0.1–320 MPa, unless noted otherwise. Positive (negative) Δg/ΔP value means equilibrium shift to the in-(Rp) (out-(Sp)) conformer upon pressurization. d Too bulky to be fully accommodated in the P[5] cavity (4.7 Å i.d.).55–58 e ΔP: 0.1–160 MPa. f ΔP: 0.1–200 MPa. | |||||||
| n-H | 30.9 | −0.2 | +0.6 | +3.4 | −0.7 | +1.4 | −2.6 |
| MCHd | ∼31 | +4.1 | +3.7 | +4.9 | +0.6 | +1.5 | +0.9 |
| CTCd | 32.5 | +1.7 | +2.0 | +2.2 | −5.1e | −4.8e | −4.7e |
| THFd | 37.4 | −1.9 | +1.2 | +3.1 | −4.9 | −2.0 | −7.1 |
| EA | 38.1 | −1.2 | +2.3 | +2.4 | +3.4 | +2.4 | −0.2 |
| CHLd | 39.1 | −0.8 | +3.4 | +0.8 | −0.0 | −2.8f | −2.9 |
| DCM | 41.1 | −3.6 | −1.9 | −3.9 | +3.0 | +5.9 | +3.0 |
| AN | 46.0 | −3.4 | −1.6 | −3.7 | +3.5 | +15.6 | +4.2 |
To better understand the interaction of solvent molecules with the P[5] cavity, we attempted to determine the association constant (Ka) of the relevant solvents with 1,4-diethoxyP[5] (a model host with comparable cavity parameters) in MCH (a nonpolar/non-solvating molecule that is oversized and hence impenetrable into the P[5] cavity).43 As shown in Table S3,† AN and DCM turned out to have 1–2 orders of magnitude higher affinities toward the P[5] cavity (Ka = 50–550 M−1) than the other solvents (Ka = 4–18 M−1), which well rationalizes the rich population of the out-(Sp) conformer in these two solvents at 0.1 MPa. In the other solvents of lower P[5] affinities, MUJ2 includes the subring in its own cavity to form the in-(Rp) conformer and hence exhibits positive g3100.1 MPa factors at 0.1 MPa.
Possessing a polyether subring, MUJ1 and MUJ3 afford twice larger g3100.1 MPa factors of −3.4 to −3.9 × 10−3 than MUJ2 in both DCM and AN (Table 1), indicating enriched populations of the out-(Sp)-conformers. This enrichment is most probably facilitated by the stabilization of the out-conformers through the solvation of the excluded polyether subring as well as the solvent inclusion in the P[5] cavity that is common to all the subring-excluded MUJs. In less polar solvents of lower P[5] affinities, the g310 factor of MUJ3, though not exactly the same in magnitude, shares the same (positive) sign with that of MUJ2, while the g310 factor of MUJ1 with the opposite sign behaves very differently in n-H, THF, EA, and CHL, implying nontrivial effects of the smaller subring on the in–out equilibrium. These observations and considerations led us to a tentative conclusion that the pressure-driven chirality inversion from out-(Sp)- to in-(Rp)-MUJ2 observed in AN and DCM was caused by self-inclusion of the dodecamethylene-subring accompanied by the exclusion of polar solvent molecule(s) originally residing in the P[5] cavity.
In order to substantiate the above hypothesis and quantitatively discuss the pressure effects on the in–out conformer equilibrium, we wanted to estimate the in/out ratio or the equilibrium constant (Kin/out = [in]/[out]) from the observed g310 factor. This should be made possible if we could experimentally determine the g310 factors for both of the pure in-(Rp)- and out-(Sp)-MUJs, or if we could determine one of them and could claim gin310 = −gout310.
1,4-Dicyanobutane (DCB) is one of the strongest-binding guests for P[5] hosts,59 and hence the addition of a large excess amount of DCB to an MUJ solution is expected to drive the subring out of the P[5] cavity. Upon addition of DCB of up to 2000 equivalents, the g310 factor gradually increased to reach a plateau in all the solvents. The ultimate values thus attained (Table S4†) are regarded as the g310 factors for pure out-(Sp)-conformers of MUJs. However, these gout310 factors for MUJ1–MUJ3 are not close to each other, but depend on the subring incorporated in all the solvents used, thus being reduced by 20–30% on going from MUJ3 to MUJ1 and by 15–37% by increasing the solvent polarity (except CTC, which causes 46–47% reduction for unclear reasons; see Table S4†). These results imply that the gin310 factors for pure in-(Rp)-MUJs, if properly estimated, are also significantly affected by the internal and external factors, which means that postulating gin310 = −gout310 is unrealistic.
Since experimentally determining the gin310 factors for pure in-(Rp)-MUJs was infeasible due to the lack of appropriate methods for completely driving the subring into the P[5] cavity, we semi-quantitatively discuss the pressure effects on the MUJ conformation. For this purpose, we simply define the overall pressure susceptivity of the chiroptical parameter g310 as Δg/ΔP = (g310Pmax − g310Pmin)/(Pmax – Pmin), despite that the pressure dependence is not necessarily homogeneous over the entire pressure range examined (Fig. S4–S72†).
The pressure susceptivities (Δg/ΔP) thus evaluated for MUJ2 (Table 1) are large positive (indicating a strong drive to the in-(Rp)-conformer upon pressurization) particularly in DCM and AN (amounting to +5.9 and +15.6 TPa−1, respectively), but are much smaller positive or even negative, varying from +2.4 to −4.8 TPa−1, in other solvents. We now comprehend why MUJ2 achieves the pressure-induced chirality inversion in DCM and AN. Thus, the small negative initial g3100.1 MPa values (−1.9 and −1.6 × 10−3) are readily cancelled out upon pressurization by the large positive Δg/ΔP values (+5.9 and +15.6 TPa−1), eventually leading to a sign inversion within the pressure range employed. In contrast, the Δg/ΔP value has the same sign with g3100.1 MPa in n-H, MCH, and EA and no sign inversion is anticipated to occur upon pressurization, or is not sufficiently large to overwhelm the g3100.1 MPa factor even at the highest pressure applied in CTC and THF.
In polar DCM and AN, MUJ1 and MUJ3 less sensitively responded to pressure than MUJ2 to give much smaller Δg/ΔP values of +3.0 to +4.2 TPa−1 (Table 1), for which the out-to-in equilibrium shift hindered by the solvation of the polyether subring that stabilizes the out-(Sp)-conformer should be responsible. Among the less polar solvents, CTC, THF, and CHL consistently afford negative Δg/ΔP values for all the MUJs. Since the molar volume of the out-(Sp)-MUJ, particularly when solvated, is considered to be larger than that of the in-(Rp)-MUJ, the in-to-out equilibrium shift upon pressurization observed in CTC, THF, and CHL is unusual, indicating that the shift to the out-(Sp)-conformer under pressure is driven not by the volume difference but by other mechanisms.
CTC, THF, and CHL are too bulky to be fully accommodated in the P[5] cavity but could “perch” on the wide-opening ethoxy-decorated portal of the MUJ. Indeed, THF was found to be weakly bound to DEP[5] with Ka = 4.12 M−1 in MCH at 0.1 MPa by isothermal titration calorimetric (ITC) studies (Table S3†). Although the calorimetric titration at the same DEP[5] concentration (1 mM) did not produce any detectable heat upon addition of CHL or CTC, the NMR spectrum of DEP[5] (4–4.3 mM) in MCH-d14 showed small downfield shifts (0.10–0.13 ppm) of the aromatic and ethoxy protons in the presence of 2.8 M CHL (Fig. S79 and Table S5†); the least-square-means fit of the chemical shift changes to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry afforded Ka = 0.63 ± 0.02 M−1 (Fig. S80†). In contrast, the addition of 5.18 M CTC caused slight upfield shifts (0.005–0.045 ppm) of the same protons, which are substantially smaller in magnitude and opposite in direction when compared with those observed upon addition of CHL (Table S5†) and AN (Table S7†). We conclude therefore that the slight shifts observed upon addition of CTC are irrelevant to the complexation but caused by the change in medium polarity. This conclusion is compatible with the very resembling pressure dependence behaviors commonly observed for all the MUJs examined (Fig. 2 and Table 1). These results reveal that DEP[5] weakly interacts with the seemingly oversized guests (THF and CHL, but not CTC), probably at its ethoxy-decorated portal. Combining this fact and the significant CD spectral changes of MUJs observed upon addition of CHL at elevated pressures (Fig. 2 and S22, S25, and S28†), we deduce that the supramolecular interaction of MUJs with the modestly oversized molecules through portal perching is promoted under pressure.
1 stoichiometry afforded Ka = 0.63 ± 0.02 M−1 (Fig. S80†). In contrast, the addition of 5.18 M CTC caused slight upfield shifts (0.005–0.045 ppm) of the same protons, which are substantially smaller in magnitude and opposite in direction when compared with those observed upon addition of CHL (Table S5†) and AN (Table S7†). We conclude therefore that the slight shifts observed upon addition of CTC are irrelevant to the complexation but caused by the change in medium polarity. This conclusion is compatible with the very resembling pressure dependence behaviors commonly observed for all the MUJs examined (Fig. 2 and Table 1). These results reveal that DEP[5] weakly interacts with the seemingly oversized guests (THF and CHL, but not CTC), probably at its ethoxy-decorated portal. Combining this fact and the significant CD spectral changes of MUJs observed upon addition of CHL at elevated pressures (Fig. 2 and S22, S25, and S28†), we deduce that the supramolecular interaction of MUJs with the modestly oversized molecules through portal perching is promoted under pressure.
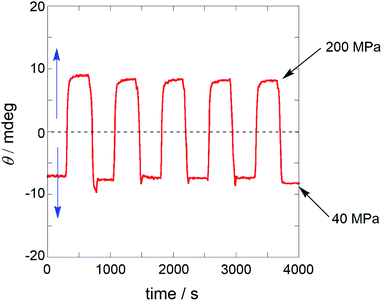
The wide dynamic range, quick and reversible response to pressure change, and dynamic sign inversion of the CD signal prompted us to scrutinize the capability of MUJ2 as a supramolecular chiroptical switching device driven by pressure.60–63 Thus, a dilute solution of MUJ2 in AN was subjected to repeated pressurization–depressurization cycles between 40 and 200 MPa to afford the alternating positive and negative ellipticities of comparable intensities with good reproducibility, as shown in Fig. 3.
Conclusions
In this study to elucidate the pressure dependence behaviors of conformationally flexible planar chiral cyclophano-pillar[5]arenes (MUJs), we have shown that the in–out conformer equilibrium of MUJs is highly susceptive to hydrostatic pressure particularly in polar solvents that possess strong affinities to the pillar[5]arene cavity to achieve the pressure-driven chirality switching and the largest ever reported jump of anisotropy factor. These unique features of MUJs open a new avenue for the multidimensional control of molecular machines and advanced piezo-sensitive materials by using pressure, temperature, and solvents, and also help us understand and utilize the pressure effects on natural and artificial supramolecular systems.64–66Author contributions
G. F., C. Y. and Y. I. initiated the project. J. Y., H. M., C. X. and W. W. conceived and designed the experiments, analysed the data and prepared the manuscript, with input from all the authors. J. Y. conducted the chemical synthesis. G. F. and H. M. performed CD and UV-vis spectral analysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the support of this work by the National Natural Science Foundation of China (No. 92056116, 21871194, 21572142), National Key Research and Development Program of China (No. 2017YFA0505903), Key R & D project of Science & Technology Department of Sichuan Province (2019YJ0160, 2019YJ0090, and 2017SZ0021), and State Key Laboratory of Fine Chemicals (KF 1508), Comprehensive Training Platform of Specialized Laboratory, College of Chemistry and Prof. Peng Wu of Analytical & Testing Center, Sichuan University. G. F. appreciates the generous supports by Grant-in-Aid for Scientific Research (B) (No. 19H02746) from the Japan Society for the Promotion of Science (JSPS) and Japan Science and Technology Agency (JST), PRESTO (No. JPMJPR17PA).References
- A. Coskun, M. Banaszak, R. D. Astumian, J. F. Stoddart and B. A. Grzybowski, Chem. Soc. Rev., 2012, 41, 19–30 RSC.
- S. Kassem, A. T. L. Lee, D. A. Leigh, V. Marcos, L. I. Palmer and S. Pisano, Nature, 2017, 549, 374–378 CrossRef CAS.
- K. Zhu, G. Baggi and S. J. Loeb, Nat. Chem., 2018, 10, 625–630 CrossRef CAS.
- J. J. D. de Jong, L. N. Lucas, R. M. Kellogg, J. H. van Esch and B. L. Feringa, Science, 2004, 304, 278–281 CrossRef CAS.
- Z. Zheng, Y. Li, H. K. Bisoyi, L. Wang, T. J. Bunning and Q. Li, Nature, 2016, 531, 352–356 CrossRef CAS.
- M. D. Poli, W. Zawodny, O. Quinonero, M. Lorch, S. J. Webb and J. Clayden, Science, 2016, 352, 575–580 CrossRef.
- M. Fujiki, J. Am. Chem. Soc., 2000, 122, 3336–3343 CrossRef CAS.
- T. Ooi, Science, 2011, 331, 1395–1396 CrossRef CAS.
- S. Zahn and J. W. Canary, Science, 2000, 288, 1404–1407 CrossRef CAS.
- H. Goto and E. Yashima, J. Am. Chem. Soc., 2002, 124, 7943–7949 CrossRef CAS.
- J. W. Canary, Chem. Soc. Rev., 2009, 38, 747–756 RSC.
- E. Ohta, H. Sato, S. Ando, A. Kosaka, T. Fukushima, D. Hashizume, M. Yamasaki, K. Hasegawa, A. Muraoka, H. Ushiyama, K. Yamashita and T. Aida, Nat. Chem., 2011, 3, 68–73 CrossRef CAS.
- Y. J. Zhang, T. Oka, R. Suzuki, J. T. Ye and Y. Iwasa, Science, 2014, 344, 725–728 CrossRef CAS.
- H. Liang, B. Hua, F. Xu, L.-S. Gan, L. Shao and F. Huang, J. Am. Chem. Soc., 2020, 142, 19772–19778 CrossRef CAS.
- V. V. Borovkov, T. Harada, Y. Inoue and R. Kuroda, Angew. Chem., Int. Ed., 2002, 41, 1378–1381 CrossRef CAS.
- Y. Qiu, P. Chen, P. Guo, Y. Li and M. Liu, Adv. Mater., 2008, 20, 2908–2913 CrossRef CAS.
- G. Haberhauer, Angew. Chem., Int. Ed., 2010, 49, 9286–9289 CrossRef CAS.
- E. Lee, H. Ju, I.-H. Park, J. H. Jung, M. Ikeda, S. Kuwahara, Y. Habata and S. S. Lee, J. Am. Chem. Soc., 2018, 140, 9669–9677 CrossRef CAS.
- Y.-F. Yang, W.-B. Hu, L. Shi, S.-G. Li, X.-L. Zhao, Y. A. Liu, J.-S. Li, B. Jiang and W. Ke, Org. Biomol. Chem., 2018, 16, 2028–2032 RSC.
- F. Zhao, Y. Zhao, H. Cheng and L. Qu, Angew. Chem., Int. Ed., 2015, 54, 14951–14955 CrossRef CAS.
- Y. Zhang, J. Yu, H. N. Bomba, Y. Zhu and Z. Gu, Chem. Rev., 2016, 116, 12536–12563 CrossRef CAS.
- Y. Ishijima, H. Imai and Y. Oaki, Chem, 2017, 3, 509–521 CAS.
- Y. Sagara, N. Tamaoki and G. Fukuhara, ChemPhotoChem, 2018, 2, 959–963 CrossRef CAS.
- T. Kosaka, S. Iwai, G. Fukuhara, Y. Imai and T. Mori, Chem.–Eur. J., 2019, 25, 2011–2018 CrossRef CAS.
- S. Takahashi and N. Sugimoto, Angew. Chem., Int. Ed., 2013, 52, 13774–13778 CrossRef CAS.
- A. Krzyżaniak, J. Barciszewski, J. P. Fürste, R. Bald, V. A. Erdmann, P. Salański and J. Jurczak, Int. J. Biol. Macromol., 1994, 16, 159–162 CrossRef.
- K. Heremans and L. Smeller, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 1998, 1386, 353–370 CrossRef CAS.
- K. Ariga, Y. Terasaka, D. Sakai, H. Tsuji and J. Kikuchi, J. Am. Chem. Soc., 2000, 122, 7835–7836 CrossRef CAS.
- B. Neumann and P. Pollmann, Phys. Chem. Chem. Phys., 2000, 2, 4784–4792 RSC.
- Y. Nagata, R. Takeda and M. Suginome, Chem. Commun., 2015, 51, 11182–11185 RSC.
- Y. Wang, X. Tan, Y.-M. Zhang, S. Zhu, I. Zhang, B. Yu, K. Wang, B. Yang, M. Li, B. Zou and S. X.-A. Zhang, J. Am. Chem. Soc., 2015, 137, 931–939 CrossRef CAS.
- C. Liu, G. Xiao, M. Yang, B. Zou, Z.-L. Zhang and D.-W. Pang, Angew. Chem., Int. Ed., 2018, 57, 1893–1897 CrossRef CAS.
- Y. Inoue, E. Matsushima and T. Wada, J. Am. Chem. Soc., 1998, 120, 10687–10696 CrossRef CAS.
- M. Kaneda, S. Asaoka, H. Ikeda, T. Mori, T. Wada and Y. Inoue, Chem. Commun., 2002, 1272–1273 RSC.
- C. Yang, A. Nakamura, G. Fukuhara, Y. Origane, T. Mori, T. Wada and Y. Inoue, J. Org. Chem., 2006, 71, 3126–3136 CrossRef CAS.
- C. Yang, T. Mori, Y. Origane, Y. H. Ko, N. Selvapalam, K. Kim and Y. Inoue, J. Am. Chem. Soc., 2008, 130, 8574–8575 CrossRef CAS.
- M. Gao, W. Zhang and L. Zhang, Nano Lett., 2018, 18, 4424–4430 CrossRef CAS.
- T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS.
- D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721–9723 CrossRef CAS.
- Y. Ma, Z. Zhang, X. Ji, C. Han, J. He, Z. Abliz, W. Chen and F. Huang, Eur. J. Org. Chem., 2011, 2011, 5331–5335 CrossRef CAS.
- X.-B. Hu, Z. Chen, L. Chen, L. Zhang, J.-L. Hou and Z.-T. Li, Chem. Commun., 2012, 48, 10999–11001 RSC.
- T. Ogoshi, T. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS.
- K. Jie, Y. Zhou, E. Li, R. Zhao and F. Huang, Angew. Chem., Int. Ed., 2018, 57, 12845–12849 CrossRef CAS.
- H. Zhang, Z. Liu and Y. Zhao, Chem. Soc. Rev., 2018, 47, 5491–5528 RSC.
- E. Li, Y. Zhou, R. Zhao, K. Jie and F. Huang, Angew. Chem., Int. Ed., 2019, 58, 3981–3985 CrossRef CAS.
- P. Xin, H. Kong, Y. Sun, L. Zhao, H. Fang, H. Zhu, T. Jiang, J. Guo, Q. Zhang, W. Dong and C.-P. Chen, Angew. Chem., Int. Ed., 2019, 58, 2779–2784 CrossRef CAS.
- W. Yang, K. Samanta, X. Wan, T. U. Thikekar, Y. Chao, S. Li, K. Du, J. Xu, Y. Gao, H. Zuilhof and A. C.-H. Sue, Angew. Chem., Int. Ed., 2020, 59, 3994–3999 CrossRef CAS.
- T. Ogoshi, K. Masaki, R. Shiga, K. Kitajima and T. Yamagishi, Org. Lett., 2011, 13, 1264–1266 CrossRef CAS.
- T. Ogoshi, T. Akutsu, D. Yamafuji, T. Aoki and T. Yamagishi, Angew. Chem., Int. Ed., 2013, 52, 8111–8115 CrossRef CAS.
- S.-H. Li, H.-Y. Zhang, X. Xu and Y. Liu, Nat. Commun., 2015, 6, 7590 CrossRef.
- J. Yao, W. Wu, W. Liang, Y. Feng, D. Zhou, J. J. Chruma, G. Fukuhara, T. Mori, Y. Inoue and C. Yang, Angew. Chem., Int. Ed., 2017, 56, 6869–6873 CrossRef CAS.
- C. Fan, J. Yao, G. Li, C. Guo, W. Wu, D. Su, Z. Zhong, D. Zhou, Y. Wang, J. J. Chruma and C. Yang, Chem.–Eur. J., 2019, 25, 12526–12537 CrossRef CAS.
- J. Ji, Y. Li, C. Xiao, G. Cheng, K. Luo, Q. Gong, D. Zhou, J. J. Chruma, W. Wu and C. Yang, Chem. Commun., 2020, 56, 161–164 RSC.
- C. Xiao, W. Wu, W. Liang, D. Zhou, K. Kanagaraj, G. Cheng, D. Su, Z. Zhong, J. J. Chruma and C. Yang, Angew. Chem., Int. Ed., 2020, 59, 8094–8098 CrossRef CAS.
- F. Guo, Y. Sun, B. Xi and G. Diao, Supramol. Chem., 2018, 30, 81–92 CrossRef CAS.
- K. Jie, Y. Zhou, E. Li and F. Huang, Acc. Chem. Res., 2018, 51, 2064–2072 CrossRef CAS.
- M. Panneerselvam, M. D. Kumar, M. Jaccob and R. V. Solomon, ChemistrySelect, 2018, 3, 1321–1334 CrossRef.
- C. Schönbeck, H. Li, B.-H. Han and B. W. Laursen, J. Phys. Chem. B, 2015, 119, 6711–6720 CrossRef.
- Y. Wang, G. Ping and C. Li, Chem. Commun., 2016, 52, 9858–9872 RSC.
- D. Fichou, C. Hubert and F. Garnier, Adv. Mater., 1995, 7, 914–917 CrossRef.
- B. Onida, L. Borello, S. Fiorilli, B. Bonelli, C. O. Areán and E. Garrone, Chem. Commun., 2004, 2496–2497 RSC.
- T. Kosaka, Y. Inoue and T. Mori, J. Phys. Chem. Lett., 2016, 7, 783–788 CrossRef CAS.
- T. Kosaka, S. Iwai, Y. Inoue, T. Moriuchi and T. Mori, J. Phys. Chem. A, 2018, 122, 7455–7463 CrossRef CAS.
- D. Bartlett, M. Wright, A. A. Yayanos and M. Silverman, Nature, 1989, 342, 572–574 CrossRef CAS.
- B. Fromy, E. Lingueglia, D. Sigaudo-Roussel, J. L. Saumet and M. Lazdunski, Nat. Med., 2012, 18, 1205–1207 CrossRef CAS.
- W.-Z. Zeng, K. L. Marshall, S. Min, I. Daou, M. W. Chapleau, F. M. Abboud, S. D. Liberles and A. Patapoutian, Science, 2018, 362, 464–467 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc06988d |
| This journal is © The Royal Society of Chemistry 2021 |