Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Construction of congested Csp3–Csp3 bonds by a formal Ni-catalyzed alkylboration†
Amit Kumar
Simlandy
 ,
Stephen R.
Sardini
and
M. Kevin
Brown
,
Stephen R.
Sardini
and
M. Kevin
Brown
 *
*
Department of Chemistry, Indiana University, 800E. Kirkwood Ave, Bloomington, IN 47401, USA. E-mail: brownmkb@indiana.edu
First published on 9th March 2021
Abstract
Through the combination of a Ni-catalyzed alkene alkenylboration followed by hydrogenation, the synthesis of congested Csp3–Csp3-bonds can be achieved. Conditions have been identified that allow for the use of both alkenyl-bromides and -triflates. In addition, the hydrogenation creates another opportunity for stereocontrol, thus allowing access to multiple stereoisomers of the product. Finally, the method is demonstrated in the streamlined synthesis of a biologically relevant molecule.
Introduction
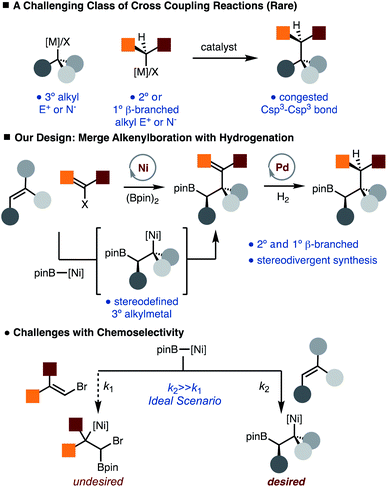
Recent studies have demonstrated that molecules with an increased proportion of Csp3 centers can often result in improved pharmacological properties (i.e. “escape from flatland”).1 Therefore, development of methods that facilitate the synthesis of Csp3–Csp3 bonds is of value. While much progress has been made in the development of alkyl–alkyl cross-coupling reactions, synthesis of congested Csp3–Csp3 bonds remains a formidable challenge (Scheme 1).2 In particular, generation of products that would arise from the direct coupling of 2° and 3° alkyl fragments is not known. In addition, use of 1° β-branched alkyl electrophiles is also known to be challenging.3 Therefore, introduction of protocols that would achieve the synthesis of these congested bonds would provide an important tool in the construction of complex Csp3 rich molecules.Our lab has recently disclosed the Ni-catalyzed arylboration of unactivated alkenes.4,5 The value of these methods lies in that simple starting materials (alkenes, diboron reagents, and carbon-based electrophiles) are converted to more complex structures with control of diastereoselectivity and regioselectivity in one step, and the reactivity of the resulting C–B bond, which can be easily transformed into C–O, C–N and C–C bonds thus allowing for diverse product formation.6 One of the key aspects of the Ni-catalyzed arylboration reaction is that sterically demanding di- and tri-substituted unactivated alkenes can be used. In these reactions, a stereodefined tertiary alkyl-Ni-complex is generated by borylnickelation of an alkene, which undergoes facile reaction with an arylbromide.5 To address the aforementioned challenge of making Csp3–Csp3 bonds, we envisioned a net alkylboration that would merge an alkenylboration and subsequent hydrogenation (Scheme 1).7–9 This strategy is appealing as the hydrogenation event offers an additional point of stereocontrol such that diverse products can be generated from a common set of starting materials. While coupling of 3° alkyl and aryl fragments is known,10 the use of alkenyl partners that would allow for synthesis of congested Csp3–Csp3 bonds by subsequent hydrogenation is exceedingly rare.11,12 The outlined approach does bring to light a chemoselectivity challenge in that conditions must be tuned to favor borylnickelation of the alkene rather than alkenyl bromide (Scheme 1).
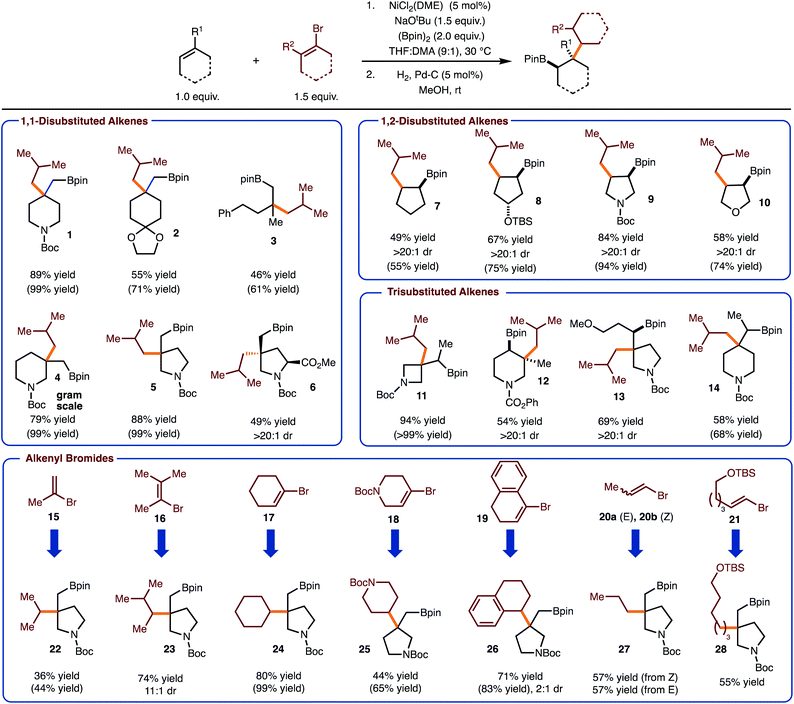
Under conditions optimized for arylboration of alkenes,5c it was identified that alkenyl halides could be used to deliver the desired alkenylboration products. Furthermore, hydrogenation with Pd/C proceeded in high yield to generate the products of a net alkylboration. The use of 1-bromo-2-methylpropene allowed for the introduction of an isobutyl group with a variety of alkenes (products 1–14). Both sterically demanding trisubstituted and 1,1-disubstituted alkenes function well in the process to generate a congested C–C bond between a quaternary carbon and a β-branched primary carbon. A focus of these efforts was on the synthesis of saturated nitrogen containing heterocycles, which are of value in medicinal chemistry.13 Particularly notable are examples 12 and 13 as these are generated as single observable diastereomers. In addition, reaction to produce 6, occurred with high selectivity for addition on the face opposite the ester. Reactions of more strained alkenes generally result in higher yield (compare product 11 with 13/14). Finally, with 1-bromo-2-methylpropene the reaction of cyclic 1,2-disubstiuted alkenes were also investigated, which allowed for the synthesis of syn-1,2-substituted 5-membered ring carbo- and heterocycles.
With respect to the alkenyl bromide component, the use of all substitution patterns worked. In the case of 15–19 the coupling proceeded to prepare the sterically congested bond of coupling between 2° and 3° fragments. In the case of products 23 and 26, the hydrogenation reaction led to formation of diastereomers with variable selectivity. The formal introduction of a 1° alkyl group can also be achieved with use of bromides 20a/b and 21. With the former example, both E and Z-alkenyl bromide work with equal efficiency. In addition, functional groups such as acetals (product 2) and silyl ethers were tolerated (product 28). Finally, the reaction can be performed on gram scale as demonstrated in the synthesis of 4.
With the data gathered in Scheme 2, some general trends are revealed regarding chemoselectivity of [Ni]-Bpin addition. In general, the use of sterically demanding tri- and tetra-substituted alkenyl bromides led to formation of the product in the highest yields. In these cases, it is likely that k1 is reduced due to steric hindrance, thus allowed k2 to predominate (Scheme 1). For reactions with disubstituted alkenyl bromides 15, 20–21, competitive borylation of the alkene was observed. Despite this challenge, products 22 and 27–28 could still be formed in acceptable yields. However, it is notable that in most cases the addition of [Ni]-Bpin proceeds with high chemoselectivity for addition to the unactivated alkene in preference to the alkenyl bromide.
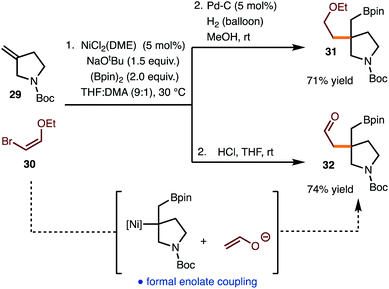
The reaction of commercially available bromo enol ether 30 was also investigated (Scheme 3). Standard hydrogenation of the alkenylboration product led to the formation of 31. Alternatively, hydrolysis of the alkenylboration product allowed for the formation of aldehyde 32, which represents the formal coupling of an enolate with the generated alkyl Ni-complex.
While the use of alkenyl bromides is convenient due to commercial availability, the use of enol-triflates would also be of value as they are readily prepared from the corresponding aldehydes or ketones. Under the optimized conditions, however, only 60% of 35 was observed (Table 1, entry 1). We hypothesized that bromide ion may be important for reactivity. Thus, sodium bromide was added, however, only a modest increase in yield was observed (Table 1, entry 2). Interestingly, when NiBr2(DME) was evaluated, a significant increase in yield relative to use of NiCl2(DME) was observed (Table 1, entry 3). Based on the observation that additives impacted the reaction yield, other salts were investigated, which led to the finding that using NaBF4 led to the highest yield of product (Table 1, entry 9). At this stage, the role of NaBF4 is not clear. It should also be noted that the use of NaBF4 was explored in reactions of the alkenyl bromides; however, no beneficial effect was observed.
| Entry | Ni-catalyst | Additive | Yielda (%) |
|---|---|---|---|
| a Yield determined by analysis of the unpurified reaction mixture with an internal standard. b 30 mol % additive. c 60 mol % additive. d Yield in parentheses is of isolated product after silica gel column chromatography. | |||
| 1 | NiCl2(DME) | None | 60 |
| 2 | NiCl2(DME) | NaBrb | 64 |
| 3 | NiBr2(DME) | None | 77 |
| 4 | NiBr2(DME) | NaClc | 79 |
| 5 | NiBr2(DME) | NaBrc | 21 |
| 6 | NiBr2(DME) | NaOTf | 87 |
| 7 | NiBr2(DME) | NaPF6 | 52 |
| 8 | NiBr2(DME) | NaSbF6 | 19 |
| 9 | NiBr 2 (DME) | NaBF 4 | 91 (70) |
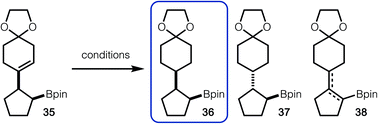
For hydrogenation of 35 with Pd/C, the formation of isomers 36, 37 and 38 were observed (Table 2, entry 1).14 It is likely that anti-isomer 37 is formed after hydrogenation of an in situ generated tetrasubstituted alkene. Use of Crabtree's catalyst did lead to suppressed formation of anti-isomer 37, however alkene isomers (38) were still observed (Table 2, entry 2). Finally, it was discovered that HAT hydrogenation allowed for exclusive formation of 36, without undesired isomerization (Table 2, entry 3).15
| Entry | Conditions | Yielda (%) (36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38) 38) |
|---|---|---|
| a Yield determined by analysis of the unpurified reaction mixture by 1H NMR with an internal standard. b Yield of isolated product after silica gel column chromatography. | ||
| 1 | 5 mol % Pd–C, H2 (balloon), EtOAc, rt | 95% yield (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
| 2 | 5 mol % Crabtree's cat., H2 (balloon), CH2Cl2, 0 °C | 84% yield (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 3 | Mn(dpm)3, PhSiH2(Oi-Pr), TBHP, hexanes, rt | 66% yieldb (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
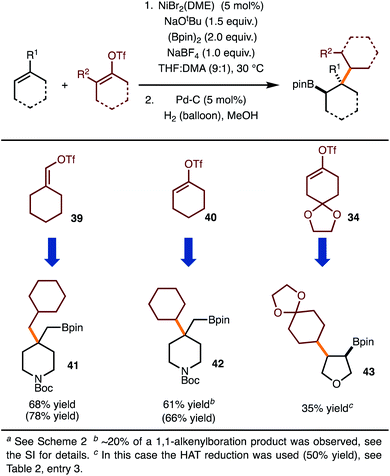
Under the optimal conditions for coupling with alkenyl triflates, several examples were investigated (Scheme 4). The use of cyclohexenyl triflate 39 allowed for the formation of 41, whereas use of triflate 40 led to synthesis of 42. For the synthesis of 43, the moderate yield was the result of a 50% yield in the hydrogenation step.
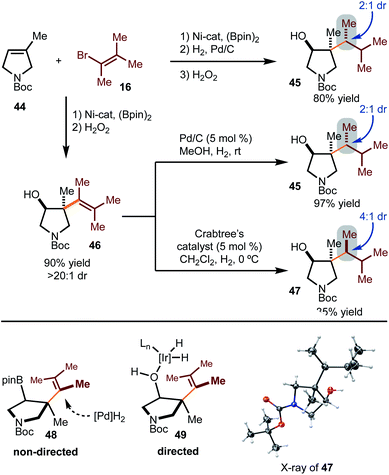
Hydrogenation with substrates that would result in diastereomers was probed more deeply (Scheme 5). Hydrogenation of the alkenylboration product derived from 44 and 16 gave rise to 45 as the major diastereomer in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Other hydrogenation conditions were attempted, but poor reactivity was observed. Hydrogenation of the corresponding alcohol 46 was also probed. Under heterogeneous conditions, the same major diastereomer (45) was formed as that observed in the reduction of the Bpin-derived substrate. Based on the stereochemistry of product 45, reduction likely occurs from the least hindered face, as shown in model 48. On the other hand, directed hydrogenation of the alcohol with Crabtree's catalyst led to formation of the opposite diastereomer (47), likely via intermediate 49.16 It is important to note that while the selectivities are modest, this strategy demonstrates that tuning of conditions can allow for stereodivergent synthesis. In addition, these examples demonstrate that three contiguous stereogenic centers can be prepared from simple components in a modular fashion.
1 dr. Other hydrogenation conditions were attempted, but poor reactivity was observed. Hydrogenation of the corresponding alcohol 46 was also probed. Under heterogeneous conditions, the same major diastereomer (45) was formed as that observed in the reduction of the Bpin-derived substrate. Based on the stereochemistry of product 45, reduction likely occurs from the least hindered face, as shown in model 48. On the other hand, directed hydrogenation of the alcohol with Crabtree's catalyst led to formation of the opposite diastereomer (47), likely via intermediate 49.16 It is important to note that while the selectivities are modest, this strategy demonstrates that tuning of conditions can allow for stereodivergent synthesis. In addition, these examples demonstrate that three contiguous stereogenic centers can be prepared from simple components in a modular fashion.
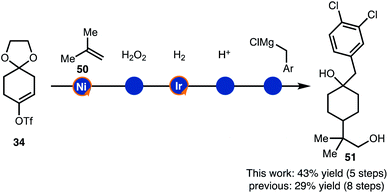
Finally, the alkenylboration/hydrogenation sequence was used in the synthesis of drug like intermediates (Scheme 6). Compound 51 was prepared though a brief sequence of five steps in 43% overall starting from 34 and 50. Thus, the demonstrated strategy offers an alternative to the established route that required eight steps.17
Conclusions
In summary, the synthesis of sterically congested Csp3–Csp3 bonds by a formal alkylboration of unactivated alkenes is reported. The process was made possible by combining a Ni-catalyzed alkenylboration followed by hydrogenation. In addition, the hydrogenation could be tuned to achieve stereodivergent synthesis. Through the development of this process, we have demonstrated the utility of Ni-catalyzed carboboration for the generation of molecular complexity with high Csp3 content.Author contributions
A. K. S., S. R. S and M. K. B. designed the study. A. K. S. and S. R. S. performed the experiements. A. K. S. and M. K. B. wrote the paper with input from S. R. S.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Indiana University and the NIH (R35GM131755) for financial support. This project was partially funded by the Vice Provost for Research through the Research Equipment Fund and the NSF MRI program, CHE-1726633 and CHE-1920026. We thank Dr Maren Pink and Dr Veronica Carta of the IU Molecular Structure Center for acquisition of X-ray crystal structure data. Support for the acquisition of the Bruker Venture D8 diffractometer through the Major Scientific Research Equipment Fund from the President of Indiana University and the Office of the Vice President for Research is gratefully acknowledged.Notes and references
- (a) F. Lovering, MedChemComm, 2013, 4, 515 RSC; (b) F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef CAS PubMed.
- J. Choi and G. C. Fu, Science, 2017, 356(6334), eaaf7230 CrossRef PubMed.
- For an example, see: S. A. Green, T. R. Huffman, R. O. McCourt, V. van der Puyl and R. A. Shenvi, J. Am. Chem. Soc., 2019, 141, 7709–7714 CrossRef CAS PubMed.
- For recent reviews, see: (a) A. Whyte, A. Torelli, B. Mirabi, A. Zhang and M. A. Lautens, ACS Catal., 2020, 10, 11578 CrossRef CAS; (b) W. Xue and M. Oestreich, ACS Cent. Sci., 2020, 6, 1070–1081 CrossRef CAS PubMed; (c) Z. Liu, Y. Gao, T. Zeng and K. M. Engle, Isr. J. Chem., 2020, 60, 219–229 CrossRef CAS PubMed; (d) J. Derosa, V. T. Tran, V. A. van der Puyl and K. M. Engle, Aldrichimica Acta, 2018, 51, 21–32 CAS.
- (a) S. Joung, A. M. Bergmann and M. K. Brown, Chem. Sci., 2019, 10, 10944–10947 RSC; (b) L.-A. Chen, A. R. Lear, P. Gao and M. K. Brown, Angew. Chem., Int. Ed., 2019, 58, 10956–10960 CrossRef CAS PubMed; (c) S. R. Sardini, A. L. Lambright, G. L. Trammel, H. M. Omer, P. Liu and M. K. Brown, J. Am. Chem. Soc., 2019, 141, 9391–9400 CrossRef PubMed; (d) K. M. Logan, S. R. Sardini, S. D. White and M. K. Brown, J. Am. Chem. Soc., 2018, 140, 159–162 CrossRef CAS PubMed.
- C. Sandford and V. K. Aggarwal, Chem. Commun., 2017, 53, 5481–5494 RSC.
- (a) N.-Y. Wu, X.-H. Xu and F.-L. Qing, ACS Catal., 2019, 9, 5726–5731 CrossRef CAS; (b) Y. Li, H. Pang, D. Wu, Z. Li, W. Wang, H. Wei, Y. Fu and G. Yin, Angew. Chem., Int. Ed., 2019, 58, 8872–8876 CrossRef CAS PubMed; (c) J. Royes, S. Ni, A. Farré, E. La Cascia, J. J. Carbó, A. B. Cuenca, F. Maseras and E. Fernández, ACS Catal., 2018, 8, 2833–2838 CrossRef CAS; (d) B. Chen, P. Cao, Y. Liao, M. Wang and J. Liao, Org. Lett., 2018, 20, 1346–1349 CrossRef CAS PubMed; (e) Y.-J. Zuo, X.-T. Chang, Z.-M. Hao and C.-M. Zhong, Org. Biomol. Chem., 2017, 15, 6323–6327 RSC; (f) I. Kageyuki, I. Osaka, K. Takaki and H. Yoshida, Org. Lett., 2017, 19, 830–833 CrossRef CAS PubMed; (g) H. Iwamoto, S. Akiyama, K. Hayama and H. Ito, Org. Lett., 2017, 19, 2614–2617 CrossRef CAS PubMed; (h) K. Hayama, K. Kubota, H. Iwamoto and H. Ito, Chem. Lett., 2017, 46, 1800–1802 CrossRef CAS; (i) J. Cui, H. Wang, J. Song, X. Chi, L. Meng, Q. Liu, D. Zhang, Y. Dong and H. Liu, Org. Biomol. Chem., 2017, 15, 8508–8512 RSC; (j) A. Parra, A. López, S. Díaz-Tendero, L. Amenós, J. L. G. Ruanoa and M. Tortosa, Synlett, 2015, 26, 494–500 CrossRef CAS; (k) I. Kageyuki, H. Yoshida and K. Takaki, Synthesis, 2014, 46, 1924–1932 CrossRef; (l) H. Yoshida, I. Kageyukia and K. Takaki, Org. Lett., 2013, 15, 952–955 CrossRef CAS PubMed; (m) K. Kubota, E. Yamamoto and H. Ito, J. Am. Chem. Soc., 2013, 135, 2635–2640 CrossRef CAS PubMed; (n) C. Zhong, S. Kunii, Y. Kosaka, M. Sawamura and H. Ito, J. Am. Chem. Soc., 2010, 132, 11440–11442 CrossRef CAS PubMed; (o) H. Ito, T. Toyoda and M. Sawamura, J. Am. Chem. Soc., 2010, 132, 5990–5992 CrossRef CAS PubMed; (p) H. Ito, Y. Kosaka, K. Nonoyama, Y. Sasaki and M. Sawamura, Angew. Chem., Int. Ed., 2008, 47, 7424–7427 CrossRef CAS PubMed.
- W. Su, T.-J. Gong, X. Lu, M.-Y. Xu, C.-G. Yu, Z.-Y. Xu, H.-Z. Yu, B. Xiao and Y. Fu, Angew. Chem., Int. Ed., 2015, 54, 12957 CrossRef CAS PubMed.
- Our attempts for the use alkylhalides for the direct synthesis of the product have not yet been successful.
- (a) Z. Wu, T. Si, G. Xu, B. Xu and W. Tang, Chin. Chem. Lett., 2019, 30, 597–600 CrossRef CAS; (b) X. Wang, G. Ma, Y. Peng, C. E. Pitsch, B. J. Moll, T. D. Ly, X. Wang and H. Gong, J. Am. Chem. Soc., 2018, 140, 14490–14497 CrossRef CAS PubMed; (c) S. Ando, M. Mawatari, H. Matsunaga and T. Ishizuka, Tetrahedron Lett., 2016, 57, 3287–3290 CrossRef CAS; (d) S. Thapa, A. Kafle, S. K. Gurung, A. Montoya, P. Riedel and R. Giri, Angew. Chem., Int. Ed., 2015, 54, 8236–8240 CrossRef CAS PubMed; (e) A. Joshi-Pangu and M. R. Biscoe, Synlett, 2012, 2012, 1103–1107 Search PubMed; (f) C. Lohre, T. Dröge, C. Wang and F. Glorius, Chem.–Eur. J., 2011, 17, 6052–6055 CrossRef CAS PubMed; (g) A. Joshi-Pangu, C.-Y. Wang and M. R. Biscoe, J. Am. Chem. Soc., 2011, 133, 8478–8481 CrossRef CAS PubMed; (h) L. Hintermann, L. Xiao and A. Labonne, Angew. Chem., Int. Ed., 2008, 47, 8246–8250 CrossRef CAS PubMed; (i) T. W. Bell, L. Y. Hu and S. V. Patel, J. Org. Chem., 1987, 52, 3847–3850 CrossRef CAS.
- J. T. Edwards, R. R. Merchant, K. S. McClymont, K. W. Knouse, T. qin, L. R. Malins, B. Vokits, S. A. Shaw, D.-H. Bao, F.-L. Wei, T. Zhou, M. D. Eastgate and P. S. Baran, Nature, 2017, 545, 213–219 CrossRef CAS PubMed.
- For representative examples, for coupling of 1-alkenyl and tertiary groups, see (a) W. Dai, H. Shi, X. Zhao and S. Cao, Org. Lett., 2016, 18, 4284–4287 CrossRef CAS PubMed; (b) G. Cahiez, O. Gager, J. Buendia and C. Patinote, Chem.–Eur. J., 2012, 18, 5860–5863 CrossRef CAS PubMed; (c) G. Cahiez and H. Avedissian, Synthesis, 1998, 1998, 1199–1205 CrossRef; (d) H. Tamio, K. Mitsuo, Y. Kan-ichi and K. Makoto, Chem. Lett., 1980, 9, 767–768 CrossRef; (e) R. S. Smith and J. K. Kochi, J. Org. Chem., 1976, 41, 502–509 CrossRef CAS.
- (a) R. D. Taylor, M. MacCoss and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845–5859 CrossRef CAS PubMed; (b) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed.
- M. Krel, J.-Y. Lallemand and F. Guillou, Synlett, 2005, 13, 2043–2046 Search PubMed.
- (a) K. Iwasaki, K. K. Wan, A. Oppedisano, S. W. M. Crossley and R. A. Shenvi, J. Am. Chem. Soc., 2014, 136, 1300–1303 CrossRef CAS PubMed; (b) S. M. King, X. Ma and S. B. Herzon, J. Am. Chem. Soc., 2014, 136, 6884 CrossRef CAS PubMed.
- J. J. Verendel, O. Pàmies, M. Diéguez and P. G. Andersson, Chem. Rev., 2014, 114, 2130–2169 CrossRef CAS PubMed.
- N. B. Zuckerman, A. S. Myers, T. K. Quan, W. M. Bray, R. S. Lokey, G. A. Hartzog and J. P. Konopelski, ChemMedChem, 2012, 7, 761–765 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2062906. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc00900a |
| This journal is © The Royal Society of Chemistry 2021 |