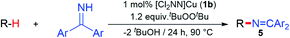Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Copper(II) ketimides in sp3 C–H amination†
Isuri U.
Jayasooriya
 ,
Abolghasem (Gus)
Bakhoda‡
,
Abolghasem (Gus)
Bakhoda‡
 ,
Rachel
Palmer
,
Kristi
Ng
,
Nour L.
Khachemoune
,
Rachel
Palmer
,
Kristi
Ng
,
Nour L.
Khachemoune
 ,
Jeffery A.
Bertke
,
Jeffery A.
Bertke
 and
Timothy H.
Warren§
and
Timothy H.
Warren§
 *
*
Department of Chemistry, Georgetown University, Box 571227-1227, Washington, DC 20057, USA. E-mail: warre155@msu.edu
First published on 5th November 2021
Abstract
Commercially available benzophenone imine (HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2) reacts with β-diketiminato copper(II) tert-butoxide complexes [CuII]–OtBu to form isolable copper(II) ketimides [CuII]–N
CPh2) reacts with β-diketiminato copper(II) tert-butoxide complexes [CuII]–OtBu to form isolable copper(II) ketimides [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2. Structural characterization of the three coordinate copper(II) ketimide [Me3NN]Cu–N
CPh2. Structural characterization of the three coordinate copper(II) ketimide [Me3NN]Cu–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 reveals a short Cu-Nketimide distance (1.700(2) Å) with a nearly linear Cu–N–C linkage (178.9(2)°). Copper(II) ketimides [CuII]–N
CPh2 reveals a short Cu-Nketimide distance (1.700(2) Å) with a nearly linear Cu–N–C linkage (178.9(2)°). Copper(II) ketimides [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 readily capture alkyl radicals R˙ (PhCH(˙)Me and Cy˙) to form the corresponding R–N
CPh2 readily capture alkyl radicals R˙ (PhCH(˙)Me and Cy˙) to form the corresponding R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 products in a process that competes with N–N coupling of copper(II) ketimides [CuII]–N
CPh2 products in a process that competes with N–N coupling of copper(II) ketimides [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 to form the azine Ph2C
CPh2 to form the azine Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N
N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2. Copper(II) ketimides [CuII]–N
CPh2. Copper(II) ketimides [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2 serve as intermediates in catalytic sp3 C–H amination of substrates R–H with ketimines HN
CAr2 serve as intermediates in catalytic sp3 C–H amination of substrates R–H with ketimines HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2 and tBuOOtBu as oxidant to form N-alkyl ketimines R–N
CAr2 and tBuOOtBu as oxidant to form N-alkyl ketimines R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2. This protocol enables the use of unactivated sp3 C–H bonds to give R–N
CAr2. This protocol enables the use of unactivated sp3 C–H bonds to give R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2 products easily converted to primary amines R–NH2via simple acidic deprotection.
CAr2 products easily converted to primary amines R–NH2via simple acidic deprotection.
Introduction
Transition metal-catalysed sp3 C–H amination protocols have gained immense attention in the synthetic community over the past couple of decades.1–4 A majority of these protocols proceed via metal–nitrene2,5 [M]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR′ or metal–amide [M]–NR′R′′ intermediates.1,6 Extensive studies on such intermediates and underlying mechanisms have paved the way towards more efficient sp3 C–H amination protocols.1
NR′ or metal–amide [M]–NR′R′′ intermediates.1,6 Extensive studies on such intermediates and underlying mechanisms have paved the way towards more efficient sp3 C–H amination protocols.1
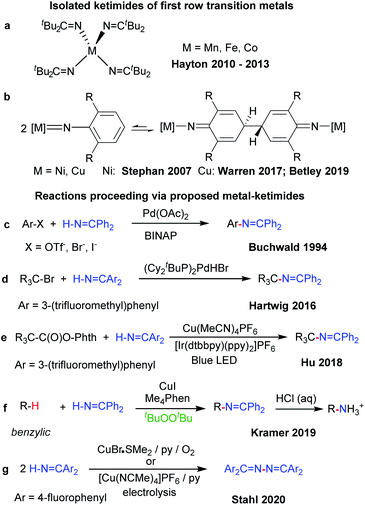
Related metal–ketimide [M]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR′R′′ intermediates, however, have received less attention in C–H amination chemistry. The strong metal–Nketimide interaction makes ketimides effective spectator ligands. For instance, ketimides stabilize high valent homoleptic Mn(IV),7 Fe(IV)8 and Co(IV)9 complexes (Fig. 1a). In some cases, ketimides can also form via nickel and copper arylimido/nitrene intermediates [M]
CR′R′′ intermediates, however, have received less attention in C–H amination chemistry. The strong metal–Nketimide interaction makes ketimides effective spectator ligands. For instance, ketimides stabilize high valent homoleptic Mn(IV),7 Fe(IV)8 and Co(IV)9 complexes (Fig. 1a). In some cases, ketimides can also form via nickel and copper arylimido/nitrene intermediates [M]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr via C–C coupling at the para-position of the aryl nitrene ligand (Fig. 1b). While this reactivity was initially uncovered with nickel β-diketiminato complexes,10 reversible C–C bond formation/cleavage in related copper complexes provides access to terminal copper nitrenes [Cu]
NAr via C–C coupling at the para-position of the aryl nitrene ligand (Fig. 1b). While this reactivity was initially uncovered with nickel β-diketiminato complexes,10 reversible C–C bond formation/cleavage in related copper complexes provides access to terminal copper nitrenes [Cu]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr that participate in sp3 C–H amination.11,12
NAr that participate in sp3 C–H amination.11,12
Fewer examples of ketimides exist, however, in which the ketimide ligand serves as a reactive functional group in discrete transition metal complexes.13 Metal ketimide intermediates have been proposed in several Pd-catalysed cross-coupling reactions of aryl (Fig. 1c)14 and alkyl halides (Fig. 1d)15 with benzophenone imine. Cu-catalysed photoredox cross-coupling reactions of redox-active alkyl esters (Fig. 1e)16 and Cu-catalysed benzylic sp3 C–H amination with benzophenone imine (Fig. 1f)17 are among other examples that may be mediated by metal–ketimide intermediates. Moreover, Stahl and colleagues have proposed copper(II) ketimides in the N–N oxidative coupling of imines Ar2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH to azines Ar2C
NH to azines Ar2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N
N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2 under aerobic or electrocatalytic conditions (Fig. 1g).18,19
CAr2 under aerobic or electrocatalytic conditions (Fig. 1g).18,19
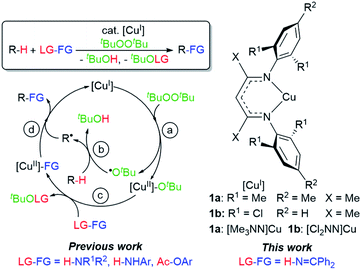
Herein we describe discrete first-row transition metal–ketimide complexes intimately involved in C–H amination chemistry. Building upon the Kharasch–Sosnovsky reaction,20–22 we previously demonstrated that copper(II) alkyl amides [CuII]–NHR′,23 anilides [CuII]–NHAr,6,24 and aryloxides [CuII]–OAr25 serve as key intermediates in a radical relay protocol for sp3 C–H functionalisation (Fig. 2). Formed via acid–base6,23,24 or transesterification25 reactions between [CuII]–OtBu with H-FG or Ac-FG reagents, these copper(II) complexes [CuII]–FG capture sp3-C radicals R˙ generated via H-atom abstraction from R–H to furnish the functionalized product R-FG. We anticipated that the relatively high acidity of the imine N–H bond26 coupled with a preference for binding at copper with softer N-donors should enable the formation of [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2 species from [CuII]–OtBu complexes and HN
CAr2 species from [CuII]–OtBu complexes and HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 allow for an examination of copper(II) ketimides in C–H amination catalysis.
CPh2 allow for an examination of copper(II) ketimides in C–H amination catalysis.
Results and discussion
Synthesis and characterization of copper(II) ketimides
Monitored by UV-vis spectroscopy, addition of benzophenone imine (1 equiv.) to a solution of [Me3NN]Cu-OtBu (2a) in toluene at −80 °C results in decay of the characteristic UV-vis absorption of 2a at 470 nm with growth of a new band at 570 nm (Fig. S2†). Performed on a preparative scale, this new species [Me3NN]Cu–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 (3a) may be isolated as dark purple crystals from pentane at −35 °C in 78% yield (Fig. 3a).
CPh2 (3a) may be isolated as dark purple crystals from pentane at −35 °C in 78% yield (Fig. 3a).
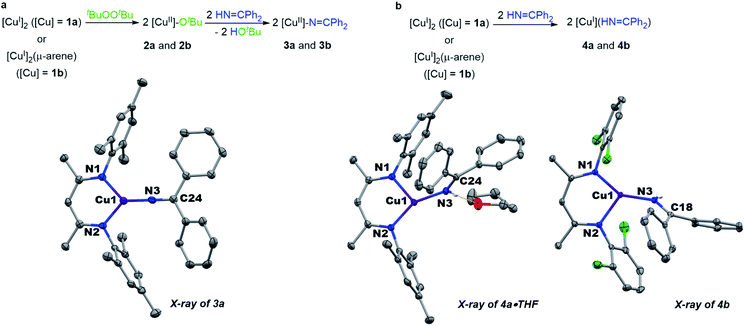 | ||
| Fig. 3 (a) Synthesis and structure of copper(II) ketimides. (b) Synthesis and structure of copper(I) imine adducts. | ||
The X-ray crystal structure of [Me3NN]Cu–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 (3a) (Fig. 3a) reveals the Cu–Nketimide distance of 1.700(2) Å, significantly shorter than the Cu–N bond found in the copper(II) amide [Cl2NN]Cu–NHAd (1.839(9) Å)23 and copper(II) anilide [Cl2NN]Cu–NHArCl3 (1.847(3) Å).6 Copper(II) ketimide 3a possesses a nearly linear Cu–N3–C24 angle of 178.9(2)°. The short Cu–Nketimide distance and linear Cu–N3–C24 angle support effective sp hybridization at the ketimide N atom. These values remarkably differ from those in the homoleptic copper(I) ketimide [Cu–N
CPh2 (3a) (Fig. 3a) reveals the Cu–Nketimide distance of 1.700(2) Å, significantly shorter than the Cu–N bond found in the copper(II) amide [Cl2NN]Cu–NHAd (1.839(9) Å)23 and copper(II) anilide [Cl2NN]Cu–NHArCl3 (1.847(3) Å).6 Copper(II) ketimide 3a possesses a nearly linear Cu–N3–C24 angle of 178.9(2)°. The short Cu–Nketimide distance and linear Cu–N3–C24 angle support effective sp hybridization at the ketimide N atom. These values remarkably differ from those in the homoleptic copper(I) ketimide [Cu–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2]4 with bridging ketimide ligands that lead to a square-like tetrameric structure with Cu–N distances 1.847(2)–1.861(2) Å and Cu–N–Cu angles of 94.17(9)–98.25(9).27 To outline differences between coordination of anionic ketimide ligands and their neutral ketimine counterparts, we prepared the corresponding benzophenone imine adducts [Me3NN]Cu(NH
CPh2]4 with bridging ketimide ligands that lead to a square-like tetrameric structure with Cu–N distances 1.847(2)–1.861(2) Å and Cu–N–Cu angles of 94.17(9)–98.25(9).27 To outline differences between coordination of anionic ketimide ligands and their neutral ketimine counterparts, we prepared the corresponding benzophenone imine adducts [Me3NN]Cu(NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2) (4a) and [Cl2NN]Cu(NH
CPh2) (4a) and [Cl2NN]Cu(NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2) (4b) (Fig. 3b). These copper(I) complexes feature substantially longer Cu–Nketimine distances of 1.8940(14) and 1.8937(14) Å. These ketimine adducts 4a and 4b each exhibit a pronounced bend in the Cu–ketimide linkage with Cu–N–C angles of 132.68(12) and 130.25(12)° consistent with sp2 hybridization at N.
CPh2) (4b) (Fig. 3b). These copper(I) complexes feature substantially longer Cu–Nketimine distances of 1.8940(14) and 1.8937(14) Å. These ketimine adducts 4a and 4b each exhibit a pronounced bend in the Cu–ketimide linkage with Cu–N–C angles of 132.68(12) and 130.25(12)° consistent with sp2 hybridization at N.
UV-vis analysis of copper(II) ketimide [Me3NN]Cu–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 (3a) reveals the presence of a single low energy absorption band at 570 nm (ε = 1910 M−1 cm−1) in toluene at room temperature. The EPR spectrum of 3a in a mixture of toluene and pentane at room temperature shows a signal centred at giso = 2.081 with very well resolved coupling to 63/65Cu (ACu = 298.0 MHz) and additional hyperfine modelled with three equivalent 14N nuclei (AN = 35.0 MHz) (Fig. S13†). The related copper(II) ketimide [Cl2NN]Cu–N
CPh2 (3a) reveals the presence of a single low energy absorption band at 570 nm (ε = 1910 M−1 cm−1) in toluene at room temperature. The EPR spectrum of 3a in a mixture of toluene and pentane at room temperature shows a signal centred at giso = 2.081 with very well resolved coupling to 63/65Cu (ACu = 298.0 MHz) and additional hyperfine modelled with three equivalent 14N nuclei (AN = 35.0 MHz) (Fig. S13†). The related copper(II) ketimide [Cl2NN]Cu–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 (3b) prepared from [Cl2NN]Cu-OtBu (2b) and HN
CPh2 (3b) prepared from [Cl2NN]Cu-OtBu (2b) and HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 exhibits a similar spectroscopic profile. The UV-vis spectrum of [Cl2NN]Cu–N
CPh2 exhibits a similar spectroscopic profile. The UV-vis spectrum of [Cl2NN]Cu–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ph2 (3b) exhibits a single absorption at 520 nm (ε = 3120 M−1 cm−1) in toluene at room temperature and possesses a similar isotropic EPR spectrum to that of 3a (Fig. S14†). Unfortunately, the greater thermal sensitivity of [Cl2NN]Cu–N
Ph2 (3b) exhibits a single absorption at 520 nm (ε = 3120 M−1 cm−1) in toluene at room temperature and possesses a similar isotropic EPR spectrum to that of 3a (Fig. S14†). Unfortunately, the greater thermal sensitivity of [Cl2NN]Cu–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ph2 (3b) has precluded its crystallographic characterization.
Ph2 (3b) has precluded its crystallographic characterization.
DFT calculations reveal remarkably high unpaired electron density on the ketimide N atom of both 3a (0.58) and 3b (0.61) (Fig. 4 and S23†). These values are significantly higher than values reported for related three coordinate β-diketiminato Cu(II) anilides [CuII]–NHAr (0.23–0.25)6 and a copper(II) amide [CuII]–NHAd (0.49).23 We rationalize this as a result of a 2-center 3-electron π interaction between the highest energy d orbital at the copper(II) center destabilized by the β-diketiminato N-donors and a p orbital of the sp-hybridized ketimide N atom (Fig. 4a). In addition, the orthogonal orientation of the Cu–Nketimide π-interaction relative to the conjugated ketimide N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 π system further limits the delocalization of unpaired electron density away from the ketimide N atom (Fig. 4b and c).
CPh2 π system further limits the delocalization of unpaired electron density away from the ketimide N atom (Fig. 4b and c).
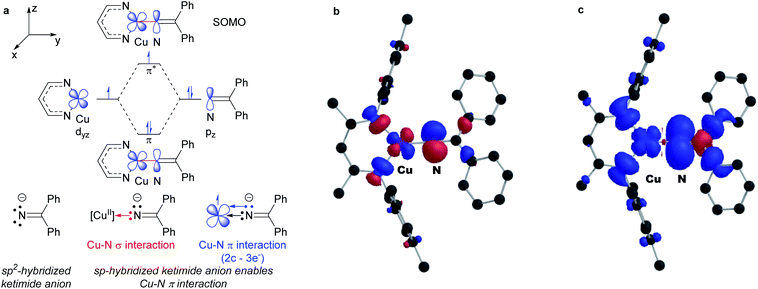 | ||
| Fig. 4 (a) Electronic structure of copper(II) ketimides. (b) SOMO and (c) spin density plot of copper(II) ketamide 3a (net spin α: blue, net spin β: red, 0.001 isospin value). | ||
Copper(II) ketimide reactivity: radical capture and N–N bond formation
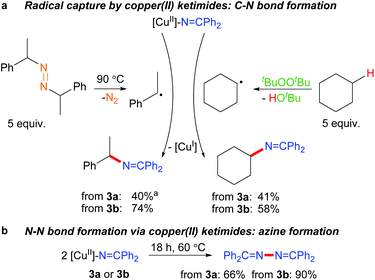
The ability of many β-diketiminato copper(II) complexes to participate in catalytic sp3 C–H functionalisation via radical relay (Fig. 2) encouraged us to assess the reactivity of copper(II) ketimides 3 towards alkyl radicals. We find that [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 species 3a and 3b capture alkyl radicals R˙ to provide the corresponding R–N
CPh2 species 3a and 3b capture alkyl radicals R˙ to provide the corresponding R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 products (Fig. 5a). [CuI] is anticipated to form in these radical capture reactions that correspond to step d in the radical relay catalytic cycle (Fig. 2). For instance, reaction of 3a and 3b with (E/Z)-azobis(α-phenylethane) at 90 °C that generates the benzylic radical PhCH(˙)Me upon heating provides the alkylated imine PhCH(N
CPh2 products (Fig. 5a). [CuI] is anticipated to form in these radical capture reactions that correspond to step d in the radical relay catalytic cycle (Fig. 2). For instance, reaction of 3a and 3b with (E/Z)-azobis(α-phenylethane) at 90 °C that generates the benzylic radical PhCH(˙)Me upon heating provides the alkylated imine PhCH(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2)Me in 40% and 74% yields, respectively. Generation of Cy˙ radicals in the presence of 3a and 3b by heating tBuOOtBu in cyclohexane (via H-atom abstraction by tBuO˙ radicals) provides Cy-N
CPh2)Me in 40% and 74% yields, respectively. Generation of Cy˙ radicals in the presence of 3a and 3b by heating tBuOOtBu in cyclohexane (via H-atom abstraction by tBuO˙ radicals) provides Cy-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 in 58% and 41% yields, respectively.
CPh2 in 58% and 41% yields, respectively.
Upon heating to 60 °C, copper(II) ketimides 3a and 3b undergo N–N coupling to form benzophenone azine Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N
N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 isolated in 66% and 90% yields, respectively (Fig. 5b). This represents a competing reaction for radical capture at copper(II) ketimides 3a and 3b.
CPh2 isolated in 66% and 90% yields, respectively (Fig. 5b). This represents a competing reaction for radical capture at copper(II) ketimides 3a and 3b.
Copper(II) ketimides in sp3 C–H amination
With a fundamental understanding of copper(II) ketimide formation and reactivity, we explored these complexes in catalytic C–H amination via radical relay. Using ethylbenzene as a model R–H substrate, we screened a modest range of copper(I) β-diketiminato catalysts 1 that possess different electronic and steric properties (Table 1). The catalyst [Cl2NN]Cu (1b) provides the highest yield compared to more electron-rich (1a and 1c) and electron-poor (1d) catalysts. Increasing the tBuOOtBu oxidant amount does not significantly improve the yield. Lowering the temperature from 90 °C reduces the yield drastically (Table S1†), possibly due to binding of the ketimine HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2 to the copper(I) catalyst (Fig. 3b) that inhibits tBuOOtBu activation.28
CAr2 to the copper(I) catalyst (Fig. 3b) that inhibits tBuOOtBu activation.28
| Entry | Catalyst | (X, R1, R2) | Yield (%) |
|---|---|---|---|
| a Conditions: 50 equiv. R–H. All yields determined by 1H NMR | |||
| 1 | [Me3NN]Cu 1a | (Me, Me, Me) | 34 |
| 2 | [Cl2NN]Cu 1b | (Me, Cl, H) | 65 |
| 3 | [tPr2NN]Cu 1c | (Me, tPr, H) | 30 |
| 4 | [Cl2NNF6]Cu 1d | (CF3, Cl, H) | 42 |
While (1-(tert-butoxy)ethyl)benzene forms in trace amounts via C–H etherification,28 the azine Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N
N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 is the main byproduct in these catalytic C–H amination reactions, representing non-productive consumption of H–N
CPh2 is the main byproduct in these catalytic C–H amination reactions, representing non-productive consumption of H–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2. In a previous study of C–H amination with anilines H2NAr employing the [Cl2NN]Cu/tBuOOtBu catalyst system, electron-poor anilines provided the highest yields in the face of competing diazene ArN
CPh2. In a previous study of C–H amination with anilines H2NAr employing the [Cl2NN]Cu/tBuOOtBu catalyst system, electron-poor anilines provided the highest yields in the face of competing diazene ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr formation.24 Copper(II) anilido intermediates [CuII]–NHAr serve as intermediates in C–H amination with anilines H2NAr; those derived from electron-poor anilines H2NAr (e.g. Ar = 2,4,6-Cl3C6H2) proved more resistant to reductive bimolecular N–N bond formation.6,24
NAr formation.24 Copper(II) anilido intermediates [CuII]–NHAr serve as intermediates in C–H amination with anilines H2NAr; those derived from electron-poor anilines H2NAr (e.g. Ar = 2,4,6-Cl3C6H2) proved more resistant to reductive bimolecular N–N bond formation.6,24
To examine whether similar electronic changes in the ketimine H–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2 could similarly promote more efficient catalysis, we explored two electron-poor ketimine derivatives H–N
CAr2 could similarly promote more efficient catalysis, we explored two electron-poor ketimine derivatives H–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2 (Ar = 4-CF3C6H4 and 4-FC6H4) in C–H amination (Table 2). Although the p-CF3 substituted imine provides a higher C–H amination yield with cyclohexane (C–H BDE = 97 kcal mol−1),29 the increase in yield is modest with the benzylic substrate ethylbenzene (C–H BDE = 87 kcal mol−1).29 No significant differences were observed between benzophenone imine and the p-F substituted analogue.
CAr2 (Ar = 4-CF3C6H4 and 4-FC6H4) in C–H amination (Table 2). Although the p-CF3 substituted imine provides a higher C–H amination yield with cyclohexane (C–H BDE = 97 kcal mol−1),29 the increase in yield is modest with the benzylic substrate ethylbenzene (C–H BDE = 87 kcal mol−1).29 No significant differences were observed between benzophenone imine and the p-F substituted analogue.
| Entry | Ar | Yield (%) | |
|---|---|---|---|
| a Conditions: 10 equiv. R–H, 1.2 equiv. tBuOOtBu, 1 mol% [Cl2NN]Cu, 90 °C, 24 h. Yields are determined by 1H NMR. | |||
| 1 |
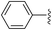
|
44 (5a) | 40 (5b) |
| 2 |
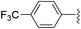
|
51 (5a-CF3) | 56 (5b-CF3) |
| 3 |
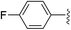
|
36 (5a-F) | 39 (5b-F) |
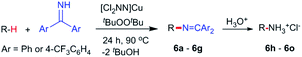
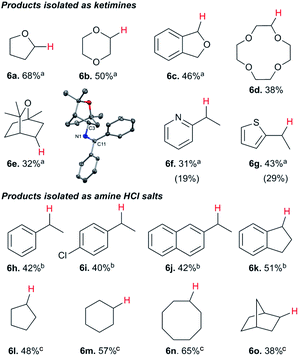
While electron-poor imines can give somewhat higher C–H amination yields, we most broadly examined the commercially available H–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 to survey the scope of R–H substrates in sp3 C–H amination (Table 3). Ethers such as THF, 1,4-dioxane, or even 12-crown-4 undergo C–H amination at the α-carbon in relatively high yields (6a–6d). Amination of the benzylic secondary C–H bonds in heteroaromatic substrates occurs (6f–6g), though yields may be lower due to the possibility of coordination of these substrates and/or products to the copper(I) centre that can decrease the rate of reoxidation with tBuOOtBu.28 Aromatic substrates with benzylic C–H bonds undergo C–H amination in moderate to high yields (6h–6k). Cycloalkanes with stronger, unactivated sp3 C–H bonds give moderate yields with electron-poor ketimine HN
CPh2 to survey the scope of R–H substrates in sp3 C–H amination (Table 3). Ethers such as THF, 1,4-dioxane, or even 12-crown-4 undergo C–H amination at the α-carbon in relatively high yields (6a–6d). Amination of the benzylic secondary C–H bonds in heteroaromatic substrates occurs (6f–6g), though yields may be lower due to the possibility of coordination of these substrates and/or products to the copper(I) centre that can decrease the rate of reoxidation with tBuOOtBu.28 Aromatic substrates with benzylic C–H bonds undergo C–H amination in moderate to high yields (6h–6k). Cycloalkanes with stronger, unactivated sp3 C–H bonds give moderate yields with electron-poor ketimine HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr′2 (Ar′ = 4-CF3C6H4) (6l–6o). The bicyclic eucalyptol undergoes C–H amination in 32% yield (6e). These aminated products may be isolated either as synthetically versatile protected primary amines R–N
CAr′2 (Ar′ = 4-CF3C6H4) (6l–6o). The bicyclic eucalyptol undergoes C–H amination in 32% yield (6e). These aminated products may be isolated either as synthetically versatile protected primary amines R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2via column chromatography (6a–6g) or as the primary ammonium salts [R–NH3]Cl via deprotection upon simple acidic work up (6h–6o) under mild conditions. The potential to use recovered benzophenone from deprotection of ketimine products and azine byproducts to regenerate the Ph2C
CPh2via column chromatography (6a–6g) or as the primary ammonium salts [R–NH3]Cl via deprotection upon simple acidic work up (6h–6o) under mild conditions. The potential to use recovered benzophenone from deprotection of ketimine products and azine byproducts to regenerate the Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH starting material30 enhances the overall atom economy of this amination protocol.
NH starting material30 enhances the overall atom economy of this amination protocol.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2a
CAr2a
Conclusions
The isolation of mononuclear copper(II) ketimides [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 reveals the role that they play as intermediates in sp3 C–H amination. These reactive intermediates readily form via acid–base exchange between [CuII]–OtBu and HN
CPh2 reveals the role that they play as intermediates in sp3 C–H amination. These reactive intermediates readily form via acid–base exchange between [CuII]–OtBu and HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2, amenable to spectroscopic and structural investigation. Importantly, [CuII]–N
CPh2, amenable to spectroscopic and structural investigation. Importantly, [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 complexes efficiently intercept alkyl radicals R˙ generated via H-atom abstraction by tBuO˙ from substrates R–H that ultimately enable the C–H amination of unactivated sp3 C–H substrates. DFT analysis reveals a significant amount of unpaired electron density at the ketimide N atom of 0.58 and 0.61 e− for [Me3NN]Cu–N
CPh2 complexes efficiently intercept alkyl radicals R˙ generated via H-atom abstraction by tBuO˙ from substrates R–H that ultimately enable the C–H amination of unactivated sp3 C–H substrates. DFT analysis reveals a significant amount of unpaired electron density at the ketimide N atom of 0.58 and 0.61 e− for [Me3NN]Cu–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 (3a) and [Cl2NN]Cu–N
CPh2 (3a) and [Cl2NN]Cu–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 (3b) (Fig. 4 and S23†), respectively, opening a facile pathway for C–N bond formation with radicals R˙ to form R–N
CPh2 (3b) (Fig. 4 and S23†), respectively, opening a facile pathway for C–N bond formation with radicals R˙ to form R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 products (Fig. 5a). Moreover, this spin density at the ketimide N-atom likely facilitates N–N bond formation via copper(II) ketimides [CuII]–N
CPh2 products (Fig. 5a). Moreover, this spin density at the ketimide N-atom likely facilitates N–N bond formation via copper(II) ketimides [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 to give the azine Ph2C
CPh2 to give the azine Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N
N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 (Fig. 5b), a competing pathway in sp3 C–H functionalisation. Use of the more electron-poor ketimine HN
CPh2 (Fig. 5b), a competing pathway in sp3 C–H functionalisation. Use of the more electron-poor ketimine HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr′ (Ar′ = 4-CF3C6H4) extends the scope of catalysis to unactivated sp3 C–H bonds in cycloalkanes (Table 3; entries 6l–6o). Nonetheless, facile N–N bond formation also by copper(II) ketimides [CuII]–N
CAr′ (Ar′ = 4-CF3C6H4) extends the scope of catalysis to unactivated sp3 C–H bonds in cycloalkanes (Table 3; entries 6l–6o). Nonetheless, facile N–N bond formation also by copper(II) ketimides [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2 underscores the role that they may play in the (electro)catalytic copper(II) promoted oxidative N–N coupling of benzophenone imine to form benzophenone azine (Fig. 1g).18
CAr2 underscores the role that they may play in the (electro)catalytic copper(II) promoted oxidative N–N coupling of benzophenone imine to form benzophenone azine (Fig. 1g).18
Experimental section
Detailed experimental procedures are provided in the ESI.†Data availability
All synthetic procedures, characterization data, spectroscopic data, computational data, supplementary figures and tables, and detailed crystallographic information can be found in the ESI.† Crystallographic data are available via the Cambridge Crystallographic Data Centre (CCDC): 1940417, 1945374, 1940418, 1945375, 1940420, 2035780.Author contributions
I. U. J. and A. B. prepared and characterized the metal complexes, I. U. J. performed reactivity and computational studies with metal complexes, I. U. J., R. P., K. N., and N. L. K. carried out catalytic amination experiments, isolating and characterizing organic products, J. A. B. solved and refined X-ray diffraction data, T. H. W. guided the research and assisted with data analysis, I. U. J. and T. H. W. wrote the manuscript with input from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to NSF (CHE-1665348 and CHE-1955942) for support of this work.Notes and references
- Y. Park, Y. Kim and S. Chang, Chem. Rev., 2017, 117, 9247–9301 CrossRef CAS PubMed.
- R. T. Gephart III and T. H. Warren, Organometallics, 2012, 31, 7728–7752 CrossRef.
- P. Gandeepan, T. Muller, D. Zell, G. Cera, S. Warratz and L. Ackermann, Chem. Rev., 2019, 119, 2192–2452 CrossRef CAS PubMed.
- H. Yi, G. Zhang, H. Wang, Z. Huang, J. Wang, A. K. Singh and A. Lei, Chem. Rev., 2017, 117, 9016–9085 CrossRef CAS PubMed.
- D. Intrieri, P. Zardi, A. Caselli and E. Gallo, Chem. Commun., 2014, 50, 11440–11453 RSC.
- E. S. Jang, C. L. McMullin, M. Kass, K. Meyer, T. R. Cundari and T. H. Warren, J. Am. Chem. Soc., 2014, 136, 10930–10940 CrossRef CAS PubMed.
- R. A. Lewis, G. Wu and T. W. Hayton, Inorg. Chem., 2011, 50, 4660–4668 CrossRef CAS PubMed.
- R. A. Lewis, G. Wu and T. W. Hayton, J. Am. Chem. Soc., 2010, 132, 12814–12816 CrossRef CAS PubMed.
- R. A. Lewis, S. P. George, A. Chapovetsky, G. Wu, J. S. Figueroa and T. W. Hayton, Chem. Commun., 2013, 49, 2888–2890 RSC.
- G. Bai and D. W. Stephan, Angew. Chem., Int. Ed., 2007, 46, 1856–1859 CrossRef CAS PubMed.
- A. G. Bakhoda, Q. Jiang, J. A. Bertke, T. R. Cundari and T. H. Warren, Angew. Chem., Int. Ed., 2017, 56, 6426–6430 CrossRef CAS PubMed.
- K. M. Carsch, I. M. Dimucci, D. A. Iovan, A. Li, S.-L. Zheng, C. J. Titus, S. J. Lee, K. D. Irwin, D. Nordlund, K. M. Lancaster and T. A. Betley, Science, 2019, 365, 1138–1143 CrossRef CAS PubMed.
- Y. Kondo, H. Morimoto and T. Ohshima, Chem. Lett., 2020, 49, 497–504 CrossRef CAS.
- J. P. Wolfe, J. Åhman, J. P. Sadighi, R. A. Singer and S. L. Buchwald, Tetrahedron Lett., 1997, 38, 6367–6370 CrossRef CAS.
- D. M. Peacock, C. B. Roos and J. F. Hartwig, ACS Cent. Sci., 2016, 2, 647–652 CrossRef CAS PubMed.
- R. Mao, J. Balon and X. Hu, Angew. Chem., Int. Ed., 2018, 57, 9501–9504 CrossRef CAS PubMed.
- S. Kramer, Org. Lett., 2019, 21, 65–69 CrossRef CAS PubMed.
- M. C. Ryan, Y. J. Kim, J. B. Gerken, F. Wang, M. M. Aristov, J. R. Martinelli and S. S. Stahl, Chem. Sci., 2020, 11, 1170–1175 RSC.
- F. Wang, J. B. Gerken, D. M. Bates, Y. J. Kim and S. S. Stahl, J. Am. Chem. Soc., 2020, 142, 12349–12356 CrossRef CAS PubMed.
- M. S. Kharasch and G. Sosnovsky, J. Am. Chem. Soc., 1958, 80, 756 CrossRef CAS.
- M. S. Kharasch, G. Sosnovsky and N. C. Yang, J. Am. Chem. Soc., 1959, 81, 5819–5824 CrossRef CAS.
- D. J. Rawlinson and G. Sosnovsky, Synthesis, 1972, 1, 1–28 CrossRef.
- S. Wiese, Y. M. Badiei, R. T. Gephart, S. Mossin, M. S. Varonka, M. M. Melzer, K. Meyer, T. R. Cundari and T. H. Warren, Angew. Chem., Int. Ed., 2010, 49, 8850–8855 CrossRef CAS PubMed.
- R. T. Gephart III, D. L. Huang, M. J. Aguila, G. Schmidt, A. Shahu and T. H. Warren, Angew. Chem., Int. Ed., 2012, 51, 6488–6492 CrossRef PubMed.
- T. K. Salvador, C. H. Arnett, S. Kundu, N. G. Sapiezynski, J. A. Bertke, M. Raghibi Boroujeni and T. H. Warren, J. Am. Chem. Soc., 2016, 138, 16580–16583 CrossRef CAS PubMed.
- F. G. Bordwell and G. Z. Ji, J. Am. Chem. Soc., 1991, 113, 8398–8401 CrossRef CAS.
- R. A. D. Soriaga, S. Javed and D. M. Hoffman, J. Cluster Sci., 2010, 21, 567–575 CrossRef CAS.
- R. T. Gephart, 3rd, C. L. McMullin, N. G. Sapiezynski, E. S. Jang, M. J. Aguila, T. R. Cundari and T. H. Warren, J. Am. Chem. Soc., 2012, 134, 17350–17353 CrossRef PubMed.
- Y.-R. Luo, Handbook of Bond Dissociation Energies in Organic Compounds, CRC Press, Boca Raton, 2002 Search PubMed.
- A. K. H. Hayashi, M. Katayama, K. Kawasaki and T. Okazaki, Ind. Eng. Chem. Prod. Res. Dev., 1976, 15, 299–303 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1940417, 1940418, 1940420, 1945374, 1945375 and 2035780. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc01990b |
| ‡ Current Address: Department of Chemistry, Towson University, 8000 York Road, Towson, MD, 21252, USA. |
| § Current Address: Department of Chemistry, Michigan State University, 578 S. Shaw Lane, East Lansing, MI 48824, USA. |
| This journal is © The Royal Society of Chemistry 2021 |