Open Access Article
Open Access ArticleWhole-cell screening of oxidative enzymes using genetically encoded sensors†
Tsvetan
Kardashliev
 *a,
Alexandra
Weingartner‡
b,
Elvira
Romero§
c,
Ulrich
Schwaneberg
*a,
Alexandra
Weingartner‡
b,
Elvira
Romero§
c,
Ulrich
Schwaneberg
 b,
Marco
Fraaije
b,
Marco
Fraaije
 c,
Sven
Panke
a and
Martin
Held
c,
Sven
Panke
a and
Martin
Held
 *a
*a
aDepartment of Biosystems Science and Engineering, ETH Zurich, Mattenstrasse 26, 4058 Basel, Switzerland. E-mail: heldma@ethz.ch; tsvetank@ethz.ch
bInstitute of Biotechnology, RWTH Aachen University, Worringerweg 3, 52074 Aachen, Germany
cFaculty of Science and Engineering, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands
First published on 29th October 2021
Abstract
Biocatalysis is increasingly used for synthetic purposes in the chemical and especially the pharmaceutical industry. Enzyme discovery and optimization which is frequently needed to improve biocatalytic performance rely on high-throughput methods for activity determination. These methods should ideally be generic and applicable to entire enzyme families. Hydrogen peroxide (H2O2) is a product of several biocatalytic oxidations and its formation can serve as a proxy for oxidative activity. We designed a genetically encoded sensor for activity measurement of oxidative biocatalysts via the amount of intracellularly-formed H2O2. A key component of the sensor is an H2O2-sensitive transcriptional regulator, OxyR, which is used to control the expression levels of fluorescent proteins. We employed the OxyR sensor to monitor the oxidation of glycerol to glyceraldehyde and of toluene to o-cresol catalysed by recombinant E. coli expressing an alcohol oxidase and a P450 monooxygenase, respectively. In case of the P450 BM3-catalysed reaction, we additionally monitored o-cresol formation via a second genetically encoded sensor based on the phenol-sensitive transcriptional activator, DmpR, and an orthogonal fluorescent reporter protein. Single round screens of mutant libraries by flow cytometry or by visual inspection of colonies on agar plates yielded significantly improved oxidase and oxygenase variants thus exemplifying the suitability of the sensor system to accurately assess whole-cell oxidations in a high-throughput manner.
Introduction
Several enzyme class 1 (EC 1) members enable highly selective oxidations1 and are increasingly perceived as advanced catalysts for chemical production.2,3 The catalytic properties of oxidative enzymes are undoubtedly unique,4 yet the performance of wildtype biocatalysts is frequently insufficient for preparative synthesis.5,6 However, enzyme features such as operational stability, reaction rates, and substrate specificity can be tailored in rounds of random or (semi-) rational diversification of amino acid sequences followed by activity screening of the generated protein sequence space.7,8 As a rule of thumb, in protein engineering campaigns the likelihood of discovering an improved variant is proportional to the total number of variants tested. Thus, detection methods with both high discriminatory power and compatibility with high-throughput screening workflows are essential for the successful discovery and engineering of industrial biocatalysts.9,10Oxidative enzymes are structurally- and mechanistically diverse but a common feature of many is the use of molecular oxygen for catalysis.2 Enzymatic activation of oxygen is mediated by prosthetic groups/co-factors, most commonly flavins or metal ions coordinated by porphyrin or proteinaceous sulfur.2,11,12 A frequently observed byproduct of these reactions is hydrogen peroxide (H2O2). For example, P450 monooxygenases form H2O2 when substrate binding and electron transfer from NAD(P)H to the prosthetic group are not well coordinated.13 In the case of oxidases, molecular oxygen acts as a terminal electron acceptor and H2O2 forms along with the organic oxidation product in stoichiometric amounts.14 Because of its ubiquity, H2O2 formation can be considered a quasi-generic indicator of oxidative enzyme activity.9,15–17
Enzymatic H2O2 formation is most commonly assayed by coupling of the H2O2-generating reaction to peroxidase-catalysed oxidation of fluorogenic or chromogenic indicators.17,18 In addition to considerable assay complexity requiring multiple liquid handling steps, the diffusive nature of the indicators makes them unsuitable for single-cell analyses and limits the plating density of screens executed on a solid support.
A more elegant means for H2O2 detection directly within living cells can be achieved by a genetically encoded probe termed HyPer.19–21 HyPer makes use of permuted fluorophores (cpXFP) integrated into the regulatory domain of E. coli's master regulator of oxidative stress, OxyR.22 Oxidation of cysteine residues of the OxyR domain by H2O2 triggers a conformational change of cpXFP leading to a shift of the fluorescence spectrum that is indicative for cellular H2O2 levels. The HyPer sensor is especially suited for monitoring changes of the redox status of cells and cellular compartments but, due to the highly dynamic nature of the signal, is less suitable for enzyme screening applications where cumulative and irreversible reporters are preferred.
Here, we report the design and application of a genetically encoded transcription factor biosensor for detection of the intracellular H2O2 levels in E. coli developed specifically for enzyme screening applications. The suitability of the sensor for high-throughput enzyme screening was demonstrated with two E. coli whole-cell biocatalysts harbouring an alcohol oxidase or a cytochrome P450 monooxygenase, both of which generate H2O2 in the course of glycerol oxidation and toluene hydroxylation, respectively. We succeeded in rapidly isolating oxidase and monooxygenase variants with markedly increased activity towards non-natural substrates from large enzyme variant libraries, in screens on the level of colonies grown on a solid support and at single-cell level using flow cytometry. The collected data demonstrates the broad scope, versatility, and compatibility of the presented H2O2-sensitive sensor with state-of-the-art high-throughput sampling protocols.
Results and discussion
Biosensor development and characterization
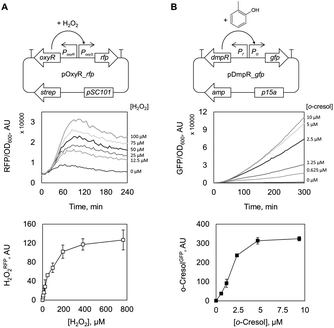
To non-invasively assess the oxidative activity of recombinant E. coli catalysts, we built an H2O2-sensitive genetically encoded sensor based on the master regulator protein of oxidative stress in E. coli, OxyR, and a cognate promoter, PoxyS.22 At elevated intracellular H2O2 levels, OxyR is altered by oxidation of a cysteine residue (C199)23 and interacts with promoter PoxyS to modulate transcription.24 We introduced the regulatory gene and the regulated promoter upstream of the genes encoding for green or red fluorescent proteins, GFP or RFP, to give plasmids pOxyR_gfp and pOxyR_rfp (Fig. 1A and Table S1†) that generate H2O2RFP or H2O2GFP fluorescence signals as outputs, respectively. As a pilot test, we supplied increasing amounts of H2O2 to E. coli BW25113/pOxyR_rfp cells grown to mid-logarithmic phase and recorded H2O2RFP fluorescence change over time (Fig. S1A†). As H2O2 readily diffuses across the bacterial cell envelope,25 H2O2RFP signal increased rapidly upon peroxide addition and reached a plateau within 180 minutes. The magnitude of the signal correlated well over an external concentration range of 5–100 μM H2O2, and non-linearly even up to 400 μM (Fig. 1A) which is consistent with both, high sensitivity and broad dynamic range of the sensor.Biosensor-mediated detection of oxidase activity
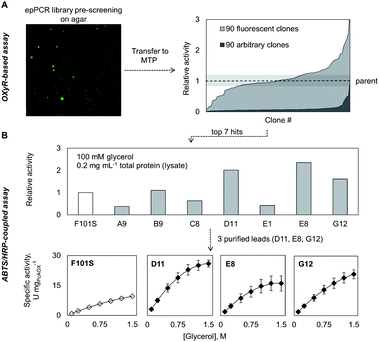
Next, we investigated whether the OxyR sensor can be used to monitor intracellular H2O2 formation. For this purpose, we introduced plasmids pBAD_pcaox encoding variants of the glycerol oxidase from Phanerochaete chrysosporium (PcAOX, EC 1.1.3.13)12 into E. coli BW25113 ΔglpK/pOxyR_gfp. The resulting strain converts glycerol via the PcAOX enzyme as they no longer produce the housekeeping glycerol kinase GlpK.26 Two PcAOX variants, wild type (WT) and variant F101S displaying 15-fold higher turnover frequency of glycerol,12 were used to assess the H2O2GFP signal at increasing glycerol concentrations in a microtiter plate assay. After incubation with glycerol, cells expressing PcAOX F101S displayed the highest H2O2GFP signal which could be unambiguously distinguished from that of cells harbouring the WT enzyme. The H2O2GFP signal of PcAOX F101S-containing cells was on average 3.5-fold higher than that of cells containing the WT enzyme (Fig. S2A†) while the signal of the negative control (cells transformed with the same vector backbone but without a glycerol oxidase gene) remained essentially unchanged. As the cellular contents of the WT protein and variant F101S were comparable (Fig. S2B†), we concluded that the difference in H2O2GFP fluorescence output could only arise from the dissimilar glycerol-oxidizing capacity of the strains at H2O2 liberation rates as low as 0.2 s−1 (PcAOX WT12).We additionally tested the performance of the OxyR sensor in another frequently used high-throughput assay format – cell colonies grown on solid support (Fig. 2A, bottom panel). To that end, E. coli ΔglpK/pOxyR_gfp expressing either PcAOX WT or variant F101S were grown on nutrient agar supplemented with glycerol until visible colonies were formed. When the plates were exposed to light for excitation of the H2O2GFP fluorescence, the two variants could be unambiguously distinguished by naked eye from cells that did not express the recombinant oxidase (Fig. 2A). The highly cell-contained nature of the signal allows for plating densities not realizable with assays relying on diffusive indicator substances and halo-formation as a readout.
Biosensor-based detection of monooxygenase activity
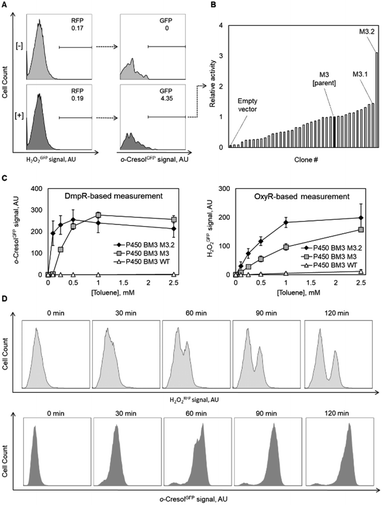
Following the encouraging results with alcohol oxidase as catalyst, we attempted to monitor the catalytic performance of a P450 monooxygenase in the course of an aromatic hydroxylation reaction using the OxyR sensor. Uncoupling, an unproductive reaction pathway commonly observed in P450 monooxygenase in the course of conversion of non-natural substrates,14 results in H2O2 formation at the catalytic center. Since P450 enzymes are rarely completely uncoupled with substrates that fit in their active site, H2O2 formation could to a certain extend serve as an indicator of the rate at which substrates are oxidized. To improve the confidence that we indeed monitor the productive P450 hydroxylation reaction (in this example, o-cresol synthesis), a second genetically encoded sensor based on the transcription factor DmpR from Pseudomonas sp.27 was implemented (Fig. 1B). DmpR-based sensors have been previously used for screening of hydrolase28 and lyase29 mutant libraries but, to the best of our knowledge, not for monooxygenases. Given its high sensitivity and capacity to discriminate between toluene and its phenolic derivatives,30 DmpR appeared suitable for detection of o-cresol formed upon hydroxylation of toluene by cells that express the monooxygenase P450 BM3 from Bacillus megaterium (EC 1.14.14.1).31 We first increased the intracellular concentration of the DmpR regulator protein encoded on plasmid pDmpR_gfp by modifying the transcription of the dmpR gene via rational engineering of the Pr promoter (Fig. 1B and Table S2†) as reported elsewhere.32 As a result, the o-cresol-triggered GFP signal (o-cresolGFP) in the presence of 10 μM o-cresol could be unambiguously measured within 60 minutes of incubation as compared to the >180 minutes required for the sensor employing the native Pr promoter (Fig. S1C†).Next, the H2O2RFP and o-cresolGFP signals were used to simultaneously assess the formation of both enzymatic products. The plasmids encoding the two genetically encoded sensors were introduced into E. coli BL21 (DE3) lacIq1 harboring either P450 BM3 WT (encoded on pALXtreme-1a_p450_wt) or P450 BM3 M3 (on pALXtreme-1a_p450_m3). The two enzymes have been shown to exhibit considerably different H2O2 and o-cresol formation rates with toluene as substrate.31,33 Product formation was accurately reflected by o-cresolGFP and H2O2RFP signals measured on a single-cell level by flow cytometry (Fig. 2 and S3†). The cellular content of P450 BM3 WT and M3 monooxygenases were comparable (Fig. S6A†), which corroborates the notion that the observed differences in fluorescence are indeed resulting from the dissimilar catalytic performance of the two enzymes (Fig. 4C).
Taken together, the results illustrate that the proposed P450 monooxygenase activity determination method employing two biosensors allows to simultaneously monitor the intracellular production of hydroxylated organic products and H2O2, and as such it has the potential to facilitate the isolation of improved enzyme variants.
Biosensor-based screening of alcohol oxidase libraries
As a first proof-of-principle screening application, we applied the OxyR sensor for pre-screening of a random mutagenesis library of PcAOX F101S aiming at enriching mutants with elevated glycerol-oxidizing capacity. Initially, we set out to measure H2O2 formation as indicated by H2O2GFP signal using flow cytometry. By applying model libraries (mixtures of active and inactive E. coli strains at defined ratios), we could demonstrate efficient enrichment of the active strain during sorting (Fig. S2C†). However, treatment of E. coli cells with propidium iodide (PI, a DNA stain readily taken up only by damaged or dead cells34) indicated a correlation of H2O2GFP and PI-stain signals. This suggests that the extra H2O2 generated as a result of the recombinant oxidase activity damages E. coli cells. It is also likely that those cells expressing the most active oxidase variants would be most severely affected. To mitigate this effect, we chose to perform the screening on agar plates. In addition to being simple to use (“plate, wait, and see”) and easily parallelizable, this format allows maintaining high survival even for E. coli cells producing highly active oxidase variants (Fig. S4†). After incubating E. coli cells carrying mutagenized variants of the plasmid encoding pcaox gene on nutrient agar supplemented with 25 mM glycerol (20-fold lower than the reported KM of F101S), we visually inspected the plates and picked 90 clones displaying bright H2O2GFP fluorescence. After their re-cultivation in liquid medium supplemented with glycerol, 85% of these strains exhibited H2O2GFP fluorescence between one and threefold the signal of the E. coli synthesizing the parent enzyme (±20%) (Fig. 3A). This indicated that below 15% of the picked E. coli strains were falsely assigned. As a control, 90 arbitrarily selected strains (colonies picked irrespective of the H2O2GFP fluorescence) were also assayed. Less than 5% of these strains displayed an H2O2GFP signal similar to that of E. coli cells synthesizing the parental enzyme while the remaining ones were not distinguishable from the negative control (cells that do not express an oxidative enzyme). We could therefore efficiently enrich clones of elevated oxidative capacity. To verify this, we proceeded with the seven PcAOX F101S variants that triggered the strongest H2O2GFP fluorescence, isolated the plasmids, and determined the pcaox gene sequence. Each of the variants carried a unique set of mutations (Table S2†). The PcAOX mutants were overproduced in an E. coli Top10 and oxidase activities in cell-free extracts (i.e., activities were normalized to total protein content in the lysate not to the cell-specific oxidase content thereby selecting for variants that were produced well and displayed in vitro activity) were determined using an ABTS/HRP-coupled assay (Fig. 3B and S3†). The three PcAOX variants that displayed the highest activity in lysate (D11, E8 and G12) were purified (Fig. S5A†) and their specific activity on glycerol was measured. All three variants showed improved initial activity on glycerol with variant displaying D11 displaying about 3-fold higher specific activity as compared to the parent enzyme, PcAOX F101S (Fig. 3B, bottom panel).Biosensor-based screening of monooxygenase libraries
As a final proof-of-concept application, we set out to isolate more active monooxygenases variants for aromatic hydroxylation of toluene using the OxyR and DmpR sensor pair by screening of a P450 BM3 M3 library. We performed biotransformation experiments with a pooled population of recombinant E. coli BL21 (DE3) lacIq1 expressing a P450 BM3 M3 multi-site saturation mutagenesis library in liquid medium and simultaneously recorded H2O2RFP and o-cresolGFP signals at single-cell level by flow cytometry. Notably, low substrate load (0.1–0.2 mM toluene) and short incubation times (≤60 minutes) were applied to minimize unspecific background drift of o-cresolGFP signal occurring at higher concentrations and/or longer incubation times (Fig. 4D). The reason for the deterioration of the o-cresolGFP signal under these conditions was not fully elucidated. We suspect that because small phenolic compounds can rapidly penetrate bacterial membranes,35,36 the concentration of newly formed o-cresol steadily increased in the liquid medium to the point at which the highly-sensitive DmpR-based sensor was triggered in the entire E. coli population. Such a signal drift was significantly less pronounced for the H2O2RFP signal. This is because H2O2 can react with and oxidize cellular components (e.g. lipids, DNA, proteins, antioxidant molecules such as glutathione)37 and is actively degraded by several housekeeping enzymes (catalases and peroxidases)38 and only little H2O2 presumably diffuses out of the cells.39 Accordingly, we sorted cells based on a high H2O2RFP signal as a primary criterion and a high o-cresolGFP signal as a secondary criterion. We argue that the combination and hierarchy of both signals provide means to lessen the effect associated with single-cell noise and background drift caused by membrane-permeable products (Fig. 4D). We spotted 384 gated E. coli cells on nutrient agar and obtained 42 colonies after overnight incubation (11% recovery). The o-cresolGFP signal of individual E. coli strains in the presence of toluene was re-assessed in a microtiter plate assay and indicated that half of the clones displayed considerably lower signals in comparison to the parent (Fig. 4B). This result indicates a substantial degree of incorrect assignment likely due to the aforementioned background drift and noise inherent to single-cell measurements40 which could not be entirely prevented. The signal of the remainder of clones was in the range of the parent and one variant (M3.2) outstandingly displayed a 3-fold higher o-cresolGFP signal than the parent. We selected M3.2 along with variant M3.1 featuring the second highest o-cresolGFP fluorescence in re-screening assay and determined the DNA sequence of both genes. The enzymes were purified and characterized with respect to NADPH consumption (indicative of the overall O2 turnover frequency in the presence of toluene) and o-cresol formation (productive reaction). The in vitro results (Table 1) recorded for M3.1 indicated 30% reduced activity to relative the parent at the substrate concentration (1 mM) used in the whole-cell re-screening assay. However, M3.2 displayed 11- and 1.7-fold increased product formation rates at low (1 mM) and high (10 mM) toluene, respectively. In addition, the degree of coupling of M3.2 was comparable to that of the parent at 1 mM and approximately 14% lower at 10 mM toluene. The in vivo performance of this mutant and its parent measured with the genetically encoded sensors in a microtiter plate assay (Fig. 4C) correlated well with the in vitro measurements. Taken together our results indicate that in a microtiter assay, the H2O2RFP and o-cresolGFP signals are suited for assignment of catalytic capacity of P450 BM3s within the complex environment provided by E. coli's cytosol and that the genetically encoded sensors can be employed with moderate success for high-throughput screening at a single cell level.| P450 BM3 | Mutationsa | Toluene conc.c | NADPH oxidationd | Coupling efficiencye | o-Cresol formationf |
|---|---|---|---|---|---|
| a The underlined mutations have been discovered in the course of this work. b From Dennig et al., 2013.31 c mM. d μmol μmolP450−1 min−1. e %. f μmol μmolP450−1 min−1. | |||||
| WTb | — | 1 | 24 ± 1 | 10 | 2 |
| 10 | — | — | — | ||
| M3 | R48S Y51W A330F I401M | 1 | 89 ± 11 | 32 ± 2 | 29 |
| 10 | 652 ± 83 | 48 ± 3 | 313 | ||
| M3.1 |
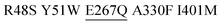
|
1 | 63 ± 6 | 19 ± 1 | 18 |
| 10 | 45 ± 12 | 24 ± 11 | 11 | ||
| M3.2 |
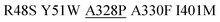
|
1 | 1066 ± 6 | 29 ± 3 | 309 |
| 10 | 1609 ± 91 | 34 ± 1 | 547 | ||
To demonstrate the crucial importance of the H2O2RFP signal for flow cytometric sorting of P450 BM3 M3 libraries, a conversion of toluene by a one-to-one mixture of cells expressing P450 BM3 wildtype and the isolated variant P450 BM3 M3.2 was prepared and both H2O2RFP and o-cresolGFP signals were recorded over two hours (Fig. 4D). The result demonstrates that the two strains could be unambiguously distinguished based on the H2O2RFP but not based on o-cresolGFP. This suggests that the o-cresol concentration in the culture broth gradually increased thereby activating the DmpR circuit and therefore o-cresolGFP in all cells while the amount of secreted H2O2 by cells expressing P450 BM3 M3.2 was not high enough to trigger a strong H2O2RFP response in cells expressing the less active or inactive enzyme variants.
Conclusions
The genetically encoded sensors for high-throughput screening presented here enable reliable measurements of intracellular H2O2 formed in the course of alcohol oxidase and P450 monooxygenase catalysed reactions formed as co-product or side product, respectively. H2O2 has previously been shown to serve as an indicator for benchmarking of the performance of oxidative enzymes in enzyme-coupled assays. Classically, H2O2 can be assayed in the presence of a peroxidase (e.g. horse reddish peroxidase) which uses H2O2 to oxidize dyes such as 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)18 or AmplexRed.17 The change of the spectral properties of the dyes can easily be recorded by plate readers or even by eye thereby allowing their application in a high-throughput setup. However, the supporting procedures include multiple manipulation steps for e.g. cell lysis and liquid handling, which complicates the workflow and limits the overall throughput.17,41Due to high degree of signal dynamics and a low signal-to-noise ratio barely exceeding an order of magnitude, the HyPer sensor design can only do so much for enzyme screening applications, in which integration times are short and the signal needs to be preserved for an extended period (in the range of hours) even if the reaction has been discontinued (e.g. to allow sample transfer or its pre-analytic storage). The OxyR sensor as reported here allows recording of H2O2-triggered signals over a wide dynamic range covering at least two orders of magnitude. When integrated directly into recombinant hosts expressing libraries of H2O2-liberating oxidative enzymes, the sensor proved compatible with several of the most frequently used platforms for high-throughput enzyme screening, i.e. plating on solid support followed by visual inspection of colonies, microtiter plate reader and flow cytometric measurements.
In summary, we present a method for activity assessment of oxidative enzymes directly in recombinant E. coli expression hosts via fluorescence measurements. The latter readouts are most frequently used for the execution of high-throughput screening protocols employed for biocatalyst optimization by directed evolution.42,43 The application of the genetically encoded OxyR sensor for enzyme screening provides advantages over existing approaches9 as it is economical (no need for chemical reagents), simpler to use (reduced number of manipulation steps, no cell lysis or compartmentalization required), and scalable (enabling ultra-high throughput). The OxyR sensor can therefore be readily used for activity screening of oxidating biocatalysts provided that the substrates can be taken up by cells.
The high modularity of the OxyR sensor system ought to allow its application for detection of H2O2 liberated in the course of other enzyme catalysed reactions (in particular, for flavin- or heme-containing biocatalysts mainly found in EC 1 but also in EC 2 (ref. 44) and EC 4 (ref. 45)) and may potentially be engineered to record signals generated in other recombinant microbial hosts such as P. putida, B. subtilis, C. glutamicum.
Data availability
Data are available within the article or its ESI materials.† The data that cannot be found in the article or in ESI materials† are available on request from the authors.Author contributions
TK, MH and SP conceived the project; TK and MH designed the experiments; TK performed the majority of the experiments; TK and MH analysed the data and wrote the manuscript; AW performed in vitro characterization of cytochrome P450 enzymes and ER contributed to oxidase engineering experiments. All authors discussed the results and commented on the manuscript.Conflicts of interest
The authors declare the following competing financial interest(s): Elvira Romero is currently a Postdoctoral Researcher at AstraZeneca R&D Gothenburg.Acknowledgements
The research for this work received funding from the European Union project ROBOX (grant agreement no. 635734) under EU’s Horizon 2020 Programme Research and Innovation actions H2020-LEIT BIO-2014-1. This document reflects only the author's view and the Agency is not responsible for any use that may be made of the information it contains.Notes and references
- S. G. Burton, Trends Biotechnol., 2003, 21, 543–549 CrossRef CAS PubMed.
- J. Dong, E. Fernández-Fueyo, F. Hollmann, C. Paul, M. Pasic, S. Schmidt, Y. Wang, S. Younes and W. Zhang, Angew. Chem., Int. Ed., 2018, 57, 9238–9261 CrossRef CAS PubMed.
- C. A. Martinez and S. G. Rupasinghe, Curr. Top. Med. Chem., 2013, 13, 1470–1490 CrossRef CAS PubMed.
- R. A. Sheldon and J. M. Woodley, Chem. Rev., 2018, 118, 801–838 CrossRef CAS PubMed.
- S. Martínez Cuesta, S. A. Rahman, N. Furnham and J. M. Thornton, Biophys. J., 2015, 109, 1082–1086 CrossRef PubMed.
- S. Wu, R. Snajdrova, J. C. Moore, K. Baldenius and U. T. Bornscheuer, Angew. Chem., Int. Ed., 2021, 60, 88–119 CrossRef CAS PubMed.
- U. T. Bornscheuer, G. W. Huisman, R. J. Kazlauskas, S. Lutz, J. C. Moore and K. Robins, Nature, 2012, 485, 185–194 CrossRef CAS PubMed.
- F. H. Arnold, Angew. Chem., Int. Ed., 2018, 57, 4143–4148 CrossRef CAS PubMed.
- C. K. Longwell, L. Labanieh and J. R. Cochran, Curr. Opin. Biotechnol., 2017, 48, 196–202 CrossRef CAS PubMed.
- G. Qu, A. Li, Z. Sun, C. G. Acevedo-Rocha and M. T. Reetz, Angew. Chem., Int. Ed., 2020, 59, 2–30 CrossRef.
- J. Lawrence Que, J. Chem. Soc., Dalton Trans., 1997, 0, 3933–3940 Search PubMed.
- Q.-T. Nguyen, E. Romero, W. P. Dijkman, S. P. de Vasconcellos, C. Binda, A. Mattevi and M. W. Fraaije, Biochemistry, 2018, 57, 6209–6218 CrossRef CAS PubMed.
- Y. V. Grinkova, I. G. Denisov, M. A. McLean and S. G. Sligar, Biochem. Biophys. Res. Commun., 2013, 430, 1223–1227 CrossRef CAS PubMed.
- D. Holtmann and F. Hollmann, ChemBioChem, 2016, 17, 1391–1398 CrossRef CAS PubMed.
- J. B. Lim and H. D. Sikes, Protein Eng., Des. Sel., 2015, 28, 79–83 CrossRef CAS PubMed.
- M. J. Weissenborn, S. Notonier, S.-L. Lang, K. B. Otte, S. Herter, N. J. Turner, S. L. Flitsch and B. Hauer, Chem. Commun., 2016, 52, 6158–6161 RSC.
- A. Debon, M. Pott, R. Obexer, A. P. Green, L. Friedrich, A. D. Griffiths and D. Hilvert, Nat. Catal., 2019, 2, 740–747 CrossRef CAS.
- R. Prodanović, W. L. Ung, K. Ilić Đurđić, R. Fischer, D. A. Weitz and R. Ostafe, Molecules, 2020, 25, 2418 CrossRef PubMed.
- V. V. Belousov, A. F. Fradkov, K. A. Lukyanov, D. B. Staroverov, K. S. Shakhbazov, A. V. Terskikh and S. Lukyanov, Nat. Methods, 2006, 3, 281–286 CrossRef CAS PubMed.
- D. S. Bilan and V. V. Belousov, Antioxid. Redox Signaling, 2015, 24, 731–751 CrossRef PubMed.
- Y. G. Ermakova, D. S. Bilan, M. E. Matlashov, N. M. Mishina, K. N. Markvicheva, O. M. Subach, F. V. Subach, I. Bogeski, M. Hoth, G. Enikolopov and V. V. Belousov, Nat. Commun., 2014, 5, 5222–5225 CrossRef CAS PubMed.
- B. González-Flecha and B. Demple, J. Bacteriol., 1999, 181, 3833–3836 CrossRef PubMed.
- I. Jo, I. Y. Chung, H. W. Bae, J. S. Kim, S. Song, Y. H. Cho and N. C. Ha, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 6443–6448 CrossRef CAS PubMed.
- L. A. Tartaglia, C. J. Gimeno, G. Storz and B. N. Ames, J. Biol. Chem., 1992, 267, 2038–2045 CrossRef CAS.
- G. P. Bienert, J. K. Schjoerring and T. P. Jahn, Biochim. Biophys. Acta, Biomembr., 2006, 1758, 994–1003 CrossRef CAS PubMed.
- T. Baba, T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner and H. Mori, Mol. Syst. Biol., 2006, 2, 2006–2008 CrossRef PubMed.
- V. Shingler, M. Bartilson and T. Moore, J. Bacteriol., 1993, 175, 1596–1604 CrossRef CAS PubMed.
- S.-L. Choi, E. Rha, S. J. Lee, H. Kim, K. Kwon, Y.-S. Jeong, Y. H. Rhee, J. J. Song, H.-S. Kim and S.-G. Lee, ACS Synth. Biol., 2014, 3, 163–171 CrossRef CAS PubMed.
- K. K. Kwon, D. H. Lee, S. J. Kim, S. L. Choi, E. Rha, S. J. Yeom, B. Subhadra, J. Lee, K. J. Jeong and S. G. Lee, Sci. Rep., 2018, 8, 1–9 Search PubMed.
- V. Shingler and T. Moore, J. Bacteriol., 1994, 176, 1555–1560 CrossRef CAS PubMed.
- A. Dennig, N. Lülsdorf, H. Liu and U. Schwaneberg, Angew. Chem., Int. Ed., 2013, 52, 8459–8462 CrossRef CAS PubMed.
- T. del Peso-Santos, L. M. D. Bernardo, E. Skärfstad, L. Holmfeldt, P. Togneri and V. Shingler, Nucleic Acids Res., 2011, 39, 5853–5865 CrossRef CAS PubMed.
- A. M. Weingartner, D. F. Sauer, G. V. Dhoke, M. D. Davari, A. J. Ruff and U. Schwaneberg, Appl. Microbiol. Biotechnol., 2018, 102, 9657–9667 CrossRef CAS PubMed.
- R. López-Amorós, J. Comas and J. Vives-Rego, Appl. Environ. Microbiol., 1995, 61, 2521–2526 CrossRef PubMed.
- H. Keweloh, G. Weyrauch and H.-J. Rehm, Appl. Microbiol. Biotechnol., 1990, 33, 66–71 CrossRef CAS PubMed.
- M. S. Roberts, E. J. Triggs and R. A. Anderson, Nature, 1975, 257, 225–227 CrossRef CAS PubMed.
- J. A. Imlay, Annu. Rev. Microbiol., 2003, 57, 395–418 CrossRef CAS PubMed.
- L. C. Seaver and J. A. Imlay, J. Bacteriol., 2001, 183, 7173–7181 CrossRef CAS PubMed.
- S. Mishra and J. Imlay, Arch. Biochem. Biophys., 2012, 525, 145–160 CrossRef CAS PubMed.
- L. K. Flachbart, S. Sokolowsky and J. Marienhagen, ACS Synth. Biol., 2019, 8, 1847–1857 CrossRef CAS PubMed.
- L. K. Morlock, D. Böttcher and U. T. Bornscheuer, Appl. Microbiol. Biotechnol., 2018, 102, 985–994 CrossRef CAS PubMed.
- U. T. Bornscheuer, B. Hauer, K. E. Jaeger and U. Schwaneberg, Angew. Chem., Int. Ed., 2019, 58, 36–40 CrossRef CAS PubMed.
- M. D. Truppo, ACS Med. Chem. Lett., 2017, 8, 476–480 CrossRef CAS PubMed.
- Z. Wang, A. Chernyshev, E. M. Koehn, T. D. Manuel, S. A. Lesley and A. Kohen, FEBS J., 2009, 276, 2801–2810 CrossRef CAS PubMed.
- M. P. Torrens-Spence, P. Liu, H. Ding, K. Harich, G. Gillaspy and J. Li, J. Biol. Chem., 2013, 288, 2376–2387 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc02578c |
| ‡ Current address: Richter Pharma AG, Durisolstraße 14, 4600 Wels, Austria. |
| § Current address: Compound Synthesis and Management, Discovery Sciences, AstraZeneca R&D Gothenburg, Sweden, 1 Pepparedsleden, Mölndal, SE-431 83, Sweden. |
| This journal is © The Royal Society of Chemistry 2021 |

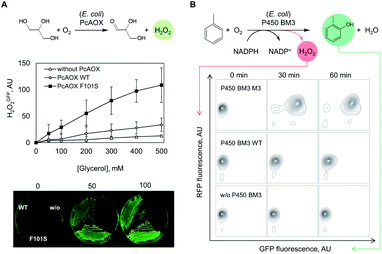
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 events shown for each time point) are presented in 2D probability contour plots.
000 events shown for each time point) are presented in 2D probability contour plots.