Open Access Article
Open Access ArticleNoria and its derivatives as hosts for chemically and thermally robust Type II porous liquids†
Francesca M.
Alexander
 a,
Sergio F.
Fonrouge
b,
José L.
Borioni
b,
Mario G.
Del Pópolo
a,
Sergio F.
Fonrouge
b,
José L.
Borioni
b,
Mario G.
Del Pópolo
 b,
Peter N.
Horton
c,
Simon J.
Coles
b,
Peter N.
Horton
c,
Simon J.
Coles
 c,
Benjamin P.
Hutchings
a,
Deborah E.
Crawford
d and
Stuart L.
James
c,
Benjamin P.
Hutchings
a,
Deborah E.
Crawford
d and
Stuart L.
James
 *a
*a
aSchool of Chemistry and Chemical Engineering, Queen's University Belfast, David Keir Building, Stranmillis Road, Belfast, BT7 1NN, UK. E-mail: s.james@qub.ac.uk
bICB-CONICET, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Cuyo, Padre Jorge Contreras 1300, Mendoza, M5502 JMA, Argentina
cEPSRC National Crystallography Service, School of Chemistry, Faculty of Engineering and Physical Sciences, University of Southampton, Southampton, SO17 1BJ, UK
dSchool of Chemistry and Bioscience, University of Bradford, Richmond Road, Bradford, BD7 1DP, UK
First published on 14th October 2021
Abstract
Porous Liquids (PLs) are a new class of material that possess both fluidity and permanent porosity. As such they can act as enhanced, selective solvents and may ultimately find applications which are not possible for porous solids, such as continuous flow separation processes. Type II PLs consist of empty molecular hosts dissolved in size-excluded solvents and to date have mainly been based on hosts that have limited chemical and thermal stability. Here we identify Noria, a rigid cyclic oligomer as a new host for the synthesis of more robust Type II PLs. Although the structure of Noria is well-documented, we find that literature has overlooked the true composition of bulk Noria samples. We find that bulk samples typically consist of Noria (ca. 40%), a Noria isomer, specifically a resorcinarene trimer, “R3” (ca. 30%) and other unidentified oligomers (ca. 30%). Noria has been characterised crystallographically as a diethyl ether solvate and its 1H NMR spectrum fully assigned for the first time. The previously postulated but unreported R3 has also been characterised crystallographically as a dimethyl sulfoxide solvate, which confirms its alternative connectivity to Noria. Noria and R3 have low solubility which precludes their use in Type II PLs, however, the partially ethylated derivative Noria-OEt dissolves in the size-excluded solvent 15-crown-5 to give a new Type II PL. This PL exhibits enhanced uptake of methane (CH4) gas supporting the presence of empty pores in the liquid. Detailed molecular dynamics simulations support the existence of pores in the liquid and show that occupation of the pores by CH4 is favoured. Overall, this work revises the general accepted composition of bulk Noria samples and shows that Noria derivatives are appropriate for the synthesis of more robust Type II PLs.
Introduction
Porosity is a useful fundamental characteristic of materials that has led to a variety of large-scale applications.1,2 Conventionally, permanent porosity has only been associated with solid state materials. This is intuitively reasonable since the relatively static nature of solids at the molecular level can enable empty cavities to persist. Porosity in liquids has conventionally been limited to the small, transient and irregular voids that are generated between molecules of the liquid as they tumble.3–5 However, strategies for generating large, permanent and well-defined pores in liquids have recently been proposed and demonstrated.6,7 For example, if discrete host molecules with rigid structures can be dissolved in solvents which cannot enter the host's cavities, then the resulting solutions will have persistent empty pores. The resulting counter-intuitive materials are termed Porous Liquids (PLs), with those based on empty hosts dissolved in size-excluded solvents categorised as Type II PLs. The selection/design of appropriate hosts for generating Type II PLs is not trivial since several criteria must be satisfied.6 Specifically, the host must, (i) be rigid (i.e., not collapse in the absence of a guest) so that its cavity persists, (ii) sterically exclude the solvent used (which also makes the choice of solvent a critical part of the design of the PL), (iii) be highly soluble in the solvent used if useful bulk properties (e.g., gas solubility) are to be maximised. To date, only a limited number of host types have been experimentally demonstrated for the formation of Type II PLs. They include T-symmetry imino-spherand organic cages7,8 tetrahedral Zn(II) coordination cages9 and Cu(II)24 ‘nanoball’ coordination cages (MOP-18).10 Notably, such hosts all have limited chemical stability in solution (e.g., to water, acids, and bases). This is in contrast to Type III PLs, i.e., those composed of microporous solids dispersed in size-excluded liquids, which exhibit high chemical and thermal stability.11–14 Therefore, developing Type II PLs that are more chemically robust as well as economical to prepare would be a significant step toward enabling applications for these materials. Chemically robust macrocyclic hosts such as calix[n]arenes,15 cucurbiturils16 and pillar[n]arenes17 have been extensively investigated for various applications but not in the context of PLs. Drawbacks of these hosts in this regard variously include insufficient rigidity and/or solubility. However, further host molecules have been developed from calix[n]arenes such as cavitands, carcerands and more recently the paddle-wheel shaped host referred to as Noria.18a Noria does have potential for the synthesis of Type II PLs since in addition to having a significant central cavity (ca. 5 Å in diameter), it has an apparently rigid structure as well as 24 external hydroxyl groups which can be functionalised to provide high solubility. Also, it is reported to be easily prepared in one step from inexpensive starting materials (resorcinol and 1,5-pentanedial).18aIn this paper, we explore the use of Noria and its derivatives for the formation of Type II PLs. To this end, we have revisited its synthesis and characterisation, and have obtained detailed insight into the true composition of bulk samples of Noria (as obtained by the standard literature procedure) for the first time. In particular, material prepared by the standard literature method was found to consist of only ca. 40% Noria, with 30% being an additional high symmetry species identified as a resorcinarene trimer, “R3”, which is an isomer of Noria and 30% other oligomers. Noria at higher purity (ca. 60%) has been obtained and its proton nuclear magnetic resonance (1H NMR) spectrum fully assigned for the first time. The previously postulated but unreported isomer R3 has also been fully characterised including an X-ray crystal structure. We have also investigated the more soluble derivative, Noria-OEt, in which 12 ethyl substituents are scrambled over the 24 external O sites, for the synthesis of PLs.19 Experimental gas uptake measurements and detailed molecular dynamics (MD) modelling suggest that Noria-OEt dissolved in 15-crown-5 is indeed a new chemically and thermally robust Type II PL.
Results and discussion
The structure of Noria and the composition of bulk Noria samples – previous work
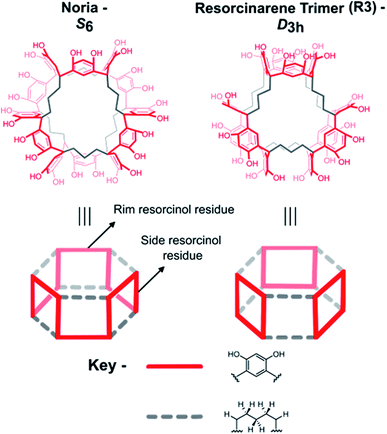
Kudo et al. first synthesised Noria in 2006 by the acid-catalysed co-condensation of resorcinol and 1,5-pentanedial.18a They also reported two derivatives, Noria-BOC and Noria-COOEt, in which the 24 external –OH groups were functionalised with tert-butoxycarbonyl or ethoxycarbonyl groups, respectively.18a Through single crystal X-ray analysis of Noria-BOC, the core connectivity of the Noria host was established to have the S6 symmetry topology depicted in Fig. 1.In fact, Kudo et al. had expected to obtain an alternative structure, a resorcinarene trimer herein denoted “R3”, which is a structural isomer of Noria (Fig. 1).18a Subsequently other analogous trimeric structures with methylene chains of different lengths (R–(CH2)n–R, n = 5, 7, 9)18b as well as dimeric structures with even-number methylene chains have been reported in the literature.18c–e Although Noria and R3 have different topologies with regard to the positions of the resorcinol and 1,5-pentanedial residues, they each have high symmetry (S6 and D3h respectively) and remarkably similar overall structures in that each has shallow cavities around the periphery and a single larger 5 Å hydrophobic cavity at the centre. Further characterisation of Noria by Kudo et al. included 1H NMR spectroscopy, infrared (IR) spectroscopy and mass spectrometry (MS).18a However, for the 1H NMR spectrum only ranges of chemical shift rather than specific values were quoted for each chemically distinct proton. In 2010 the 1H NMR spectrum itself was reported, which is reproduced with the assignment ranges indicated in Fig. 2a.20
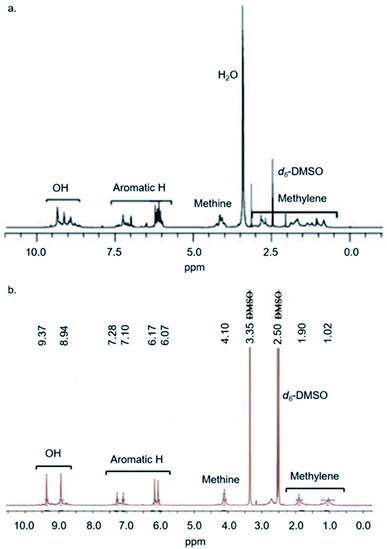 | ||
| Fig. 2 (a) 1H NMR spectrum of Noria reported by Kudo et al., adapted from ref. 20. (b) 1H NMR spectrum of Noria reported by Zhai et al., adapted from ref. 22. Note the peak at 3.35 ppm may be due to water. | ||
The 1H NMR spectrum shown in Fig. 2(a) is too broad and complex to be consistent with the crystallographically determined S6 topology of Noria, which would have, for example, only two chemically distinct –OH groups, and four chemically distinct ArH protons due to the presence of only two chemically distinct (‘rim’ and ‘side’) aromatic rings. In the 1H NMR spectrum, all proton environments manifest as broad features upon which several sharper peaks are superimposed. Computational prediction of the 1H NMR spectra by Peerannawar and Gejji et al.21 also supports the assertion that the 1H NMR spectrum of Noria should be far simpler than observed. Further, in 2018, Zhai et al. reported a 1H NMR spectrum (Fig. 2(b)) which is indeed simpler and consistent with the S6 Noria structure.22 For example, it shows the expected two –OH signals and four ArH signals, together with some impurities. However, this spectrum was not fully interpreted, especially regarding the methylene groups of the 1,5-pentanedial residues. Also, no purification procedure was given, so it is not clear how an apparently purer bulk material was obtained than in the preceding reports. In 2009, Tian et al. determined the structure of Noria itself by single crystal X-ray diffraction25 and found the same S6 topology as in the crystal structure of Noria-BOC as determined by Kudo et al.18a Analysis by powder X-ray diffraction25 has shown that bulk samples of Noria are amorphous. Derivatives of Noria in which the –OH protons are substituted with various R groups have also been reported.23,24 In all cases found in the literature, 1H NMR data for bulk samples of Noria derivatives have also exhibited broad signals that are not consistent with pure, high-symmetry products. Very recently, a 1H NMR spectrum has been reported by Shimoyama et al.,27 which is consistent with the S6 symmetry, but with different chemical shifts to the 1H NMR spectrum reported by Zhai et al. (see ESI, S2.14†).22 Despite the overall lack of definitive characterisation of bulk Noria samples, in terms of the functional porosity of bulk Noria samples, gas sorption isotherms do point to significant microporosity consistent with the nature of Noria as a rigid host with a central cavity.25,26
Overall, the existing literature can be summarised as follows: (1) there is clear evidence for the generally accepted S6 core connectivity of Noria (from X-ray crystal structures of Noria and Noria-BOC),25,18a (2) despite this, in all but two cases,22,27 solution state 1H NMR spectra of bulk Noria samples are inexplicably broad and contain too many peaks than can be accounted for by this S6 topology structure, (3) the two better-resolved 1H NMR spectra reported by Zhai et al. and Shimoyama et al. differ to each other in terms of chemical shifts suggesting that they were observing different species.22,27 This points to bulk samples of Noria and its derivatives having greater structural/compositional complexity than is apparent from the two crystal structure determinations and which is generally recognised in the literature i.e., bulk samples of Noria seem to be crude.
Revision of the composition of Noria bulk samples – this work
In this work, our aim was to develop Type II PLs based on Noria. We therefore began by re-synthesising Noria according to the original literature method described by Kudo et al. and obtained bulk samples with 1H NMR spectra similar to the broad and complex ones reported in the literature which are not consistent with a pure S6 Noria structure.18a,20 As previously mentioned, a recent paper published by Shimoyama et al.27 stated that bulk Noria samples were comprised of a mixture of species, however, no detail was given as to what these species were, other than that they may be polymeric byproducts. The lack of consistency regarding the 1H NMR spectrum of Noria found in the literature was concerning and necessitated systematic studies into the true nature of bulk Noria samples. Variable temperature 1H NMR experiments were firstly conducted to probe the possibility of dynamic behaviour (see ESI Fig. S30†). However, the spectra did not change significantly between 25 and 120 °C (in deuterated dimethyl sulfoxide (d6-DMSO)). Therefore, the complexity and broadness were concluded to indicate that a mixture of species was indeed present, in agreement with Shimoyama et al.27 Liquid chromatography-mass spectrometry (LCMS) analysis was inconclusive on this point because of low solubility in appropriate solvents such as acetonitrile, and we therefore investigated various bulk separation and purification procedures to elucidate the composition (Fig. 3).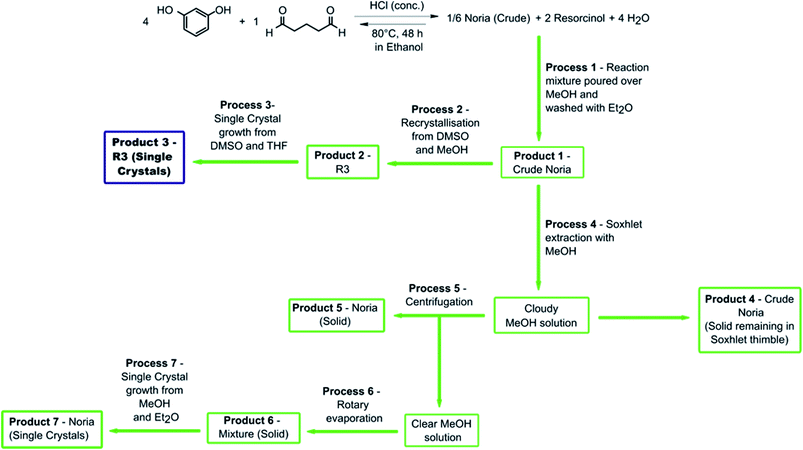 | ||
| Fig. 3 A flowchart showing the procedures applied and products obtained in the purification of crude Noria. Note that the blue box highlights R3, a previously unreported cyclic molecular host and structural isomer of Noria. Further details can be found in the ESI.† | ||
These procedures generated six solid samples (Products 1–7) as follows:
Product 1: (crude Noria) Noria was synthesised according to the literature procedure of Kudo et al.18a and was obtained as a yellow powder. As stated above, 1H NMR spectra were broad and contained too many signals (see below), similar to the spectrum reported by Kudo et al.18a,20
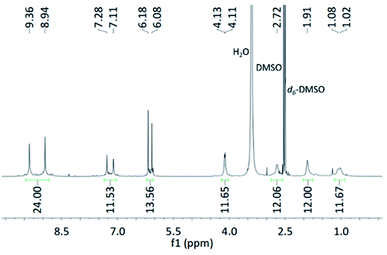
Product 2: (recrystallised material) Product 1 was recrystallised from DMSO and MeOH (process 2 in Fig. 3) to give a cream-coloured powder. This procedure was used because Noria single crystals were reportedly grown from DMSO/MeOH.25 Also, in the 1H NMR spectrum of the apparently purer material reported by Zhai et al.22 (Fig. 2(b)) a peak due to H6-DMSO is present in addition to residual D/H-DMSO (d6-DMSO was used as the 1H NMR solvent) suggesting that the purification procedure they used involved DMSO. The 1H NMR spectrum of Product 2 (see Fig. 4) did indeed match well with that reported by Zhai et al.22 with all major peaks matching in terms of chemical shift, multiplicity, and integration. There are some impurities present according to the 1H NMR spectrum, and we thus estimate (by peak integration) the level of purity of Product 2 to be >70%.
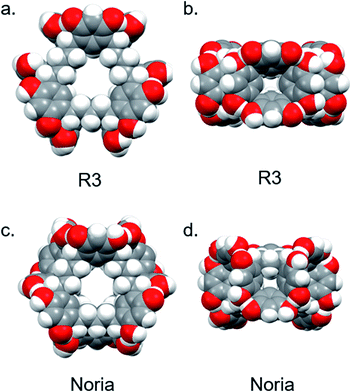
Product 3: (single crystals of R3) Product 2 was washed with dimethylformamide (DMF) and the residue crystallised over several weeks by vapour diffusion of tetrahydrofuran (THF) into a DMSO solution to give colourless plate-like single crystals suitable for X-ray analysis (Product 3). Interestingly, the structure was determined not to be that of Noria but instead R3 (Fig. 5(a) and (b)).
Product 3 crystallises as a DMSO solvate in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Although there are several solvent molecules present in the structure some were too disordered to be suitably modelled and therefore solvent masking was employed. Notable features of the R3 molecules are the resorcinarene trimer connectivity with idealised D3h symmetry. In contrast to the sinusoidal chain of aryl rings in Noria the aryl rings in R3 are arranged into three resorcinol tetramers such that three large, well-defined concavities exist on the outside surface (Fig. 5(a)), each occupied by a single DMSO molecule. There is a central cavity which can accommodate a sphere of diameter 4.88 Å as inferred from the distance from molecular centroid to the nearest H atom, taking into account the van der Waals radius. This cavity is also occupied by DMSO and is very similar in size and shape to the cavity of Noria (see below).There is extensive intramolecular hydrogen bonding between the hydroxyl groups of neighbouring aryl rings, as well as intermolecular hydrogen bonding between R3 molecules such that they form dimers within the crystal (Fig. S48†). The dimers are interspersed by further solvent molecules to form columns. These columns pack in a hexagonal arrangement with additional solvent molecules between them (Fig. S48†). Within the R3 molecules all bond lengths and angles are unremarkable. Some of the single crystals of R3 were dissolved in d6-DMSO and 1H NMR spectroscopy confirmed that it matched the material from which it was crystallised (Product 2) confirming that Product 2 is definitively R3. The purity was higher than Product 2 and estimated to be ca. 90% (see ESI Section S2.3†). Therefore, the 1H NMR spectrum in the report of Zhai et al.22 relates to R3 rather than Noria. The 1H NMR spectrum of Product 2 is fully consistent with the crystallographically-determined D3h symmetry of R3 (for example there are two –OH signals and four aromatic (ArH) signals, corresponding to the two chemically distinct resorcinol residues (refer to Fig. 4). Further, we have fully assigned all peaks in the spectrum including the more complex region due to the methylene protons of the 1,5-pentanedial residues for the first time. In particular, the signal at 2.72 ppm, which was previously not assigned as a product peak,22 was in fact shown by 2D NMR experiments to belong to the C5 chain (see ESI Section S2.2, Fig. S5–S10†). Four resonances in the alkyl region can be fully accounted for by the expected inequivalence of mutually geminal protons in CH2 groups in the C5 chain. In particular, the central CH2 group resonates at 1.02 and 1.08 ppm and its neighbouring CH2 units at 1.91 and 2.72 ppm (the methylene protons were assigned on the basis of the angular dependence of vicinal coupling constants). The CH methine resonance appears at 4.12 ppm. The matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrum was also consistent with the structure of R3, showing clear peaks for the ions [M + Na]+, [M + Na + DCTB]+ and [M + Na + 2DCTB]+ (DCTB = trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenylidene]malononitrile matrix) (see ESI Fig. S11 and S12†).
. Although there are several solvent molecules present in the structure some were too disordered to be suitably modelled and therefore solvent masking was employed. Notable features of the R3 molecules are the resorcinarene trimer connectivity with idealised D3h symmetry. In contrast to the sinusoidal chain of aryl rings in Noria the aryl rings in R3 are arranged into three resorcinol tetramers such that three large, well-defined concavities exist on the outside surface (Fig. 5(a)), each occupied by a single DMSO molecule. There is a central cavity which can accommodate a sphere of diameter 4.88 Å as inferred from the distance from molecular centroid to the nearest H atom, taking into account the van der Waals radius. This cavity is also occupied by DMSO and is very similar in size and shape to the cavity of Noria (see below).There is extensive intramolecular hydrogen bonding between the hydroxyl groups of neighbouring aryl rings, as well as intermolecular hydrogen bonding between R3 molecules such that they form dimers within the crystal (Fig. S48†). The dimers are interspersed by further solvent molecules to form columns. These columns pack in a hexagonal arrangement with additional solvent molecules between them (Fig. S48†). Within the R3 molecules all bond lengths and angles are unremarkable. Some of the single crystals of R3 were dissolved in d6-DMSO and 1H NMR spectroscopy confirmed that it matched the material from which it was crystallised (Product 2) confirming that Product 2 is definitively R3. The purity was higher than Product 2 and estimated to be ca. 90% (see ESI Section S2.3†). Therefore, the 1H NMR spectrum in the report of Zhai et al.22 relates to R3 rather than Noria. The 1H NMR spectrum of Product 2 is fully consistent with the crystallographically-determined D3h symmetry of R3 (for example there are two –OH signals and four aromatic (ArH) signals, corresponding to the two chemically distinct resorcinol residues (refer to Fig. 4). Further, we have fully assigned all peaks in the spectrum including the more complex region due to the methylene protons of the 1,5-pentanedial residues for the first time. In particular, the signal at 2.72 ppm, which was previously not assigned as a product peak,22 was in fact shown by 2D NMR experiments to belong to the C5 chain (see ESI Section S2.2, Fig. S5–S10†). Four resonances in the alkyl region can be fully accounted for by the expected inequivalence of mutually geminal protons in CH2 groups in the C5 chain. In particular, the central CH2 group resonates at 1.02 and 1.08 ppm and its neighbouring CH2 units at 1.91 and 2.72 ppm (the methylene protons were assigned on the basis of the angular dependence of vicinal coupling constants). The CH methine resonance appears at 4.12 ppm. The matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrum was also consistent with the structure of R3, showing clear peaks for the ions [M + Na]+, [M + Na + DCTB]+ and [M + Na + 2DCTB]+ (DCTB = trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenylidene]malononitrile matrix) (see ESI Fig. S11 and S12†).
The above crystallographic and NMR spectrometric analysis not only suggests that previous literature in which the same 1H NMR data was assigned to the S6 Noria structure (with which it is also consistent) should be reconsidered,22 but also reveals that the crude Noria material as reported in the literature more generally actually consists substantially (ca. 30%) of the isomeric structure R3 (see further discussion below).
Soxhlet extraction of Product 1 with MeOH was also investigated as an alternative purification method. This generated four additional samples, Products 4–7:
Product 4: solid remaining in the Soxhlet thimble.
Product 5: a precipitate in the MeOH extract that was collected by centrifugation.
Product 6: material dissolved in the MeOH extract supernatant that was isolated by evaporation of the solvent.
Product 7: single crystals grown from Product 6 by vapour diffusion of Et2O into a MeOH solution.
Products 4–7 were analysed by 1H NMR spectroscopy (see ESI Sections S2.4, S2.5, S2.6 and S2.7†).
Products 4 and 6 were both found to be similar in composition to Product 1 (i.e., crude Noria) as indicated by their similar 1H NMR spectra.
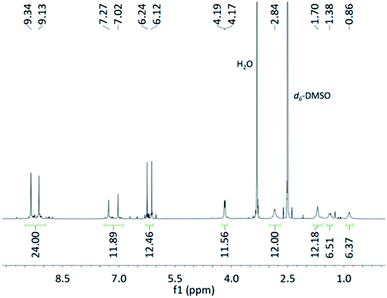
Since Product 6 had high solubility in MeOH, and Noria is reported to have low solubility in MeOH,20 we attempted to grow single crystals of this apparently unknown material by vapour diffusion of Et2O into a MeOH solution in the expectation of obtaining a new, non-Noria species. Single crystals (Product 7) were obtained over the course of several weeks but surprisingly, the X-ray structure determination revealed that Product 7 was Noria (Fig. 5(c) and (d), ESI Section S2.7†), obtained as a MeOH–Et2O solvate. The same overall topology with S6 symmetry is observed as in the previous structure determinations of Noria and Noria-BOC mentioned above. Within the structure all bond lengths and angles are unremarkable, and the central cavity can accommodate a sphere of diameter 4.98 Å as inferred from the distance from molecular centroid to the nearest H atom, taking into account the van der Waals radius. Thus, the cavities of the Noria and R3 are very similar in size. The cavity contains disordered MeOH molecules. There are additional interstitial solvent molecules, specifically one MeOH molecule which donates a hydrogen bond to an Ar–OH group of the Noria and one Et2O molecule which occupies one of the shallow external cavities. The Noria molecules are arranged in columns along the c axis which pack hexagonally (Fig. S47†). The 1H NMR spectrum of Product 7 was obtained by dissolving some single crystals in d6-DMSO and it was found to match well with the spectrum reported by Shimoyama et al.27 However, we have also now fully assigned the spectrum (see ESI Section S2.5 and S2.7†). (Fig. 6) and it should be noted that it was found to match very closely to that Product 5 in terms of chemical shifts, multiplicities, and integrations (see ESI Section S2.5†).
Although perhaps unlikely, we considered the possibility that some interconversion between Noria and R3 isomers might occur (i.e., do they form simultaneously during synthesis, or do they interconvert during the work-up?). Inspection of the 1H NMR spectrum of Product 1 reveals that both species are in fact present in the crude material (Fig. 7), suggesting that both species form during the synthesis itself.
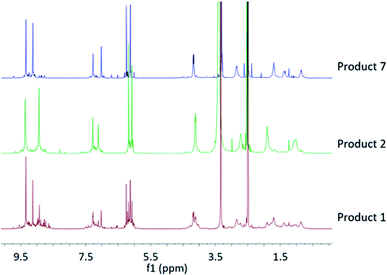 | ||
| Fig. 7 Overlay of 1H NMR spectra for Product 1, 2 and 7, showing that both Noria and R3 are present in the crude Product 1. | ||
From the 1H NMR spectrum of Product 1 (crude Noria) it is also clear that other species in addition to Noria and R3 are present. We suggest that these are further linear and/or cyclic oligomers. From the 1H NMR spectrum we estimate the relative amounts of the various species present to be 40% Noria, 30% R3 and 30% other oligomers.
In summary, the above analysis suggests the following key points:
(1) Bulk samples of Noria as obtained by the standard literature procedure, are actually crude and consist of a mixture of Noria, R3 and other oligomers.
(2) Samples consisting primarily of R3 can be obtained by recrystallisation of Product 1 from DMSO/MeOH.
(3) Single crystals of R3 can then be obtained by crystallisation from DMSO/THF.
(4) Samples consisting primarily of Noria can be obtained from Product 1 by Soxhlet extraction with MeOH.
(5) Noria single crystals can then be obtained by crystallisation using MeOH/Et2O.
Given the above revision of the composition of bulk Noria samples (crude), it is important to consider any implications for the previously observed properties of this bulk crude material in the literature. Firstly, the reported amorphous nature of the material25 is fully consistent with a mixture of isomeric and oligomeric species being present. Secondly, the ability of the bulk crude material to adsorb gases in the solid state25,26 (i.e., to exhibit microporosity) is still consistent with the revised composition since the bulk crude material consists around 70% of rigid hosts with cavities (albeit a combination of Noria and R3, rather than pure Noria) which according to X-ray analysis are likely to have similar host properties to each other. It is also conceivable that larger cyclic oligomers may be present which could also exhibit porosity. We note that there have been no detailed studies of guest inclusion with Noria in solution to date. We suggest that such studies may not have been feasible because of the ill-defined 1H NMR spectra and lack of understanding of the true composition of bulk samples. Finally, we note that the solubilities of Noria and R3 samples unsurprisingly seem to be affected by their levels of purity, with solubility generally decreasing markedly as purity increases. This variable solubility may also have hampered attempts to understand and elucidate the composition of bulk samples.
Noria as a host for Type II PLs
As noted in the introduction, for a host to be appropriate for forming Type II PLs it should be unable to collapse when empty and soluble in a size-excluded solvent. The Noria structure appears to satisfy the first criterion according to MD modelling as discussed below. Also, it shows excellent stability which is important if Type II PLs are to be considered at some point for applications. For example, it is soluble in alkaline water (pH 10) and 1H NMR spectra showed it to be unchanged after 5 days. Also, heating to 100 °C for 7 days in wet d6-DMSO showed no significant changes in 1H NMR spectra (see ESI S2.11 and S2.12†). However, it has low solubility in all solvents investigated and we therefore turned attention to the known semi-ethylated derivative Noria-OEt.19Synthesis and characterisation of Noria-OEt and formation of a Type II PL in 15-crown-5
Noria-OEt, has been synthesised previously by co-condensation of 3-ethoxyphenol and 1,5-pentanedial under acidic conditions.19 The 12 ethyl groups of Noria-OEt are expected to be scrambled over the 24 external O sites of the S6 core structure. Hence, bulk Noria-OEt is likely to exist as a complex mixture of up to 64 geometrical isomers (see ESI Fig. S36†). Although this complicates the analysis of the material, such scrambling of substituents can be an advantage in that it can lead to higher solubility.7 We repeated the published synthesis with some slight adaptations (see ESI Section S2.13†).19 Evidence for the expected product was seen in the MALDI-MS spectrum which showed expected peaks for [M]+˙, [M + Na]+, [M + K]+ as well as inclusion complexes with the DCTB matrix (see ESI Section S2.13.1, Fig. S34†). Due to the scrambling of the ethyl groups, however, the 1H NMR spectrum of Noria-OEt is expected to be complex. This was indeed the case with each chemically distinct proton environment showing as a broad feature (see ESI Section S2.13, Fig. S33†). Despite this, the relative integrations of these features matched with the expected overall structure, the spectrum closely matched that in the literature19a and there were no peaks which would suggest that other types of product might be present. It is important to consider that Noria-OEt may also contain the R3-OEt structure and other oligomers analogous to those seen in bulk samples of Noria itself. However, because of the inherent complexity of the 1H NMR spectrum due to the scrambling, it was not possible to draw firm conclusions on this from the 1H NMR spectrum alone. Further, attempts at LCMS analysis were not successful because of its low solubility in appropriate solvents such as acetonitrile. However, as noted above, both the D3h (R3) and S6 (Noria) topologies possess central cavities of similar size (see also below). Thus, if Noria-OEt were to consist of both R3-OEt and Noria-OEt, then both hosts should give rise to pores in the liquid state when dissolved in a size-excluded solvent. It was found that Noria-OEt had significant solubility (up to 80 mg mL−1) in the bulky solvent 15-crown-5. The bulky solvent 15-crown-5 has a diameter of approximately 10 Å (measured between the most distant H atoms including van der Waals radii) and a low surface curvature, which has enabled it to be used successfully as a size-excluded solvent in PLs based on imino-spherand cages and coordination cages.7,10 Consequently, it seemed likely that such 15-crown-5 solutions of Noria-OEt would constitute new Type II PLs. MD modelling and gas solubility measurements were performed to substantiate the presence of permanent pores in such solutions and to investigate more generally the structure of this putative PL phase.Molecular dynamics modelling of Noria-OEt-15-crown-5 PL
MD simulations were performed at 300 and 350 K, both at 1 atm. The liquid consisted of Noria-OEt dissolved in 15-crown-5 at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
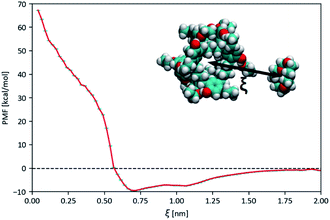
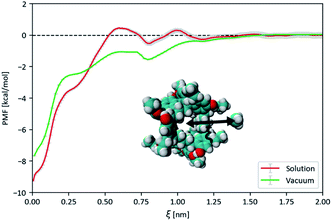
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 206 molar ratio, which corresponds to the 50 mg mL−1 concentration used in the gas solubility measurements described below (see Fig. 14). As a reference, simulations of the neat solvent were conducted at the same temperatures and pressure. Atomistic models for the Noria-OEt host and the solvent were constructed from the Optimised Potentials for Liquid Simulations (OPLS) all-atom force-field, with atomic charges obtained from ab initio Density Functional Theory (DFT) methods and equilibrium reference geometries. For simplicity, a single Noria-OEt isomer was modelled, with –OH and –OEt groups arranged in an alternating fashion on the outside of the molecule so that the OEt groups were maximally spaced. Further information on model parameters, simulation details and electronic structure calculations is provided in the ESI (S5).† As expected, throughout the MD simulation runs, 15-crown-5 solvent molecules were unable to occupy the central Noria-OEt cavity due to steric hindrance. This exclusion is reflected in the large formation energy of the Noria-OEt solvent inclusion complex, estimated with DFT methods as +90.96 kcal mol−1. Moreover, no minimum was observed below 0.6 nm in the free energy landscape of the inclusion complex, calculated by MD-based umbrella sampling simulations using the distance ξ between the centres of mass of the host and the solvent molecule as reaction coordinate (Fig. 8). The free energy curve features a global minimum at 0.71 nm, i.e., with 15-crown-5 sitting outside the cavity entrance and rises sharply as the solvent molecule enters the cavity. The maximum corresponds to a 67.28 kcal mol−1 energy difference relative to the average asymptotic value.
206 molar ratio, which corresponds to the 50 mg mL−1 concentration used in the gas solubility measurements described below (see Fig. 14). As a reference, simulations of the neat solvent were conducted at the same temperatures and pressure. Atomistic models for the Noria-OEt host and the solvent were constructed from the Optimised Potentials for Liquid Simulations (OPLS) all-atom force-field, with atomic charges obtained from ab initio Density Functional Theory (DFT) methods and equilibrium reference geometries. For simplicity, a single Noria-OEt isomer was modelled, with –OH and –OEt groups arranged in an alternating fashion on the outside of the molecule so that the OEt groups were maximally spaced. Further information on model parameters, simulation details and electronic structure calculations is provided in the ESI (S5).† As expected, throughout the MD simulation runs, 15-crown-5 solvent molecules were unable to occupy the central Noria-OEt cavity due to steric hindrance. This exclusion is reflected in the large formation energy of the Noria-OEt solvent inclusion complex, estimated with DFT methods as +90.96 kcal mol−1. Moreover, no minimum was observed below 0.6 nm in the free energy landscape of the inclusion complex, calculated by MD-based umbrella sampling simulations using the distance ξ between the centres of mass of the host and the solvent molecule as reaction coordinate (Fig. 8). The free energy curve features a global minimum at 0.71 nm, i.e., with 15-crown-5 sitting outside the cavity entrance and rises sharply as the solvent molecule enters the cavity. The maximum corresponds to a 67.28 kcal mol−1 energy difference relative to the average asymptotic value.
Furthermore, the host–solvent radial distribution function, g(r), for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 206 Noria-OEt–solvent system illustrates that solvent molecules remained outside the central cavities during the 100 ns simulations, with unbiased MD trajectories (Fig. 9).
206 Noria-OEt–solvent system illustrates that solvent molecules remained outside the central cavities during the 100 ns simulations, with unbiased MD trajectories (Fig. 9).
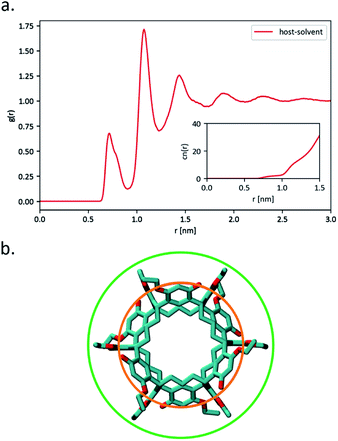
Further analysis of the host–solvent radial distribution function (Fig. 9(a)) shows two distinct solvation shells at 0.72 nm and 1.08 nm. These values highlight two structural features of interest illustrated in Fig. 9(b): the distance between the centre of the host and the solvent molecules located at any of the six lateral indentations outside Noria-OEt (orange circle), and the outer effective radius of the host as outlined by the solvent (green circle). In addition to their structural significance, both distances correspond to the minima in the PMF curve of the inclusion complex (Fig. 8). The minimum at 0.72 nm also hints that the shallow outer indentations of the Noria-OEt host accommodate some solvent molecules (∼2 on average) in the liquid phase, unlike the inner intrinsic cavity. Since solvent molecules remain outside the intrinsic cavity of a Noria-OEt host, dissolving Noria-OEt in 15-crown-5 is expected to introduce additional empty cavities in this solvent. To quantify this effect, we calculated the insertion probability P(R), which represents the likelihood of being able to insert a hard sphere of radius R at a random point in the liquid.28 Furthermore, we previously defined the relative porosity W(R) as the quotient between P(R) in the PL and in the neat solvent (at the same temperature and pressure).7 We calculated P(R) from MD simulations of both liquids at 300 and 350 K. 1000 configurations were selected and probed every 0.05 nm along the x, y and z axes to find the largest sphere that could be inserted without overlapping the van der Waals radius of any atom. The resulting W(R) curves are shown in Fig. 10.
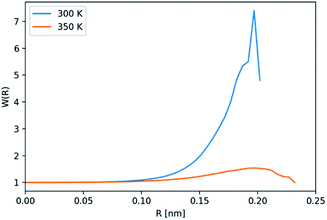 | ||
| Fig. 10 Relative porosity, W(R), of the PL obtained from MD simulations at 300 and 350 K. W(R) = PPL(R)/PSolv(R), where PPL(R) is the insertion probability for spherical probe of radius R (ref. 28) in the PL, and PSolv(R) the corresponding insertion probability in the neat solvent at the same temperature. | ||
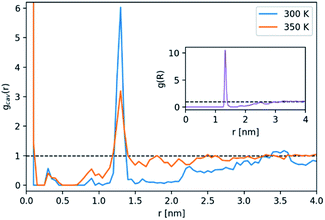
W(R) > 1 indicates that for a probe radius R, the concentration of cavities in the PL is greater than in the neat solvent. While both curves feature a maximum at approximately 0.20 nm, the increase of porosity at 300 K is much steeper. Relative to the neat solvent, the PL has seven times more cavities whose radii are roughly 0.20 nm. At 350 K, however, this relative factor is approximately 1.54. This contrast suggests that the increase in porosity in the PL stems primarily from the presence of intrinsic Noria-OEt cavities: a fixed number of intrinsic cavities has a greater effect on porosity when fewer (extrinsic) gaps exist between the solvent molecules, gaps that are more prevalent as temperature rises. Also, the peak in relative porosity occurs at the lower end of the average intrinsic cavity radius, estimated from simulations as 0.22 ± 0.02 nm, which reinforces the previous assessment. In order to assess how cavities are distributed in the space around the hosts, we calculated the radial distribution function of large cavities relative to the geometrical centre of the Noria-OEt molecules, gcav(r) (Fig. 11).
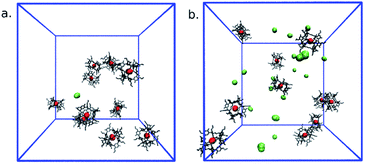
This analysis comprised 100 configurations of each of the 300 and 350 K MD simulations of the PL and was restricted to cavities whose radii were equal to or greater than 0.20 nm. As expected, the large peaks in gcav(r) for r → 0 nm stem from the fact that the host intrinsic cavity is empty. Outside the hosts, however, a large maximum was found at ∼1.3 nm for both temperatures. As evidenced by the host–host g(r) included as an inset in Fig. 11, this peak arises from Noria-OEt dimers that remained stable throughout the simulations. Other than this maximum, no discernible trends in cavity distribution were observed beyond the effective radius of the hosts. In particular, there are no large cavities localised in the outer indentations of Noria-OEt. Fig. 12(a) and (b) illustrate the distribution of cavities in configurations selected from simulations at 300 and 350 K. Red and green surfaces indicate empty cavities inside or outside the hosts, respectively.
Overall, clearly the MD simulations reveal relevant details of the liquid structure, a key aspect being that 15-crown-5 is an appropriate size-excluded solvent for the formation a Type II PL based on Noria-OEt.
Methane solubility measurements
The existence of empty pores in PLs can lead to increased gas solubility as shown previously for Type II PLs based on other hosts.7,8,10 For the methane (CH4) solubility experiments with the suspected Noria-OEt PL, a high-pressure gas rig with gas flow monitoring was used as described elsewhere.14 Solubilities were measured at 1, 2, 3, 4 and 5 bar at 303.15 K. Gas flow data confirmed that the system reached equilibrium well within the 1 hour period allowed for equilibration at each pressure. Data from three runs were averaged and compared with those for pure 15-crown-5 as a control (Fig. 13).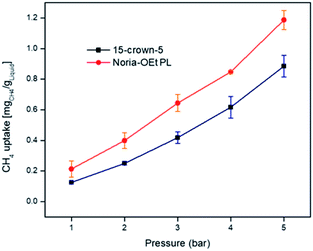 | ||
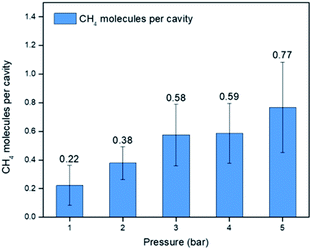
| Fig. 13 CH4 uptake at 303.15 K for 15-crown-5 average compared to Noria-OEt in 15-crown-5 (50 mg mL−1) average (1–5 bar). | ||
The presence of Noria-OEt clearly increases the CH4 solubility, e.g., at 1 bar from 0.13 mg g−1 for 15-crown-5 to 0.21 mg g−1 for the Noria-OEt PL. Although modest in comparison to more concentrated Type II PLs,7 this increase is consistent with the lower concentration of the host species in this case. The excess solubility corresponds to ca. 0.22 CH4 molecules per intrinsic cavity at 1 bar (see Fig. 14), which is an intuitively reasonable level of occupancy. As the pressure increases, the gas solubility remains consistently greater in the PL and reaches ca. 0.77 CH4 molecules per cavity at 5 bar.
As a further control experiment, CH4 solubility in a solution of 3-ethoxyphenol in 15-crown-5 was measured under similar conditions to check for the possibility that the increase in CH4 solubility may be due to the presence of aromatic groups rather than actual intrinsic cavities. As shown in Fig. S41 in the ESI,† no increase in CH4 solubility was observed, supporting the existence of cavities as being critical. Overall, the results from the gas uptake studies show that a solution of Noria-OEt in 15-crown-5, at a concentration of 50 mg mL−1, does indeed exhibit an increase in CH4 uptake compared to the solvent alone, and that the increase is in line with the expected level of porosity. Therefore, these measurements provide experimental evidence that this solution is a new Type II PL.
MD modelling of the PL with dissolved CH4
To characterise the affinity of CH4 for the Noria-OEt host, DFT-based geometry optimisation methods were used to calculate the formation energy of the host–CH4 complex in vacuum. This complex was stable, with a favourable formation energy of −7.05 kcal mol−1. In addition, the process of insertion of CH4 into a host both in solution and vacuum was analysed through MD simulations and umbrella sampling. Fig. 15 shows the resulting free energy profiles at 350 K and 1 atm for the inclusion of a CH4 molecule inside a single Noria-OEt molecule. For the system in solution, 341 15-crown-5 molecules were used.The reaction coordinate, ξ, was defined as the distance between the host–guest centres of mass. In both landscapes, global minima occur at the centre of each intrinsic cavity and illustrate a strong tendency to form inclusion complexes of Noria-OEt and CH4. Small minima are also observed at 0.8 nm, a distance that matches the valley between the first two peaks of the host–solvent g(r) in Fig. 9(a). The inclusion of CH4 into the Noria-OEt cage was more favourable in the liquid phase, i.e., when the solvent was present, than in vacuum, suggesting that 15-crown-5 pushes the gas into the cages. This is further confirmed by Fig. S44,† which shows the free energy profile for inserting a single CH4 molecule inside a 15-crown-5 liquid slab, starting from vacuum. The resulting solvation free energy of CH4 in the pure solvent at 350 K was 1.17 kcal mol−1.
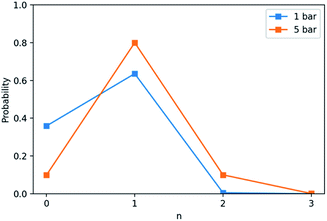
Further MD simulations were conducted on two PLs loaded with CH4 and run at 350 K and either 1 or 5bar. Each system comprised 10 Noria-OEt hosts dissolved in 2060 15-crown-5 molecules, and the amount of CH4 was set to correspond approximately to the experimental solubilities at each pressure: 7 gas molecules for the system at 1 bar pressure, and 36 molecules for the liquid at 5 bar. During the simulations, CH4 remained predominantly inside the Noria-OEt intrinsic cavities. This is illustrated by the occupation probabilities, p(n), shown in Fig. 16, where n represents the number of CH4 molecules within 0.61 nm of the centre of mass of a host.
At 1 bar, hosts were 64% likely to be occupied, slightly below the maximum of 70% set by the 0.7 CH4/Noria-OEt ratio. At 5 bar, occupation was 90% likely. Interestingly, at this higher pressure there was also 10% probability of double occupation, i.e., of two CH4 molecules being included in a single Noria-OEt cavity. The affinity of CH4 to the Noria-OEt hosts is also reflected in large occupancy times, defined as the average span of time that a Noria-OEt hosts at least one CH4 molecule without intermission. These values were 32.1 and 32.2 ns for the simulations at 1 and 5 bar, respectively.
Conclusions
In conclusion we have shown that Noria derivatives can be used for the synthesis of Type II PLs. As part of this work, we have substantially elucidated the composition of bulk (now known to be crude) Noria samples and revised the understanding in the literature. In particular rather than being a pure material, bulk samples of Noria prepared by the standard method consist of ca. 40% Noria, 30% R3 and 30% other oligomers. The partially ethylated derivative Noria-OEt dissolves readily in the size-excluded solvent 15-crown-5 to give a new Type II PL as supported by MD simulations and CH4 solubility measurements. Such phases have the attraction of being inherently more chemically and thermally robust than Type II PLs reported to date and can also be synthesised economically. As such this represents a step toward Type II PLs which are more practical and may ultimately find applications. It also opens a new line of investigation for Type II PLs, specifically, other structures related to those here (e.g., other oligomers, alternative peripheral functionalisation etc.) could be developed to provide a new family of Type II PLs which may accelerate the development of the field.Data availability
Experimental, computational, and crystallographic data are provided in the ESI.†Author contributions
FMA conducted the synthetic and analytical work. Gas solubility measurements were done by FMA. DEC contributed substantially to the technical analysis and interpretation. SLJ supervised the project. BPH contributed to ideation. MGdP, SFF and JLB conducted the MD simulation. PNH and SJC conducted the single crystal structure determination. The paper was drafted jointly by FMA, SLJ, MGdP, SFF and JLB. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the National Mass Spectrometry Facility (NMSF) at Swansea University and in particular the useful discussions had with Dr Ann P. Hunter and Dr Mark F. Wyatt. MGDP acknowledges financial support from SECTyP-UNCUYO, ANPCyT (PICT-2015-1835), and SNCAD for computer time in the clusters Mendieta (CCAD-UNC) and Toko (UNCUYO). We also acknowledge funding from EPSRC, grant number: EP/R005540/1.Notes and references
- P. A. Wright, Chapter 1 Introduction, in Microporous Framework Solids, The Royal Society of Chemistry, 2008, pp. 1–7 Search PubMed.
- F. Romm, Microporous Media, Marcel Dekker, New York, 2004 Search PubMed.
- A. Pohorille and L. R. Pratt, J. Am. Chem. Soc., 1990, 112, 5066–5074 CrossRef CAS PubMed.
- G. Graziano, Biophys. Chem., 2003, 104, 393–405 CrossRef CAS PubMed.
- S. Sastry, T. M. Truskett, P. G. Debenedetti, S. Torquato and F. H. Stillinger, Mol. Phys., 1998, 95, 289–297 CrossRef CAS.
- (a) N. O'Reilly, N. Giri and S. L. James, Chem.–Eur. J., 2007, 13, 3020–3025 CrossRef PubMed; (b) S. L. James and B. Hutchings, in Funct. Org. Liq., ed. T. Nakanishi, Wiley-VCH, 2019, pp. 39–52 Search PubMed.
- N. Giri, M. G. Del Pópolo, G. Melaugh, R. L. Greenaway, K. Rätzke, T. Koschine, L. Pison, M. F. C. Gomes, A. I. Cooper and S. L. James, Nature, 2015, 527, 216–220 CrossRef CAS PubMed.
- K. Jie, N. Onishi, J. A. Schott, I. Popovs, D. Jiang, S. Mahurin and S. Dai, Angew. Chem., Int. Ed., 2020, 59, 2268–2272 CrossRef CAS PubMed.
- L. Ma, C. J. E. Haynes, A. B. Grommet, A. Walczak, C. C. Parkins, C. M. Doherty, L. Longley, A. Tron, A. R. Stefankiewicz, T. D. Bennett and J. R. Nitschke, Nat. Chem., 2020, 12, 270–275 CrossRef CAS PubMed.
- Z. Deng, W. Ying, K. Gong, Y.-J. Zeng, Y. Yan and X. Peng, Small, 2020, 16, 1907016 CrossRef CAS PubMed.
- M. Gomes, L. Pison, C. Červinka and A. Padua, Angew. Chem., Int. Ed., 2018, 57, 11909–11912 CrossRef PubMed.
- W. Shan, P. F. Fulvio, L. Kong, J. A. Schott, C. L. Do-Thanh, T. Tian, X. Hu, S. M. Mahurin, H. Xing and S. Dai, ACS Appl. Mater. Interfaces, 2018, 10, 32–36 CrossRef CAS PubMed.
- S. He, L. Chen, J. Cui, B. Yuan, H. Wang, F. Wang, Y. Yu, Y. Lee and T. Li, J. Am. Chem. Soc., 2019, 141, 19708–19714 CrossRef CAS PubMed.
- B. Lai, J. Cahir, M. Y. Tsang, J. Jacquemin, D. Rooney, B. Murrer and S. L. James, ACS Appl. Mater. Interfaces, 2021, 13, 932–936 CrossRef CAS PubMed.
- C. D. Gutsche, B. Dhawan, K. H. No and R. Muthukrishnan, J. Am. Chem. Soc., 1981, 103, 3782–3792 CrossRef CAS.
- S. Lim, H. Kim, N. Selvapalam, K. J. Kim, S. J. Cho, G. Seo and K. Kim, Angew. Chem., Int. Ed., 2008, 47, 3352–3355 CrossRef CAS PubMed.
- K. Jie, M. Liu, Y. Zhou, M. A. Little, A. Pulido, S. Y. Chong, A. Stephenson, A. R. Hughes, F. Sakakibara, T. Ogoshi, F. Blanc, G. M. Day, F. Huang and A. I. Cooper, J. Am. Chem. Soc., 2018, 140, 6921–6930 CrossRef CAS PubMed.
- (a) H. Kudo, R. Hayashi, K. Mitani, T. Yokozawa, N. C. Kasuga and T. Nishikubo, Angew. Chem., Int. Ed., 2006, 45, 7948–7952 CrossRef CAS PubMed; (b) D. Shimoyama, R. Sekiya, H. Kudo and T. Haino, Org. Lett., 2020, 22, 352–356 CrossRef CAS PubMed; (c) T. Nishikubo and H. Kudo, Japan PatentJP2008-280269A, 2008 Search PubMed; (d) H. Yamada, T. Ikeda, T. Mizuta and T. Haino, Org. Lett., 2012, 14, 4510–4513 CrossRef CAS PubMed; (e) D. Shimoyama, T. Ikeda, R. Sekiya and T. Haino, J. Org. Chem., 2017, 82, 13220–13230 CrossRef CAS PubMed.
- (a) N. Niina, H. Kudo and T. Nishikubo, Chem. Lett., 2009, 38, 1198–1199 CrossRef CAS; (b) N. Niina, H. Kudo, K. Maruyama, T. Kai, T. Shimokawa, H. Oizumi, T. Itani and T. Nishikubo, Polym. J., 2011, 43, 407–413 CrossRef CAS.
- H. Kudo, Y. Suyama, H. Oizumi, T. Itani and T. Nishikubo, J. Mater. Chem., 2010, 20, 4445–4450 RSC.
- S. R. Peerannawar and S. P. Gejji, Comput. Theor. Chem., 2013, 1015, 44–51 CrossRef CAS.
- Z. Zhai, C. Jiang, N. Zhao, W. Dong, H. Lan, M. Wang and Q. J. Niu, J. Mater. Chem. A, 2018, 6, 21207–21215 RSC.
- H. Kudo, D. Watanabe, T. Nishikubo, K. Maruyama, D. Shimizu, T. Kai, T. Shimokawa and C. K. Ober, J. Mater. Chem., 2008, 18, 3588–3592 RSC.
- T. Nishikubo, H. Kudo, Y. Suyama, H. Oizumi and T. Itani, J. Photopolym. Sci. Technol., 2009, 22, 73–76 CrossRef CAS.
- J. Tian, P. K. Thallapally, S. J. Dalgarno, P. B. McGrail and J. L. Atwood, Angew. Chem., Int. Ed., 2009, 48, 5492–5495 CrossRef CAS PubMed.
- R. S. Patil, D. Banerjee, C. M. Simon, J. L. Atwood and P. K. Thallapally, Chem.–Eur. J., 2016, 22, 12618–12623 CrossRef CAS PubMed.
- D. Shimoyama, R. Sekiya, H. Maekawa, H. Kudo and T. Haino, CrystEngComm, 2020, 22, 4740–4747 RSC . Note that the NMR spectrum reported in the ESI Fig. S1† may have inadvertently been incorrectly referenced and we suggest the correct chemical shifts are 1.12 ppm less than those reported..
- A. Pohorille and L. R. Pratt, J. Am. Chem. Soc., 1990, 112, 5066–5074 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2080523 and 2080524. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc03367k |
| This journal is © The Royal Society of Chemistry 2021 |