Open Access Article
Open Access ArticleTuning the porosity of triangular supramolecular adsorbents for superior haloalkane isomer separations†
Bin
Hua
a,
Yanjun
Ding
a,
Lukman O.
Alimi
a,
Basem
Moosa
a,
Gengwu
Zhang
a,
Walaa S.
Baslyman
a,
Jonathan
Sessler
 b and
Niveen M.
Khashab
b and
Niveen M.
Khashab
 *a
*a
aSmart Hybrid Materials Laboratory (SHMs), Advanced Membranes and Porous Materials Center, King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia. E-mail: niveen.khashab@kaust.edu.sa
bDepartment of Chemistry, The University of Texas at Austin, Austin, TX 78712-1224, USA
First published on 16th August 2021
Abstract
Distillation-free separations of haloalkane isomers represents a persistent challenge for the chemical industry. Several classic molecular sorbents show high selectivity in the context of such separations; however, most suffer from limited tunability or poor stability. Herein, we report the results of a comparative study involving three trianglamine and trianglimine macrocycles as supramolecular adsorbents for the selective separation of halobutane isomers. Methylene-bridged trianglamine, TA, was found to capture preferentially 1-chlorobutane (1-CBU) from a mixture of 1-CBU and 2-chlorobutane (2-CBU) with a purity of 98.1%. It also separates 1-bromobutane (1-BBU) from a mixture of 1-BBU and 2-bromobutane (2-BBU) with a purity of 96.4%. The observed selectivity is ascribed to the thermodynamic stability of the TA-based host–guest complexes. Based on single crystal X-ray diffraction analyses, a [3]pseudorotaxane structure (2TA⊃1-CBU) is formed between TA and 1-CBU that is characterized by an increased level of noncovalent interactions compared to the corresponding [2]pseudorotaxane structure seen for TA⊃2-CBU. We believe that molecular sorbents that rely on specific molecular recognition events, such as the triangular pores detailed here, will prove useful as next generation sorbents in energy-efficient separations.
Introduction
Haloalkanes are commercially important raw materials for the production of PVC, industrial lubricants, pesticides, insecticides, herbicides and many others.1 They are generally produced through the direct halogenation of alkanes to produce a mixture of isomers, which are consequently separated by energy-intensive extractive and azeotropic distillations.2–5 With an expected global market of 75.5 billion dollars by 2027, this segment of the chemical industry would benefit from economically advantageous access to 1-halo butanes such as 1-chlorobutane (1-CBU) and 1-bromobutane (1-BBU).6,7 However, the efficient separation of these products from a mixture of isomers produced upon initial halogenation still constitutes a major obstacle towards their production under distillation-free, energy-efficient conditions.High-performance molecular adsorbents for energy-saving isomer separations, including zeolites and metal–organic frameworks (MOFs), have been actively developed over the past few decades.8,9 While precise control over the pore structure and hence separation selectivity has proved challenging in the case of classic zeolites, a lack of long term stability has emerged as the main factor limiting translation of many MOF-based platforms into the area of petrochemical separations.10,11 On the other hand, considerable current effort is being focused on the development of new molecular and supramolecular adsorbents endowed with tunable structures and good stabilities; many show promise as next generation so-called smart separation materials.12–17
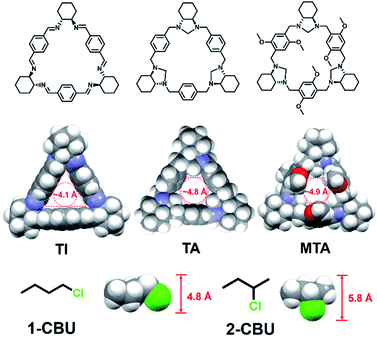
Molecular sorbents possessing triangular channels often display excellent performance as reflected in, e.g., hexane isomer and olefin/paraffin separations.18–20 Trianglamine and trianglimine macrocycles, in particular, have emerged as selective hosts for various benzene derivatives.21,22 Receptors of this generalized class are readily prepared and easy to scale up, and possess cavities whose size can be tuned through design.22–25 As detailed below, we have now investigated three different trianglamine/trianglimine macrocycles and tested their ability to separate halobutane isomers (Fig. 1). The methylene-bridged trianglamine (TA) was found to be a particularly good supramolecular adsorbent and one that allowed the facile separation of 1-chlorobutane (1-CBU), 1-bromobutane (1-BBU) and 1-chloropentane (1-CP) from mixtures of their corresponding isomers with purities of 98.1%, 96.4% and 97.1%, respectively. To the best of our knowledge, this is the first study that illustrates the importance of optimizing the pores of supramolecular adsorbents for various haloalkane separations and the origin of selectivity through the formation of pseudorotaxane assemblies.
Results and discussion
Three molecular triangle-shaped hosts with different cavity sizes were first selected as potential sorbents of chlorobutane isomers (Fig. 1). They were readily prepared via a simple condensation reaction as previously reported (Fig. S1–S4†).26 Trianglimine (TI) has a rigid triangular cavity with a diameter of ∼4.1 Å. The use of a different bridge between the aromatic subunits allowed access to a more hexagonal host, methylene-bridged trianglamine (TA), with an extended cavity whose effective size was increased to ∼4.8 Å without the loss of structural rigidity. The corresponding dimethoxylated trianglamine (MTA), containing deeper cavities compared to TA, was also prepared (Fig. S5–S7†).Guest-free TI, TA and MTA were converted to materials expected to be activated adsorptive separation materials via recrystallization and desolvation (Fig. S8–S10†).27–30 These activated TI, TA and MTA crystalline materials were characterized by powder X-ray diffraction (PXRD) analyses that served to confirm their crystallinity (Fig. S11†).31–34 Thermogravimetric analysis (TGA) of the activated TI, TA and MTA revealed no appreciable weight loss below 280 °C, thus providing support for the conclusion that these activated materials were fully desolvated (Fig. S12–S14†). N2 adsorption experiments gave BET surface areas of 1.67 m2 g−1, 3.65 m2 g−1, and 1.74 m2 g−1 for the activated TI, TA and MTA, respectively (Fig. S15–S17†). Although these values are low, previous reports on triangular macrocycles with little apparent porosity have served to reveal interesting molecular recognition features.21,22,33
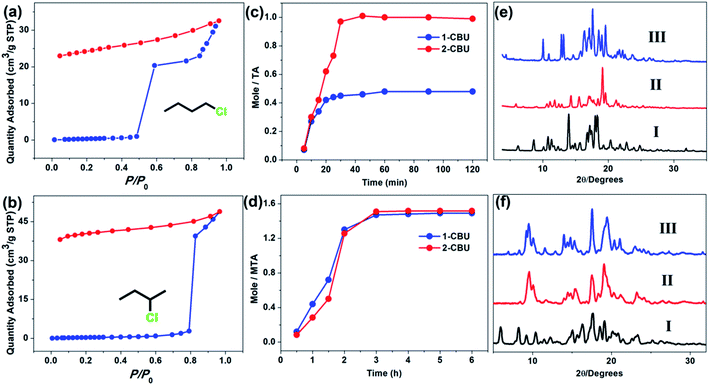
The adsorption performance of the activated TI, TA and MTA in the presence of chlorobutane isomers was tested through solid–vapor adsorption experiments. Activated TI did not prove useful as an adsorbent for either 1-CBU or 2-CBU (Fig. S18†). In contrast, the vapor sorption isotherm for activated TA revealed that the adsorption of 1-CBU and 2-CBU was subject to pronounced gate-opening behavior at P/P0 = 0.5 (for 1-CBU) and P/P0 = 0.8 (for 2-CBU) (Fig. 2a and b). Analysis of the desorption process revealed that the adsorbed 1-CBU and 2-CBU molecules were not released, even under reduced pressure. This result was interpreted in terms of the chlorobutane isomers being stably located in the cavity of TA through host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest interactions. We further investigated the binding using 1H NMR spectroscopic analysis. On this basis, we conclude that TA accommodates 1-CBU and 2-CBU differently. In the case of 1-CBU, two TA molecules bind a single 1-CBU molecule while 2-CBU binds TA with a 1
guest interactions. We further investigated the binding using 1H NMR spectroscopic analysis. On this basis, we conclude that TA accommodates 1-CBU and 2-CBU differently. In the case of 1-CBU, two TA molecules bind a single 1-CBU molecule while 2-CBU binds TA with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
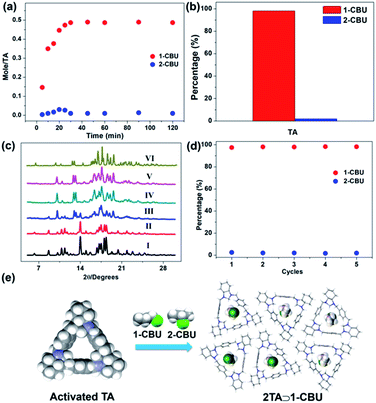
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (Fig. S19 and S20†). The chlorobutane isomers were readily taken up by TA with ca. 30 minutes being required to reach the point of saturation (Fig. 2c). The larger receptor MTA adsorbs about 1.5 equiv. of 1-CBU or 2-CBU (Fig. S21 and S22†). Time-dependent solid–vapor studies with MTA and 1-CBU and 2-CBU revealed that close to 2 hours were required to reach saturation (Fig. 2d). We rationalize this relatively slow rate (compared to TA) in terms of the increased steric bulk of the methoxy groups of MTA serving to obstruct the entry of guests into the receptor cavity.
1 stoichiometry (Fig. S19 and S20†). The chlorobutane isomers were readily taken up by TA with ca. 30 minutes being required to reach the point of saturation (Fig. 2c). The larger receptor MTA adsorbs about 1.5 equiv. of 1-CBU or 2-CBU (Fig. S21 and S22†). Time-dependent solid–vapor studies with MTA and 1-CBU and 2-CBU revealed that close to 2 hours were required to reach saturation (Fig. 2d). We rationalize this relatively slow rate (compared to TA) in terms of the increased steric bulk of the methoxy groups of MTA serving to obstruct the entry of guests into the receptor cavity.
After confirming the adsorption of chlorobutane isomers by activated TA and MTA, the adsorption mechanism was further explored.34 First PXRD was used to study the structural changes of the macrocycles before and after adsorption of 1-CBU and 2-CBU. As shown in Fig. 2e and f, the guest uptake induced a crystal to crystal transformation in these macrocycles. The PXRD pattern of activated TA drastically changed after adsorption of 2-CBU and 1-CBU (Fig. 2e, II and III). In contrast, in the case of activated MTA, almost no difference between the PXRD patterns of activated MTA after adsorption of 2-CBU or 1-CBU was seen (Fig. 2f, II and III), a finding interpreted in terms of the structures of this receptor remaining relatively unchanged upon the uptake of these two guests.
Single crystal X-ray diffraction (SCXRD) data confirmed that TA and 1-CBU yielded a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
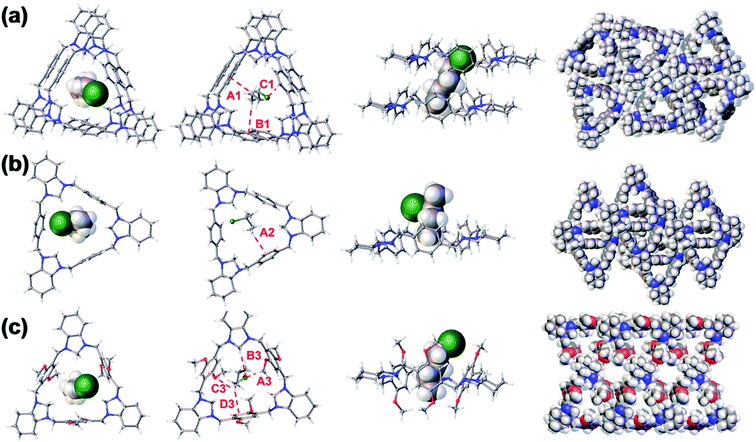
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex and that the cavity of TA matched well with 1-CBU (Fig. 3a). One 1-CBU molecule was located in the cavity formed from two TA macrocycles to produce a threaded [3]pseudorotaxane structure 2TA⊃1-CBU. Two C–H⋯π interactions and one C–H⋯Cl hydrogen bond were inferred from the metric parameters, which presumably contribute to the formation of the host–guest complex (Fig. S23†). The triangular macrocycles assemble in a 1D channel packing mode, with molecules 1-CBU aligned within the channels. Notably, the PXRD spectrum of activated TA after the uptake of 1-CBU was similar to the simulated pattern from the SCXRD data for 2TA⊃1-CBU (Fig. S24†).
1 host–guest complex and that the cavity of TA matched well with 1-CBU (Fig. 3a). One 1-CBU molecule was located in the cavity formed from two TA macrocycles to produce a threaded [3]pseudorotaxane structure 2TA⊃1-CBU. Two C–H⋯π interactions and one C–H⋯Cl hydrogen bond were inferred from the metric parameters, which presumably contribute to the formation of the host–guest complex (Fig. S23†). The triangular macrocycles assemble in a 1D channel packing mode, with molecules 1-CBU aligned within the channels. Notably, the PXRD spectrum of activated TA after the uptake of 1-CBU was similar to the simulated pattern from the SCXRD data for 2TA⊃1-CBU (Fig. S24†).
In contrast, TA and 2-CBU were seen to form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex in the solid state on the basis of an SCXRD analysis. In this case a [2]pseudorotaxane structure TA⊃2-CBU was observed (Fig. 3b). Presumably reflecting the relatively larger size of 2-CBU as compared to 1-CBU, only the methyl group on 2-CBU was seen to thread into the center of the cavity with binding driven by a single presumed C–H⋯π interaction (Fig. S25†). The complex TA⊃2-CBU does not produce a 1D channel-like structure, perhaps reflecting the fact that the adjacent TA macrocycles are staggered relative to one another. As in the case of 2TA⊃1-CBU, the PXRD pattern of activated TA after adsorption of 2-CBU (i.e., TA⊃2-CBU) proved congruent with the simulated pattern derived from the single crystal data (Fig. S26†).
1 host–guest complex in the solid state on the basis of an SCXRD analysis. In this case a [2]pseudorotaxane structure TA⊃2-CBU was observed (Fig. 3b). Presumably reflecting the relatively larger size of 2-CBU as compared to 1-CBU, only the methyl group on 2-CBU was seen to thread into the center of the cavity with binding driven by a single presumed C–H⋯π interaction (Fig. S25†). The complex TA⊃2-CBU does not produce a 1D channel-like structure, perhaps reflecting the fact that the adjacent TA macrocycles are staggered relative to one another. As in the case of 2TA⊃1-CBU, the PXRD pattern of activated TA after adsorption of 2-CBU (i.e., TA⊃2-CBU) proved congruent with the simulated pattern derived from the single crystal data (Fig. S26†).
The SCXRD structure of MTA@1-CBU was also determined. It was found that the bound 1-CBU molecules are located in the endo-cavity of MTA and in the extrinsic 1D channels (Fig. 3c and S27†). The molar ratio of MTA to 1-CBU was calculated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, which matched the results of solid–vapor adsorption experiments (Fig. S21†). The PXRD pattern of activated MTA after adsorption of 1-CBU was the same as the simulated pattern from the single crystal structural data (Fig. S28†). The SCXRD analysis of MTA@2-CBU revealed a structure very similar to that of MTA@1-CBU. The 2-CBU molecules were found in both the intrinsic and extrinsic 1D channels. Unfortunately, the SCXRD data could not be refined due to the disorder seen for the 2-CBU molecules bound in the extrinsic channels (Fig. S29 and S30†). Nevertheless, these data and the corresponding PXRD analyses confirmed that crystallinity is retained as activated MTA adsorbs 2-CBU vapor (Fig. S31†).
1.5, which matched the results of solid–vapor adsorption experiments (Fig. S21†). The PXRD pattern of activated MTA after adsorption of 1-CBU was the same as the simulated pattern from the single crystal structural data (Fig. S28†). The SCXRD analysis of MTA@2-CBU revealed a structure very similar to that of MTA@1-CBU. The 2-CBU molecules were found in both the intrinsic and extrinsic 1D channels. Unfortunately, the SCXRD data could not be refined due to the disorder seen for the 2-CBU molecules bound in the extrinsic channels (Fig. S29 and S30†). Nevertheless, these data and the corresponding PXRD analyses confirmed that crystallinity is retained as activated MTA adsorbs 2-CBU vapor (Fig. S31†).
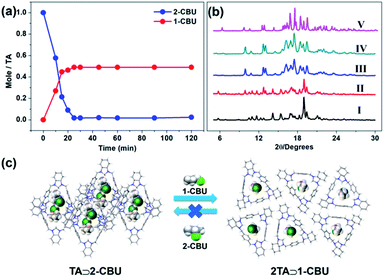
We next probed whether the crystalline forms of activated TA and MTA displayed selectivity towards a specific chlorobutane isomer. It was found that TA⊃2-CBU is transformed into 2TA⊃1-CBU upon exposure to 1-CBU vapor (Fig. 4a). At the saturation point, the content of 2-CBU in the solid was almost negligible (Fig. 4a). Time-dependent PXRD analyses proved to be consistent with the crystal-to-crystal transformation of TA⊃2-CBU into 2TA⊃1-CBU (Fig. 4b) and that the process was essentially irreversible (Fig. S32† and 4c). On this basis, we conclude that 2TA⊃1-CBU is thermodynamically more stable than TA⊃2-CBU. We believe that the relatively high number of noncovalent interactions in the [3]pseudorotaxane structure of 2TA⊃1-CBU serves to drive the equilibrium towards this dominant species.
Inspired by these initial experiments, we investigated the possibility of employing activated TA and activated MTA to separate 1-CBU from an isomeric mixture of 1-CBU and 2-CBU (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Based on NMR spectroscopic analyses associated with the solid–vapor adsorption experiments, it was found that activated MTA failed to display selectivity toward either chlorobutane isomer (Fig. S33 and S34†). Gas chromatography (GC) studies revealed that after exposure to a 1
1). Based on NMR spectroscopic analyses associated with the solid–vapor adsorption experiments, it was found that activated MTA failed to display selectivity toward either chlorobutane isomer (Fig. S33 and S34†). Gas chromatography (GC) studies revealed that after exposure to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 isomeric mixture of chlorobutane, the ratio of 1-CBU to 2-CBU in MTA was 51.5
1 isomeric mixture of chlorobutane, the ratio of 1-CBU to 2-CBU in MTA was 51.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48.5, (Fig. S35†). In marked contrast, TA can clearly discriminate 1-CBU from 2-CBU with high selectivity (Fig. S36†).
48.5, (Fig. S35†). In marked contrast, TA can clearly discriminate 1-CBU from 2-CBU with high selectivity (Fig. S36†).
We further performed time-dependent solid–vapor adsorption studies of activated TA and an isomeric mixture consisting of 1-CBU and 2-CBU. These studies revealed that the uptake of 1-CBU was essentially complete within 30 min while the amount of 2-CBU absorbed over this time proved negligible (Fig. 5a). GC analysis revealed that the percentage of 1-CBU in TA was 98.1% (Fig. 5b and S37†). Time-dependent PXRD studies revealed that after exposure to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1-CBU and 2-CBU, the final PXRD pattern was similar to that of 2TA⊃1-CBU (Fig. 5c). More importantly, activated TA could be recycled at least five times without losing its adsorption capacity and selectivity (Fig. 5d); it also proved to be stable in aqueous media (Fig. S38†).
1 mixture of 1-CBU and 2-CBU, the final PXRD pattern was similar to that of 2TA⊃1-CBU (Fig. 5c). More importantly, activated TA could be recycled at least five times without losing its adsorption capacity and selectivity (Fig. 5d); it also proved to be stable in aqueous media (Fig. S38†).
We then tested the selectivity of activated TA towards other halobutanes such as bromobutanes (BBU). Notably, activated TA can discriminate 1-bromobutane (1-BBU) from 2-bromobutane (2-BBU) as confirmed by 1H NMR and PXRD data (Fig. S39–S41†). GC results showed that the activated TA can selectively capture 1-BBU from a mixture of 1-BBU and 2-BBU with a purity of 96.4%; excellent reusability was seen again (Fig. S42 and S43†). We also investigated the ability of TA to host other haloalkanes such as 1-chloropentane (1-CP). SCXRD data showed that when exposed to 1-CP, TA forms 1D channels reminiscent of 2TA⊃1-CBU (Fig. S44†). Moreover, we further found that activated TA can also be used to separate 1-chloropentane from a mixture of 1-chloropentane and 2-chloropentane with a purity of 97.1% (Fig. S45–S47†).
Conclusion
In conclusion, we investigated the adsorption performance of three trianglamine/trianglimine macrocycles TI, TA, and MTA upon exposure to chlorobutane isomers (CBU). It was found that TI, the system with the smallest cavity size within the series, was ineffective as an absorbent for either chlorobutane isomer (i.e., 1-CBU and 2-CBU). In contrast, both TA and MTA, possessing relatively larger cavity sizes, proved to be effective adsorbents for both 1-CBU and 2-CBU. In both cases, saturation was reached in about 30 min, which is fast compared to literature reports of other molecular sorbents that can require up to 5 h to reach saturation.7 Of particular note is the finding that TA could be used to separate 1-CBU from a mixture of 1-CBU and 2-CBU with a purity of 98.1%. The selectivity is rationalized on thermodynamic grounds. Based on SCXRD analyses, a [3]pseudorotaxane structure, 2TA⊃1-CBU, is formed in the case of 1-CBU that is characterized by a greater number of noncovalent host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest interactions compared to the [2]pseudorotaxane structure seen in the case of TA⊃2-CBU. Further studies revealed that TA can also separate 1-bromobutane (1-BBU) and 1-chloropentane from mixtures of their corresponding isomers with purities of 96.4% and 97.1%, respectively. Both TA and MTA proved to be stable under conditions of use and could be recycled and reused. The fact that these systems display excellent selectivity, and are easy to prepare and subject to preparative scale up leads us to predict that sorbents with triangular channels may see use in practical applications, particularly in energy-efficient small molecule separations.
guest interactions compared to the [2]pseudorotaxane structure seen in the case of TA⊃2-CBU. Further studies revealed that TA can also separate 1-bromobutane (1-BBU) and 1-chloropentane from mixtures of their corresponding isomers with purities of 96.4% and 97.1%, respectively. Both TA and MTA proved to be stable under conditions of use and could be recycled and reused. The fact that these systems display excellent selectivity, and are easy to prepare and subject to preparative scale up leads us to predict that sorbents with triangular channels may see use in practical applications, particularly in energy-efficient small molecule separations.
Author contributions
N. M. K. concieved the concept and supervised the project. H. B. developed the concept and performed the majority of the experiments. All authors contributed to the analysis and writing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Office of Sponsored Research (CRG4) at the King Abdullah University of Science and Technology (KAUST), Saudi Arabia.References
- M. Rossberg, W. Lendle, G. Pfleiderer, A. Togel, E.-L. Dreher, E. Langer, H. Rassaerts, P. Kleinschmidt, H. Strack, R. Cook, U. Beck, K.-A. Lipper, T. R. Torkelson, E. Loser, K. K. Beutel and T. Mann, Chlorinated Hydrocarbons in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000 Search PubMed.
- J. E. Copenhaver and A. M. Whaley, Org. Synth., 1925, 5, 27 CrossRef.
- L. Vasaros, H. J. Machulla and W. Tornau, J. Chromatogr. A, 1971, 62, 458–461 CrossRef CAS.
- L. H. Brandsma and D. Verkruijsse, Preparative Polar Organometellic Chemistry I, Springer-Verlag, Berlin, 1987, ISBN 3-540-16916-4 Search PubMed.
- P. Comba and S. Wunderlich, Chem.–Eur. J., 2010, 16, 7293–7299 CrossRef CAS PubMed.
- J.-R. Wu, B. Li and Y.-W. Yang, Angew. Chem., Int. Ed., 2020, 59, 2251–2255 CrossRef CAS PubMed.
- Y. Zhou, K. Jie, R. Zhao, E. Li and F. Huang, J. Am. Chem. Soc., 2020, 142, 6957–6961 CrossRef CAS PubMed.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. Yaghi, Nature, 1999, 402, 276 CrossRef CAS.
- A. Cadiau, K. Adil, P. M. Bhatt, Y. Belmabkhout and M. Eddaoudi, Science, 2016, 353, 137–140 CrossRef CAS PubMed.
- S. M. Kuznicki, V. A. Bell, S. Mair, H. W. Hillhouse, R. M. Jacubinas, C. M. Braunbarth, B. H. Toby and M. Tsapatsis, Nature, 2001, 412, 720–724 CrossRef CAS PubMed.
- R. E. Morris and L. Brammer, Chem. Soc. Rev., 2017, 46, 5444–5462 RSC.
- M. A. Little and A. I. Cooper, Adv. Funct. Mater., 2020, 30, 1909842 CrossRef CAS.
- G. Zhang, A. H. Emwas, U. F. S. Hameed, S. T. Arold, P. Yang, A. Chen, J. F. Xiang and N. M. Khashab, Chem, 2020, 6, 1082–1096 CAS.
- W. Yang, K. Samanta, X. Wan, T. U. Thikekar, Y. Chao, S. Li, K. Du, J. Xu, Y. Gao, H. Zuilhof and A. C.-H. Sue, Angew. Chem., Int. Ed., 2019, 59, 3994–3999 CrossRef PubMed.
- L. Chen, Y. Cai, W. Feng and L. Yuan, Chem. Commun., 2019, 55, 7883–7898 RSC.
- G. Zhu, F. Zhang, M. P. Rivera, X. Hu, G. Zhang, C. W. Jones and R. P. Lively, Angew. Chem., Int. Ed., 2019, 58, 2638–2643 CrossRef CAS PubMed.
- S. Xie, S. Wu, S. Bao, Y. Wang, Y. Zheng, D. Deng, L. Huang, L. Zhang, M. Lee and Z. Huang, Adv. Mater., 2018, 30, 1800683 CrossRef PubMed.
- Z. R. Herm, B. M. Wiers, J. A. Mason, J. M. v. Baten, M. R. Hudson, P. Zajdel, C. M. Brown, N. Masciocchi, R. Krishna and J. R. Long, Science, 2013, 340, 960–964 CrossRef CAS PubMed.
- Z. Bao, J. Wang, Z. Zhang, H. Xing, Q. Yang, Y. Yang, H. Wu, R. Krishna, W. Zhou, B. Chen and Q. Ren, Angew. Chem., Int. Ed., 2018, 57, 16020–16025 CrossRef CAS PubMed.
- P. Wang, X. Chen, Q. Jiang, M. Addicoat, N. Huang, S. Dalapati, T. Heine, F. Huo and D. Jiang, Angew. Chem., Int. Ed., 2019, 58, 15922–15927 CrossRef CAS PubMed.
- A. Dey, S. Chand, L. O. Alimi, M. Ghosh, L. Cavallo and N. M. Khashab, J. Am. Chem. Soc., 2020, 142, 15823–15829 CrossRef CAS PubMed.
- A. Dey, S. Chand, B. Maity, P. M. Bhatt, M. Ghosh, L. Cavallo, M. Eddaoudi and N. M. Khashab, J. Am. Chem. Soc., 2021, 143, 4090–4094 CrossRef CAS PubMed.
- J. Gawronski, H. Kolbon, M. Kwit and A. Katrusiak, J. Org. Chem., 2000, 65, 5768–5773 CrossRef CAS PubMed.
- A. Chaix, G. Mouchaham, A. Shkurenko, P. Hoang, B. Moosa, P. M. Bhatt, K. Adil, K. N. Salama, M. Eddaoudi and N. M. Khashab, J. Am. Chem. Soc., 2018, 140, 14571–14575 CrossRef CAS PubMed.
- Y. Ding, L. O. Alimi, B. Moosa, C. Maaliki, J. Jacquemin, F. Huang and N. M. Khashab, Chem. Sci., 2021, 12, 5315–5318 RSC.
- J. Gawronski, K. Gawronska, J. Grajewski, M. Kwit, A. Plutecka and U. Rychlewska, Chem.–Eur. J., 2006, 12, 1807–1817 CrossRef CAS PubMed.
- R. S. Patil, D. Banerjee, C. Zhang, P. K. Thallapally and J. L. Atwood, Angew. Chem., Int. Ed., 2016, 55, 4523–4526 CrossRef CAS PubMed.
- T. Ogoshi, K. Saito, R. Sueto, R. Kojima, Y. Hamada, S. Akine, A. M. P. Moeljadi, H. Hirao, T. Kakuta and T.-a. Yamagishi, Angew. Chem., Int. Ed., 2018, 57, 1592–1595 CrossRef CAS PubMed.
- J.-R. Wu and Y.-W. Yang, Angew. Chem., Int. Ed., 2021, 60, 1690–1701 CrossRef CAS PubMed.
- L. J. Barbour, Chem. Commun., 2006, 11, 1163–1168 RSC.
- L. R. MacGillivray and J. L. Atwood, Chem. Commun., 1999, 2, 181–182 RSC.
- K. Jie, Y. Zhou, E. Li and F. Huang, Acc. Chem. Res., 2018, 51, 2064–2072 CrossRef CAS PubMed.
- G. Zhang, B. Hua, A. Dey, M. Ghosh, B. A. Moosa and N. M. Khashab, Acc. Chem. Res., 2021, 54, 155–168 CrossRef CAS PubMed.
- B. Moosa, L. O. Alimi, A. Shkurenko, A. Fakim, P. M. Bhatt, G. Zhang, M. Eddaoudi and N. M. Khashab, Angew. Chem., Int. Ed., 2020, 59, 21367–21371 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic files (CIF), experimental details, NMR spectra and other materials (PDF). CCDC 2056799–2056802 and 2079899. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc03509f |
| This journal is © The Royal Society of Chemistry 2021 |