Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Interaction of anions with the surface of a coordination cage in aqueous solution probed by their effect on a cage-catalysed Kemp elimination†
Michael D.
Ludden
a,
Christopher G. P.
Taylor
 a,
Max B.
Tipping
a,
Jennifer S.
Train
b,
Nicholas H.
Williams
a,
Max B.
Tipping
a,
Jennifer S.
Train
b,
Nicholas H.
Williams
 b,
Jack C.
Dorrat
c,
Kellie L.
Tuck
b,
Jack C.
Dorrat
c,
Kellie L.
Tuck
 c and
Michael D.
Ward
c and
Michael D.
Ward
 *a
*a
aDepartment of Chemistry, University of Warwick, Coventry CV4 7AL, UK. E-mail: m.d.ward@warwick.ac.uk
bDepartment of Chemistry, University of Sheffield, Sheffield S3 7HF, UK
cSchool of Chemistry, Monash University, Melbourne, VIC3800, Australia
First published on 25th October 2021
Abstract
An octanuclear M8L12 coordination cage catalyses the Kemp elimination reaction of 5-nitro-1,2-benzisoxazole (NBI) with hydroxide to give 2-cyano-4-nitrophenolate (CNP) as the product. In contrast to the previously-reported very efficient catalysis of the Kemp elimination reaction of unsubstituted benzisoxazole, which involves the substrate binding inside the cage cavity, the catalysed reaction of NBI with hydroxide is slower and occurs at the external surface of the cage, even though NBI can bind inside the cage cavity. The rate of the catalysed reaction is sensitive to the presence of added anions, which bind to the 16+ cage surface, displacing the hydroxide ions from around the cage which are essential reaction partners in the Kemp elimination. Thus we can observe different binding affinities of anions to the surface of the cationic cage in aqueous solution by the extent to which they displace hydroxide and thereby inhibit the catalysed Kemp elimination and slow down the appearance of CNP. For anions with a −1 charge the observed affinity order for binding to the cage surface is consistent with their ease of desolvation and their ordering in the Hofmeister series. With anions that are significantly basic (fluoride, hydrogen carbonate, carboxylates) the accumulation of the anion around the cage surface accelerates the Kemp elimination compared to the background reaction with hydroxide, which we ascribe to the ability of these anions to participate directly in the Kemp elimination. This work provides valuable mechanistic insights into the role of the cage in co-locating the substrate and the anionic reaction partners in a cage-catalysed reaction.
Introduction
The ability of coordination cages to act as molecular containers, binding small-molecule guests inside their central cavity,1–4 is now very well established and has led to a wide range of functional behaviours including catalysis,2 sensing,3 and transport.4 As part of our work in this area using an octanuclear cubic M8L12 cage (Fig. 1),5 we have demonstrated that the accumulation of counter-ions around the exterior surface of a cationic cage is just as important a phenomenon as the binding of neutral guest molecules inside the cavity.6–10 This is a conclusion that has also been reached by others who have demonstrated how interactions of metal/ligand cage assemblies with counter-ions at the exterior surface can play fundamentally important roles in controlling structure and speciation behaviour of cage assemblies, as well as their ability to bind guests in the central cavity.11–13 Notably, Lusby and co-workers recently demonstrated how the positive charge associated with the surface of a cage host based on poorly-coordinating anions plays a crucial role in cage-catalysed reactions by stabilising anions, enhancing the acidity of reacting partners by several pKa units;12 and a while ago Raymond and co-workers demonstrated the same effect in the opposite sense, with a highly negatively charged cage strongly stabilising (by 4 pKa units) protonation of a bound guest, leading to the possibility of acid-based catalysis even under weakly basic conditions.13 Such charge-based effects are, clearly, equally as important as the more obvious host/guest effects based on cavity size and shape when considering the properties of cage-based systems.The two types of guest binding that we have observed with our cage system in water – cavity-based binding of neutral hydrophobic organic molecules, and the binding of anions in the surface portals on the faces of the cage – have different origins with the former being substantially driven by the hydrophobic effect, and the latter by an electrostatic ion-pairing effect.5 The result is that the host cage co-locates the two guest types, bringing neutral organic species and anions into close proximity, which is the basis of catalysis of a range of reactions.5–9 The best example of cage-based catalysis we have demonstrated is the Kemp elimination: reaction of cavity-bound benzisoxazole with the shell of closely-adjacent hydroxide ions that accumulated around the cage surface, effectively giving a high local pH even when the bulk pH was modest. This effect resulted in a rate acceleration of up to 2 × 105 fold compared to the uncatalysed reaction under the same conditions. The high catalytic turnover (>100 cycles with no loss of activity) arises because the reaction product, the 2-cyanophenolate anion, is sufficiently hydrophilic to exit the cavity and preferentially reside in the external aqueous phase, thereby ensuring no loss of activity due to the product blocking the cage cavity.6
We subsequently showed that the 2-cyanophenolate anion could itself accumulate around the cage surface and act as the base to deprotonate a cavity-bound benzisoxazole molecule in an autocatalytic cycle.7 It was apparent from this work and other control experiments that the tendency of anions to accumulate around the M8L12 cage surface (which has a 16+ charge) is not just driven by electrostatic factors but has a strong hydrophobicity component, with anions that are relatively hydrophobic and weakly hydrated having a higher affinity for the cage surface compared to more hydrophilic and strongly hydrated anions.6,7 We were able to exploit this recently in development of a fluorescence-based displacement assay to evaluate the ability of different anions to bind to the cage surface.10 Fluorescein dianions at pH ≈ 8 are relatively hydrophobic because of their large aromatic surface area and bind strongly to the M8L12 cage surface in aqueous solution, completely quenching the fluorescein's fluorescence. Titrations with different analyte ions displaced the fluorescein units from the cage surface, restoring their fluorescence, to varying extents according to the binding affinity of the analyte anion: using this method we could generate an affinity order of different anions for the M8L12 cage surface.10
In this paper we show how the differing binding abilities of different anions to the M8L12 cage surface can be used to modulate the catalysis of a Kemp elimination reaction using the substrate 5-nitro-1,2-benzisoxazole (NBI) (Scheme 1).14 This is a more tractable substrate to use for this cage-catalysed reaction than the original example (unsubstituted 1,2-benzisoxazole) as the reaction product 2-cyano-4-nitrophenolate (hereafter abbreviated CNP) has a strong absorbance at around 400 nm which can be conveniently monitored using UV/Vis spectroscopy rather than requiring 1H NMR spectroscopy, allowing large numbers of experiments to be run quickly and cheaply in parallel in a plate reader. The reaction itself, it should be emphasised, is not of major significance in terms of the importance of the product generated. However it provides a convenient way to monitor the effects of anion accumulation around the cage surface on cage-catalysed reactivity,2 and is equally relevant to control of catalysis in other ‘nanoreactors’ such as micelles and vesicles where catalysis can occur on the same basis – viz. by co-locating hydrophobic (in the cavity) and anionic (at the surface) reaction partners.14a,15
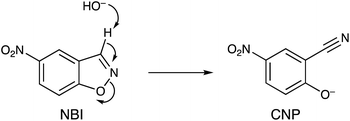 | ||
| Scheme 1 Kemp elimination reaction of 5-nitro-1,2-benzisoxazole (NBI) to generate 2-cyano-4-nitrophenolate (CNP). | ||
We found – somewhat to our surprise – that the cage-catalysed Kemp elimination of NBI occurs around the exterior surface of the cage rather than inside the cavity, as we have observed with some other substrates,8,9 despite the fact that NBI can occupy the cavity (as a crystal structure demonstrates). However, this does not hinder our ability to demonstrate how different anions affect the cage-based catalysis by displacing hydroxide ions (one of the reaction partners) from around the cage surface. This nicely illustrates the control that can be achieved in co-locating both neutral hydrophobic species and anions around the cage surface, a key component in developing further examples of cage-based catalysis.
Results and discussion
Guest binding and the structure of the cage/NBI assembly
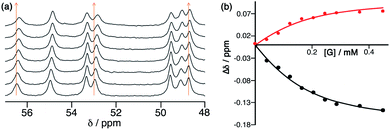
The Co8L12 octanuclear cubic cage system used in this work (denoted H or Hw, according to the absence or presence of water-solubilising hydroxymethyl groups on the exterior surface, and usually prepared as the tetrafluoroborate salt), which has formed the basis of several studies on cage-based catalysis,5–9 is shown in Fig. 1. The use of NBI for further studies of the cage-catalysed Kemp elimination reaction, as mentioned above, is pragmatically driven by the ease with which multiple datasets can be measured using a UV/Vis plate reader given the strong visible absorbance of the CNP product anion. Our first priority was to investigate its binding in the cavity of the Co8L12 cage both in solution and the solid state. A 1H NMR titration in which aliquots of NBI were added to a solution of [Co8L12]Cl16 (H·Cl16) in water7 showed a small but steady shift of several of the (paramagnetically-shifted) NMR signals, indicative of guest binding being in fast exchange on the NMR timescale; fitting the data to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm afforded a K value of 2 × 104 M−1 (Fig. 2). This is higher than we observed with unsubstituted benzisoxazole (4 × 103 M−1),6 though the counter-ion of the cage used in that experiment was different for solubility reasons which can have an effect on K values, so the two numbers are not directly comparable. In water the dominant contribution to guest binding is the hydrophobic effect,16 and it has been shown recently that nitro substituents – despite their locally dipolar nature – are not effectively hydrated and best described as ‘hydroneutral’.17 The increase in surface area of NBI compared to unsubstituted benzisoxazole will therefore increase the contact area with the hydrophobic interior cage surface and displace more water molecules from the cage cavity when it binds, so a higher binding constant for NBI binding compared to benzisoxazole can be rationalised.16c It is clear that NBI binds in fast exchange inside the cubic cage cavity in water, and that under the dilute solution conditions 1
1 binding isotherm afforded a K value of 2 × 104 M−1 (Fig. 2). This is higher than we observed with unsubstituted benzisoxazole (4 × 103 M−1),6 though the counter-ion of the cage used in that experiment was different for solubility reasons which can have an effect on K values, so the two numbers are not directly comparable. In water the dominant contribution to guest binding is the hydrophobic effect,16 and it has been shown recently that nitro substituents – despite their locally dipolar nature – are not effectively hydrated and best described as ‘hydroneutral’.17 The increase in surface area of NBI compared to unsubstituted benzisoxazole will therefore increase the contact area with the hydrophobic interior cage surface and displace more water molecules from the cage cavity when it binds, so a higher binding constant for NBI binding compared to benzisoxazole can be rationalised.16c It is clear that NBI binds in fast exchange inside the cubic cage cavity in water, and that under the dilute solution conditions 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host
1 host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest binding occurs, as we have commonly seen with many other guests.5
guest binding occurs, as we have commonly seen with many other guests.5
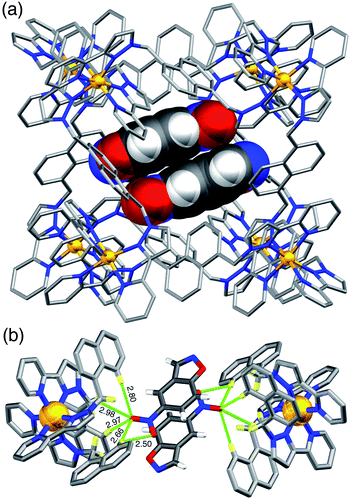
A crystal structure of the host cage complex with NBI bound in the cavity was determined (Fig. 3), with the sample prepared by the ‘crystalline sponge’ method18 that we have used before:19 a single crystal of [Co8L12](BF4)16 was immersed in a concentrated solution of NBI in MeOH for 24 hours, resulting in uptake of NBI guests into the cage cavity without loss of crystallinity. Structural analysis revealed that the cavity is occupied a stacked pair of symmetry-equivalent NBI guests (site occupancy 0.57 each) which lie across the crystallographic inversion centre at the centre of the cage molecule – an arrangement which we have seen with several other planar aromatic guests of comparable size.19 Based on a molecular volume for NBI of 144 Å3 and a cage cavity volume of 409 Å3 this leads to a cavity occupancy of 70% for the cage containing two NBI guests. This is higher than the value of ca. 55 ± 9% that is considered the optimal cavity occupancy in solution,20 but such high cavity occupancies are known in the solid state when a guest array is tightly packed because of e.g. π-stacking or hydrogen-bonding to the walls of the host,19,21 both of which occur here. We emphasise that this is not a reflection of what happens in dilute solution where – as the NMR titration showed – 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding dominates the speciation, because the crystalline sponge experiment is carried out under forcing, non-equilibrium conditions using a large excess of guest.19 In solution, even though a second guest could in principle bind, this will not be significant because – at the low concentrations used for spectroscopic measurements – K2 ≪ K1.
1 binding dominates the speciation, because the crystalline sponge experiment is carried out under forcing, non-equilibrium conditions using a large excess of guest.19 In solution, even though a second guest could in principle bind, this will not be significant because – at the low concentrations used for spectroscopic measurements – K2 ≪ K1.
Each NBI molecule is oriented such that the nitro group is directed towards one of the two fac tris-chelate vertices, which lie at either end of the long diagonal of the cubic cage array, where there is an inwardly-directed set of CH protons from the ligand set which converge to make an H-bond donor site comparable in strength to a phenol.22 There are several CH⋯O interactions between these ligand protons and the electron-rich oxygen atoms of the nitro groups (distances in Å included in Fig. 3b). This type of H-bonding interaction between the electron-rich regions of guests and the H-bond donor site on the cage interior surface is a recurrent feature of these cage/guest structures,5,19,22 and contributes substantially to the strengths of guest binding in organic solvents.23 The stacked guests are exactly parallel to one another (because of the inversion centre) with their mean planes separated by a typical π-stacking distance of 3.32 Å. We note that this orientation of the guest in the cavity is different from what we observed with unsubstituted benzisoxazole, when it was the N and O atoms of the isoxazole ring that acted as the H-bond acceptors and are docked into the fac tris-chelate H-bond donor site,6 rather than (as here, with NBI) the nitro group: neutral organic nitro groups are known to be able to act as H-bond acceptors, albeit weakly, given the negative charge density on the O atoms.24 This difference of orientation of NBI compared to benzisoxazole in the cavity turns out to be significant (see later). As usual, the anions [tetrafluoroborate, arising from use of Co(BF4)2 in the cage synthesis] occupy the windows in the centre of each face, anchored by multiple CH⋯F hydrogen-bonds to the surrounding ligand array, such that six anions surround the cavity-bound guests.5,7
Catalysis of the Kemp elimination reaction with NBI
Given that NBI does bind inside the cage cavity in aqueous solution, we expected that the cage-catalysed Kemp elimination would occur by the same mechanism that we observed with unsubstituted nitrobenzisoxazole, with the cavity-bound substrate surrounded by a high local concentration of surface-bound hydroxide ions.6 This turned out not to be the case. Initial experiments did show clearly that the cage has a catalytic effect on the reaction (Fig. 4), with the background reaction of NBI with hydroxide ions in buffered solution at pH 7 being accelerated by addition of cage Hw to an extent that is linear with concentration of cage added (i.e. the catalysed reaction is first order in catalyst; see Fig. 4c).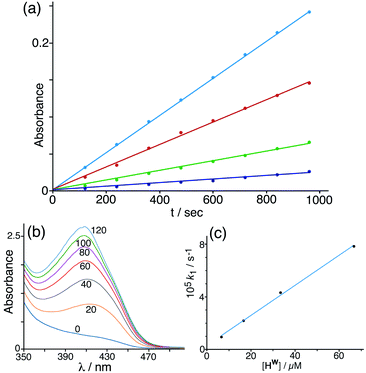 | ||
| Fig. 4 (a) Progress of the Kemp elimination reaction (background-corrected) using NBI as substrate at various catalyst concentrations (from bottom up: 6.7 μM; 16.7 μM; 33 μM; 67 μM): these show first order behaviour in NBI in the form of linear ln[NBI] vs. t plots. Reaction progress was monitored by absorbance at 408 nm. (b) Progress of a typical catalysed experiment (0.125 mM Hw) showing increasing absorbance from the CNP product with time (shown in minutes for each trace). (c) Observed reaction (initial) rate vs. catalyst concentration, confirming first order behaviour in catalyst Hw. Conditions: 298 K, pH 7; 0.25 mM NBI; varying concentrations of Hw as indicated. The slope of this line gives a value for the second-order reaction rate constant k2 of 1.18 M−1 s−1 which may be compared with the values given in Table 1 (entries a and p). | ||
Under the conditions used, the rate of the background reaction – conversion of NBI to CNP – had an observed first-order rate constant of 1.8 × 10−5 s−1; in the presence of 0.125 mM Hw, this increased to a total rate (background + catalysed) of 1.53 × 10−4 s−1 and the catalysed reaction was confirmed to be first-order in NBI based on analysis of initial rates during the first hour. Subtracting the background rate from the observed rate in the presence of Hw and dividing by catalyst concentration gives a second-order rate constant of k2 = 1.08 M−1 s−1 (Table 1, entry a) for the Hw-catalysed reaction at 298 K and pH 7. For comparison purposes this is ca. two orders of magnitude smaller than the equivalent figure for the catalysed reaction with benzisoxazole which occurs much more efficiently.6
| Entry | Added anion (as Na+ salt) | Conc./mM | k 2/M−1 s−1 |
|---|---|---|---|
| a All experiments performed at 298 K and pH 7. Experiments a–o: 0.125 mM Hw; 0.2 mM NBI. Experiments p–t: 0.05 mM Hw; 0.1 mM NBI. All rate constants derived from initial-rate data during the first 2000 seconds. Estimated errors in k2, ±5%; all plate-reader measurements from which these rate constants are derived were performed in quadruplicate and averaged (see ESI). | |||
| a | None | — | 1.08 |
| b | F− | 1.67 | 1.14 |
| c | Cl− | 1.67 | 0.81 |
| d | Br− | 1.67 | 0.29 |
| e | NO3− | 1.67 | 0.23 |
| f | IO3− | 1.67 | 0.89 |
| g | HCO3− | 1.67 | 1.51 |
| h | SO42− | 1.67 | 0.85 |
| i | F− | 16.7 | 1.60 |
| j | Cl− | 16.7 | 0.34 |
| k | Br− | 16.7 | 0.12 |
| l | NO3− | 16.7 | 0.12 |
| m | IO3− | 16.7 | 0.84 |
| n | HCO3− | 16.7 | Decomposes |
| o | SO42− | 16.7 | 0.73 |
| p | None | — | 1.24 |
| q | F− | 1.0 | 1.28 |
| r | Formate | 1.0 | 1.38 |
| s | Acetate | 1.0 | 1.54 |
| t | HCO3− | 1.0 | 2.15 |
A key observation indicates that, in this case, the catalysed reaction is not occurring in the cage cavity: blocking the cavity with an unreactive but strongly binding guest (cycloundecanone, CUD; K > 106 M−1)15 does not significantly slow down the reaction. In our earlier work, with benzisoxazole as substrate, the very fast reaction was slowed in the presence of CUD to the background rate: i.e. the catalysis was completely inhibited when the cage cavity was blocked and the substrate could not bind.6 In contrast, with NBI as substrate, the reaction continues unchanged in the presence of CUD (see ESI†). We have observed before that some other substrates undergo cage-catalysed reactions with hydroxide ions at the exterior surface of the cage: the observations described above with NBI are consistent with this type catalysis occurring.8,9 The exterior surface is just as hydrophobic as the interior surface and so will allow some aggregation with hydrophobic species such as NBI. This association will be weaker than cavity-binding as the guest is not surrounded by the host so there will be less overlap of hydrophobic host and guest surfaces compared to cavity binding. Nonetheless, this brings the substrate into contact with the high local concentration of hydroxide ions that accumulate around the cage surface for electrostatic reasons. In addition, the reaction is occurring in a more favourable solvation environment for the product, in the exterior aqueous phase rather than the interior hydrophobic cavity (though this also solvates the hydroxide ions, reducing their activity). Catalysis of this type of elimination reaction is therefore not limited to the interior cavity of the cage, although our previous work with benzisoxazole showed that catalysis on the interior was far more efficient.5,6
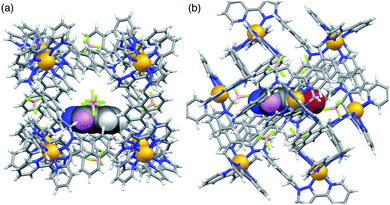
Given that NBI clearly does bind inside the cage cavity in solution, it is curious that cavity-bound catalysis does not work in the way that it does with unsubstituted benzisoxazole.6 Molecular modelling provides some insight to this. Molecular models of host cage H containing one molecule of either benzisoxazole or NBI as guest were calculated using the molecular docking program ‘GOLD’, by posing one molecule of the guest inside the cavity of a (rigid) host cage whose structure comes from crystallographic data (Fig. 5).25 With a single molecule of benzisoxazole as guest, in the energy-minimised structure the C–H proton that is removed during the Kemp elimination is directed towards a portal and is therefore accessible to a surface-bound anion (Fig. 5a). In contrast, with NBI as guest, its different orientation in the cavity – which was obtained as the minimum-energy structure from multiple different initial cage/NBI geometries, see ESI† – is associated with H-bonding of the nitro group to the cage interior surface (seen also in the crystal structure reported above, but with a stacked pair of guests). This means that the C–H proton is no longer directed towards a portal and – in this conformation of the adduct – is less accessible to a surface-bound anion (Fig. 5b). Thus, the different steric properties of the cage/guest complex could be significant here.
An additional possibility could be that, even if a cavity-bound NBI is accessible to a surface-bound hydroxide ion, the pathway to formation of the expanded, ring-opened product is inhibited in the cavity by the additional bulk of the nitro group. Similar loss of reactivity has been demonstrated for cage-bound P4, for example, arising from the fact that reaction of P4 with O2 results in initial formation of bulky intermediate species that cannot be accommodated in the confined space.26 The overall effect in our case is that catalysis does happen, but outside the cavity, and much more slowly than for the cavity-based reaction of unsubstituted benzisoxazole.6
Interaction of the product phenolate anion with the cage surface
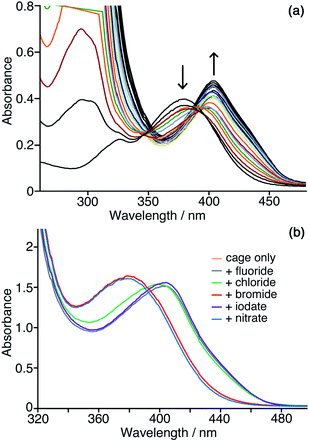
We need to highlight at this point an issue which complicates monitoring of the reaction progress using the absorbance of the CNP anion. During the catalysis studies described in this paper we noticed that the absorption maximum of the product anion CNP was red-shifted by the presence of Hw. In aqueous solution without cage present, λmax for the lowest-energy charge-transfer absorption of CNP is 379 nm: but in the presence of Hw this is red-shifted to 405 nm, which we ascribe to interaction of the anion with the cage surface at the anion-binding sites in the windows.6,7,10 This red-shift happens in exactly the same way with both Hw and the unsubstituted but otherwise isostructural cage H (Fig. 6a) indicating that the hydroxymethyl pendant groups attached to Hw are not involved in this interaction: this is consistent with the windows at the face centres being involved, as we have seen crystallographically for many small inorganic anions.5,27 Significantly, this red-shift in λmax for CNP is complete after addition of about 0.3 equivalents of either H or Hw, indicating that multiple (≈3) CNP anions can interact with a single cage molecule, consistent with surface rather than cavity binding. This is exactly consistent with the behaviour that we have seen with other large/soft aromatic anions such as fluorescein, where multiple anions can interact with the surface of a single Hw molecule depending on the concentrations of components.10We can use this red-shift to put the binding affinity of the CNP anion for the cage surface into an affinity series with other anions. Fig. 6b shows the results of adding an excess of a range of different inorganic monoanions (halides, iodate, nitrate) to a solution of 0.05 mM Hw and 0.1 mM CNP at pH 7. Initially λmax for CNP is at 405 nm, as the two equivalents of CNP are fully bound to Hw. Addition of 0.5 mM of fluoride or iodate (hydrophilic anions which bind weakly to the cage surface)10 did not change the λmax value, indicating that these anions could not displace CNP even when present in excess. Addition of 0.5 mM of bromide or nitrate – more weakly hydrophilic anions which bind more strongly to the cage surface as they are easier to desolvate10 – in contrast results in λmax for CNP shifting to 379 nm, which is the value for the free anion, indicating complete displacement. With addition of 10 equivalents of chloride (green line in Fig. 6b), an intermediate result is obtained, with a slight blue shift of λmax to 399 nm and a change in curve shape with the emergence of increased absorbance between 340–380 nm associated with some free CNP: so we can see a slight amount of displacement of CNP from the surface of Hw but it is far from complete even in the presence of a large excess of chloride, indicating that the affinity order of these two is CNP > chloride. We know from our previous work that the affinity order of inorganic monoanions for the surface of Hw is nitrate > bromide > chloride > iodate > fluoride (following the Hofmeister series).10 We can now insert CNP into that sequence to give an affinity order of anions for the cage surface of nitrate > bromide > CNP > chloride > iodate > fluoride, with the hydrophobicity and ease of desolvation of CNP positioning it between chloride and bromide in the affinity order of anions.
This shift of λmax for the CNP anion according to whether it is bound to the cage surface or free in aqueous solution – which depends on any competing anions present (Fig. 6) – affects the reaction monitoring because the proportion of bound vs. free CNP will change as the reaction proceeds, meaning that the absorbance at one fixed monitoring wavelength may not follow the Beer–Lambert law. We can partly compensate for this in two ways. Firstly, the absorbance for the catalysis reactions was monitored by recording UV/Vis spectra over the whole relevant range rather than just observing the absorbance at one wavelength, and the most appropriate monitoring wavelength was selected for each experiment calculation according to where λmax was located. Secondly, rate constants discussed in this paper are based on measurements of initial rates during the early stages of the reaction when the curves fit well to simple first-order behaviour at a given catalyst concentration, and the absorption maximum had not significantly drifted because of a change in balance between free and cage-bound CNP.
Effect of added anions on the cage-based catalysis
In Fig. 7a is shown the effect of adding a fixed concentration (1.67 mM, as the sodium salt) of a range of different anions to a catalysis reaction using 0.125 mM Hw and 0.2 mM NBI (buffered at pH 7). In the as-synthesised cage, 0.125 mM Hw (with its 16+ charge) is accompanied by 2 mM tetrafluoroborate, but hydrolysis of tetrafluoroborate following dissolution generates borate and fluoride28 which accordingly are also present as part of the baseline conditions, as is the ionic background from the 16.7 mM phosphate buffer: so the discussion of anion effects in this section is relative to the effects of this fixed background. The initial rates for the catalysed reactions in the presence of the different additional anions (with rates for the background reaction in the absence of catalysts subtracted) have been used to calculate the second-order rate constants in Table 1.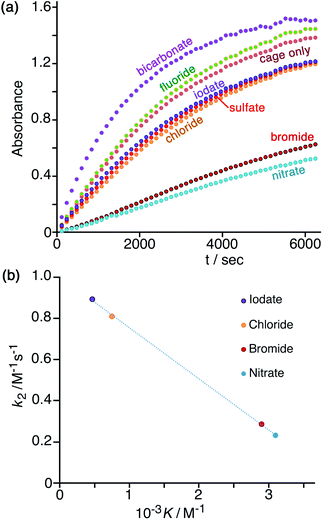 | ||
| Fig. 7 (a) Progress of cage-catalysed Kemp eliminations (background-subtracted) using NBI as substrate, monitoring formation of CNP by its absorbance in the 380–410 nm region (conditions: aqueous solution at pH 7 using 16.7 mM phosphate buffer; 0.125 mM Hw; 0.2 mM NBI; various different added anions as sodium salts, 1.67 mM. Shown are results from individual plate-reader experiments; four such repeats are averaged to give the k2 values in Table 1). (b) Correlation between reduction in second-order rate constant for catalysis (k2) in the presence of various anions (this work), based on analysis of initial rates, and the binding constant of that anion for a cage surface binding site (K, from ref. 10). | ||
We can immediately see two contrasting effects, according to how basic the added anions are. Compared to the experiment with 0.125 mM Hw on its own, addition of non-basic anions reduces the reaction rate, with – for example – addition of 1.67 mM or 16.7 mM bromide causing a decrease in k2 by factors of ca. 3 or 9 respectively (Table 1, entries d and k). This is in line with what we have observed before:7,10 bromide has a higher affinity than does hydroxide for the cationic but hydrophobic cage surface, due to its smaller desolvation enthalpy.29 Bromide therefore preferentially accumulates around the cage, reducing the local concentration of hydroxide and slowing down the reaction. The order in which the added monoanions inhibit the Kemp elimination reaction is IO3− < Cl− < Br− < NO3−, which is the same as the affinity order of these anions for the cage surface that we established recently using our recent fluorescence-displacement assay.10 In fact the correlation of the k2 value in the presence of these anions (measured in this work, Table 1) with K (the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding constant with an anion-binding site in a cage face)10 is strikingly linear (R2 > 0.999; Fig. 7b): the extent of inhibition of the surface-catalysed Kemp elimination is directly related to the affinity of the anion for the cage surface. For chloride, bromide and nitrate this is the expected order based on the Hofmeister series.30 Iodate does not figure in some lists of the Hofmeister series,30a but recently the iodate anion has been shown to be more strongly hydrated than many other oxyanions due to a highly polarised I(δ+)/O(δ−) charge distribution and therefore acts as a more powerful kosmotrope than chloride31 – exactly in agreement with what we observe.
1 binding constant with an anion-binding site in a cage face)10 is strikingly linear (R2 > 0.999; Fig. 7b): the extent of inhibition of the surface-catalysed Kemp elimination is directly related to the affinity of the anion for the cage surface. For chloride, bromide and nitrate this is the expected order based on the Hofmeister series.30 Iodate does not figure in some lists of the Hofmeister series,30a but recently the iodate anion has been shown to be more strongly hydrated than many other oxyanions due to a highly polarised I(δ+)/O(δ−) charge distribution and therefore acts as a more powerful kosmotrope than chloride31 – exactly in agreement with what we observe.
The effect of sulfate, the only 2− anion of this series, on inhibiting the cage-catalysed Kemp elimination of NBI is however out of step with the high affinity of sulfate for the cage surface that we measured earlier.10 Although sulfate is expensive to desolvate,29 it nonetheless has a high affinity for the cage surface, possibly because its high desolvation enthalpy is offset by strong electrostatic attraction between 16+ cage and 2− anion: its 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding constant to a cage surface binding site is higher than that of bromide and nitrate.10 Its ability to inhibit the Kemp elimination of NBI by accumulating around the cage surface is therefore smaller than we expected (Table 1, entries h and o) on the basis of the 1
1 binding constant to a cage surface binding site is higher than that of bromide and nitrate.10 Its ability to inhibit the Kemp elimination of NBI by accumulating around the cage surface is therefore smaller than we expected (Table 1, entries h and o) on the basis of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 anion/cage binding constants. However there will be an additional electrostatic effect in play here: whilst one sulfate dianion binds strongly to Hw, its 2− charge limits the number of additional sulfates that will approach the cage. Indeed we observed before that such effects are significant, with the trianion of hydroxypyrene-tris(sulfonate) [HPTS]3− forming a 6
1 anion/cage binding constants. However there will be an additional electrostatic effect in play here: whilst one sulfate dianion binds strongly to Hw, its 2− charge limits the number of additional sulfates that will approach the cage. Indeed we observed before that such effects are significant, with the trianion of hydroxypyrene-tris(sulfonate) [HPTS]3− forming a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 HPTS
1 HPTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hw complex under forcing conditions, but the tetra-anion [HPTS]4− forming a neutral 4
Hw complex under forcing conditions, but the tetra-anion [HPTS]4− forming a neutral 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 HPTS
1 HPTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hw complex at the same concentration.10 Thus, a small number of sulfate anions may indeed bind strongly to Hw, as the 1
Hw complex at the same concentration.10 Thus, a small number of sulfate anions may indeed bind strongly to Hw, as the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 K value suggests, but its ability to saturate the cage surface and exclude all hydroxide ions will be electrostatically inhibited, such that some hydroxide will still have access to the cage surface and facilitate the catalysed reaction with NBI, as we observe. It is notable that increasing the concentration of sulfate tenfold from 1.67 mM (Table 1, entry h) to 16.7 mM (Table 1, entry o) has very little additional effect on the inhibition.
1 K value suggests, but its ability to saturate the cage surface and exclude all hydroxide ions will be electrostatically inhibited, such that some hydroxide will still have access to the cage surface and facilitate the catalysed reaction with NBI, as we observe. It is notable that increasing the concentration of sulfate tenfold from 1.67 mM (Table 1, entry h) to 16.7 mM (Table 1, entry o) has very little additional effect on the inhibition.
Finally, these experiments are carried out in phosphate buffer, which is a mixture of mono- and dianionic inorganic phosphate, and so these specific electrostatic effects of more highly charged anions are already present in the background baseline activity. Hence, the specific electrostatic impact of dianionic sulfate (relative to the other anions) may already be present, and so the additional impact is much reduced under these conditions as sulfate substitutes for dianionic phosphate. In contrast to the effects of the above anions which all have an inhibitory effect, addition of a basic anion such as HCO3−increases the catalysed reaction rate, with k2 increasing slightly by a factor of ca. 1.5 (Table 1, entry g) by addition of 1.67 mM NaHCO3. Given that the solution is buffered at pH 7 this cannot be ascribed to a pH change on addition of NaHCO3, but it can plausibly be ascribed to the fact that the HCO3− ions that will accumulate around the cage surface can act as bases in the Kemp elimination in a way that bromide, nitrate etc. do not. This result suggests that HCO3− ions deprotonate NBI and initiate the Kemp elimination more rapidly than do hydroxide ions. This of course is inconsistent with the relative pKa values of these anions: but it is consistent with the lower hydration enthalpy of HCO3− resulting in a higher local concentration of it around the cage surface than the more strongly solvated HO− ions can achieve.29 An experiment in the absence of cage (Fig. 8a) showed that this effect does not arise from the anions alone but requires the additional presence of Hw to bring the anions into close proximity with the NBI substrate, co-locating the two reaction partners. A 50% increase in reaction rate is small, likely because of (i) the high background concentration of other anions from the buffer and cage counter-ions, and (ii) the fact that the reaction is happening at the cage exterior surface where hydroxide ions can also access the substrate. However the fundamental difference between (i) the weakly accelerating behaviour of HCO3−, and (ii) the inhibitory nature of other non-basic anions, on the catalysis by Hw is very clear.
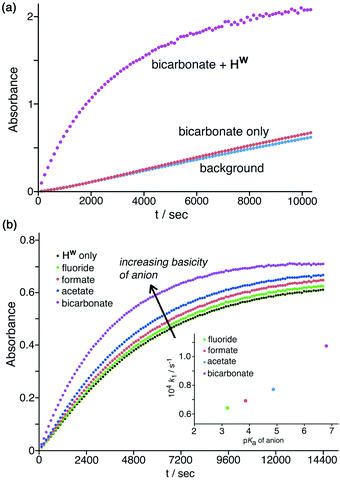 | ||
| Fig. 8 Effects of anion basicity on catalysis reaction rates. Shown are results from individual plate-reader experiments; four such repeats are averaged to give the k2 values in Table 1. (a) Experiment showing how added HCO3− has no effect on the background reaction rate when Hw is not present; both Hw and HCO3− need to be present to see the additional rate-accelerating effect. Conditions: 0.2 mM NBI; 0.125 mM Hw; pH 7 using 16.7 mM phosphate buffer. (b) Reaction progress curves (pH 7; 298 K; 0.05 mM Hw; 0.1 mM NBI) for the cage-catalysed Kemp elimination with NBI in the absence of any added anion, and then with added fluoride, formate, acetate or hydrogen carbonate as their sodium salts (1.0 mM) [inset: correlation of catalysed pseudo first-order reaction rates with pKa of added anion]. | ||
Similarly, addition of fluoride causes a small increase in reaction rate (Fig. 7a and 8b) which we ascribe to its weak basicity, as discussed above for HCO3−, though the effect is smaller (addition of 1.67 mM and then 16.7 mM fluoride results in increases in k2 by ca. 5% and then 50% respectively; Table 1, entries b and i). We investigated this effect of the basicity of the added anion further using the simple carboxylates acetate and formate in addition to fluoride and HCO3−, giving a pKa range for the added anions ranging from 3.2 (fluoride), 3.8 (formate), 4.8 (acetate), and 6.4 (HCO3−) (Table 1, entries p–t). With the same concentration of added anion, all resulted in an increase in the catalysed reaction rate compared to the reaction rate in the presence of Hw alone, with a clear correlation between anion basicity and increase in reaction rate (Fig. 8b). The particularly substantial effect of HCO3− may be because of the presence of traces of the more powerful base carbonate (pKa 10.3). Although at pH 7 there should be very little of this present in bulk solution, the stabilising effect of the 16+ charge of the cage moves this pKa value for surface-bound carbonate downwards,10 meaning that the rate-enhancing effect of added NaHCO3 may include a contribution from carbonate as well as from hydrogen carbonate. As mentioned above the absolute effects are small – a difference of >3 pKa units between different anions results in just a factor of 2 difference in initial catalysed rates, as shown in Fig. 8b, and reasons for this have been suggested. However the trend is again clear and confirms the role of Hw in solution in co-locating the NBI substrate and the anion which acts as base to initiate the Kemp elimination reaction.
The differing effects of the halides (chloride and bromide retarding the catalysis by displacing hydroxide from around the cage surface, but fluoride accelerating the catalysis because of its weakly basic nature) are clearly illustrated in Fig. 9 which shows the effects of adding up to 200 equivalents of these anions in small portions. On incremental additions of fluoride the reaction rate steadily increases until it has approximately doubled after 200 equivalents of fluoride are added. In contrast there are obvious reductions in reaction rate as more and more chloride or bromide are added, with bromide having a greater effect for the reasons discussed earlier. Overall these observations confirm the presence of two distinct effects on the catalysis associated with accumulation of anions around the surface of Hw: (i) the generally inhibiting effect on the Kemp elimination associated with displacement of hydroxide, to an extent depending on the binding affinity of the anions for the cage surface; but (ii) an accelerating effect in those cases where the new anion can itself act as base, as shown by the relationship between the magnitude of this effect and the anion basicity (Fig. 8b and 9a).
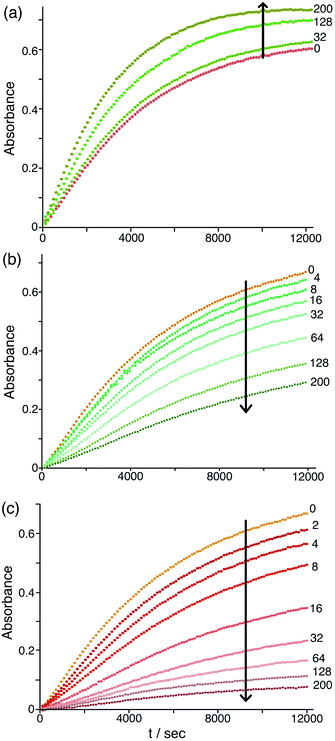 | ||
| Fig. 9 Effect of increasing concentrations of halide ions [(a) fluoride; (b) chloride; (c) bromide] on the progress of the Hw-catalysed Kemp elimination reaction of NBI (background subtracted), monitoring formation of CNP by its absorbance in the 380–410 nm region. Conditions: 16.7 mM pH 7 aqueous phosphate buffer; 0.05 mM Hw; 0.1 mM NBI; concentration of added halide, 0.05–10 mM. The numbers on the curves are numbers of equivalents of added halide ion per cage. Shown are results from individual plate-reader experiments; four such repeats are averaged to give the k2 values in Table 1. | ||
One final point to note in this section is that we can clearly see, in those cases when the reaction is most inhibited (notably in the presence of chloride, nitrate or bromide; e.g. in Fig. 7a and 9b, c), a slightly sigmoidal component to the reaction progress curve. This could be indicative of an autocatalytic7,32 mechanism becoming significant in which the product of the reaction – which here would be the CNP anion, itself a ‘soft’ (hence, weakly solvated) but weakly basic33 anion – acting as the base to deprotonate another equivalent of starting material and propagate the reaction, as we saw before with our cavity-bound Kemp elimination catalysis.7 In such situations the reaction accelerates as the product (the catalyst) accumulates, until substrate runs out, resulting in the characteristic sigmoidal shape for the reaction progress profile.32 However we can conclusively rule this out here. A simple test for autocatalysis is that adding a small amount of product to the start of the reaction should accelerate the reaction, but that does not happen: instead, addition of increasing amounts of CNP at the start of the reaction progressively inhibits it, on the same basis as the other anions which displace hydroxide from around the cage surface. So autocatalysis is not happening here, presumably because of the poor basicity of the CNP anion.33 Instead we ascribe the small sigmoidal contribution in the reaction profiles when the reaction is particularly strongly inhibited to the change in λmax of the CNP absorption maximum that we discussed earlier. A change in balance between cage-bound and free CNP as the reaction proceeds could lead to the extinction coefficient at the monitoring wavelength increasing slightly as the reaction proceeds which would give this effect. This vindicates our decision to use initial rates as the basis for comparison between the effects of different anions, which are the values used in Table 1: general trends associated with the effects of different added anions are quite clear.
Conclusion
In contrast to our previously reported cavity-catalysed Kemp elimination using the cage Hw as catalyst and benzisoxazole as substrate,6,7 use of NBI as substrate resulted in much lower catalytic rate enhancement with the reaction occurring outside the cage cavity, at the exterior surface which is nonetheless both hydrophobic and cationic and therefore provides a locus for co-location of the hydrophobic NBI substrate and the anionic reaction partner (normally hydroxide). We could therefore use the reaction rate as a way to monitor the affinity of the cage surface for different types of added anion, as accumulation of other anions around the cage surface displaces hydroxide and slows down the surface-catalysed reactions. We observed that for a range of simple mono-anions the affinity for the cage surface followed the Hofmeister series, with more weakly solvated anions (e.g. bromide, nitrate) having a higher affinity for binding to the cage surface10 and therefore causing a greater degree of inhibition of the Kemp elimination. In opposition to this effect, if the added anions are themselves basic, their accumulation around the cage surface can accelerate the reaction to an extent related to their basicity. Although these added anions (fluoride, acetate, hydrogen carbonate etc.) are weaker bases than hydroxide they are present in the reaction medium at much higher concentrations than hydroxide at pH 7, resulting in a high local concentration close to the NBI substrate around the cage surface, accounting for this observation. The CNP anion itself can bind to the cage surface, more strongly than chloride but less strongly than bromide, as demonstrated by UV/Vis spectroscopy; the absence of autocatalysis suggests that it is too weakly basic to complete significantly with hydroxide.Overall the role of the cage in bringing hydrophobic organic species and anions into proximity, which is the basis of the catalytic effects that we have previously observed,6–9 is very clear, which is useful to know considering the possibilities for catalysis that arise from accumulation of phenolate anions at the cage surface. The nucleophilicity of phenolate anions, for example, may provide the basis for reactions with a cavity-bound electrophile if multiple phenolates can surround a cavity-bound guest giving a high local concentration.7 More generally we can imagine that any of a vast range of reactions between a neutral/hydrophobic organic substrate in the cavity, and an anionic nucleophile with a high local concentration due to accumulation at the cage surface, are ripe targets to investigate for catalysis in this way.
Experimental
Samples of H (used for the crystalline sponge experiment)34 and Hw (used for solution catalysis studies)16b were prepared as previously described. Inorganic salts used to evaluate anion binding affinities were purchased from Sigma-Aldrich and used as delivered. Catalysis studies were carried out at 298 K using a BMG ClarioStar plate reader with 96-well plates by measuring UV/Vis spectra of the emerging 2-cyano-4-nitrophenolate anion (350–500 nm) and then monitoring the reaction by taking the absorbance at the maximum which shifts slightly between experiments according to the nature of other anions present (see main text). The extinction coefficient of the CNP absorption maximum was taken as 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 cm−1 irrespective of whether CNP is free or cage-bound; the small error introduced by this (see Fig. 6b) is subsumed in the ±5% estimated uncertainty for the rate constant values in Table 1. Samples for catalysis studies were prepared as described in the relevant figure captions and buffered at pH 7 using 16.7 mM phosphate buffer. Each dataset reported is based on the average of four individual measurements with different samples. The starting material NBI was prepared according to the literature procedure.14c The ESI contains the data used for the rate constant calculations in Table 1.
800 M−1 cm−1 irrespective of whether CNP is free or cage-bound; the small error introduced by this (see Fig. 6b) is subsumed in the ±5% estimated uncertainty for the rate constant values in Table 1. Samples for catalysis studies were prepared as described in the relevant figure captions and buffered at pH 7 using 16.7 mM phosphate buffer. Each dataset reported is based on the average of four individual measurements with different samples. The starting material NBI was prepared according to the literature procedure.14c The ESI contains the data used for the rate constant calculations in Table 1.
Information on the crystal properties, data collection and refinement parameters associated with the structure determination of the H/NBI host–guest complex is collected in Table 2. The data collection was performed in Experiment Hutch 1 of beamline I-19 at the UK Diamond Light Source synchrotron facility,35 using methodology, data processing and software outlined previously.19 CCDC deposition number: 2107397.†
| a The value of R1 is based on ‘observed’ data with I > 2σ(I); the value of wR2 is based on all data. | |
|---|---|
| Complex | H·NBI1.14 |
| Formula | C374.84H392.48B14.2Cl1.8Co8F56.8N74.24O34.36 |
| Molecular weight | 8255.24 |
| T/K | 100(1) |
| Radiation wavelength/Å | Synchrotron (0.6889) |
| Crystal system | Monoclinic |
| Space group | C2/c |
| a/Å | 33.12481(19) |
| b/Å | 30.0412(2) |
| c/Å | 40.1650(3) |
| β/° | 95.9731(6) |
| V/Å3 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 751.6(3) 751.6(3) |
| Z | 4 |
| ρ/g cm−3 | 1.379 |
| Crystal size/mm3 | 0.13 × 0.10 × 0.08 |
| μ/mm−1 | 0.409 |
| Data, restraints, parameters | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 327, 6797, 2523 327, 6797, 2523 |
| Final R1, wR2a | 0.0886, 0.3254 |
| Largest diff. peak/hole/e Å−3 | 1.33/−0.74 |
Data availability
Data that is not in the ESI is available from the corresponding author (MDW) on request.Author contributions
Synthesis: MDL, JST. Binding constant measurement: JST. X-Ray crystallography: CGPT. Plate reader and UV/Vis spectroscopic measurements: MDL, MBT, JD, KLT. Data analysis: MDL, NHW, KLT. Manuscript preparation: MDW, NHW, MDL. Project conception and supervision: MDW.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank EPSRC for a PhD studentship to MDL (grant number EP/N509796/1); the Universities of Warwick and Monash University for a ‘Monash/Warwick Alliance’ Accelerator grant to MDW and KLT; and the Royal Society for an International Exchange grant IES/R3/170177 to MDW.Notes and references
- (a) T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001 CrossRef CAS PubMed; (b) T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734 CrossRef CAS PubMed; (c) R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810 CrossRef CAS PubMed; (d) M. M. J. Smulders, I. A. Riddell, C. Browne and J. R. Nitschke, Chem. Soc. Rev., 2013, 42, 1728 RSC; (e) D. Zhang, T. K. Ronson and J. R. Nitschke, Acc. Chem. Res., 2018, 51, 2423 CrossRef CAS PubMed; (f) H. Vardhan, M. Yusubov and F. Verpoort, Coord. Chem. Rev., 2016, 306, 171 CrossRef CAS.
- (a) Y. Fang, J. A. Powell, E. Li, Q. Wang, Z. Perry, A. Kirchon, X. Yang, Z. Xiao, C. Zhu, L. Zhang, F. Huang and H.-C. Zhou, Chem. Soc. Rev., 2019, 48, 4707 RSC; (b) C. J. Brown, F. D. Toste, R. G. Bergman and K. N. Raymond, Chem. Rev., 2015, 115, 3012 CrossRef CAS PubMed; (c) M. Yoshizawa, J. K. Klosterman and M. Fujita, Angew. Chem., Int. Ed., 2009, 48, 3418 CrossRef CAS PubMed; (d) M. Otte, ACS Catal., 2016, 6, 6491 CrossRef CAS; (e) C. M. Hong, R. G. Bergman, K. N. Raymond and F. D. Toste, Acc. Chem. Res., 2018, 51, 2447 CrossRef CAS PubMed; (f) W.-X. Gao, H.-N. Zhang and G.-X. Jin, Coord. Chem. Rev., 2019, 386, 69 CrossRef CAS.
- (a) Y. Sun, C. Chen, J. Liu and P. J. Stang, Chem. Soc. Rev., 2020, 49, 3889 RSC; (b) M. Zhang, M. L. Saha, M. Wang, Z. Zhou, B. Song, C. Lu, X. Yan, X. Li, F. Huang, S. Yin and P. J. Stang, J. Am. Chem. Soc., 2017, 139, 5067 CrossRef CAS PubMed; (c) C.-L. Liu, R.-L. Zhang, C.-S. Lin, L.-P. Zhou, L.-X. Cai, J.-T. Kong, S.-Q. Yang, K.-L. Han and Q.-F. Sun, J. Am. Chem. Soc., 2017, 139, 12474 CrossRef CAS PubMed; (d) J. Wang, C. He, P. Wu, J. Wang and C. Duan, J. Am. Chem. Soc., 2011, 133, 12402 CrossRef CAS PubMed; (e) Y. Jiao, J. Wang, P. Wu, L. Zhao, C. He, J. Zhang and C. Duan, Chem.–Eur. J., 2014, 20, 2224 CrossRef CAS PubMed; (f) X.-Z. Li, L.-P. Zhou, L.-L. Yan, D.-Q. Yuan, C.-S. Lin and Q.-F. Sun, J. Am. Chem. Soc., 2017, 139, 8237 CrossRef CAS PubMed.
- (a) A. B. Grommett and J. R. Nitschke, J. Am. Chem. Soc., 2017, 139, 2176 CrossRef PubMed; (b) A. Garci, J.-P. Mbakidi, V. Chaleix, V. Sol, E. Orhan and B. Therrien, Organometallics, 2015, 34, 4138 CrossRef CAS; (c) T. Grancha, A. Carné-Sánchez, L. Hernández-López, J. Albalad, I. Imaz, J. Juanhuix and D. Maspoch, J. Am. Chem. Soc., 2019, 141, 18349 CrossRef CAS PubMed; (d) A. M. Castilla, T. K. Ronson and J. R. Nitschke, J. Am. Chem. Soc., 2016, 138, 2342 CrossRef CAS PubMed; (e) S. Löffler, J. Lübben, L. Krause, D. Stalke, B. Dittrich and G. H. Clever, J. Am. Chem. Soc., 2015, 137, 1060 CrossRef PubMed; (f) B.-N. T. Nguyen, A. B. Grommett, A. Tron, M. C. A. Georges and J. R. Nitschke, Adv. Mater., 2020, 32, 1907241 CrossRef CAS PubMed.
- (a) M. D. Ward, C. A. Hunter and N. H. Williams, Acc. Chem. Res., 2018, 51, 2073 CrossRef CAS PubMed; (b) M. D. Ward, C. A. Hunter and N. H. Williams, Chem. Lett., 2017, 46, 2 CrossRef CAS.
- W. Cullen, M. C. Misuraca, C. A. Hunter, N. H. Williams and M. D. Ward, Nat. Chem., 2016, 8, 231 CrossRef CAS PubMed.
- W. Cullen, A. J. Metherell, A. B. Wragg, C. G. P. Taylor, N. H. Williams and M. D. Ward, J. Am. Chem. Soc., 2018, 140, 2821 CrossRef CAS PubMed.
- C. G. P. Taylor, A. J. Metherell, S. P. Argent, F. M. Ashour, N. H. Williams and M. D. Ward, Chem.–Eur. J., 2020, 26, 3065 CrossRef CAS PubMed.
- C. Mozaceanu, C. G. P. Taylor, J. R. Piper, S. P. Argent and M. D. Ward, Chemistry, 2020, 2, 22 CrossRef CAS.
- M. D. Ludden and M. D. Ward, Dalton Trans., 2021, 50, 2782 RSC.
- (a) F. J. Rizzuto, W. Y. Wu, T. K. Ronson and J. R. Nitschke, Angew. Chem., Int. Ed., 2016, 55, 7958 CrossRef CAS PubMed; (b) I. A. Riddell, M. M. J. Smulders, J. K. Clegg, Y. R. Hristove, B. Breiner, J. D. Thoburn and J. R. Nitschke, Nat. Chem., 2012, 4, 751 CrossRef CAS PubMed; (c) N. Mihara, T. K. Ronson and J. R. Nitschke, Angew. Chem., Int. Ed., 2019, 58, 12497 CrossRef CAS PubMed; (d) C. Sgarlata, J. S. Mugridge, M. D. Pluth, B. E. F. Tiedemann, V. Zito, G. Arena and K. N. Raymond, J. Am. Chem. Soc., 2010, 132, 1005 CrossRef CAS PubMed; (e) B. Li, B. Zheng, W. Zhang, D. Zhang, X.-J. Yang and B. Wu, J. Am. Chem. Soc., 2020, 142, 6304 CrossRef CAS PubMed; (f) A. C. Stelson, C. M. Hong, M. C. Groenenboom, C. A. E. Little, J. C. Booth, N. D. Orloff, R. G. Bergman, K. N. Raymond, K. A. Schwarz, F. D. Toste and C. J. Long, Commun. Chem., 2019, 2, 54 CrossRef.
- J. Wang, T. A. Young, F. Duarte and P. J. Lusby, J. Am. Chem. Soc., 2020, 142, 17743 CrossRef CAS PubMed.
- (a) M. D. Pluth, R. G. Bergman and K. H. Raymond, Science, 2007, 316, 85 CrossRef CAS PubMed; (b) M. D. Pluth, R. G. Bergman and K. N. Raymond, Acc. Chem. Res., 2009, 42, 1650 CrossRef CAS PubMed.
- (a) J. E. Klijn and J. B. F. N. Engberts, J. Am. Chem. Soc., 2003, 125, 1825 CrossRef CAS PubMed; (b) J. E. Klijn and J. B. F. N. Engberts, Org. Biomol. Chem., 2004, 2, 1789 RSC; (c) F. Hollfelder, A. J. Kirby, D. S. Tawfik, K. Kikuchi and D. Hilvert, J. Am. Chem. Soc., 2000, 122, 1022 CrossRef CAS.
- C. A. Bunton, F. Nome, F. H. Quina and L. S. Romsted, Acc. Chem. Res., 1991, 24, 357 CrossRef CAS.
- (a) S. Turega, W. Cullen, M. Whitehead, C. A. Hunter and M. D. Ward, J. Am. Chem. Soc., 2014, 136, 8475 CrossRef CAS PubMed; (b) M. Whitehead, S. Turega, A. Stephenson, C. A. Hunter and M. D. Ward, Chem. Sci., 2013, 4, 2744 RSC; (c) A. J. Metherell, W. Cullen, N. H. Williams and M. D. Ward, Chem.–Eur. J., 2018, 24, 1554 CrossRef CAS PubMed.
- N. Sagawa and T. Shikata, Phys. Chem. Chem. Phys., 2014, 16, 13262 RSC.
- (a) M. Hoshino, A. Khutia, H. Xing, Y. Inokuma and M. Fujita, IUCrJ, 2016, 3, 139 CrossRef CAS PubMed; (b) K. Rissanen, Chem. Soc. Rev., 2017, 46, 2638 RSC.
- C. G. P. Taylor, S. P. Argent, M. D. Ludden, J. R. Piper, C. Mozaceanu, S. A. Barnett and M. D. Ward, Chem.–Eur. J., 2020, 26, 3054 CrossRef CAS PubMed.
- (a) M. R. Ams, D. Ajami, S. L. Craig, J. S. Yang and J. Rebek, J. Am. Chem. Soc., 2009, 131, 13190 CrossRef CAS PubMed; (b) S. Mecozzi and J. Rebek, Chem.–Eur. J., 1998, 4, 1016 CrossRef CAS; (c) J. Rebek, Acc. Chem. Res., 2009, 42, 1660 CrossRef CAS PubMed.
- (a) R. Puttreddy, N. K. Beyeh, E. Kalenius, R. H. A. Ras and K. Rissanen, Chem. Commun., 2016, 52, 8115 RSC; (b) W. Zhang, D. Yang, J. Zhao, L. Hou, J. L. Sessler, X.-J. Yang and B. Wu, J. Am. Chem. Soc., 2018, 140, 5248 CrossRef CAS PubMed; (c) R. Djemili, L. Kocher, S. Durot, A. Peuronen, K. Rissanen and V. Heitz, Chem.–Eur. J., 2019, 25, 1481 CrossRef CAS PubMed.
- A. J. Metherell and M. D. Ward, Dalton Trans., 2016, 45, 16096 RSC.
- S. Turega, M. Whitehead, B. R. Hall, A. J. H. M. Meijer, C. A. Hunter and M. D. Ward, Inorg. Chem., 2013, 52, 1122 CrossRef CAS PubMed.
- (a) C. R. Shugrue, J. R. DeFrancisco, A. J. Metrano, B. D. Brink, R. S. Nomoto and B. R. Linton, Org. Biomol. Chem., 2016, 14, 2223 RSC; (b) T. R. Kelly and M. H. Kim, J. Am. Chem. Soc., 1994, 116, 7072 CrossRef CAS; (c) C. V. K. Sharma and G. R. Desiraju, J. Chem. Soc., Perkin Trans. 2, 1994, 2345 RSC.
- (a) W. Cullen, S. Turega, C. A. Hunter and M. D. Ward, Chem. Sci., 2015, 6, 2790 RSC; (b) https://www.ccdc.cam.ac.uk/solutions/csd-discovery/components/gold/, last accessed 11/5/2021.
- P. Mal, B. Breiner, K. Rissanen and J. R. Nitschke, Science, 2009, 324, 1697 CrossRef CAS PubMed.
- M. D. Ludden, C. G. P. Taylor and M. D. Ward, Chem. Sci., 2021, 12, 12640 RSC.
- (a) R. E. Mesmer, K. M. Palen and C. F. Baes Jr, Inorg. Chem., 1973, 12, 89 CrossRef CAS; (b) S. Radosavljević, V. Ŝćepanović, S. Stević and D. Milojković, J. Fluorine Chem., 1979, 13, 465 CrossRef.
- D. W. Smith, J. Chem. Educ., 1977, 54, 540 CrossRef CAS.
- (a) B. Kang, H. Tang, Z. Zhao and S. Song, ACS Omega, 2020, 5, 6229 CrossRef CAS PubMed; (b) W. Kunz, P. Lo Nostro and B. W. Ninham, Curr. Top. Colloid Interface Sci., 2004, 9, 1 CrossRef CAS.
- (a) A. P. dos Santos, A. Diehl and Y. Levin, Langmuir, 2010, 26, 10778 CrossRef CAS PubMed; (b) M. Baer, V.-T. Pham, J. L. Fulton, G. K. Schenter, M. Balasubramanian and C. J. Mundy, J. Phys. Chem. Lett., 2011, 2, 2650 CrossRef CAS.
- (a) A. J. Bissette and A. J. Fletcher, Angew. Chem., Int. Ed., 2013, 52, 12800 CrossRef CAS PubMed; (b) A. Vidonne and D. Philp, Eur. J. Org. Chem., 2009, 593 CrossRef CAS; (c) P. Schuster, Monatsh. Chem., 2019, 150, 763 CrossRef CAS; (d) E. A. Wintner and J. Rebek Jr, Acta Chem. Scand., 1996, 50, 469 CrossRef CAS.
- M. D. Liptak, K. C. Gross, P. G. Seybold, S. Feldgus and G. C. Shields, J. Am. Chem. Soc., 2002, 124, 6421 CrossRef CAS PubMed.
- I. S. Tidmarsh, T. B. Faust, H. Adams, L. P. Harding, L. Russo, W. Clegg and M. D. Ward, J. Am. Chem. Soc., 2008, 130, 15167 CrossRef CAS PubMed.
- D. R. Allan, H. Nowell, S. A. Barnett, M. R. Warren, A. Wilcox, J. Christensen, L. K. Saunders, A. Peach, M. T. Hooper, L. Zaja, S. Patel, L. Cahill, R. Marshall, S. Trimnell, A. J. Foster, T. Bates, S. Lay, M. A. Williams, P. V. Hathaway, G. Winter, M. Gerstel and R. W. Wooley, Crystals, 2017, 7, 336 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed breakdown of rate constant calculations from plate reader experiments, and some additional figures. CCDC 2107397. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc04887b |
| This journal is © The Royal Society of Chemistry 2021 |