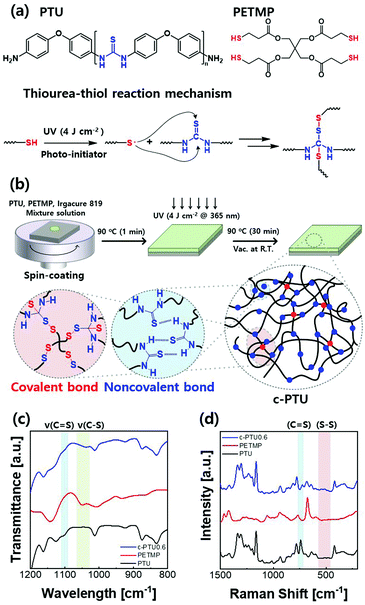
A dual cross-linked aromatic polythiourea gate dielectric with multifunctional capabilities for organic field-effect transistors†
Sungmi
Yoo‡
a,
Hyunjin
Park‡
 a,
Yong Seok
Kim
ab,
Jong Chan
Won
ab,
Dong-Gyun
Kim
a,
Yong Seok
Kim
ab,
Jong Chan
Won
ab,
Dong-Gyun
Kim
 *ab and
Yun Ho
Kim
*ab and
Yun Ho
Kim
 *ab
*ab
aAdvanced Materials Division, Korea Research Institute of Chemical Technology, 141 Gajeong-ro, Yuseong-gu, Daejeon 34114, Republic of Korea. E-mail: dgkim@krict.re.kr; yunho@krict.re.kr
bAdvanced Materials and Chemical Engineering, KRICT School, University of Science and Technology, 217 Gajeong-ro, Yuseong-gu, Daejeon 34114, Republic of Korea
First published on 12th December 2020
Abstract
A multifunctional gate dielectric material based on dual cross-linked aromatic polythiourea (c-PTU) networks for organic field-effect transistors is presented. Tailoring the dual covalent and noncovalent cross-links in the c-PTU gate dielectric enables all the advantageous capabilities of excellent insulating properties, including high capacitance, good solvent resistance and thermal stability, as well as low-temperature solution processing and photo-patterning.
Organic field-effect transistors (OFETs) have received considerable attention due to their critical roles in the development of advanced electronic devices, such as E-papers, embedded displays, smart labels, and wearable electronics.1 Based on the intrinsic characteristics of flexibility, low-cost manufacturing, simple and facile fabrication, and lightweight, many efforts have been made to improve OFET performance by synthesizing a new class of organic semiconductors (OSCs),2 adopting better device structures,3 and developing fabrication techniques.4 As one of the essential elements of OFETs, the device performance can also be significantly controlled by the nature of the gate dielectric because it affects the growth of OSC layers, charge transport, electrical stability, and threshold voltage of OFETs by contacting directly with the active layer.5 Conventional inorganic gate dielectric materials, such as silicon dioxide and silicon nitride, have been widely used in OFETs to achieve the desired electrical characteristics.6 However, they often suffer from their intrinsic brittleness and poor processability in terms of processing temperature and the deposition manner.7 Thus, research on organic gate dielectrics to replace inorganic gate dielectrics is becoming increasingly important for emerging flexible and printable electronic applications.8
There are several requirements of organic gate dielectrics that need to be considered for the fabrication and utilization of OFETs. Low-temperature solution processability of organic gate dielectrics is one of the most advantageous capabilities over the inorganic counterparts because it allows low-cost processing of large-area devices in the manners of spin-coating, printing, or meniscus-guided coating.9 Another requirement is the chemical resistance to endure organic solvent-based post-wet processes, which prevents damage to organic gate dielectric layers.10 Cross-linking via thermal annealing or photo-irradiation is a facile method to satisfy the necessity of solvent resistance.11 In comparison to thermal cross-linking, photo-cross-linking is more preferred due to the low-temperature processing for the integration of inexpensive plastic substrates and the photo-patternability for lower drain–source current at the off-state.12 In addition to the processability, organic gate dielectrics should meet the essential requirements of dielectric and electrical properties, such as a high breakdown voltage to reduce the dielectric layer thickness and a high dielectric constant to enhance the gate modulation.13 Moreover, the thermal and surface properties of organic gate dielectrics should be also controlled to optimize the device performance.7,14 While numerous research studies on organic gate dielectrics have shown substantial progress for meeting some of the above specific requirements,14 it is still difficult to achieve a combination of all the important characteristics in terms of processing and physical properties in a single organic gate dielectric material.
Since the discovery of intriguing electrical properties of aromatic polythiourea (PTU), such as a high breakdown strength, low dielectric loss, and high electrical energy density, by Zhang et al.,15 PTU-based dielectric materials have been extensively studied.16 Such high energy-storable dielectric characteristics of the aromatic PTU originate from the high dipole moment (≈4.89 D) of the thiourea unit as well as its hydrogen (H)-bonding capability.17 Different from semicrystalline polyurea analogues, the PTU is an amorphous and glassy polymer with good solubility in common organic solvents, thus enabling defect-free film (≈1–5 μm thick) formation via simple solution-processing for better performance.15,16a,b While there have been many studies on the utilization of aromatic PTU for high-voltage and high-energy-density capacitor applications,15,16a,b,e,18 as far as we know, there has been no report on its gate dielectric applications for OFETs. For use as a high-capacitance gate dielectric, the aromatic PTU should maintain its electrical insulating properties even with much thinner thicknesses of around several hundreds of nanometers, but such thin film characteristics of the PTU have not been clearly described in the literature. Thus, we sought to explore its use in the preparation of novel gate dielectric thin films for OFETs. In particular, based on the reactivity between thiourea units and radical species,19 the potential to achieve multifunctional capabilities by forming dual cross-linked PTU networks with thiol-based cross-linkers has been explored in detail.
Herein, we report a new class of dual cross-linked polymeric gate dielectric materials, consisting of densely H-bonding thiourea units and covalently cross-linked thiourea–thiol linkages (Fig. 1a and b). The aromatic PTU and thiol-based covalent cross-linker, pentaerythritol tetrakis(3-mercaptopropionate) (PETMP), are highly soluble in common organic solvents, thus enabling simple and facile solution-based fabrication processes, including spin-coating, low-temperature thermal treatment, and subsequent photo-cross-linking (Fig. 1b). Once cross-linked, the cross-linked PTU (c-PTU) possesses good solvent resistance, which allows additional solution-based surface modifications such as metal–oxide-assisted surface treatment (MAST).20 By tailoring the chemical composition of the networks, we have controlled their overall properties, consequently achieving the combination of outstanding electrical insulation (Ebd > 5 MV cm−1, Jleak = 3.37 × 10−9 A cm−2 at 5 MV cm−1, where Ebd and Jleak are the breakdown electric field and the leakage current density, respectively) and enhanced thermal stability (Tg, Td > 200 °C, where Tg and Td are the glass transition temperature and the decomposition temperature, respectively) without compromising the moderate dielectric constant (εr = 4.1) of the PTU. The c-PTU gate dielectric is also found to be employable in dinaphtho[2,3-b:2′,3′-f]thieno[3,2-b]thiophene (DNTT)-based OFETs. The DNTT-based OFETs with the MAST-applied c-PTU exhibited excellent electrical performance with a carrier mobility of 1.03 cm2 V−1 s−1, an on/off current ratio of 1.48 × 106, and a threshold voltage of −1.08 V.
The aromatic PTU in this study was synthesized via polycondensation of 1,1′-thiocarbonyldiimidazole (TCDI) and 4,4′-oxydianiline (ODA) (Fig. S1, ESI†). 1H nuclear magnetic resonance (NMR) and gel permeation chromatography (GPC) revealed that the polymer was successfully synthesized (Fig. S2 and S3, ESI†). The number-average molecular weight (Mn,GPC) and molecular weight dispersity (Đ), determined via GPC, were 12700 g mol−1 and 1.92, respectively.
The dual cross-linked PTU networks (c-PTU#) are composed of covalently cross-linked PTU and PETMP moieties (Fig. 1b), wherein the molar feed ratio of the thiol group of PETMP to the thiourea group of PTU (# = [thiol]/[thiourea]) was varied from 0.2 to 1.0 at 0.2 intervals to optimize the dielectric properties of c-PTUs (Table S1, ESI†). The c-PTU thin films were prepared via simple spin-coating of the PTU, PETMP, and Irgacure® 819 (a photo-initiator) mixture dissolved in N-methyl-2-pyrrolidone (NMP) on a glass substrate, followed by drying on a hot plate at 90 °C for 1 min and subsequent ultraviolet (UV, 4 J cm−2 at 365 nm) irradiation at room temperature (Fig. 1b). Additional thermal treatment (at 90 °C for 30 min) and drying under vacuum (at room temperature overnight) were applied to completely remove any residual solvent before use. In addition to the H-bonding characteristic,17b,c a thiourea functional group is known to react with radical species (Fig. 1a),19,21 although such chemistry has not yet been utilized for the preparation of PTU-based dual cross-linked networks. The reaction between the thiourea units of PTU and thiyl radicals of PETMP under UV irradiation can be confirmed via Fourier-transform infrared (FTIR) spectroscopy, where the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S stretching vibration peak of PTU at 1100 cm−1 decreases and the characteristic peak of C–S of PETMP shifts from 1052 cm−1 to 1037 cm−1, with the disappearance of the S–H stretching vibration at 2550 cm−1 in PETMP (Fig. 1c and Fig. S4, ESI†).18,22 The characteristic peak at around 3310 cm−1, corresponding to the NH deformation vibration of nonlinearly H-bonded thiourea units, is still observed in the FTIR spectrum of c-PTU (Fig. S4, ESI†), thus attesting to the presence of the amorphous H-bonded PTU chains in the networks.17b,c Raman spectroscopy also confirms the reaction from the decrease of C
S stretching vibration peak of PTU at 1100 cm−1 decreases and the characteristic peak of C–S of PETMP shifts from 1052 cm−1 to 1037 cm−1, with the disappearance of the S–H stretching vibration at 2550 cm−1 in PETMP (Fig. 1c and Fig. S4, ESI†).18,22 The characteristic peak at around 3310 cm−1, corresponding to the NH deformation vibration of nonlinearly H-bonded thiourea units, is still observed in the FTIR spectrum of c-PTU (Fig. S4, ESI†), thus attesting to the presence of the amorphous H-bonded PTU chains in the networks.17b,c Raman spectroscopy also confirms the reaction from the decrease of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S peaks at 740 cm−1 and the appearance of a S–S peak at 520 cm−1 (Fig. 1d).23
S peaks at 740 cm−1 and the appearance of a S–S peak at 520 cm−1 (Fig. 1d).23
The covalently cross-linked network structure of the c-PTUs can also be estimated by their enhanced solvent resistance and thermal stability. All the c-PTUs with different feed ratios of PETMP and PTU, except for c-PTU0.2, are found to be insoluble when immersed in NMP (Fig. S5, ESI†). Thermogravimetric analysis (TGA) shows that c-PTU0.6 exhibits insignificant weight loss until above 200 °C, with 5% weight loss occurring near 215 °C, which is around 20 °C higher than that of the PTU (Fig. S6a, ESI†). Moreover, the glass transition temperatures (Tg) of both PTU and c-PTU0.6 are not observed before their thermal decomposition (Fig. S6b, ESI†). Even with the cross-linking of PTU by PETMP, the amorphous nature of the PTU matrix is still preserved (Fig. S7, ESI†).
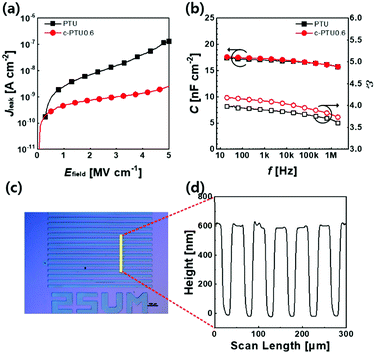
Metal–insulator–metal (MIM) capacitors were fabricated to evaluate the dielectric properties of PTU and c-PTU thin films in terms of breakdown electric field (Ebd) and dielectric constant (εr), determined by measuring leakage current density (Jleak) and capacitance (C), respectively. Among the c-PTUs (≈310–340 nm thick) with different PTU and PETMP compositions, we focus on c-PTU0.6 because it shows the lowest Jleak of 1.36 × 10−9 A cm−2 at 3 MV cm−1 (Fig. S8 and Table S2, ESI†). In addition, the dielectric properties of c-PTU0.6 are found to be maintained when the thickness decreases to 166 nm (Fig. S9, ESI†).
The Jleak value of MIM capacitors with 200 nm-thick PTU and c-PTU0.6 thin films was measured as a function of electric field (Efield) in the range of 0 to 5 MV cm−1 (Fig. 2a). The MIM capacitor with c-PTU0.6 shows Jleak of 1.01 × 10−9 A cm−2 at 3 MV cm−1, which is around an order of magnitude lower than that of the capacitor with the PTU. Even with repeated preparation and measurements, the 200 nm-thick c-PTU0.6 thin films exhibit a large breakdown electric field over 5 MV cm−1 (Fig. S10, ESI†). It is worth noting here that this is a relatively large breakdown electric field among various solution-processed polymer gate dielectrics such as poly(4-vinylphenol) (PVP), polyimide and polyvinyl alcohol (PVA) and is comparable to those of vacuum-processed ones such as poly-para-xylylene) (Table S3, ESI†). The dielectric constant (εr), which is one of the most important factors that determines device performance with gate modulation, was extracted from the capacitance of MIM capacitors as a function of frequency (Fig. 2b). At 1 kHz, the c-PTU0.6 thin film shows a εr value of 4.1, which is slightly higher than that of the PTU. This phenomenon is in contrast to conventional cross-linked polymer gate dielectrics based on PVP and PVA, which are usually cross-linked by consuming the polar moieties such as hydroxyl groups through condensation reaction, thus resulting in decreased dielectric constants (Table S4, ESI†). Despite the increase in low polarity S–S bonds with a higher cross-linking ratio, polar thiourea analogues, including thioether and ester groups, still remain, leading to higher dielectric constants regardless of the PETMP-induced cross-linking reaction.24
In addition to the reliable dielectric properties, c-PTU0.6 provides the applicability of the conventional photolithography technique owing to its excellent solvent resistance after the photo-cross-linking reaction. Photo-patterning is often required to increase the device integration density and decrease the drain–source current in the off-state.11b,c A facile photo-patterning process, including 4 J cm−2 of UV exposure (λ = 365 nm) through a shadow mask and development in NMP, can provide c-PTU thin film patterns (Fig. S11, ESI†). The optical microscope image and height profile of the photo-patterned c-PTU0.6 thin film are shown in Fig. 2c and d, respectively.
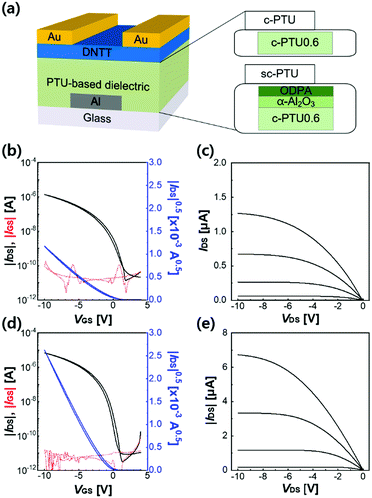
The electrical properties of c-PTU0.6 as a gate dielectric layer in OFETs were evaluated by identifying the transfer and output characteristics of DNTT-based OFETs (Fig. 3a). The electrical characteristics in terms of field-effect mobility (μ), threshold voltage (Vth), subthreshold swing (SS), and on/off current ratio (Ion/Ioff) were extracted from transfer curves in the saturation regime (Fig. 3b). The extracted μ, Vth, SS, and Ion/Ioff values are 0.13 cm2 V−1 s−1, −1.10 V, 0.82 V dec−1, and 9.51 × 104, respectively (Table 1). The transfer curve shows a reliable gate–source current (IGS) based on the excellent insulating properties of c-PTU0.6. The output curve, shown in Fig. 3c, exhibits moderate gate modulation and no considerable contact resistance issue at a low drain–source voltage (VDS), but the field-effect mobility of 0.13 cm2 V−1 s−1 is a relatively low value, compared to that of previously reported DNTT-based OFETs.25 To further improve the device performance, we have employed the MAST method to modify the interface characteristic between c-PTU and DNTT layers (Fig. S12, ESI†).20 The MAST method modifies the surface of the gate dielectric for reducing the charge trap density, which hinders charge transport at the interface related to the field-effect mobility and subthreshold swing, without sacrificing gate dielectric bulk properties (Fig. S13, ESI†).19 The covalently cross-linked system of c-PTU0.6 allows the application of the MAST technique owing to its excellent thermal and chemical resistance. Although the surface roughness (0.48 nm) of the surface-treated c-PTU0.6 (sc-PTU0.6), recorded by AFM, is somewhat higher than that (0.25 nm) of the pristine c-PTU0.6 (Fig. S14, ESI†), Ion of sc-PTU0.6 is about 5 times higher than that of c-PTU0.6 (Fig. 3d and e and Table 1). We attributed this to the larger grain boundary of DNTT on the sc-PTU0.6 gate dielectric, originating from the decreased surface energy after the surface treatment (Fig. S15 and Table S5, ESI†). As a result, the DNTT-based OFET with the sc-PTU0.6 gate dielectric exhibits a higher field-effect mobility of 1.03 cm2 V−1 s−1 and a larger Ion/Ioff ratio of 1.48 × 106 compared to that with c-PTU0.6, while the threshold voltage of −1.08 V and the SS of 0.89 V dec−1 are negligibly changed. In addition, with the MAST technique, IGS is considerably lowered by about an order of magnitude and larger gate modulation is observed in output characteristics. Thus, the solution-processed MAST technique is found to be successfully applied for the c-PTU gate dielectric by suppressing the interface charge trap density, which hinders charge transport within the active layers, as well as allowing the reduction of the gate–source leakage current. The low-temperature processability of the sc-PTU gate dielectric allowed it to be integrated with an ultra-flexible substrate, 3 μm-thick poly-para-xylylene, and the fabricated flexible DNTT-based OFET showed no obvious differences in electrical characteristics (Fig. S16 and Table S6, ESI†).
| Gate dielectric | C [nF cm−2] | μ [cm2 V−1 s−1] | V th [V] | SSd [V dec−1] | I on [A] | I off [A] | I on /I off |
|---|---|---|---|---|---|---|---|
| a Dielectric capacitance per unit area. b Average field-effect mobility of DNTT OFETs. c Threshold voltage. d Subthreshold swing. e On and off currents and on/off current ratio. | |||||||
| c-PTU0.6 (200 nm) | 17.03 | 0.13 ± 0.03 | −1.10 | 0.82 | 1.37 × 10−6 | 1.44 × 10−11 | 9.51 × 104 |
| sc-PTU0.6 (250 nm) | 15.17 | 1.03 ± 0.52 | −1.08 | 0.89 | 6.92 × 10−6 | 4.68 × 10−12 | 1.48 × 106 |
In summary, we have demonstrated that dual cross-linking based on thiourea noncovalent interactions and thiourea–thiol covalent linkages can bestow the c-PTU gate dielectric with remarkable multifunctional capabilities. Through the covalent cross-linking of the noncovalent cross-linking building block (PTU), the c-PTU dielectric exhibits much enhanced overall characteristics, including a large breakdown electric field (Ebd > 5 MV cm−1), a low leakage current density (Jleak = 3.37 × 10−9 A cm−2 at 5 MV cm−1), good solvent resistance (for post solution-processed surface treatment), and thermal stability (Tg, Td > 200 °C), while still maintaining its moderate dielectric constant (εr = 4.1). Furthermore, the low-temperature solution processability and photo-patternability, originating from non-linear arrays of H-bonded thiourea units in the PTU and their additional reactivity with radical species, are great advantages for the low-cost manufacturing of OFETs. The c-PTU dielectric, surface-treated using the MAST method, also works efficiently in DNTT-based OFETs, where they exhibit a field-effect mobility of 1.03 cm2 V−1 s−1, a subthreshold swing of 0.89 V dec−1, a threshold voltage of −1.08 V, and an on/off current ratio of 1.48 × 106. We believe that the present work provides an insight into the design and preparation of organic gate dielectric materials with advanced properties for OFET applications by taking advantage of the dual cross-linkable PTU.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Center for Advanced Soft-Electronics funded by the Ministry of Science and ICT as a Global Frontier Project (2015M3A6A5065315) and the Korea Research Institute of Chemical Technology (KRICT) core project (SS2021-20; KK2061-12).Notes and references
- (a) H. Sirringhaus, Adv. Mater., 2014, 26, 1319–1335 CrossRef CAS; (b) H. Sirringhaus, Proc. IEEE, 2009, 97, 1570–1579 CAS.
- Y. Yamashita, F. Hinkel, T. Marszalek, W. Zajaczkowski, W. Pisula, M. Baumgarten, H. Matsui, K. Müllen and J. Takeya, Chem. Mater., 2016, 28, 420–424 CrossRef CAS.
- J. Kwon, Y. Takeda, R. Shiwaku, S. Tokito, K. Cho and S. Jung, Nat. Commun., 2019, 10, 1–10 CrossRef PubMed.
- H. Park, J. Kwon, H. Ahn and S. Jung, J. Mater. Chem. C, 2019, 7, 6251–6256 RSC.
- (a) D. Ji, T. Li, W. Hu and H. Fuchs, Adv. Mater., 2019, 31, 1–19 Search PubMed; (b) H. Dong, X. Fu, J. Liu, Z. Wang and W. Hu, Adv. Mater., 2013, 25, 6158–6183 CrossRef CAS PubMed.
- (a) A. Facchetti, Mater. Today, 2007, 10, 28–37 CrossRef CAS; (b) C. Reese and Z. Bao, Mater. Today, 2007, 10, 20–27 CrossRef CAS.
- H. Chen, W. Zhang, M. Li, G. He and X. Guo, Chem. Rev., 2020, 120, 2879–2949 CrossRef CAS PubMed.
- (a) H. Park, J. Kwon, B. Kang, W. Kim, Y.-H. Kim, K. Cho and S. Jung, ACS Appl. Mater. Interfaces, 2018, 10, 24055–24063 CrossRef CAS PubMed; (b) H. Park, S. Yoo, H. Ahn, J. Bang, Y. Jeong, M. Yi, J. C. Won, S. Jung and Y. H. Kim, ACS Appl. Mater. Interfaces, 2019, 11, 45949–45958 CrossRef CAS PubMed.
- Y. Diao, L. Shaw, Z. Bao and S. C. B. Mannsfeld, Energy Environ. Sci., 2014, 7, 2145–2159 RSC.
- (a) F. Zhang, H. Zhang, L. Zhu, L. Qin, Y. Wang, Y. Hu, Z. Lou, Y. Hou and F. Teng, J. Mater. Chem. C, 2019, 7, 4004–4012 RSC; (b) H. Park, H. Ahn, J. Kwon, S. Kim and S. Jung, ACS Appl. Mater. Interfaces, 2018, 10, 37767–37772 CrossRef CAS PubMed.
- (a) M. E. Roberts, N. Queralto, S. C. B. Mannsfeld, B. N. Reinecke, W. Knoll and Z. Bao, Chem. Mater., 2009, 21, 2292–2299 CrossRef CAS; (b) J. M. Won, H. J. Suk, D. Wee, Y. H. Kim, J. W. Ka, J. Kim, T. Ahn, M. H. Yi and K. S. Jang, Org. Electron. Phys. Mater. Appl., 2013, 14, 1777–1786 CAS; (c) M. J. Kim, M. Lee, H. Min, S. Kim, J. Yang, H. Kweon, W. Lee, D. H. Kim, J.-H. Choi, D. Y. Ryu, M. S. Kang, B. Kim and J. H. Cho, Nat. Commun., 2020, 11, 1520 CrossRef CAS PubMed.
- (a) K. Pei, M. Chen, Z. Zhou, H. Li and P. K. L. Chan, ACS Appl. Electron. Mater., 2019, 1, 379–388 CrossRef CAS; (b) F. Huang, Y. Xu, Z. Pan, W. Li and J. Chu, IEEE Electron Device Lett., 2020, 41, 1082–1085 CAS.
- B. Nketia-Yawson, S. J. Kang, G. D. Tabi, A. Perinot, M. Caironi, A. Facchetti and Y. Y. Noh, Adv. Mater., 2017, 29, 1605685 CrossRef PubMed.
- (a) B. Nketia-Yawson and Y.-Y. Noh, Adv. Funct. Mater., 2018, 28, 1802201 CrossRef; (b) S. Casalini, C. A. Bortolotti, F. Leonardi and F. Biscarini, Chem. Soc. Rev., 2017, 46, 40–71 RSC; (c) H. Park, S. Yoo, M. H. Yi, Y. H. Kim and S. Jung, Org. Electron., 2019, 68, 70–75 CrossRef CAS; (d) Y. Wang, X. Huang, T. Li, L. Li, X. Guo and P. Jiang, Chem. Mater., 2019, 31, 2212–2240 CrossRef CAS.
- S. Wu, W. Li, M. Lin, Q. Burlingame, Q. Chen, A. Payzant, K. Xiao and Q. M. Zhang, Adv. Mater., 2013, 25, 1734–1738 CrossRef CAS PubMed.
- (a) Q. Burlingame, S. Wu, M. Lin and Q. M. Zhang, Adv. Energy Mater., 2013, 3, 1051–1055 CrossRef CAS; (b) S. Wu, Q. Burlingame, Z.-X. Cheng, M. Lin and Q. M. Zhang, J. Electron. Mater., 2014, 43, 4548–4551 CrossRef CAS; (c) A. Mannodi-Kanakkithodi, G. M. Treich, T. D. Huan, R. Ma, M. Tefferi, Y. Cao, G. A. Sotzing and R. Ramprasad, Adv. Mater., 2016, 28, 6277–6291 CrossRef CAS PubMed; (d) V. Sharma, C. Wang, R. G. Lorenzini, R. Ma, Q. Zhu, D. W. Sinkovits, G. Pilania, A. R. Oganov, S. Kumar, G. A. Sotzing, S. A. Boggs and R. Ramprasad, Nat. Commun., 2014, 5, 1–8 Search PubMed; (e) Y. Feng, Y. Hasegawa, T. Suga, H. Nishide, L. Yang, G. Chen and S. Li, Macromolecules, 2019, 52, 8781–8787 CrossRef CAS.
- (a) W. D. Kumler and G. M. Fohlen, J. Am. Chem. Soc., 1942, 64, 1944–1948 CrossRef CAS; (b) Y. Yanagisawa, Y. Nan, K. Okuro and T. Aida, Science, 2018, 359, 72–76 CrossRef CAS PubMed; (c) E. J. Cha, D. S. Lee, H. Kim, Y. H. Kim, B. G. Kim, Y. Yoo, Y. S. Kim and D.-G. Kim, RSC Adv., 2019, 9, 15780–15784 RSC; (d) S. Yoo, D.-G. Kim, T. Ha, J. C. Won, K.-S. Jang and Y. H. Kim, ACS Appl. Mater. Interfaces, 2018, 10, 32462–32470 CrossRef CAS PubMed.
- R. Ma, V. Sharma, A. F. Baldwin, M. Tefferi, I. Offenbach, M. Cakmak, R. Weiss, Y. Cao, R. Ramprasad and G. A. Sotzing, J. Mater. Chem. A, 2015, 3, 14845–14852 RSC.
- I. I. Kandror, B. V. Kopylova and R. K. Freidlina, Sulfur Rep., 1984, 3, 289–316 CrossRef CAS.
- S. Kim, T. Ha, S. Yoo, J.-W. Ka, J. Kim, J. C. Won, D. H. Choi, K.-S. Jang and Y. H. Kim, Phys. Chem. Chem. Phys., 2017, 19, 15521–15529 RSC.
- S. Zeng, L. Li, J. Yu, N. Wang and S. Chen, Electrochim. Acta, 2018, 263, 53–59 CrossRef CAS.
- (a) P. W. Loscutoff, H.-B.-R. Lee and S. F. Bent, Chem. Mater., 2010, 22, 5563–5569 CrossRef CAS; (b) K. Strzelec, N. Bączek, S. Ostrowska, K. Wąsikowska, M. I. Szynkowska and J. Grams, C. R. Chim., 2012, 15, 1065–1071 CrossRef CAS.
- (a) J. Coates, Encyclopedia of Analytical Chemistry, 2004, pp. 1–23 Search PubMed; (b) S. Raju, R. Muralidharan and H. Krishnan, Optik, 2016, 127, 3620–3623 CrossRef CAS; (c) R. Le Parc, V. T. Freitas, P. Hermet, A. M. Cojocariu, X. Cattoën, H. Wadepohl, D. Maurin, C. H. Tse, J. R. Bartlett, R. A. S. Ferreira, L. D. Carlos, M. Wong Chi Man and J.-L. Bantignies, Phys. Chem. Chem. Phys., 2019, 21, 3310–3317 RSC; (d) M. M. Coleman, M. Sobkowiak, G. J. Pehlert, P. C. Painter and T. Iqbal, Macromol. Chem. Phys., 1997, 198, 117–136 CrossRef CAS; (e) T. Szmechtyk, N. Sienkiewicz and K. Strzelec, Polym. Bull., 2018, 75, 149–165 CrossRef CAS.
- J. S. Kwon, H. W. Park, D. H. Kim and Y.-J. Kwark, ACS Appl. Mater. Interfaces, 2017, 9, 5366–5374 CrossRef CAS PubMed.
- T. Yamamoto and K. Takimiya, J. Am. Chem. Soc., 2007, 129, 2224–2225 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0tc03617j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |