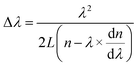Individual concave twin ZnO microdisks with optical resonances†
Yumin
Chen
 *abcd,
Zhen
Liu
b,
Xiaohui
Qiu
bd and
Xinfeng
Liu
*abcd,
Zhen
Liu
b,
Xiaohui
Qiu
bd and
Xinfeng
Liu
 *bd
*bd
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China. E-mail: yuminchen@iue.ac.cn
bCAS Key Laboratory of Standardization and Measurement for Nanotechnology, CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing 100190, P. R. China. E-mail: liuxf@nanoctr.cn
cCenter for Excellence in Regional Atmospheric Environment, Key Laboratory of Urban Pollutant Conversion, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, China
dUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 29th November 2021
Abstract
We synthesized concave twin ZnO microdisks, whose outer surfaces are entirely enclosed by high-energy facets. Different from hexagonal planar WGM microdisks, individual concave twin ZnO microdisks show Fabry–Pérot resonances and anisotropic photoluminescence properties at room temperature.
Crystals enclosed by high-energy facets have attracted keen attention because of their unique structures (high-energy facets, surface cavities and sharp corners/edges), extraordinary optical, catalytic, and sensing properties, and so on.1–5 Compared with flat or spherical structures, concave crystals with unique surface structures might provide the potential opportunity to enrich the diversity of photo-matter interactions; however, the optical properties of concave semiconductor crystals have been seldom studied.6,7 Challenges still remain to chemically synthesize concave crystals enclosed by high-energy surfaces according to Wulff theorem and the majority of previous research studies have focused on various metal nanocrystals and their alloys.8–10 Compared with metal nanocrystals, compound crystals completely enclosed by regular high-energy surfaces are harder to synthesize, since the complex covalent bonds and diverse crystal structures make their surface structure control more difficult.11–13 Herein, we synthesized concave twin ZnO microdisks and further investigated the photoluminescence properties of individual concave twin microdisks at room temperature.
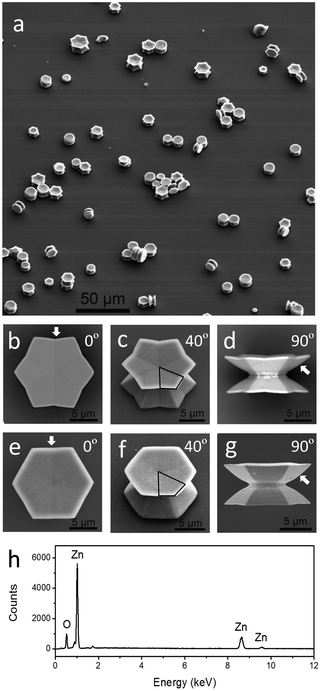
A typical synthesis was performed in a Teflon-line stainless-steel autoclave containing 4 mmol zinc acetate dehydrate, 2 mmol sulfur and 10 mL of dodecyl amine at 120 °C for 1 h and then at 220 °C for 9 h. The as-prepared precipitate products (ZnO microdisks) were separated from the upper colloids (ZnS nanoparticles) and cleaned before structure characterization and optical measurements. More experimental details can be found in the ESI.† The structure and composition characterizations of the upper ZnS colloids are shown in Fig. S1 of the ESI.† A large-scale scanning electron microscopy (SEM) image of the precipitate gives an overview of the ZnO twin microdisks which stand or side-lie on the surface (Fig. 1a). It clearly presents a unique structure that two well-defined concave microdisks back-to-back stack in pairs through a narrow joint face along the [0001] direction. These two stacked microdisks nearly have the same size, and they symmetrically distribute on the two sides of the notch. According to the projections along the [0001] direction, twin microdisks can be classified into two categories: dodecagonal twin microdisks and hexagonal twin microdisks. Their distinctive structural features were clearly revealed by the SEM images of the individual twin microdisks, recorded from various viewing angles relative to the [0001] direction. From the top viewport, the edge numbers of the ZnO microdisks are clearly displayed in the SEM images (Fig. 1b and e). The projected cross section of the dodecagonal microdisk along the [0001] direction is not an absolutely convex dodecagon; instead, convex and concave angles repeat alternately (Fig. 1b). When the inner concavity of the edge is quite small, the projected cross section of the microdisks turns into hexagonal as shown in Fig. 1e. Careful observation reveals that the edge of the hexagonal is not perfectly straight but slightly concave inwards as marked by the arrow. The hexagonal microdisk can be regarded as a specific dodecagonal microdisk which has a very small concavity on the edge. Viewing from 40°, the concave features of the microdisks are clearly displayed in Fig. 1c and f. Whether dodecagonal or hexagonal twin microdisks, their concave top and bottom surfaces both consist of six non-coplanar high-index flat facets as the black frames highlighted. The side views of twin microdisks indicate that their outer side surfaces are entirely enclosed by high-energy curved facets confirmed by the curved side contour lines marked by the arrows (Fig. 1d and g). EDS (energy dispersive spectroscopy, as shown in Fig. 1h) analysis confirms that the chemical composition of the precipitate produced is ZnO. In summary, the surfaces of the concave ZnO twin microdisks are completely enclosed by planar and curved high-energy facets, without any low-index facets such as (0001) facets.14
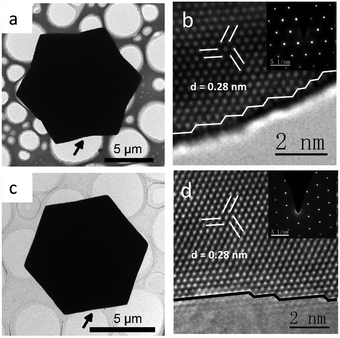
The high-index feature and the single crystal structure of the concave ZnO twin microdisks were further confirmed by HRTEM (high resolution transmission electron microscopy) and SAED (selected area electron diffraction). The HRTEM image taken from the margin of the microdisk projected along the [0001] direction, as marked by the arrow in Fig. 2a, reveals that the edge of the dodecagonal microdisk is made up of high-index facets with high-density atomic steps (Fig. 2b). The high-index feature of the hexagonal microdisks was also confirmed at the atomic level, even though the edge of the hexagonal microdisk is close to a straight line from a macro perspective (Fig. 2d). Despite the surface of the ZnO concave twin microdisks being enclosed by high-energy facets, the single crystal quality of the face-centered cubic structure was confirmed by lattice fringes with a d-spacing of 0.280 nm (Fig. 2b and d) and the inserted electronic diffraction patterns projected along the [0001] direction.
The unique morphologies of concave twin ZnO microdisks are severely affected by the crystal growth dynamics.15–17 The coordination of dodecyl amine with the Zn2+(0001) surface hinders the surface from further growth, resulting in the formation of a plate structure.16 Moreover, the further self-assembly of the dodecyl amine monolayer adsorbed on the two flat (0001) surfaces causes the oriented attachment of ZnO plates along the [0001] direction and the formation of the twin structure.17 In addition, Li et al. have reported that etching of the O2−(0001) surface by NH4+ will cause a hole or concave structure, which is also suitable for our concave ZnO case.16 We also found that bringing in a competitive reaction to produce ZnS is very crucial for the formation of well-defined concave structures. A series of control experiments (Fig. S2–S4, ESI†) have demonstrated that the morphologies of the ZnO microdisks are strongly affected by the S/Zn ratio and the reaction time. The statistical analysis results in Fig. S5 (ESI†) show that the morphology evolution from round to hexagonal and then to dodecagonal microdisks is pushed forwards by increasing the S/Zn ratio or the reaction time. The large S/Zn ratio or the reaction time promotes the improvement of the concavity of the microdisks. As shown in Fig. S6 (ESI†), nearly all ZnO microdisks can be controlled to dodecagonal plates through adjusting the S/Zn ratio and the reaction time. A possible schematic crystal growth process of concave twin ZnO microdisks is shown in Fig. S7 (ESI†).
ZnO is a direct wide band-gap (3.4 eV at room temperature) material with a relatively large exciton binding energy (60 meV). The PL spectra of individual ZnO concave microdisks were investigated by using a commercial confocal Raman microscope with excitation at 325 nm using a He–Cd laser. The measurement focus on individual microdisks could more accurately reflect the relationship between the structure and optical properties; moreover, the uniformity of the microdisks is not requested. Fig. 3a displays a set of PL spectra when the incident exciting laser with various power densities was focused on the center of the dodecagonal microdisk along the [0001] direction. A series of sharp resonance peaks coupled to the broad background fluorescence emissions which are generated from surface states. The fluorescence resonances indicate that twin ZnO microdisks are potential optical cavities.18–20 The resonances will be affected by the upper and lower parts of this special structure. Since the upper and lower facets are not very flat, it means that some of the photons could not be confined by the optical cavity. We can see weak resonance peaks superimposed onto a very broad emission peak. The light intensities of broad background fluorescence and small resonance peaks both increase when the exciting laser power densities are gradually increased from 0.0318 kW cm−2 to 3.18 kW cm−2. The positions of all the resonance peaks are invariable at the different excited powers, but the spacing between the neighboring peaks gradually increases at the high wavelength side of the spectra. In optical microcavities, the mode spacing Δλ is given by
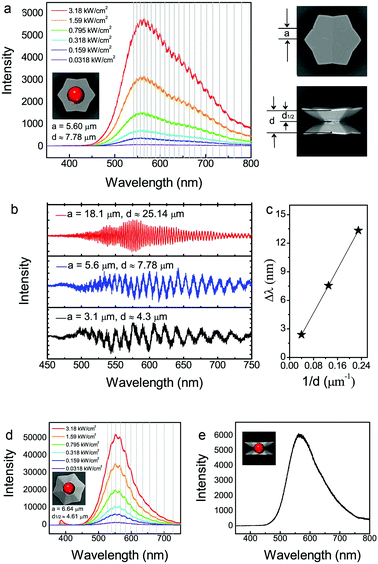 | ||
| Fig. 3 Photoluminescence (PL) spectra of individual dodecagonal concave ZnO microdisks at room temperature. (a) Pump density dependent PL spectra of dodecagonal concave twin microdisks (light irradiation parallel to the [0001] direction of the microdisks). The center position of the incident exciting laser projection was marked by a red ball. (b) Size dependent PL spectra of dodecagonal concave twin microdisks. The incident exciting light was irradiated parallel to the [0001] direction and focused on the center of the microdisks. (c) The relationship between Δλ and 1/d derived from Fig. S4b (ESI†). (d) Pump density dependent PL spectra of dodecagonal concave single-microdisks (incident light is parallel to the [0001] direction from the backside of the single-microdisk). The center position of the incident exciting laser projection was marked by a red ball. (e) PL spectrum of dodecagonal concave twin microdisks when the light irradiation direction is perpendicular to the [0001] direction. The center position of the incident exciting laser projection was marked by a red ball. Note: the values of the edge length (a) and the height of the concave dodecagonal ZnO microdisks (d and d1/2) were shown near their corresponding PL spectra. | ||
To confirm the resonance mode, the size-dependent photoluminescence spectrum of individual dodecagonal microdisks was also investigated (Fig. 3b). The modulation of fluorescence resonance on the emission spectra could be better analyzed by removing the fluorescent background.22 The resonator peak spacings (Δλ) are 2.40 nm, 7.55 nm and 13.34 nm around 550 nm for the three microdisks with heights of 25.14 μm, 7.78 μm and 4.3 μm, respectively. The linear relationship between Δλ and 1/d indicates that a large microdisk leads to a smaller interference peak spacing and higher interference orders (Fig. 3c). To further confirm that the resonant peaks are ascribed to the FP cavity modes instead of WMG modes, we choose a dodecagonal single-microdisk (half of twin microdisk) to verify its resonance mode. Compared with twin microdisks having comparative sizes, the mode spacing of the single-microdisk will basically remain invariable in the WGM mode and become double in the FP mode. Fig. 3d shows that the measured peak spacing of the single microdisk (a = 6.64 μm) is bigger than that of the twin microdisk (a = 5.60 μm), so we can qualitatively judge that the WGM modes are not suitable for describing the resonance in concave ZnO microdisks. The quantitative calculation revealed that the effective cavity length is close to the height of a single-microdisk (d1/2), which further confirmed that concave ZnO microdisks are FP resonators.
When the excited laser irradiates from the direction perpendicular to the [0001] direction of ZnO microdisks, photoluminescence resonances become not so obvious (Fig. 3e). The curvature difference between two top-bottom concave faces and two curved side faces accounts for the difference of the amplitude when excited from the directions parallel or perpendicular to the [0001] direction of ZnO microdisks. In a word, the anisotropic photoluminescence resonance excited from two orthogonal directions is attributed to the unique structure of the concave twin microdisks.
The hexagonal microdisk can be regarded as a specific dodecagonal microdisk which has a very small concavity on the edge. In the same way, the PL spectra of individual hexagonal ZnO concave microdisks were also investigated. Photoluminescence resonance phenomena also appeared in the hexagonal concave microdisks (Fig. S8a, ESI†). The cavity length of the hexagonal concave microdisk is consistent with the height of the hexagonal microdisks, suggesting that hexagonal ZnO concave microdisks are also FP resonators. Size-dependent FP resonances were also observed in the hexagonal concave microdisks (Fig. S8b, ESI†), and the linear relationship between Δλ and 1/d was displayed in Fig. S8c (ESI†). Similarly, the optical properties of a hexagonal single-microdisk (half of twin microdisk) were also checked to further confirm that the FP mode appears in the hexagonal concave ZnO microdisks (Fig. S8d, ESI†). The effective cavity length is close to the height of a single-microdisk (h1/2), indicating that concave hexagonal ZnO microdisks are also FP resonators like concave dodecagonal ZnO microdisks. The anisotropic photoluminescence resonance also appear in hexagonal ZnO microdisks when the excited lasers illuminate from the direction parallel or perpendicular to the [0001] direction of the ZnO microdisks (Fig. S8a and e, ESI†).
It is worth mentioned that the dodecagonal and hexagonal concave twin ZnO microdisks are potential FP optical cavities, though the most reported hexagonal ZnO structures are WGM resonators.23–26 We think that the WGM mode disappears since the concave structure of the top-bottom faces prevents the occurrence of the total internal reflection in the WGM mode; as a result, the FP mode becomes the dominant resonance mode. The difference in the curvature of the FP resonance mirrors accounts for the anisotropic resonance excited from two orthogonal directions, i.e. parallel or perpendicular to the [0001] direction of ZnO microdisks.
In summary, we synthesized concave twin ZnO microdisks enclosed with planar and curved high-energy surfaces through bringing in a competitive reaction. Different from hexagonal planar WGM microdisks, individual concave ZnO microdisks show potential as FP optical resonators due to their unique concave structure. Besides flat or spherical optical resonators, the unique concave crystals with high-energy surfaces enrich the diversity of photo-matter interactions of semiconductor resonators. Moreover, the twin microdisk cavities have potential applications in fields such as sensors through precisely controlling the shape and size of the microdisks.
We thank Prof. Chunxiang Xu, Mr Feifei Qin (Southeast University), Prof. Guocong Guo, Ms Jingjing Fu, Prof. Qingdong Zheng (Fujian Institute of Research on the Structure of Matter, CAS) and Prof. Chen Wang, Dr Jun Zhang (National Center for Nanoscience and Technology, China) for their help or advice. Ministry of Science and Technology (2017YFA0205004), National Natural Science Foundation of China (22073022, 11874130, 12074086), NSF of Fujian Province (2018J01024), the FJIRSM&IUE Joint Research Fund (RHZX-2019-001) and Central Government Guides Local Funds for Science and Technology Development (2020L3023) are acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- N. Tian, Z.-Y. Zhou, S.-G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732 CrossRef CAS PubMed.
- M. Iqbal, Y. Bando, Z. Sun, K. C.-W. Wu, A. E. Rowan, J. Na, B. Y. Guan and Y. Yamauchi, Adv. Mater., 2021, 33, 2004554 CrossRef CAS PubMed.
- Y. Shi, Z. Lyu, M. Zhao, R. Chen, Q. N. Nguyen and Y. Xia, Chem. Rev., 2021, 121, 649 CrossRef CAS PubMed.
- J. W. Hong, S. Lee, Y. W. Lee and S. W. Han, J. Am. Chem. Soc., 2012, 134, 4565 CrossRef CAS PubMed.
- X. Han, X. Han, L. Sun, S. Gao, L. Li, Q. Kuang, Z. Xie and C. Wang, Chem. Commun., 2015, 51, 9612 RSC.
- X. Xia, J. Zeng, B. McDearmon, Y. Zheng, Q. Li and Y. Xia, Angew. Chem., Int. Ed., 2011, 50, 12542 CrossRef CAS.
- X. Kang, Q. Ruan, H. Zhang, F. Bao, J. Guo, M. Tang, S. Cheng and J. Wang, Nanoscale, 2017, 9, 5879 RSC.
- Y. Chen, Q. Chen, S. Peng, Z. Wang, G. Lu and G. Guo, Chem. Commun., 2014, 50, 1662 RSC.
- Y. Chong, X. Dai, G. Fang, R. Wu, L. Zhao, X. Ma, X. Tian, S. Lee, C. Zhang, C. Chen, Z. Chai, C. Ge and R. Zhou, Nat. Commun., 2018, 9, 4861 CrossRef.
- S. Luo and P. Shen, ACS Nano, 2017, 11, 11946 CrossRef CAS PubMed.
- Q. Kuang, X. Wang, Z. Jiang, Z. Xie and L. Zheng, Acc. Chem. Res., 2014, 47, 308 CrossRef CAS PubMed.
- J. Hu, H. He, L. Li, X. Zhou, Z. Li, Q. Shen, C. Wu, A. Asiri, Y. Zhou and Z. Zou, Chem. Commun., 2019, 55, 4777 RSC.
- Y. Xu, M. Cao and Q. Zhang, Mater. Chem. Front., 2021, 5, 151 RSC.
- S. Yang, B. Yang, L. Wu, Y. Li, P. Liu, H. Zhao, Y. Yu, X. Gong and H. Yang, Nat. Commun., 2014, 5, 5355 CrossRef PubMed.
- C. Xu, X. W. Sun, Z. L. Dong, Y. P. Cui and B. P. Wang, Cryst. Growth Des., 2007, 7, 541 CrossRef CAS.
- F. Li, Y. Ding, P. Gao, X. Xin and Z. Wang, Angew. Chem., Int. Ed., 2004, 43, 5238 CrossRef CAS PubMed.
- T. Zhang, W. Dong, M. K. Brewer, S. Koner, R. N. Njabon and Z. R. Tian, J. Am. Chem. Soc., 2006, 128, 10960 CrossRef CAS.
- J. Li, Y. Lin, J. Lu, C. Xu, Y. Wang, Z. Shi and J. Dai, ACS Nano, 2015, 9, 6794 CrossRef CAS.
- X. Liu, Q. Zhang, Q. Xiong and T. Sum, Nano Lett., 2013, 13, 1080 CrossRef CAS.
- N. Wang, J. Dong, Y. Yang, Y. Zhang, X. He, C. Wang, B. Li and G. Yang, Adv. Mater., 2011, 23, 2937 CrossRef CAS PubMed.
- H. Dong, Z. Chen, L. Sun, W. Xie, H. Tan, J. Lu, C. Jagadish and X. Shen, J. Mater. Chem., 2010, 20, 5510 RSC.
- X. Wang, Q. Liao, Q. Kong, Y. Zhang, Z. Xu, X. Lu and H. Fu, Angew. Chem., Int. Ed., 2014, 53, 5863 CrossRef CAS PubMed.
- C. Xu, J. Dai, G. Zhu, G. Zhu, Y. Lin, J. Li and Z. Shi, Laser Photonics Rev., 2014, 8, 469 CrossRef CAS.
- R. Chen, B. Ling, X. Sun and H. Sun, Adv. Mater., 2011, 23, 2199 CrossRef CAS PubMed.
- D. J. Gargas, M. C. Moore, A. Ni, S. Chang, Z. Zhang, S. Chung and P. Yang, ACS Nano, 2010, 4, 3270 CrossRef CAS PubMed.
- Y. Yang, Y. Zhang, N. Wang, C. Wang, B. Li and G. Yang, Nanoscale, 2011, 3, 592 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supporting data. See DOI: 10.1039/d1cc05332a |
| This journal is © The Royal Society of Chemistry 2022 |