Self-contained photo-acid generators with high quantum yields triggered by photo-cyclization†
Zixuan
Xu
,
Zhaoyu
Wang
,
Yanyun
Zhang
,
Xiaokang
Yao
,
Meijuan
Ding
,
Huifang
Shi
* and
Zhongfu
An
 *
*
Key Laboratory of Flexible Electronics (KLOFE) & Institute of Advanced Materials (IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing Tech University (NanjingTech), 30 South Puzhu Road, Nanjing, 211816, P. R. China. E-mail: iamzfan@njtech.edu.cn; iamhfshi@njtech.edu.cn
First published on 4th November 2022
Abstract
We present a novel series of neutral photo-acid generators (PAGs) based on carbazole derivatives. A photo-induced 6π-electrocyclization reaction of carbazole derivatives triggers the subsequent release of halogen acids. With UV irradiation, PAGs spontaneously release acid molecules quantitatively forming polyaromatic compounds. To our knowledge, it is considered the highest quantum yield (over 85%) among Brønsted PAGs.
Photo-acid generators (PAGs) which release various acids upon light irradiation have received extensive attention in recent years owing to their widespread applications, such as microlithographic imaging,1,2 photocuring,3 3D printing, cationic polymerization,4 and photodynamic therapy,5etc. At present, there are mainly two types of PAGs: ionic and non-ionic PAGs.6,7 Diphenylio donium and triarylsulfonium salts are representative ionic PAGs.8–12 Non-ionic PAGs, such as N-oxy imidesulfonate derivatives have also been applied in photoresists for many years.13,14 However, the majority of reported PAGs undergo homolytic bond cleavage reaction after light irradiation, resulting in the generation of photo-generated radical species.14,15 These active species need to extract hydrogen atoms from solvent molecules or other hydrogen sources to generate a Brønsted acid.12 Meanwhile, they are also involved in the photo-redox catalytic reaction, resulting in complicated fragments remaining in the systems.16,17 Recently, a number of PAGs that do not require external proton source have been developed, which effectively overcome the above shortcomings of PAGs.18–24 For instance, Liao et al. reported photo-chromic merocyanine derivatives, which exhibited dynamic photo-isomerization accompanied with changes in acidity.25 Kawai's group has developed a series of efficient PAGs by exploiting the reactivity of highly sensitive photochromic terarylenes. The terarylene-based PAGs exhibited relatively high photo-chemical quantum yields up to 70%.4,26,27 Owing to the quantitative release of alcohols, carboxylic acids, sulfonic acids, and even triflic acid, terarylene-based PAGs were successfully used in the field of polymerizations and organic catalytic reactions.4 Sardon's group developed a series of PAGs based on anthrone and anthraquinone, which were able to produce tricarboxylic acid, p-toluenesulfonic acid and methanesulfonic acid to trigger ring opening polymerization (ROP) of cyclic esters under 365 nm UV irradiation.21 To date, self-contained PAGs still face the problem of a slow photochemical reaction rate and relatively low photo-chemical quantum yields. In addition, these material systems are very limited, which are not conducive to further chemical modification to optimize photophysical properties.
It is well known that carbazole has a strong absorption of ultraviolet light.28–35 Moreover, terarylene-based PAGs react at the thiophene group after UV illumination, forming a closed-loop structure and releasing acid. Therefore, we hypothesized that modified halogenated thiophene on carbazole group, might be a new class of self-contained PAGs with fast photochemical reaction rate.
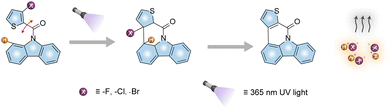
Herein, a series of self-contained PAGs were designed and synthesized (CzF, CzCl, and CzBr, Fig. 1). Their chemical structures were characterized by 1H and 13C NMR spectroscopies and mass spectra (Fig. S1–S6 and S11–S13, ESI†). Under UV irradiation, they achieved acid generation companied with a photochromic behavior. These compounds undergo 6π-electro pericyclization reaction located at hydrogen (H) from carbazole group and conjugate base (X) pair from a thiophene group upon photo-irradiation, spontaneously eliminating an acid molecule (HX), producing a structurally stable polycycle compound (CzC). Moreover, carbazole-based PAGs have good thermal stability and higher photochemical quantum yield (Φ > 85%). To the best of our knowledge, it is the highest value among the reported self-contained PAGs. Due to the unique photochemical process, this strategy provides a guidance for the subsequent development of PAG materials.
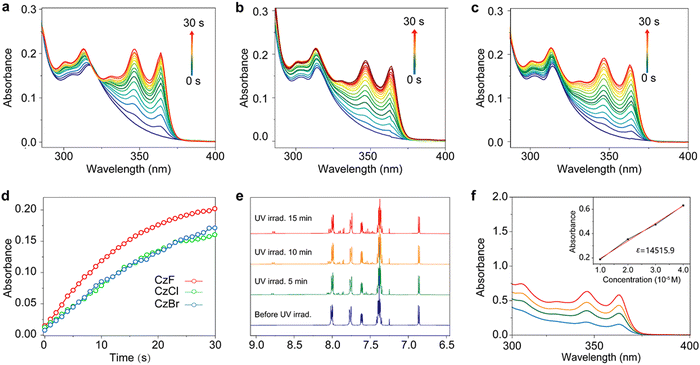
Firstly, the photophysical properties of the carbazole derivatives were studied before and after photo-irradiation. Under irradiation by a 365 nm UV light lamp, the compounds exhibit visual photochromic behaviours.36–38 With the extension of irradiation time, the PL spectra of the three compounds changed with the emission intensity at around 390 nm increased obviously, as shown in Fig. S15 (ESI†). Impressively, the products after illumination make the pH test paper turn red, indicating the generation of acids (Fig. S16, ESI†). Like the photoluminescence behaviour, UV absorption of PAGs varied similarly under the UV irradiation. Without UV light irradiation, there exist peaks at around 305 nm and shoulders at 318 nm for the absorption spectra. With the prolongation of UV irradiation time, new absorption peaks appear at 345 and 365 nm gradually (Fig. 2a–c). It is worth noting that the photo-response rates are very fast, and the chemical equilibriums can be realized within 30 s. The linear fitting curves between irradiation time and absorption intensity shows that the reaction rate of CzF is faster than that of CzCl and CzBr (Fig. 2d). We also characterized the reaction kinetics of CzF by 1H NMR. Under continuous UV irradiation, a series of new peaks appear at around 7.8, 7.9, 8.1 and 8.8 ppm (Fig. 2e). After purification of the products, we found that it underwent a 6π-electro cyclization reaction during UV irradiation (Fig. S7, S8, S14 and S17, ESI†), resulting in the formation of stable polycyclic compound CzC and halogen acids. At different concentrations, the results show that the concentration of CzC and the absorption intensity exhibit a good linear relationship. The molar absorption coefficient of CzC in THF was calculated to be (1.45 ± 0.005) × 105 cm−1 M−1 (Fig. 2f). The photochemical quantum yields of CzF, CzCl and CzBr are 93%, 86% and 85% respectively. Subsequently, the thermal stability of PAGs was investigated by thermogravimetric analysis (TGA) and differential scanning calorimetric (DSC). As shown in Fig. S18a and b (ESI†), these PAGs present excellent thermal decomposition temperatures (over 200 °C, Fig. S18b, ESI†). The DSC curves reveal that their melting point relationship is CzF > CzCl > CzBr (Fig. S18a, ESI†).
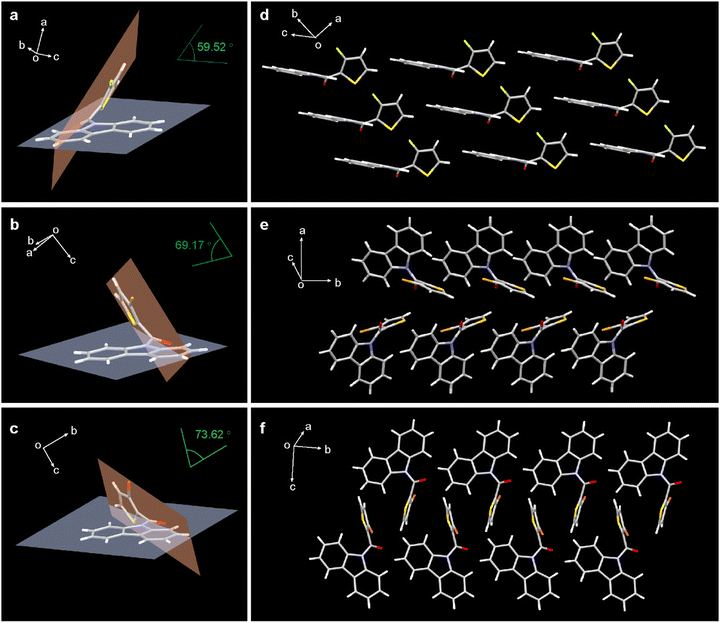
To gain insight into the mechanism for acid generation under UV-light irradiation in crystal, the molecular conformations and stacking were investigated through single crystal analysis. All the three compounds showed twisted molecular configurations and the dihedral angles between the carbazole and thiophene planes increased as the halogen atomic radius increases (Fig. 3a–c). Driving by UV light, the molecular rotor rotates to induce that carbazole and the thiophene units are in the same plane. Thus, large closed-loop intermediates are formed, which is beneficial to rapidly release halogen acids.
Additionally, the packing modes of their single crystals are also different. As shown in Fig. 3d–f, the carbazole groups are arranged in parallel and exhibit strong π⋯π stacking for CzF. While for CzCl and CzBr, the carbazole groups show zipper arrangement. Therefore, it is reasonably speculated that the more planar the PAG molecules, the smaller the driving force required for rotation, and thus the higher photochemical quantum yield of CzF. Moreover, we carefully analysed the intermolecular interactions in single crystals. In CzF crystal, the molecules were rigidly restricted by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯π (3.188 Å), π⋯π (3.374–3.385 Å), C–F⋯H–C (2.616 Å), C–H⋯π (2.854 Å) interactions, which contribute to restricting molecular motions for good thermo-stability. In comparison, there are fewer molecular interactions in CzCl and CzBr (Fig. S19, ESI†), resulting in less compact molecular packing and lower melting points.
O⋯π (3.188 Å), π⋯π (3.374–3.385 Å), C–F⋯H–C (2.616 Å), C–H⋯π (2.854 Å) interactions, which contribute to restricting molecular motions for good thermo-stability. In comparison, there are fewer molecular interactions in CzCl and CzBr (Fig. S19, ESI†), resulting in less compact molecular packing and lower melting points.
Subsequently, we further conducted a series of control experiment on the mechanism of 6π-electro pericyclization for acid generation by PAGs. Firstly, we synthesized an isomer of CzBr, named CzBr-2 (Fig. 4a). By analysing the 1H NMR spectra of CzBr-2 before and after UV irradiation, it was found that their 1H NMR signals were completely consistent (Fig. 4b), indicating that CzBr-2 did not undergo photochemical reaction. That means the electro pericyclization here is hardly realized due to the strong molecular ring tension of a 7-membered ring. Therefore, we concluded that the substituent position of halogen atom played a decisive role in the photo-acid generation.
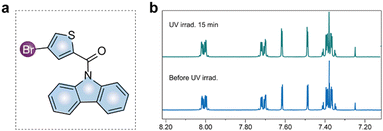 | ||
| Fig. 4 (a) The chemical structure of contrastive molecule (CzBr-2); (b) 1H NMR spectral change of CzBr-2 upon UV (365 nm) irradiation in CDCl3. | ||
In conclusion, we have successfully designed and synthesized a series of PAGs with high photochemical quantum yields of over 85%. To the best of our knowledge, this is the highest value compared with the reported PAGs (Fig. S20, ESI†). The experimental results proved that these PAGs underwent 6π-electrocyclization reaction and produced hydrohalic acids under UV illumination.
X-ray single crystal analysis showed that the smaller the dihedral angle between carbazole and thiophene units, the larger the photo-acid quantum yield. The more intermolecular interactions are, the higher the thermal stability is of the PAGs. This study presents a convenient and effective method to design and synthesize efficient Brønsted PAGs, which will provide a guideline for the study of photo-acid generators in the future.
Financial support from the National Natural Science Foundation of China (Grants No. 21975120, 62134007 and 21875104), the fund for Talented of Nanjing Tech University (No. 201983), and Young Scientific and Technological Talents Project of Jiangsu Association for Science and Technology (JSTJ-2020-026). Jiangsu Postdoctoral Research Funding Program (No. 2021K582C).
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. M. Wallraff and W. D. Hinsberg, Chem. Rev., 1999, 99, 1801–1822 CrossRef CAS PubMed.
- B. D. Gates, Q. Xu, M. Stewart, D. Ryan, C. G. Willson and G. M. Whitesides, Chem. Rev., 2005, 105, 1171–1196 CrossRef CAS PubMed.
- J.-P. Fouassier, Photoinitiation, photopolymerization, and photocuring: fundamentals and applications, Hanser, 1995 Search PubMed.
- T. Nakashima, K. Tsuchie, R. Kanazawa, R. Li, S. Iijima, O. Galangau, H. Nakagawa, K. Mutoh, Y. Kobayashi, J. Abe and T. Kawai, J. Am. Chem. Soc., 2015, 137, 7023–7026 CrossRef CAS PubMed.
- X. Yue, C. O. Yanez, S. Yao and K. D. Belfield, J. Am. Chem. Soc., 2013, 135, 2112–2115 CrossRef CAS PubMed.
- J. V. Crivello, Adv. Polym. Sci., 1984, 62, 1–48 CrossRef CAS.
- F. Ortica, C. Coenjarts, J. C. Scaiano, H. Liu, G. Pohlers and J. F. Cameron, Chem. Mater., 2001, 13, 2297–2304 CrossRef CAS.
- J. V. Crivello and J. H. W. Lam, J. Polym. Sci., Polym. Chem. Ed., 1996, 34, 3231–3253 CrossRef CAS.
- W. Zhou, S. M. Kuebler, K. L. Braun, T. Yu, J. K. Cammack, C. K. Ober, J. W. Perry and S. R. Marder, Science, 2002, 296, 1106–1109 CrossRef CAS PubMed.
- K. J. Schafer, J. M. Hales, M. Balu, K. D. Belfield, E. W. Van Stryland and D. J. Hagan, J. Photochem. Photobiol., A, 2004, 162, 497–502 CrossRef CAS.
- T. Y. Yu, C. K. Ober, S. M. Kuebler, W. H. Zhou, S. R. Marder and J. W. Perry, Adv. Mater., 2003, 15, 517–521 CrossRef CAS.
- W. Zhou, S. M. Kuebler, D. Carrig, J. W. Perry and S. R. Marder, J. Am. Chem. Soc., 2002, 124, 1897–1901 CrossRef CAS PubMed.
- P. Yang and W. T. Yang, Chem. Rev., 2013, 113, 5547–5594 CrossRef CAS PubMed.
- C. J. Martin, G. Rapenne, T. Nakashima and T. Kawai, J. Photochem. Photobiol., C, 2018, 34, 41–51 CrossRef CAS.
- J. V. Crivello and E. Reichmanis, Chem. Mater., 2014, 26, 533–548 CrossRef CAS.
- D. A. Nicewicz and D. W. C. MacMillan, Science, 2008, 322, 77–80 CrossRef CAS PubMed.
- J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC.
- X. Zhang, S. Hu, Q. Ma and S. Liao, Polym. Chem., 2020, 11, 3709–3715 RSC.
- X. Zhang, Q. Ma, Y. Jiang, S. Hu, J. Li and S. Liao, Polym. Chem., 2021, 12, 885–892 RSC.
- N. Guy, O. Giani, S. Blanquer, J. Pinaud and J.-J. Robin, Prog. Org. Coat., 2021, 153, 106159 CrossRef CAS.
- X. Lopez de Pariza, E. Cordero Jara, N. Zivic, F. Ruipérez, T. E. Long and H. Sardon, Polym. Chem., 2021, 12, 4035–4042 RSC.
- M. J. Supej, E. A. McLoughlin, J. H. Hsu and B. P. Fors, Chem. Sci., 2021, 12, 10544–10549 RSC.
- N. Zivic, P. K. Kuroishi, F. Dumur, D. Gigmes, A. P. Dove and H. Sardon, Angew. Chem., Int. Ed., 2019, 58, 10410–10422 CrossRef CAS PubMed.
- M. Valle, M. Ximenis, X. Lopez de Pariza, J. M. W. Chan and H. Sardon, Angew. Chem., Int. Ed., 2022, 61, e202203043 CrossRef CAS PubMed.
- Z. Shi, P. Peng, D. Strohecker and Y. Liao, J. Am. Chem. Soc., 2011, 133, 14699–14703 CrossRef CAS PubMed.
- R. Mizutsu, R. Asato, C. J. Martin, M. Yamada, Y. Nishikawa, S. Katao, M. Yamada, T. Nakashima and T. Kawai, J. Am. Chem. Soc., 2019, 141, 20043–20047 CrossRef CAS PubMed.
- R. Li, T. Nakashima, R. Kanazawa, O. Galangau and T. Kawai, Chem. – Eur. J., 2016, 22, 16250–16257 CrossRef CAS PubMed.
- H. L. Lee, S. O. Jeon, I. Kim, S. C. Kim, J. Lim, J. Kim, S. Park, J. Chwae, W.-J. Son, H. Choi and J. Y. Lee, Adv. Mater., 2022, 34, 2202464 CrossRef CAS PubMed.
- A. Obolda, Q. Peng, C. He, T. Zhang, J. Ren, H. Ma, Z. Shuai and F. Li, Adv. Mater., 2016, 28, 4740–4746 CrossRef CAS PubMed.
- K. Li, Y. Zhu, B. Yao, Y. Chen, H. Deng, Q. Zhang, H. Zhan, Z. Xie and Y. Cheng, Chem. Commun., 2020, 56, 5957–5960 RSC.
- C. Tang, R. Bi, Y. Tao, F. Wang, X. Cao, S. Wang, T. Jiang, C. Zhong, H. Zhang and W. Huang, Chem. Commun., 2015, 51, 1650–1653 RSC.
- F. Lombeck, H. Komber, D. Fazzi, D. Nava, J. Kuhlmann, D. Stegerer, K. Strassel, J. Brandt, A. D. de Zerio Mendaza, C. Müller, W. Thiel, M. Caironi, R. Friend and M. Sommer, Adv. Energy Mater., 2016, 6, 1601232 CrossRef.
- J. Ouyang, F. Wu, X. Zhao and X. Yang, Small, 2022, 18, 2201769 CrossRef CAS PubMed.
- O. G. Poluektov, J. Niklas, K. L. Mardis, S. Beaupré, M. Leclerc, C. Villegas, S. Erten-Ela, J. L. Delgado, N. Martín, A. Sperlich and V. Dyakonov, Adv. Energy Mater., 2014, 4, 1301517 CrossRef.
- Y. Kawanishi, Y. Segawa, K. Mutoh, J. Abe and Y. Kobayashi, Chem. Commun., 2022, 58, 4997–5000 RSC.
- Y. Li, F. Gu, B. Ding, L. Zou and X. Ma, Sci. China: Chem., 2021, 64, 1297–1301 CrossRef CAS.
- F. Hu, M. Cao, X. Ma, S. H. Liu and J. Yin, J. Org. Chem., 2015, 80, 7830–7835 CrossRef CAS PubMed.
- W. Miao, S. Wang and M. Liu, Adv. Funct. Mater., 2017, 27, 1701368 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, Fig. S1–S20 and Table S1. CCDC 2203143, 2202708 and 2202707. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc04941d |
| This journal is © The Royal Society of Chemistry 2022 |