Open Access Article
Open Access ArticleElectronic, steric and catalytic properties of N-heterocyclic carbene rhodium(I) complexes linked to (metallo)porphyrins†
Ludivine
Poyac
a,
Stefano
Scoditti
 b,
Xavier
Dumail
a,
Michel
Granier
a,
Sébastien
Clément
b,
Xavier
Dumail
a,
Michel
Granier
a,
Sébastien
Clément
 a,
Rafael
Gramage-Doria
a,
Rafael
Gramage-Doria
 c,
Charles H.
Devillers
c,
Charles H.
Devillers
 d and
Sébastien
Richeter
d and
Sébastien
Richeter
 *a
*a
aICGM, Univ Montpellier, CNRS, ENSCM, Montpellier 34293, France. E-mail: sebastien.richeter@umontpellier.fr
bDepartment of Chemistry and Chemical Technologies, Univ of Calabria, Via P. Bucci, Rende 87036, Italy
cUniv Rennes, CNRS, ISCR-UMR 6226, Rennes, F-35000, France
dICMUB UMR 6302, CNRS, Univ Bourgogne Franche-Comté, 9 avenue Alain Savary, Dijon 21078, France
First published on 7th November 2022
Abstract
Electronic and steric properties of NHC ligands functionalized with porphyrins were investigated. When porphyrins are used as NHC-wingtips, nickel(II) in the macrocyle significantly improves the catalytic activity of the neighbouring NHC-Rh(I) complex in the conjugate addition of phenylboronic acid to cyclohexen-2-one.
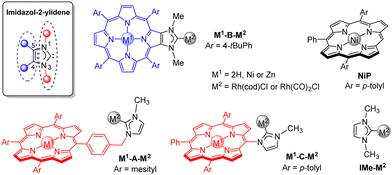
N-Heterocyclic carbenes (NHCs) are ubiquitous ligands in homogenous transition metal catalysis.1 Rationalizing catalytic properties of NHC-metal complexes mainly consists in investigating electronic and steric properties of NHCs. Imidazol-2-ylidenes are among the most commonly used NHCs in catalysis. Their electronic properties are affected by the substituents on C4 and C5 atoms and by the wingtip groups on N1 and N3 atoms, while their steric properties are mainly affected by the size and the rigidity of the wingtip groups oriented toward the metal centre (Fig. 1).2 Descriptors like Tolman electronic parameter (TEP) and percent of buried volumes (%Vbur) are routinely used to quantify electronic and steric properties of NHCs, respectively.2,3
Several NHCs linked to porphyrins were reported4 and used in organocatalysis5 and transition metal catalysis.6 Here, we propose to investigate electronic, steric and catalytic properties of three types of NHC-Rh(I) complexes linked to porphyrins, namely M1-A-M2, M1-B-M2 and M1-C-M2, where M1 is the metal in the porphyrin core (2H, Zn or Ni) and M2 is the outer Rh(cod)Cl (cod = 1,5-cyclooctadiene) or Rh(CO)2Cl complex (Fig. 1). In complexes M1-A-M2, porphyrins and NHCs are separated by benzyl groups. In complexes M1-B-M2, porphyrins and NHCs are fused together and the C4 and C5 atoms of NHCs correspond to two neighbouring β-pyrrolic carbon atoms. This rigid structure ensures that the inner metal M1 is remote from the outer metal M2. In complexes M1-C-M2, porphyrins are wingtips of NHCs and both are linked together through the formation of Cmeso–NNHC bonds. In this case, inner metal M1 and outer metal M2 are in close proximity (∼5–6 Å) and cooperativity between them may be observed. For example, the inner metal M1 can be used as binding site to fix substrates near the peripheral catalytic site in order to increase catalyst activity and/or selectivity.7 Finally, 1,3-dimethylimidazol-2-ylidene ligand IMe is used as reference NHC. (NHC)Rh(cod)Cl and (NHC)Rh(CO)2Cl complexes were used to assess steric and electronic properties, respectively. Then, catalytic properties of (NHC)Rh(cod)Cl complexes were investigated for the conjugate addition of phenylboronic acid to cyclohexen-2-one.
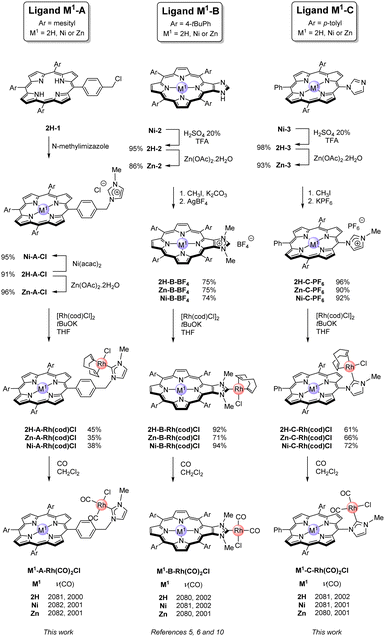
Imidazolium salts used as NHC precursors were obtained following synthetic strategies depicted in Scheme 1. Imidazolium salts M1-A-Cl were obtained by first synthesizing the free-base meso 5-(4’-chloromethylphenyl)-10,15,20-tris(mesityl)porphyrin 2H-1.8 SN reaction with N-methylimidazole afforded imidazolium salt 2H-A-Cl. Then, metalation reactions afforded the corresponding Ni(II) and Zn(II) porphyrins (Ni-A-Cl and Zn-A-Cl). Imidazolium salts M1-B-BF4 and M1-C-PF6 were obtained by first synthesizing the corresponding Ni(II) porphyrins bearing an imidazole ring fused to a pyrrole (Ni-2) or on a meso position (Ni-3) following our reported procedures.9 Then, demetalation (TFA/H2SO4 20%) and remetalation with Zn(II) afforded the corresponding free-base porphyrins (2H-2 and 2H-3) and Zn(II) porphyrins (Zn-2 and Zn-3), respectively. SN reactions of these porphyrins with CH3I and anion metathesis reactions afforded imidazolium salts M1-B-BF4 and M1-C-PF6 (M1 = 2H, Ni or Zn). Complexes M1-A-Rh(cod)Cl, M1-B-Rh(cod)Cl and M1-C-Rh(cod)Cl (M1 = 2H, Ni or Zn) were obtained by reacting imidazolium salts with tBuOK and [Rh(cod)Cl]2 (Scheme 1).5,6,10 Formation of the CNHC–Rh bonds was confirmed by the absence of the signals of the imidazolium protons in their 1H NMR spectra and by the characteristic doublets observed at δ ∼ 180–190 ppm in their 13C{1H} NMR spectra. Characteristic signals due to coordinated cod ligands were also observed by 1H NMR spectroscopy.
Measuring CO stretching wavenumbers of (NHC)Rh(CO)2Cl complexes by FTIR spectroscopy allowed to assess the electronic properties of NHCs and determine their TEP values.11 Complexes M1-A-Rh(CO)2Cl, M1-B-Rh(CO)2Cl and M1-C-Rh(CO)2Cl were obtained by bubbling CO in solutions of the corresponding (NHC)Rh(cod)Cl complexes in CH2Cl2 (Scheme 1, these complexes were generated in situ and not isolated). Two CO stretching wavenumbers were observed in the FTIR spectra of (NHC)Rh(CO)2Cl complexes confirming the cis geometry of all Rh(I) complexes (Scheme 1) and allowing the determination of TEP values (Table 1), which are in the same range (2052.6–2053.4 cm−1) and similar to the TEP value of IMe (2053.0 cm−1). These data show that the localization of the porphyrin has no or very weak inductive effect on the NHCs. Likewise, porphyrins exert similar inductive electronic effects, irrespective of the nature of the inner metal M1 used in this study (M1 = 2H, Ni or Zn). It is thus reasonable to consider that all NHCs presented here transfer similar amounts of electron density to Rh(I).
| Entry | NHC ligand | TEPa (cm−1) | %Vburb | % conv.c (% yield) 60 °C | % conv.c (% yield) 100 °C |
|---|---|---|---|---|---|
| a ν(CO) (cm−1) measured in CH2Cl2 and TEP values calculated using the equation: TEP = 0.8001 × νav(CO) + 420 cm−1, see ref. 10. b %Vbur values calculated with the SambVca 2.1 tool and the standard inputs given in Fig. 3. c Reaction conditions: 0.5 mmol of cyclohexen-2-one, 1.1 mmol of phenylboronic acid and 0.09 mmol of KOH in 2 mL of toluene with 0.2% mol catalyst loading, 60 or 100 °C, under argon. Conversions (% conv.) and yields (% yield) after 3 hours were determined by gas chromatography (GC) using anisole as internal standard and are the average values of three independent and reproducible experiments. | |||||
| 1 | IMe | 2053.0 | 26.0 | 41 (40) | 63 (58) |
| 2 | 2H-A | 2052.6 | 31.0 | 38 (26) | 55 (38) |
| 3 | Zn-A | 2053.4 | 31.0 | 37 (27) | 51 (45) |
| 4 | Ni-A | 2053.4 | 30.9 | 38 (25) | 56 (44) |
| 5 | 2H-B | 2053.0 | 26.9 | 30 (19) | 45 (34) |
| 6 | Zn-B | 2052.6 | 27.0 | 37 (33) | 55 (51) |
| 7 | Ni-B | 2053.4 | 26.8 | 33 (26) | 52 (46) |
| 8 | 2H-C | 2053.4 | 32.4 | 14 (4) | 46 (35) |
| 9 | Zn-C | 2052.6 | 31.5 | 13 (7) | 58 (52) |
| 10 | Ni-C | 2053.0 | 32.0 | 12 (1) | 91 (87) |
Steric pressures exerted by NHCs in the primary coordination sphere of Rh(I) were assessed by calculating their %VBur values and their steric maps to figure out ligands anisotropy. For this purpose, DFT calculations were first performed to optimize geometries of complexes M1-A-Rh(cod)Cl, M1-B-Rh(cod)Cl and M1-C-Rh(cod)Cl (see ESI†). Then, %Vbur values (Table 1) and steric maps presenting the buried space around Rh(I) along the CNHC–Rh(I) axes (Fig. 2 and ESI†) were calculated with SambVca 2.1.12 NHCs M1-A have %Vbur values in the range of 30.9–31.0% (Table 1, entries 2–4), but these values should be taken with caution since steric pressure is due to benzyl groups (yellow-orange area on the left side of Ni-A in Fig. 2). Indeed, only one conformation is used to calculate %Vbur values and steric maps, but benzylic sp3 carbon atoms ensure enough flexibility to adapt the steric bulk and conformations with %Vbur as low as ∼26.5% are possible.13 NHCs M1-B possess %Vbur values in the range of 26.8–27.0% (Table 1, entries 5–7) and similar to the %Vbur value of NHC IMe (26.0%). Steric maps of NHCs M1-B and IMe are similar because the steric pressure is due to the two N-methyl groups of the NHCs (see Ni-B and IMe in Fig. 2).
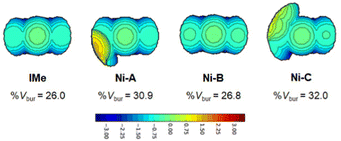 | ||
| Fig. 2 Steric maps and %Vbur values of NHCs IMe, Ni-A, Ni-B and Ni-C calculated with SambVca 2.1 with the following standard inputs: bondi radii 1.17 Å, Rh–CNHC 2.00 Å, rsphere = 3.50 Å, mesh spacing 0.10 Å, H atoms excluded, see ref. 11. | ||
As expected, NHCs M1-C with porphyrin wingtips are more bulky with %Vbur in the range of 31.5–32.4% (Table 1, entries 8–10). The broad greenish areas on the left side of their steric maps are due to the porphyrin cores (see Ni-C in Fig. 2). Thus, it is clear that porphyrins exert similar steric pressure in the primary coordination sphere of Rh(I), irrespective of the nature of the inner metal M1 used in this study (M1 = 2H, Ni or Zn). However, M1 may impact the secondary coordination sphere of Rh(I) by modifying the shape of the porphyrin core.14 To illustrate this point, DFT optimized structures of complexes M1-C-Rh(cod)Cl are represented in Fig. 3. Porphyrins of complexes 2H-C-Rh(cod)Cl and Zn-C-Rh(cod)Cl are rather flat (free base porphyrin is slightly saddle-shaped), while Ni(II) porphyrin in complex Ni-C-Rh(cod)Cl adopts a ruffled distortion in agreement with X-ray structures of similar NHCs reported by Ruppert, Weiss and coworkers.15 It is also noticeable that the ruffled distortion of the porphyrin has two major consequences: the Ni(II) porphyrin wraps the Rh(I) complex along its C5–C15 axis and both metal ions get closer. Indeed, the Rh–Ni distance of ∼5.08 Å in complex Ni-C-Rh(cod)Cl is slightly shorter than the Rh–Zn distance of ∼5.25 Å in complex Zn-C-Rh(cod)Cl (Fig. 3).
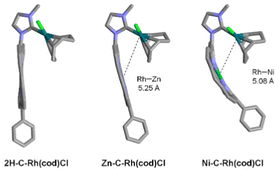 | ||
| Fig. 3 DFT optimized structures of complexes M1-C-Rh(cod)Cl with M1 = 2H, Zn and Ni (meso p-tolyl groups and H omitted for clarity). | ||
Catalytic properties of (NHC)Rh(cod)Cl complexes were investigated in the conjugate addition of phenylboronic acid to cyclohexen-2-one.16 Reactions were performed for 3 hours in toluene at 60 and 100 °C in the presence of KOH and catalyst loading of 0.2 mol% (Table 1), and were monitored by GC and UV-vis absorption spectroscopy (see ESI†). We observed that complexes IMe-Rh(cod)Cl (entry 1), M1-A-Rh(cod)Cl (entries 2–4) and M1-B-Rh(cod)Cl (entries 5–7) afforded rather similar reaction outcomes and time profiles (see ESI†), although slightly better conversion and yield were obtained with IMe-Rh(cod)Cl. Thus, porphyrins and their inner metal ions have no or very weak influence on the catalytic properties of the corresponding Rh(I) complexes. It can be explained by the fact that NHCs IMe, M1-A and M1-B possess similar electronic properties. Likewise, steric properties of NHCs IMe and M1-B are also very similar. In the case of NHCs M1-A, flexibility is ensured by benzylic sp3 carbon atoms to adapt the steric bulk (vide supra) and porphyrins are too far from Rh(I) complexes to modulate the catalytic activity.
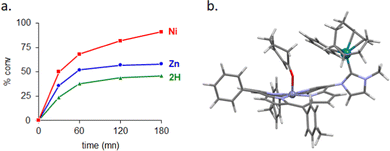
Interesting catalytic properties were observed for the three complexes M1-C-Rh(cod)Cl (Table 1, entries 8–10). At 60 °C, they are significantly less active compared to the other catalysts (lower conversions and yields) and it may be attributed to the stronger steric pressure induced by the porphyrin wingtips. At 100 °C, catalytic activity of complexes M1-C-Rh(cod)Cl depends on the nature of M1 in the order 2H < Zn < Ni (see time profiles of the reactions in Fig. 4(a)). Complex Ni-C-Rh(cod)Cl is surprisingly more active with conversion and yield of 91% and 87%, respectively (Table 1, entry 10). This complex is approximately two times more active than complex 2H-C-Rh(cod)Cl. Rate enhancement is only observed when the Ni(II) porphyrin is used as NHC wingtip. Indeed, there is no significant rate enhancement if the Ni(II) porphyrin and the NHC are not connected together (see reaction catalysed by IMe-Rh(cod)Cl + NiPvs. reaction catalysed by IMe-Rh(cod)Cl alone in the ESI†) or if they are connected but distant from each other.
Thermodynamic stability of complexes 2H-C-Rh(cod)Cl and Ni-C-Rh(cod)Cl presenting the lowest and highest catalytic activities, respectively, was investigated. For this purpose, they were treated with KOH (100 eq.) in toluene at 100 °C and reaction mixtures were analyzed by 13C{1H} NMR spectroscopy (see ESI†). Complex 2H-C-Rh(cod)Cl is stable and no significant change could be observed after 3 hours. Its lower catalytic activity does not therefore seem to be due to its lower thermodynamic stability although its decomposition in the presence of the reaction substrates (phenylboronic acid and cyclohexen-2-one) cannot be totally excluded to explain the significant drop in catalytic activity after 1 hour. Complex Ni-C-Rh(cod)Cl is also stable for at least 30 minutes suggesting that its high initial catalytic activity is due to its kinetic activity. We also observed that complex Ni-C-Rh(cod)Cl slowly decomposed over time. Indeed, characteristic signals of carbene and cod ligands disappeared after 3 hours. This gradual decomposition may also explain the redshift of the Soret absorption band observed by UV-vis absorption spectroscopy after 1 hour reaction (see ESI†).
It is obvious that Ni(II) plays a significant role in the enhancement in the reaction rate, but this effect cannot be attributed to changes in inductive electronic effects or steric pressures exerted by NHCs, as previously shown. Although the exact mechanism of this cooperative effect between Rh(I) and Ni(II) remains unclear at this stage, we believe that the ruffled porphyrin core and the short Rh–Ni distance of ∼5 Å play significant roles in substrate-preorganization and reaction rate enhancement (Fig. 4(b)).17 Extending the scope of the reaction to other substrates and detailed kinetic studies will further be needed to understand the exact role of Ni(II) in this reaction.
In summary, catalytic properties of molecular systems combining porphyrins and (NHC)Rh(cod)Cl complexes were investigated in the conjugate addition of phenylboronic acid to cyclohexen-2-one. Preliminary data show that catalytic activity of Rh(I) complexes is modulated by the metal in the porphyrin core when the macrocycle is used as NHC wingtip. In this case, we have shown that NHCs with free-base, Zn(II) and Ni(II) porphyrin wingtips transfer similar amount of electron density to Rh(I) and exert similar steric pressure in the primary coordination sphere of Rh(I). However, we found that Ni(II) in the porphyrin macrocycle dramatically increases the catalytic activity of the neighbouring Rh(I) complex. NHC-metal complexes with porphyrin wingtips are thus capable of cation-tunable reactivity and additional studies are underway to understand this phenomenon. Supramolecular catalysis and fine tuning of the catalytic activity of other NHC-metal complexes with porphyrins in close proximity are also actively explored in our group.18,19
We are grateful for the financial support of the French Agence Nationale de la Recherche (Grants ANR-19-CE07-0009 and ANR-19-CE07-0039), the University of Montpellier, the University of Rennes 1 and the Centre National de la Recherche Scientifique (CNRS). S. Scoditti is grateful for the financial support of Calabria Region (project POR Calabria-FSE/FESR 2014–2020).
Conflicts of interest
There are no conflicts to declare.Notes and references
- F. Glorius, N-Heterocyclic Carbenes in Catalysis – An Introduction, in, N-Heterocyclic Carbenes in Transition Metal Catalysis. Topics in Organometallic Chemistry, Springer, Berlin, Heidelberg, 2006, vol. 21 CrossRef CAS PubMed; S. Díez-González, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612–3676 CrossRef CAS PubMed; P. de Frémont, N. Marion and S. P. Nolan, Coord. Chem. Rev., 2009, 253, 862–892 CrossRef; M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef PubMed; E. Peris, Chem. Rev., 2018, 118, 9988–10031 CrossRef PubMed.
- D. G. Gusez, Organometallics, 2009, 28, 6458–6461 CrossRef.
- H. V. Huynh, Chem. Rev., 2018, 118, 9457–9492 CrossRef CAS PubMed; S. Díez-González and S. P. Nolan, Coord. Chem. Rev., 2007, 251, 874–883 CrossRef; D. J. Nelson and S. P. Nolan, Chem. Soc. Rev., 2013, 42, 6723–6753 RSC; A. Gómez-Suárez, D. J. Nelson and S. P. Nolan, Chem. Commun., 2017, 53, 2650–2660 RSC.
- J.-F. Longevial, C. Rose, L. Poyac, S. Clément and S. Richeter, Eur. J. Inorg. Chem., 2021, 776–791 CrossRef CAS.
- J.-F. Lefebvre, M. Lo, J.-P. Gisselbrecht, O. Coulembier, S. Clément and S. Richeter, Chem. – Eur. J., 2013, 19, 15652–15660 CrossRef CAS PubMed.
- J.-F. Lefebvre, J.-F. Longevial, K. Molvinger, S. Clément and S. Richeter, C. R. Chimie, 2016, 19, 94–102 CrossRef CAS.
- J. Trouve, P. Zardi, S. Al-Shehimy, T. Roisnel and R. Gramage-Doria, Angew. Chem., Int. Ed., 2021, 60, 18006–18013 CrossRef CAS PubMed; P. Zardi, T. Roisnel and R. Gramage-Doria, Chem. – Eur. J., 2019, 25, 627–634 Search PubMed.
- J. S. Lindsey, I. C. Schreiman, H. C. Hsu, P. C. Kearney and A. M. Marguerettaz, J. Org. Chem., 1987, 52, 827–836 CrossRef CAS.
- J.-F. Lefebvre, D. Leclercq, J.-P. Gisselbrecht and S. Richeter, Eur. J. Org. Chem., 2010, 1912–1920 CrossRef CAS; C. H. Devillers, S. Hebié, D. Lucas, H. Cattey, S. Clément and S. Richeter, J. Org. Chem., 2014, 79, 6424–6434 CrossRef PubMed.
- J.-F. Lefebvre, M. Lo, D. Leclercq and S. Richeter, Chem. Commun., 2011, 47, 2976–2978 RSC.
- C. A. Tolman, Chem. Rev., 1977, 77, 313–348 CrossRef CAS; A. R. Chianese, X. Li, M. C. Janzen, J. W. Faller and R. H. Crabtree, Organometallics, 2003, 22, 1663–1667 CrossRef; T. Dröge and F. Glorius, Angew. Chem., Int. Ed., 2010, 49, 6940–6952 CrossRef PubMed; S. Wolf and H. Plenio, J. Organomet. Chem., 2009, 694, 1487–1492 CrossRef.
- L. Falivene, Z. Cao, A. Petta, L. Serra, A. Poater, R. Oliva, V. Scarano and L. Cavallo, Nat. Chem., 2019, 11, 872–879 CrossRef CAS PubMed.
- %Vbur calculated with the X-ray crystal structure of a Rh(I) complex with 1-benzyl-3-methylimidazol-2-ylidene ligand in its coordination sphere, see the following reference: M. C. Cassani, M. A. Brucka, C. Femoni, M. Mancinelli, A. Mazzanti, R. Mazzoni and G. Solina, New J. Chem., 2014, 38, 1768–1779 RSC.
- S. J. Kingsbury and M. O. Senge, Coord. Chem. Rev., 2021, 431, 213760 CrossRef.
- J. Haumesser, J.-P. Gisselbrecht, J. Weiss and R. Ruppert, Chem. Commun., 2012, 48, 11653–11655 RSC; J. Haumesser, J.-P. Gisselbrecht, L. Karmazin-Brelot, C. Bailly, J. Weiss and R. Ruppert, Organometallics, 2014, 33, 4923–4930 CrossRef CAS.
- B. J. Truscott, G. C. Fortman, A. M. Z. Slawin and S. P. Nolan, Org. Biomol. Chem., 2011, 9, 7038–7041 RSC; S. Ruiz-Botella and E. Peris, ChemCatChem, 2018, 10, 1874–1881 CrossRef CAS; I. Bratko, G. Guisado-Barrios, I. Favier, S. Mallet-Ladeira, E. Teuma, E. Peris and M. Gómez, Eur. J. Org. Chem., 2014, 2160–2167 CrossRef; I. Peñafiel, I. M. Pastor, M. Yus, M. A. Esteruelas and M. Oliván, Organometallics, 2012, 31, 6154–6161 CrossRef; C. Mejuto, G. Guisado-Barrios and E. Peris, Organometallics, 2014, 33, 3205–3211 CrossRef; B. Ramasamy, A. P. Prakasham, M. K. Gangwar and P. Ghosh, ChemistrySelect, 2019, 4, 8526–8533 CrossRef.
- N. Abuhafez, A. Perennes and R. Gramage-Doria, Synthesis, 2022, A-I Search PubMed; M. Dommaschk, C. Schütt, S. Venkataramani, U. Jana, C. Näther, F. D. Sönnichsen and R. Herges, Dalton Trans., 2014, 43, 17395–17405 RSC; M. P. Byrn, C. J. Curtis, Y. Hsiou, S. I. Khan, P. A. Sawin, S. K. Tendick, A. Terzis and C. E. Strouse, J. Am. Chem. Soc., 1993, 115, 9480–9497 CrossRef CAS.
- L. Kovbasyuk and R. Krämer, Chem. Rev., 2004, 104, 3161–3187 CrossRef CAS PubMed; C. G. Oliveri, P. A. Ulmann, M. J. Wiester and C. A. Mirkin, Acc. Chem. Res., 2008, 41, 1618–1629 CrossRef PubMed; M. J. Wiester, P. A. Ulmann and C. A. Mirkin, Angew. Chem., Int. Ed., 2011, 50, 114–137 CrossRef PubMed; M. Raynal, P. Ballester, A. Vidal-Ferran and P. W. N. M. van Leeuwen, Chem. Soc. Rev., 2014, 43, 1660–1733 RSC; M. Raynal, P. Ballester, A. Vidal-Ferran and P. W. N. M. van Leeuwen, Chem. Soc. Rev., 2014, 43, 1734–1787 RSC; V. Blanco, D. A. Leigh and V. Marcos, Chem. Soc. Rev., 2015, 44, 5341–5370 RSC; C. Yoo, H. M. Dodge and A. J. M. Miller, Chem. Commun., 2019, 55, 5047–5059 RSC; J. Trouve and R. Gramage-Doria, Chem. Soc. Rev., 2021, 50, 3565–3584 RSC.
- Examples of porphyrin-based catalysts capable of inner metal-tunable reactivity: S. Yamaguchi, T. Katoh, H. Shinokubo and A. Osuka, J. Am. Chem. Soc., 2007, 129, 6392–6393 CrossRef CAS PubMed; Y. Matano, K. Matsumoto, T. Shibano and H. Imahori, J. Porphyrins Phthalocyanines, 2011, 15, 1172–1182 CrossRef; B. M. J. M. Suijkerbuijk, S. D. Herreras Martínez, G. van Koten and K. J. M. Klein Gebbink, Organometallics, 2008, 4, 534–542 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, synthetic procedures, NMR spectra, mass spectra, computational details and catalytic studies. See DOI: https://doi.org/10.1039/d2cc05547c |
| This journal is © The Royal Society of Chemistry 2022 |