Open Access Article
Open Access ArticleSelf-flux-grown Ba4Fe4ClO9.5−x crystals exhibiting structures with tunable modulation†
Alla
Arakcheeva
 *ab,
Wen Hua
Bi
a,
Priya Ranjan
Baral
*ab,
Wen Hua
Bi
a,
Priya Ranjan
Baral
 a and
Arnaud
Magrez
a and
Arnaud
Magrez
 a
a
aSB, IPHYS, Crystal Growth Facility, Ecole Polytechnique Fédérale de Lausanne, Lausanne 1015, Switzerland. E-mail: alla.arakcheeva@epfl.ch; allaarakcheeva@gmail.com; arnaud.magrez@epfl.ch
bPhase Solutions Co Ltd, ch. des Mésanges 7, Lausanne 1012, Switzerland
First published on 21st April 2022
Abstract
The synthesis and X-ray structural study of the new family of compounds Ba4Fe4ClO9.5−x with tunable structural modulation are reported. The framework of the structure has the Ba2Fe4O9.5−x composition, with open hexagonal channels extending along the c-axis. The channels are filled with linear [Ba–Cl–Ba] triplets. The oxygen stoichiometry and the oxidation state of iron both are controlled by the redox conditions during crystal preparation. The modulation of the crystal structure arises from the distribution of the oxygen atoms in the framework and iron coordination polyhedra are a combination of FeO4-tetrahedra, FeO5-bipyramids, and FeO6-octahedra. The structure modulation also originates from the ordered or disordered distribution of the [Ba–Cl–Ba] triplets filling the channels which is also affected by the conditions of the thermal treatment of the crystals. The structure investigation reveals a composition variation from Ba4Fe4ClO9.5 (x = 0), in which Fe exhibits a 3+ oxidation state, to Ba4Fe4ClO8 (x = 1.5) with the framework built exclusively of FeO4 tetrahedra.
Introduction
Iron-based compounds are an important class of materials for a large spectrum of research areas and applications, including catalysis,1 energy storage,2 and magnetic and electric solids/devices.3,4 Three key factors of iron contribute significantly to these versatilities: the possibility of existing in a wide range of (a) oxidation states and (b) coordination numbers and (c) the ability of iron to bind to a large range of ligands. The oxidation state of Fe varies from 0 in alloys to 6+ in electrochemically grown MFeO4 with M = K2, Ba, Sr, Cu.5 The most common oxidation states are Fe2+ and Fe3+. Higher oxidation states of Fe are always obtained under special growth conditions which differ from the ones applied in this study. In addition, iron atoms can adopt different coordination numbers, namely 4 (in square planar or tetragonal geometry) and up to 8 (in dodecahedral or pseudo-cubic geometry). The prevalent coordination polyhedra are tetrahedron, octahedron, and trigonal Fe-bipyramid.6,7 Among the plethora of Fe-based compounds, about 150 inorganic or organic–inorganic hybrid oxychlorides can be found in the ICSD and COD databases.8,9 FeOCl, the simplest iron oxychloride, exhibits a two-dimensional structure.10 Structure and Fe oxidation state are controlled by the intercalation of organic molecules or of alkali atoms between the well-separated layers of FeOCl.11Among the two known examples of barium-containing iron oxychlorides, Ba3Fe2O5Cl2 is a chiral insulating magnet crystallizing in the cubic space group I213.12 The Fe3+-containing corner-sharing tetrahedra form pseudo two-dimensional 10-membered rings. This compound undergoes an antiferromagnetic (AFM) transition (TN) at 564 K and shows an AFM to weak ferromagnetic transition (TS) at 140 K, accompanied with a structural distortion. In the second example, 10H–BaFeCl0.13O3−y, cubic and hexagonal layers are stacked in the hhchc order.13 While chlorine is located only in the hexagonal layer, oxygen vacancies are distributed over both layers. The iron atoms are located in face-sharing octahedra as well as in dimers of corner-sharing tetrahedra. 10H–BaFeCl0.13O3−y has a TN of 730 K and undergoes an AFM to ferrimagnetic transition below 400 K. The high-temperature magnetism makes the barium iron oxychlorides interesting from the magnonics point of view, among other potential applications.14
Herein, we report on the synthesis and structural characterization of a new barium iron oxychloride. Ba4Fe4ClO9.5−x crystals with mixed-valence iron (2+/3+) are synthesized as black needles (Fig. 1), exhibiting a modulated crystal structure. Based on detailed single-crystal X-ray diffraction (XRD) data, we found Ba4Fe4ClO9.5−x to exhibit a modulated crystal structure with modulations depending on the conditions of crystal growth and post-growth annealing. The iron atoms are found to be in tetrahedral, bipyramidal as well as octahedral coordination by O atoms. The large hexagonal channels formed by the structure framework are filled with [Ba–Cl–Ba] linear triplets, which are mobile. Their distribution is influenced by the annealing conditions. In addition to the magnetic properties, such an open framework compound with potentially replaceable fillers could be interesting for chemical separation, for synthesis as well as for catalysis.
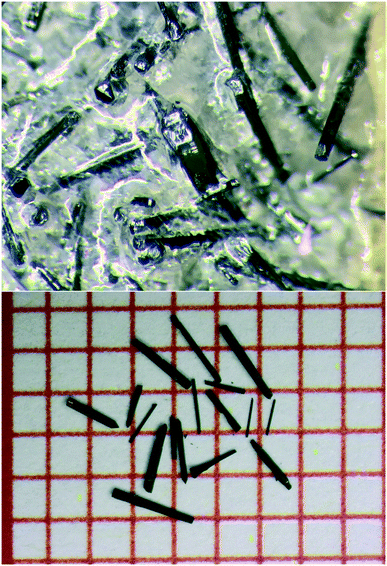 | ||
| Fig. 1 (Top) As-grown Ba4Fe4ClO9.4(1) crystals in BaCl2 flux. (Bottom) Ba4Fe4ClO9.0(1) crystals, annealed at 800 °C in an Ar/H2 flow, on a grid paper. The spacing between the lines is 1 mm. | ||
Experimental
Growth of Ba4Fe4ClO9.5−x crystals
Single crystals of Ba4Fe4ClO9.5−x were prepared in a BaCl2 flux. BaCO3, FeC2O4·2H2O, and BaCl2 were mixed in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The mixture was transferred into an alumina crucible and heated at 1000 °C for 24 hours. After cooling from 1000 °C to 900 °C at a rate of 1 °C h−1, the mixture was quenched to room temperature. In a typical growth experiment, 3.1707 g of FeC2O4·2H2O, 3.4781 g of BaCO3 and 18.3513 g of BaCl2 are mixed. From this mixture, about 1.5 g of Ba4Fe4ClO9.5−x crystals are obtained which corresponds to a yield of approximately 33%. The BaCl2 excess used as flux was subsequently washed with deionized water. The entire growth process proceeded in air. Crystals are stable in air and in water.
10. The mixture was transferred into an alumina crucible and heated at 1000 °C for 24 hours. After cooling from 1000 °C to 900 °C at a rate of 1 °C h−1, the mixture was quenched to room temperature. In a typical growth experiment, 3.1707 g of FeC2O4·2H2O, 3.4781 g of BaCO3 and 18.3513 g of BaCl2 are mixed. From this mixture, about 1.5 g of Ba4Fe4ClO9.5−x crystals are obtained which corresponds to a yield of approximately 33%. The BaCl2 excess used as flux was subsequently washed with deionized water. The entire growth process proceeded in air. Crystals are stable in air and in water.
As shown in Fig. 1, black needles with lengths of up to a few millimetres were obtained. The Ba/Fe/Cl stoichiometry is confirmed to be 4.00(4)/4.0(2)/1.0(1), by X-ray fluorescence (XRF).
Annealing of Ba4Fe4ClO9.5−x crystals in different conditions
In order to modify the oxygen stoichiometry and concurrently the oxidation state of Fe, the needles were annealed in different conditions: (i) in air at 400 °C for 16 hours, (ii) in a continuous flow of O2 (20 sccm) at 500 °C for 16 hours, (iii) in vacuum (0.1 mbar) at 800 °C for 16 hours, (iv) in an Ar/H2 flow (80 sccm/20 sccm) at 500 °C for 16 hours.The needles remained unchanged after annealing (Fig. 1). Independently of the redox conditions, the composition 4.0(2)/4.0(1)/1.00(3) was confirmed by XRF measurements.
XRF experiments
XRF measurements were performed using an OrbisPC Micro EDXRF analyzer system, equipped with a Rh micro-focus X-ray tube (50 kV and 1 mA), a 30 μm poly-capillary X-ray optics, and an Apollo XRF-ML50 silicon drift detector with an energy resolution lower than 135 eV. All the XRF measurements reported here were performed under vacuum conditions.XRD experiments
Crystals with suitable size were selected and mounted on a goniometer head with cryo-loops. All data sets were collected at room temperature and 100 K using a Rigaku Synergy-I XtaLAB X-ray diffractometer, equipped with a Mo micro-focusing source (λKα = 0.71073 Å) and a HyPix-3000 hybrid pixel array detector. The temperature was controlled by a Cryostream 800 from Oxford Cryosystems Ltd. CrysAlisPRO15 and JANA2006 software16 were used for the processing of raw experimental data and structural refinements, respectively. Structure solutions were obtained with the Superflip program.17 Other experimental details are listed in Tables S1 and S2.† Further experimental details are available as CIF files with CSD numbers 2126985–2126989 for the average structure and with CSD numbers 2127041 and 2127080 for the modulated structures. The DIAMOND program from Crystal Impact was used to draw the crystal structures.18Results and discussion
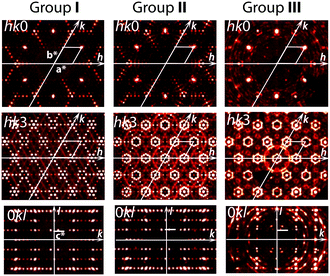
The crystal structures of the as-grown crystals and those annealed under different redox conditions have been analyzed by using: (i) reciprocal space reconstructions based on the single-crystal XRD experiments; (ii) refinements of the average structure for the modulated crystals; and (iii) refinements of the modulated structures using the superspace symmetry and a supercell presentation when possible.Analyses of the XRD reciprocal space reconstructions
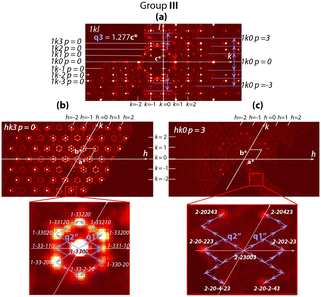
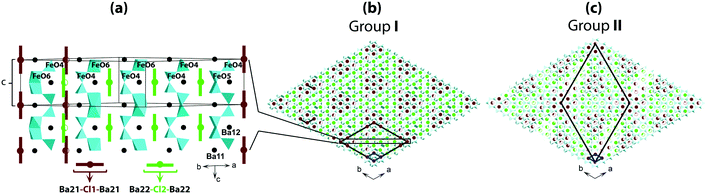
Reconstruction of the reciprocal space cross sections, which are typical for crystals prepared under different redox conditions, is shown in Fig. 2 and with more details in Fig. S1.† Three different groups can be distinguished. Group I (Fig. 2) includes the crystals prepared under oxidizing or neutral conditions, namely as-grown crystals, crystals annealed in O2, in air and in vacuum at T < 600 °C. Group II (Fig. 2) corresponds to the crystals annealed in vacuum at T > 800 °C. Crystals belonging to these two groups have similar composition, i.e. Ba4Fe4ClO9.4(1), but different crystal structure modulation. Group III (Fig. 2) contains crystals annealed under reducing atmosphere (Ar + H2). The oxygen stoichiometry of group III crystals is reduced to 9.0(1).Four significant conclusions can be drawn from the observations of the XRD patterns.
– First, all the patterns point towards hexagonal symmetry of the crystals, irrespective of the group.
– Second, the strongest reflections observed for all patterns are identical. They define the basic hexagonal unit cell (a = b ≈ 5.77 Å and c ≈ 10.0 Å) with the reciprocal parameters a*, b* and c* shown in Fig. 2.
– Third, the system of the weaker satellite reflections resulting from structure modulations is specific for each group. Namely, the satellite reflections for groups I and II suggest some modulations only in the hexagonal plane (Groups I and II, Fig. 2), while the satellite reflections of group III indicate an additional modulation along the c-axis (0kl cross sections in Fig. 2).
– Fourth, crystals belonging to these three groups can readily be distinguished by partial atomic disorder in their modulated structures. This is proved by the significant difference in diffuse scattering between the three groups (Fig. 2; more details can be seen in Fig. S1 and S2†).
Average structure determination and description
The average structure with the hexagonal unit cell parameters a ≈ 5.77 Å and c ≈ 10.0 Å was initially refined in the P63/mmc space group for all the crystals. However, the presence of weak, but clearly visible reflections 00l and hhl with l = 2n + 1 for all the crystals, contradict the 63-screw axis and the c glide plane, respectively. Consequently, lower symmetries were tested, and the best solution was obtained with the P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) m2 space group with the best reliability factor RI = 0.032–0.040. Experimental details for the average structure determination and refinement of Ba4Fe4ClO9.5−x are listed in Table S1† for crystals of groups I, II and III. The refinement results point to an oxygen stoichiometry which is group dependent. As expected from the growth and annealing conditions, crystals treated in oxidizing or neutral atmosphere (groups I and II) present a higher oxygen stoichiometry, x = 9.4(1), than that of group III crystals, x = 9.0(1), treated in reducing atmosphere.
m2 space group with the best reliability factor RI = 0.032–0.040. Experimental details for the average structure determination and refinement of Ba4Fe4ClO9.5−x are listed in Table S1† for crystals of groups I, II and III. The refinement results point to an oxygen stoichiometry which is group dependent. As expected from the growth and annealing conditions, crystals treated in oxidizing or neutral atmosphere (groups I and II) present a higher oxygen stoichiometry, x = 9.4(1), than that of group III crystals, x = 9.0(1), treated in reducing atmosphere.
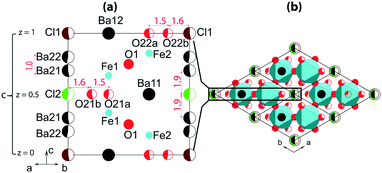
Fig. 3 shows a sketch of the average structure. The atomic sites of the structure are shown in Fig. 3a; details are listed in Table S3† for crystals prepared at different conditions. The main framework of the structure is made of Fe-polyhedra sharing vertices and faces. Half of Ba atoms are embedded in this framework of Ba2Fe4O9.5−x composition. The rest of the Ba atoms and Cl atoms are located in hexagonal channels (Fig. 3b). The atomic sites of the framework are fully occupied, with the exception of O22a and O22b at z = 0, and O21a and O21b at z = 0.5. For both z = 0 and z = 0.5, the oxygen “a” and “b” sites can only be alternately occupied due to the short 1.5 Å distance between them (red color in Fig. 3a). However, at least one O atom either “a” or “b” must be present at both z = 0 and z = 0.5 in order to complete the coordination polyhedron for each of the Fe atoms. The expected polyhedra are FeO4-tetrahedron, FeO5-bipyramid or FeO6-octahedron. The partial ordering of these O atoms is one of the main reasons for the formation of modulated structures.
Another possible reason for the structural modulation is the ordering of the filler in the channels, where the Ba- and Cl-sites are only partially occupied due to the short interatomic distance between them (red color in Fig. 3a). Cl1 and Cl2 can only be associated with two Ba21 and two Ba22, respectively, with Cl–Ba distance close to 3 Å, so that the channels can locally contain either [Ba21–Cl1–Ba21] or [Ba22–Cl2–Ba22] triplets. According to the refinement, the occupation of each triplet is close to 0.5 (Table S3†) corresponding to a total of one triplet per unit cell. An ordered distribution of the triplets can only be achieved in a modulated structure, the existence of which is confirmed by satellite reflections in the reciprocal space maps. The presence of diffuse scattering, as shown in Fig. 2, and S1 and S2,† points towards partial disorder in the distribution of the triplets.
Prediction of crystal compositions with unmodulated structure
In the following paragraphs, possible prototypes of completely ordered structures compatible with the average structure model without modulation are discussed.The first prototype is based on a framework built exclusively with FeO4 tetrahedra (right-hand sub-cell in Fig. 4). The corresponding chemical formula Ba4Fe4ClO8 imposes a ratio of 1/3 between Fe3+/Fe2+. A higher Fe2+ content, corresponding to an O stoichiometry lower than 8, is not possible as Fe cannot accommodate a coordination number lower than 4. We hypothesise that Ba4Fe4ClO8 could be produced in highly reducing conditions to maintain the average 2.25 oxidation state of Fe.
The second prototype is Ba4Fe4ClO9 (left-hand sub-cell in Fig. 4). Two out of the four tetrahedra are replaced by edge-sharing FeO5 trigonal bipyramids. The prototype composition imposes the Fe3+/Fe2+ ratio to be 3/1.
The framework composition and its charge in the Ba4Fe4ClO8 and Ba4Fe4ClO9 structures, [Ba2Fe32+Fe13+O8]3− and [Ba2Fe12+Fe33+O9]3−, respectively, are compatible with the presence of one [Ba–Cl–Ba]3+ triplet per unit cell in the hexagonal channels.
The maximum oxygen stoichiometry of 9.5 is obtained with all Fe atoms having the 3+ oxidation state. The corresponding framework with the [Ba2Fe43+O9.5]3− formula would also accommodate one [Ba2Cl]3+ triplet per unit cell in the channels. This is confirmed experimentally by the average structure refinement of crystals from both groups I and II resulting in a composition close to Ba4Fe4ClO9.5. However, the oxygen distribution cannot be completely ordered because of the short O–O as well as Ba–Cl distances as discussed in the average structure description. As concluded from the average structure refinement, the structure requires a description in a supercell or with an incommensurately modulated model. An example of a hypothetical 5a × 5b × 1c supercell is shown in Fig. 4. It corresponds to a modulated structure with an average oxygen stoichiometry close to 9.5.
In conclusion, the stoichiometry of Ba4Fe4ClO9.5−x is limited to 0 ≤ x ≤ 1.5. In the structures, a partial disorder in the distribution of the O atoms as well as the [Ba–Cl–Ba] triplets is also possible. This is discussed in more details in the following section, along with the analysis of the diffuse scattering observed in the XRD patterns.
Structural order/disorder versus crystal treatments
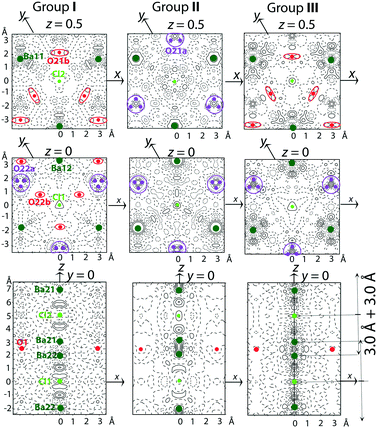
As can be observed in Fig. 2 and S1 and S2,† the diffuse scattering depends on the crystal post-growth processing conditions. The evolution of this diffuse scattering as seen by comparing the XRD patterns of groups I and II (annealed at 600 °C versus 800 °C) confirms a higher structural disorder by increasing annealing temperature in either oxygen-containing or neutral atmosphere. On the other hand, an opposite effect is seen when crystals are annealed under reducing atmosphere. Diffuse circular lines characteristic of groups I and II in hk3 reciprocal plane transform into diffuse maxima which are characteristic of group III. This means that annealing under reducing atmosphere tends towards an improved ordering of the [Ba–Cl–Ba] triplets in the channels as well as of the O atoms in the atomic planes perpendicular to the hexagonal c-axis.The residual electron density maps calculated from the average structure refinement (Fig. 5) confirm the conclusions drawn from the diffuse scattering analysis. Moreover, they provide additional information for understanding the atomic order/disorder.
In Fig. 5, the positions of Ba and Cl atoms in the channels and O atoms in the framework are shown in the maps of the residual electron density. For group I (Fig. 5), a weak density wave of ±0.5 e Å−3 in the vicinity of Ba11 and Ba12 atoms indicates an anharmonic atomic displacement (ADP). In the channels, broad maxima between Ba21, Ba22, Cl1 and Cl2 point to some disorder in the distribution of [Ba–Cl–Ba] triplets. The weak maxima of 0.5 e Å−3 near O21b and split O22b can be associated with a statistical displacement of the atoms or a position modulation of the sites which are partly vacant.
For group II (Fig. 5), the main difference from group I is an increase in the disordered distribution of [Ba–Cl–Ba] triplets in the channels. The triplet disorder affects the disorder of O atoms at z = 0 and z = 0.5 due to the Cl–O distance, which should be about 3.0 Å.
For group III (Fig. 5), the sharp maxima between Ba and Cl atoms indicate that both the triplet occupancy and position modulation in the channels are possible. In addition, some order of the triplet distribution along the c-axis is confirmed by the presence of satellite reflections in the 0kl sections of the reciprocal space (group III in Fig. 2).
The conclusions of the diffuse scattering characteristics (Fig. 2 and S1 and S2†) and the residual electron density (Fig. 5) analysis can be summarized as follows: (i) the distribution of [Ba–Cl–Ba] triplets in the channels contributes to both the structural modulations (ordering of the triplets) and diffuse scattering (disordering of the triplets); (ii) structural disorder increases with an increase in the crystal post-growth processing temperature under oxidizing and neutral conditions; (iii) the reduction of the oxygen stoichiometry imposed by the annealing in reducing atmosphere improves the ordering of the structure.
Superspace group determination for compounds treated in different conditions
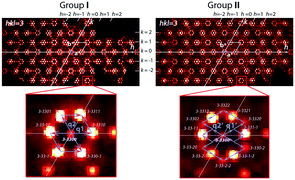
Based on the unit cell of the average structure and consideration of the satellites, the following superspace groups are determined.For crystals of group I, two modulation vectors, q1 = 1/5a* and q2 = 1/5b*, were found. Fig. 6 (see also Fig. S3a†) illustrates the reflection indexing. The absence of satellite extinction determines a unique (3 + 2)-dimension superspace group P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) m2 (α,0,0)000(−α,α,0)00 (No. 187.2.82.3).19 The value of α = 1/5, obtained as a result of the q-vector refinements during the integration of reflections, points to a commensurately modulated structure, which is therefore described with the hexagonal unit cell parameter a′ = a × 5 = 5.77 × 5 = 28.5 Å.
m2 (α,0,0)000(−α,α,0)00 (No. 187.2.82.3).19 The value of α = 1/5, obtained as a result of the q-vector refinements during the integration of reflections, points to a commensurately modulated structure, which is therefore described with the hexagonal unit cell parameter a′ = a × 5 = 5.77 × 5 = 28.5 Å.
For group II crystals (Fig. 6, group II), additional weak reflections impose two additional q-vectors, q1′ = 1/15a* + 1/15b* and q2′ = −2/15a* + 1/15b*, which are related to the previous ones as q1′ + q2′ = q1 and 2q2′ + q1′ = q2. Fig. 6, group II (see also Fig. S3b†) illustrates the relationship between the q-vectors and the indexation of reflections. Correspondingly, the superspace group was identified as (3 + 2)-dimension P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) m2 (α,α,0)000(−2α,α,0)00 (No. 187.2.83.4).19 The value of the refinement coefficient α = 1/15 points to the commensurately modulated hexagonal structure with a = 5.77 × 15 = 85.5 Å.
m2 (α,α,0)000(−2α,α,0)00 (No. 187.2.83.4).19 The value of the refinement coefficient α = 1/15 points to the commensurately modulated hexagonal structure with a = 5.77 × 15 = 85.5 Å.
The reciprocal space structure of group III is characterized by more complex patterns of satellite reflections (Fig. 7 and S4†).
In addition to the satellites defined by the refined vectors q1′′ = 1/16a* + 1/16b* and q2′′ = −2/16a* + 1/16b*, which are similar to q1′ and q2′, a third vector q3 = 1.277c* appears. It indicates additional structure modulation along the hexagonal c-axis. The satellite diffraction spots and those assigned to the average structure (Fig. 2 and 7 and S4†) are consistent with the (3 + 3)-dimension superspace group P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) m2 (α,α,0)000(−2α,α0)000(00γ)000 (No. 187.3.200.3)19 with the commensurate coefficient α = 1/16 and incommensurate coefficient γ = 1.277. Accordingly, a = 5.77 × 16 = 93.3 Å.
m2 (α,α,0)000(−2α,α0)000(00γ)000 (No. 187.3.200.3)19 with the commensurate coefficient α = 1/16 and incommensurate coefficient γ = 1.277. Accordingly, a = 5.77 × 16 = 93.3 Å.
Refinement of the Ba4Fe4ClO9.5−x modulated structures
Using the identified superspace group, the commensurately modulated structure was refined for as-grown crystals at 100 K (group I). The refinement resulted in rather good characteristics: R(all) = 0.050; wR2(all) = 0.117; R(main) = 0.030; wR2(main) = 0.072. However, the harmonic approximation needs to be applied to refine the occupancy modulation of the [Ba–Cl–Ba] triplets, as well as the O atoms. The refined occupancy varies continuously from −0.7 to 1.3 which does not provide accurate information about the occupation of either the triplets or the O21 and O22 atoms.Therefore, the supercell approximation was imposed for the structural refinement in order to estimate the O atom occupancy and, as a consequence, the oxidation state of Fe atoms. The refinement of the supercell structure was successful for the as-grown crystals and the ones annealed at 400 °C in air, at 500 °C in O2, as well as at 550 °C in vacuum. As seen in Fig. 8, the a lattice parameter is 5 times larger than that of the average structure. The details of our structural refinements given in Table S2† include the as-grown crystal using both the superspace and supercell approach, and for a crystal annealed at 400 °C in air using the supercell approximation. A CIF file corresponding to the refinement for the as-grown crystal using the supercell approximation is available with CSD number 2127041 as a representative example for group I. Illustrations of the structure are shown in Fig. 8a and b.
It can be seen that the distribution of the [Ba–Cl–Ba] triplets is partially ordered. However, the alternate occupancy of the triplets along the c-axis confirms the distribution to be partially disordered which is evident from the diffusion scattering observed in the reciprocal space reconstructions (Fig. 2, group I; and S1 and S2†). The refined chemical formula Ba4Fe4ClO9.4(1) leads to an average oxidation state of Fe to be 2.95, close to the theoretical maximum of 3.0 for iron.
While refining the structure of the crystal annealed at 800 °C in vacuum (group II), significant diffuse scattering (Fig. 2, group II) creates a big challenge to obtain a reliable refinement. In contrast to the [Ba–Cl–Ba] triplet ordering, which was successfully used, any attempt to apply the ordering of O atoms at z = 0 and 0.5 using the superspace approach was unsuccessful. Refinement in the supercell was unfeasible due to the number of refined parameters in the modulated structure which is 15 × 15 = 225 times larger than the unit cell of the average structure. Fig. 8c shows a comparison between the resulting supercell structure and the average structure. A statistical distribution of the O atoms was used, and an anharmonic approximation of their atomic displacement was applied. The best result (Table S2†), which is only an approximation of the distribution of O atoms in the crystal structure of group II, confirms an increase in the disorder of [Ba–Cl–Ba] triplets in the channels (Fig. 7c). The corresponding CIF file is available with number 2127080.
The refinement of the modulated structure of crystals annealed under reducing conditions (group III) is still in progress. However, the refined composition gives an oxygen stoichiometry close to 9.0(1). It would imply that the average oxidation state of Fe is 2.75. 25% of the Fe3+ in the as-grown crystals was reduced to Fe2+. From the refinement of the average structure, it can be claimed that the specific features of the modulated structure and its symmetry are due to partial ordering of the [Ba–Cl–Ba] triplets in the channels. In addition, although the composition of group III crystals is similar to that of the predicted non-modulated Ba4Fe4ClO9 compound, the annealing of higher oxygen-stoichiometric crystals, under reducing conditions, does not yield crystals with an ordered structure.
Powder diffraction characteristics of Ba4Fe4ClO9.5−x
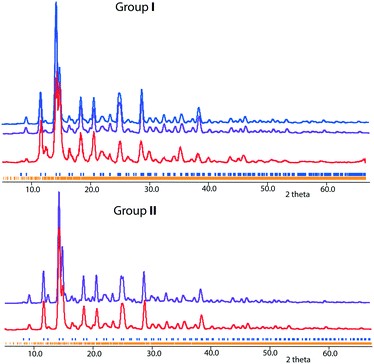
For groups I and II compositions, theoretical powder XRD patterns were simulated using the models obtained from single-crystal XRD experiments. As shown in Fig. 9, the experimental patterns match quite well with the simulated ones. In addition, the powder XRD pattern of group III crystals is shown in Fig. S5.† The theoretical XRD patterns of the predicted ordered Ba4Fe4ClO8 and Ba4Fe4ClO9 are shown in Fig. S6.†Summary and outlook
Summary
– We report on the synthesis of a new family of compounds, Ba4Fe4ClO9.5−x, in which the oxygen stoichiometry and Fe oxidation state depend on the conditions of the crystal preparation as well as redox conditions during post-growth annealing.– The framework of this hexagonal structure has the composition Ba2Fe4O9.5−x with x varying from experimentally obtained 0 to the theoretical prediction of 1.5. The structure forms open channels running parallel to the c-axis. The channels are filled with relatively mobile linear [Ba–Cl–Ba] triplets.
– Annealing the crystals in air, oxygen or vacuum at temperatures up to 600 °C does not influence the oxygen stoichiometry and oxidation state of the crystals. The composition of the crystals is found to be Ba4Fe4ClO9.4(1). However, these thermal treatments modify the structural modulation which originates in the oxygen distribution in the framework. Fe can be found in different coordination polyhedra (namely, FeO4-tetrahedra, FeO5-bipyramids, and FeO6-octahedra). In the framework channels, linear [Ba–Cl–Ba] triplet distribution can be found to be partially ordered. Such a partial ordering is also responsible for the modulation found in the crystal structure of Ba4Fe4ClO9.5−x.
– Annealing the crystals in Ar–H2 reduces the oxygen stoichiometry. The resulting composition thus becomes Ba4Fe4ClO9.0(1). Fe is in a mixed oxidation state (1 Fe2+ and 3 Fe3+). In addition to the lower O content, the structure of Ba4Fe4ClO9 is characterized by a higher ordering of [Ba–Cl–Ba] triplets than in as-grown crystals or crystals annealed in vacuum, in air or in oxygen.
– Based on our presented results, the predicted ordered Ba4Fe4ClO8 crystal structure will contain 3 Fe2+ and 1 Fe3+. Iron atoms will be exclusively found in FeO4 tetrahedra.
Outlook
The crystal growth and the post-growth annealing processes have induced modifications into the structure of Ba4Fe4ClO9.5−x. This illustrates the flexibility of the crystal structure. The framework is stable with different polyhedra as well as with Fe2+ and Fe3+ in variable ratio. Therefore, the framework can vary from [Ba2Fe43+O8]0 or [Ba2Fe42+O8]4− when built of FeO4-tetrahedra only to [Ba2Fe43+O12]8− or [Ba2Fe42+O12]12− when built of FeO6-octahedra only. In the channels created by the framework, fillers are mobile. The [Ba–Cl–Ba]3+ triplets reported in the structure can potentially be substituted by other species with a charge varying between 0 and +12 to compensate the negative charge of the framework. From these perspectives, the discovery and description of the Ba4Fe4ClO9.5−x structure could be the first of a new family of compounds with similar framework, with a range of fillers in the channels and, therefore, variable chemical and physical properties.Author contributions
Priya Ranjan Baral and Arnaud Magrez grew and annealed the crystals. Arnaud Magrez performed the XRF analysis. Wen-Hua Bi performed the single-crystal XRD experiments. The structure refinements were performed by Alla Arakcheeva. All authors wrote and commented on the paper.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The funding of this research was provided by the Swiss National Science Foundation (SNSF) Sinergia network NanoSkyrmionics (grant no. CRSII5-171003).Notes and references
- R. A. Singer, S. Monfette, D. Bernhardson, S. Tcyrulnikov, A. K. Hubbel and E. C. Hansen, Org. Process Res. Dev., 2021, 25, 1802 CrossRef CAS.
- L. Lv, M. Peng, L. Wu, Y. Dong, G. You, Y. Duan, W. Yang, L. He and X. Liu, Nanoscale Res. Lett., 2021, 16, 138 CrossRef CAS PubMed.
- W. Eerenstein, N. D. Mathur and J. F. Scott, Nature, 2006, 442, 759 CrossRef CAS PubMed.
- A. Kreisel, P. J. Hirschfeld and B. M. Andersen, Symmetry, 2020, 12(9), 1402 CrossRef CAS.
- M. Koltypin, S. Licht, I. Nowik, R. T. Vered, E. Levi, Y. Gofer and D. Aurbach, J. Electrochem. Soc., 2006, 153, A32 CrossRef CAS.
- I. Orlov, L. Palatinus, A. Arakcheeva and G. Chapuis, Acta Crystallogr., Sect. B: Struct. Sci., 2007, 63, 703 CrossRef CAS PubMed.
- A. V. Arakcheeva and O. G. Karpinskii, Sov. Phys. - Crystallogr., 1990, 35(5), 683 Search PubMed.
- NIST Inorganic Crystal Structure Database, NIST Standard Reference Database Number 3, National Institute of Standards and Technology, Gaithersburg MD, 20899, DOI:10.18434/M32147, (retrieved [Dec. 07, 2021]). Details in https://icsd.nist.gov/guide.html.
- S. Gražulis, A. Daškevič, A. Merkys, D. Chateigner, L. Lutterotti, M. Quirós, N. R. Serebryanaya, P. Moeck, R. T. Downs and A. LeBail, Crystallography Open Database (COD): an open-access collection of crystal structures and platform for world-wide collaboration, Nucleic Acids Res., 2012, 40, D420–D427 CrossRef PubMed , Details in https://wiki.crystallography.net/cod/citing/.
- J. Zhang, A. Wolfel, L. Li, S. van Smaalen, H. L. Williamson and R. K. Kremer, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 134428 CrossRef.
- J. Wang, Y. Wen, H. Wang, H. H. Liu and X. Yang, Prog. Chem., 2021, 33(2), 263 Search PubMed.
- N. Abe, S. Shiozawa, K. Matsuura, H. Sagayama, A. Nakao, T. Ohhara, Y. Tokunaga and T. Arima, Phys. Rev. B, 2020, 101, 180407(R) CrossRef.
- L. Serrador, M. Hernando, J. L. Martinez, J. M. Gonzalez-Calbet, A. Varela, F. J. Garcia-Garcia and M. Parras, Inorg. Chem., 2016, 55, 6261 CrossRef CAS PubMed.
- A. Barman, G. Gubbiotti, S. Ladak, A. O. Adeyeye, M. Krawczyk, J. Grafe, C. Adelmann, S. Cotofana, A. Naeemi, V. I. Vasyuchka, B. Hillebrands, S. A. Nikitov, H. Yu, D. Grundler, A. V. Sadovnikov, A. A. Grachev, S. E. Sheshukova, J.-Y. Duquesne, M. Marangolo, G. Csaba, W. Porod, V. E. Demidov, S. Urazhdin, S. O. Demokritov, E. Albisetti, D. Petti, R. Bertacco, H. Schultheiss, V. V. Kruglyak, V. D. Poimanov, S. Sahoo, J. Sinha, H. Yang, M. Münzenberg, T. Moriyama, S. Mizukami, P. Landeros, R. A. Gallardo, G. Carlotti, J.-V. Kim, R. L. Stamps, R. E. Camley, B. Rana, Y. Otani, W. Yu, T. Yu, G. E. W. Bauer, C. Back, G. S. Uhrig, O. V. Dobrovolskiy, B. Budinska, H. Qin, S. van Dijken, A. V. Chumak, A. Khitun, D. E. Nikonov, I. A. Young, B. W. Zingsem and M. Winklhofer, J. Phys.: Condens. Matter, 2021, 33, 413001 CrossRef CAS PubMed.
- Oxford Diffraction (2014), CrysAlisPRO. Agilent Technologies, Version 1.171.37.35 (release 13-08-2014 CrysAlis171.NET) (compiled Aug 13 2014, 18:06:01).
- V. Petříček, M. Dusek and L. Palatinus, Z. Kristallogr., 2014, 229, 345 Search PubMed.
- L. Palatinus and G. Chapuis, J. Appl. Crystallogr., 2007, 40, 786 CrossRef CAS.
- Diamond – Crystal and Molecular Structure Visualization, Crystal Impact – Dr. H. Putz and Dr. K. Brandenburg GbR, Kreuzherrenstr. 102, 53227 Bonn, Germany, (see: https://www.crystalimpact.com/diamond/references.htm).
- T. H. Stokes, B. J. Campbell and S. van Smaalen, Acta Crystallogr., Sect. A: Found. Crystallogr., 2011, 67, 45 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Reconstructions of the reciprocal space sections; indexing of reflexions; simulated and experimental XRD profiles; tables of experimental details for structure determination and refinements; atomic position parameters for average structures. See DOI: 10.1039/d1ce01657a |
| This journal is © The Royal Society of Chemistry 2022 |