Revealing the reason for the reversal of properties from fullerene to nonfullerene†
Jiu-Chang
Huang
a,
Ming-Yang
Li
a,
Li-Na
Wu
a,
Chun-Ni
Xiao
a and
Guang-Yan
Sun
 *ab
*ab
aDepartment of Chemistry, Faculty of Science, Yanbian University, Yanji 133002, China. E-mail: gysun@ybu.edu.cn
bFaculty of Chemical Engineering and New Energy Materials, Zhuhai College of Science and Technology, Zhuhai, Guangdong 519041, China
First published on 30th November 2021
Abstract
The existence of an inflection point between fullerene and nonfullerene molecules was demonstrated using bowl-nonfullerene models, which were proposed to explore the key points relating to the different properties. This study explains the differences in the stacking configurations of the inflection points and reveals the reason for the inversion of properties, which is caused by small structural differences.
Following the development of multifunctional nonfullerenes, the acceptor material in an active layer is no longer limited to fullerenes,1,2 and the power conversion efficiencies of bulk heterojunction (BHJ) organic solar cells (OSCs) are also continuously improving.3–6 However, a description of the charge-transfer mechanism in nonfullerene systems is still based on knowledge related to fullerene systems, without having been fully updated.7,8 This has produced a lot of issues relating to inapplicability and unreliability because of the large differences in the structures of these two material types,9,10 hindering the further improvement of materials and efficiencies. Hence, there is an urgent need to deeply understand the essential reasons for nonfullerene-based inapplicability in BHJ OSCs.
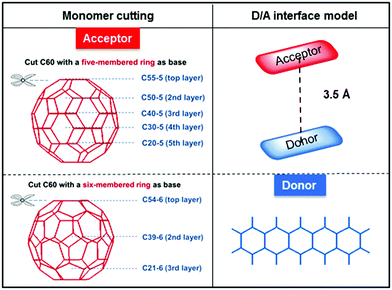
In this work, the inflection point of properties from fullerene to nonfullerene, affected by structural changes, was captured, and the way in which the dominant forces that cause differences in inflection points change was proposed. Pentacene/C60 (P/C60) was selected as a reference11–13 and two types of bowl-nonfullerene compound models were established, as shown in Fig. 1 and Fig. S1 (ESI†). Based on our previous work, the bowl-nonfullerenes were obtained via cutting C60 in a layer-by-layer fashion to visualize the total change process, based on the five-membered rings and six-membered rings of C60. According to the ring base, the bowl-nonfullerenes were divided into 5-ring and 6-ring models. Bowl-nonfullerenes have π-conjugated and curved structures, similar to C60. The quantum chemical calculation approach is shown in the Methods in Section S1 and S2 (ESI†).14,15
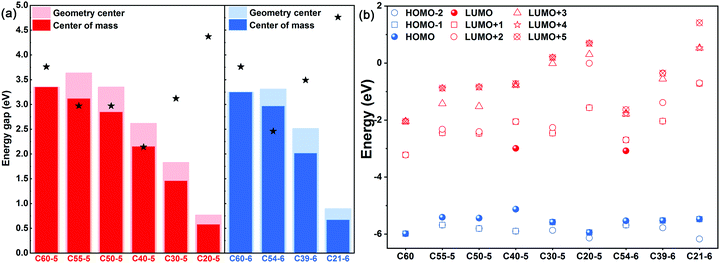
Cutting down the number of C atoms in each layer causes structural changes; the variation of molecular weights was measured firstly (Fig. S3, ESI†), and a nonlinear and decreasing tendency is seen for molecular weights. Irregular changes appear at C50-5 → C40-5 for the 5-ring model and at C54-6 → C39-6 for the 6-ring model. We speculate that there should be a potential range or specific point between C60 and bowl-nonfullerene. Herein, this is called the inflection point, and it might cause the breaking of high symmetry (Fig. 2a). The overlap between the center of mass and the geometric center of bowl-nonfullerene was used for evaluating the symmetry. Whether the 5-ring or 6-ring model is considered, overlap becomes larger from the middle to both sides, presenting an arch-shaped change, which leads to changes in stability. Therefore, C20-5 and C21-6, the smallest units, have similar stability to C60.
The break in symmetry also causes the degree of energy level degeneracy to decrease from triple to double as layer-by-layer changes are made (Fig. 2b). According to the characteristics of orbital degeneracy, the structure could be divided into LUMO+1/LUMO+2 degeneracy (C40-5 and C54-6) and LUMO/LUMO+1 degeneracy (the others). Obviously, C40-5 and C54-6 are inflection points, as could be illustrated by the frontier molecular orbitals shown in Fig. S4 (ESI†). On the one hand, differences in symmetry lead to larger differences in energy levels, which are transmitted through electronic changes. On the other hand, the shielding of the attractive potential of the nucleus is weakened via cutting the number of electrons, and the energy is reduced accordingly. For the 5-ring/6-ring model, the reason that C40-5/C54-6 is an inflection point is the important factors before and after the dominant energy level change, involving the number of electrons and symmetry/involving the symmetry and the distribution of electron clouds. In terms of the structure of the molecule itself, this apparently affects just the symmetry and energy levels of orbitals. But, in fact, these two factors further impact the optical excitation and charge-transfer characteristics.
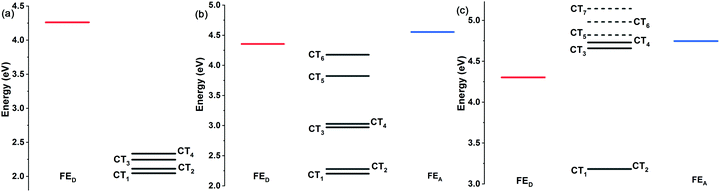
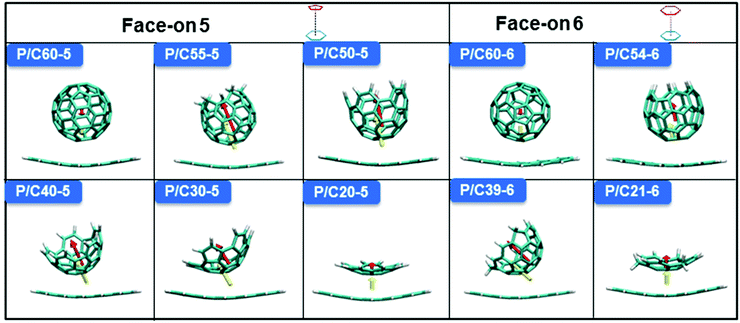
The break in symmetry also means that the energy gap shows an inverted parabolic trend, which is related to the maximum absorption wavelength (λmax). Therefore, the λmax values of C60 and bowl-nonfullerenes were characterized to visually observe the possibility of excitation (Fig. S5a, ESI†). The λmax values of the bowl-nonfullerenes are greater than that of pentacene in the face-on 5 model, except for P/C40-5 (the inflection point) and P/C20-5. A similar phenomenon exists for the face-on 6 models (face-on 5 and face-on 6 represent dimer models). To visualize the excited subjects, the energies of Frenkel-exciton (FE) states and charge-transfer (CT) states are given in Fig. 3 and Fig. S5 (ESI†). For the face-on 5 model, P/C60-5, P/C40-5, and P/C20-5 show obvious donor excitation, while the other face-on 5 dimers mainly show acceptor excitation, which is consistent with the inflection point trend from the above-discussed structural point of view. Additionally, P/C40-5 has the possibility of acceptor excitation. For the face-on 6 model, P/C60-6 and P/C21-6 show donor excitation, while P/C54-6 and P/C39-6 tend toward acceptor excitation. Although these bowl-nonfullerenes have similar accumulation sites with C60 when they are stacked with the donor, because the isotropy of C60 is broken due to the fixed cutting method, the intrinsic properties of the molecule are changed.
In fact, the structural changes relating to symmetry also affect the ability to withdraw electrons. According to the work of Troisi,16 the low-lying excited state of anionic C60 (C60−) with oscillator strengths (f) <10−3 could promote a charge separation process. To verify whether the bowl-nonfullerenes have similar anionic properties to C60−, the excitation energies of anion states were studied, as shown in Table S2 (ESI†). Firstly, the S1 state of C40-5− has higher energy, dominated by the HOMO → LUMO transition, which is not similar to C60−. The other bowl-nonfullerenes are dominated by LUMO → LUMO+1 or LUMO+2 transitions. Secondly, the f value of C54-6− is not below 10−3, which does not denote a forbidden transition, and the energy of the S1 state is not at a similar level to those of the other molecules. This indicates that the bowl-nonfullerenes with LUMO+1/LUMO+2 degeneracy could not maintain the original characteristics of C60− due to the broken symmetry. This showed that there is an inflection point for the changes in properties caused by structure, and it is speculated that the inflection point may appear upon the emergence of a molecule without LUMO/LUMO+1 degeneracy.
Apart from symmetry and isotropy, the most obvious change is the polarity of the molecule. That is, there is a change from non-polar C60 to a weakly polar bowl-nonfullerene, which was characterized based on the molecular polarity index (MPI), as shown in Fig. S6 (ESI†). As is well known, a change in polarity causes differences in electrostatic interactions.17 Therefore, we further characterized the weak intermolecular interactions to reveal the essential reason for the existence of inflection points. First, the dipole moments (Fig. 4) and electrostatic potentials (ESP, Fig. S7, ESI†) were calculated to analyze the change in electrostatic interaction upon the layer-by-layer reduction of C60. In Fig. 4, the tilt and length of the arrow indicate the direction and magnitude, respectively, and a longer arrow indicates a higher-strength the dipole moment. From P/C60-5 to P/bowl-nonfullerenes, only the dipole moment of the fullerene system points from fullerene to pentacene. For the P/bowl-nonfullerene systems, the direction of the moment upon moving from P/C55-5 to P/C40-5 is greatly changed. Moving from P/C40-5 to P/C20-5, it returns to a vertical direction, but the direction is from pentacene to the bowl-nonfullerene. The magnitude and direction of the dipole moment of P/C40-5 changes, resulting in a large difference in the stacking configuration and intermolecular interactions of the dimer, indicating that P/C40-5 is the inflection point. For the face-on 6 model, the directional change of the dipole moment is consistent with the changing trend seen for the face-on 5 model, and the intensity drops sharply for P/C39-6. According to the ESP distributions of dimers, it could be found that the positive ESP regions for bowl-nonfullerenes are distributed on H atoms, but most parts have negative ESP. There is a significant ESP difference between the larger molecular weight acceptors and pentacene. The inflection points (P/C40-5 and P/C39-6) have different trends in terms of their positive and negative ESP regions. Additionally, the ESP difference between the donor and acceptor in P/C60-6 is significantly larger than that in P/C60-5, indicating that the matching of donor/acceptor ring stacking in the model affects the electrostatic interactions.
To verify the inflection points that determine the different properties of fullerene and bowl-nonfullerenes obtained from the above conclusions and to further reveal the reasons for the altered properties between fullerene and nonfullerenes, the total intermolecular interactions and interaction energies were visualized and analyzed, as shown in Fig. 5 and Fig. S8, S9 (ESI†).
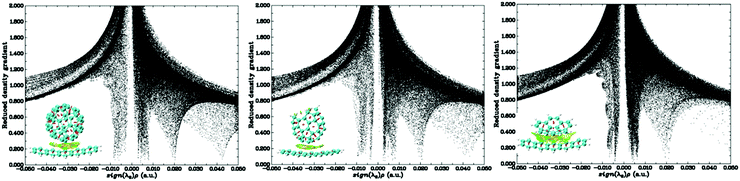 | ||
| Fig. 5 Scatter plots of sign(λ2)ρ (a.u.) vs. RDG for P/C60-5, P/C55-5, and P/C20-5 from the face-on 5 model and related colored isosurface plots. | ||
As the number of C atoms decreases, the steric hindrance between each ring decreases. The H atoms in the opened ring of the bowl-nonfullerene gradually induce a bonding effect, but this only appears in P/C55-5, P/C50-5, and P/C54-6 with larger molecular weights. Surprisingly, the decreasing interaction energy of the dimers before the inflection point causes the interaction distance to gradually decrease from P/C60-5 to P/C50-5, but the trend from P/C60-6 to P/C39-6 is opposite. This indicates that the bonding effects from H atoms in the opened ring are one of the factors that mediate the intermolecular interactions, making the inflection point (P/C40-5 and P/C39-6) abnormal. On one hand, the characteristics of the acceptor affect the intermolecular interactions that change the interaction energy and distance. On the other hand, the outcomes are also related to the matching of ring stacking, which influences whether the interaction energy and distance are positively correlated or negatively correlated. The dimers at the inflection point are affected by a variety of factors, resulting in the largest differences in distance and direction before and after configuration optimization. Through characterizing the details relating to intermolecular interactions and visualizing weak interactions, it is clear that changes in the structure and characteristics of the acceptor result in intermolecular interactions in fullerene and nonfullerene systems that cannot be generalized, directly leading to different charge-transfer mechanisms at the donor/acceptor interface.
In this work, based on two unique bowl-nonfullerene models, we found the inflection points of change from fullerene to nonfullerene that determine the differences in the properties of fullerenes and nonfullerenes. We studied the characteristics of broken C60 via cutting in a layer-by-layer manner, including isotropy, symmetry, and polarity. At the macroscopic level, depicting the molecular structure and its role in excitation and exciton dissociation can help determine the inflection point from fullerene to nonfullerene. At the fundamental level, revealing the essential reasons for the inflection point, it is obvious that structural changes lead to the emergence of crucial inflection points in terms of intermolecular interactions. Different trends are seen before and after the inflection point. We aim to illustrate the structural differences between fullerenes and high-efficiency nonfullerenes through the established bowl models. The further development of nonfullerenes should not use charge-transfer mechanisms derived from fullerenes to avoid many problems relating to inapplicability.
Jiu-Chang Huang: collected and analyzed data, manuscript; Ming-Yang Li: designed the article; Li-Na Wu: put forward some constructive suggestions for revising the paper; Chun-Ni Xiao: software, assisted in visualization; Guang-Yan Sun: reviewed and approved the manuscript.
We gratefully acknowledge financial support from the National Natural Science Foundation of China (No. 22163010), the Guangdong Basic and Applied Basic Research Foundation (Project No. 2019A1515012219), and the Innovation Ability Cultivation Project of Zhuhai College of Science and Technology (No. 2019XJCQ003). This work was funded by the “Three Levels” Talent Construction Project of Zhuhai College of Science and Technology and National Public (Technical) Service Platform for Small- and Medium-sized Enterprises.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M.-Y. Li, H. Yin and G.-Y. Sun, Appl. Mater. Today, 2020, 21, 100799 CrossRef.
- M.-Y. Sui, Z.-R. Yang, Y. Geng, G.-Y. Sun, L. H. Hu and Z.-M. Su, Sol. RRL, 2019, 3, 1900258 CrossRef CAS.
- Q. S. Liu, Y. F. Jiang, K. Jin, J. Q. Qin, J. G. Xu, W. T. Li, J. Xiong, J. F. Liu, Z. Xiao, K. Sun, S. F. Yang, X. T. Zhang and L. M. Ding, Sci. Bull., 2020, 65, 272–275 CrossRef CAS.
- C. Li, J. D. Zhou, J. L. Song, J. Q. Xu, H. T. Zhang, X. N. Zhang, J. Guo, L. Zhu, D. H. Wei, G. C. Han, J. Min, Y. Zhang, Z. Q. Xie, Y. P. Yi, H. Yan, F. Gao, F. Liu and Y. M. Sun, Nat. Energy, 2021, 6, 605–613 CrossRef CAS.
- S. H. Chen, L. W. Feng, T. Jia, J. H. Jing, Z. C. Hu, K. Zhang and F. Huang, Sci. China: Chem., 2021, 64, 1192–1199 CrossRef CAS.
- J. B. Zhao, Y. K. Li, G. F. Yang, K. Jiang, H. Lin, H. Ade, W. Ma and H. Yan, Nat. Energy, 2016, 1, 15027 CrossRef CAS.
- Y. P. Yi, V. Coropceanu and J.-L. Brédas, J. Mater. Chem., 2011, 21, 1479–1486 RSC.
- D. P. Qian, Z. L. Zheng, H. F. Yao, W. Tress, T. R. Hopper, S. Chen, S. S. Li, J. Liu, S. S. Chen, J. B. Zhang, X.-K. Liu, B. W. Gao, L. Q. Ouyang, Y. Z. Jin, G. Pozina, I. A. Buyanova, W. M. Chen, O. Inganäs, V. Coropceanu, J.-L. Brédas, H. Yan, J. H. Hou, F. L. Zhang, A. A. Bakulin and F. Gao, Nat. Mater., 2018, 17, 703–709 CrossRef CAS PubMed.
- Y. Ren, M.-Y. Li, Y.-X. Song, M.-Y. Sui, G.-Y. Sun, X.-C. Qu, P. Xie and J.-L. Lu, J. Photochem. Photobiol., A, 2021, 407, 113087 CrossRef CAS.
- M.-Y. Li, M.-Y. Sui, J.-C. Huang, F.-M. Wei, Y. Ren and G.-Y. Sun, ACS Appl. Energy Mater., 2021, 4, 8739–8744 CrossRef CAS.
- B. Yang, Y. P. Yi, C.-R. Zhang, S. G. Aziz, V. Coropceanu and J.-L. Brédas, J. Phys. Chem. C, 2014, 118, 27648–27656 CrossRef CAS.
- X.-K. Chen, M. K. Ravva, H. Li, S. M. Ryno and J.-L. Brédas, Adv. Funct. Mater., 2016, 6, 1601325 Search PubMed.
- R. E. M. Willems, S. C. J. Meskers, M. M. Wienk and R. A. J. Janssen, J. Phys. Chem. C, 2019, 123, 10253–10261 CrossRef CAS PubMed.
- M. J. Frisch, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, J. E. P. Jr., F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 09 Rev. D.01, Wallingford, CT, 2009 Search PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- T. Liu and A. Troisi, Adv. Mater., 2013, 25, 1038–1041 CrossRef CAS PubMed.
- H. F. Yao, Y. Cui, D. P. Qian, C. S. Ponseca, A. Honarfar, Y. Xu, J. M. Xin, Z. Y. Chen, L. Hong, B. W. Gao, R. N. Yu, Y. F. Zu, W. Ma, P. Chabera, T. Pullerits, A. Yartsev, F. Gao and J. H. Hou, J. Am. Chem. Soc., 2019, 141, 7743–7750 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cp04255f |
| This journal is © the Owner Societies 2022 |