First-principles calculations on the resistance and electronic properties of H2 adsorption on a CoO–SnO2 heterojunction surface†
Yunxia
He
 a,
Jing
Li
*a,
Lin
Tao
a,
Jing
Li
*a,
Lin
Tao
 *b,
Shuai
Nie
a,
Timing
Fang
c,
Xitao
Yin
*b,
Shuai
Nie
a,
Timing
Fang
c,
Xitao
Yin
 d and
Qi
Wang
a
d and
Qi
Wang
a
aSchool of Materials and Metallurgy, University of Science and Technology Liaoning, Anshan 114051, Liaoning, China. E-mail: lijing_as321@163.com
bSchool of Chemical Engineering, University of Science and Technology Liaoning, Anshan 114051, Liaoning, China. E-mail: taolin_phd@163.com
cSchool of Chemistry and Chemical Engineering, Qingdao University, Qingdao 266071, Shandong, China
dSchool of Physics and Optoelectronic Engineering, Ludong University, Yantai 264000, Shandong, China
First published on 24th November 2021
Abstract
Compared with pure metal oxides, heterojunctions greatly change the response to gas by the synergistic effect of the interface. In this work, density functional theory was used to reveal the adsorption performance of H2 on the heterojunction under oxygen conditions. First, we determined the most reasonable heterojunction structure based on the adhesion work. According to the adsorption energy, the presence of SnO2(100)(I)/CoO(110)(II) made the adsorption of H2 more stable. The DOS results showed that the resistance of the heterojunction increased with H2 adsorption, following the same trend as that of CoO(110) with H2 adsorption, although that of the heterojunction increased more. The electron density and electron density difference indicated that the heterojunction improved the reaction between H2 and oxygen ions on CoO(110). However, the resistance of CoO(110)(II)/SnO2(100)(II) increased after H2 adsorption, contrary to the resistance change of SnO2(100). Besides, the bonding energy between H2 and the adsorption site became worse. The above results demonstrated that the presence of the heterojunction could indeed change the response trend and the adsorption behavior of H2. Interestingly, the adsorption sites and effects of H2 were different when two metal oxides were used as the substrate of the heterojunction, respectively.
1. Introduction
Semiconductor metal oxides as a material for chemical gas sensors play a significant role thanks to their abundance, low cost, and easy manufacture. Among these semiconductor metal oxide gas sensors, compounds made of adsorbed air oxygen ions and the target gas on the surface cause a change in the electric resistance of the materials. These semiconductor metal oxide gas devices have sensitivity to various toxic, flammable, and explosive gases, and are widely applied in various fields.1–4However, metal oxide gas devices suffer from the effects of baseline resistance drift and from poisoning interactions. These problems make it progressively more and more difficult for metal oxide surfaces to approach reactive gases.5,6 SnO2 semiconductors are among the best n-type metal oxide gas-sensing materials for gas-sensitive devices owing to their wide band gap, excellent chemical stability, and prominent sensitivity.7 However in practical applications, the gas sensitivity to H2 still needs to be improved for the accurate identification of H2. Surface modification8–10 is the most commonly used approach to enhance the H2 sensitivity.11 However, the response effect is not obvious. Decorating H2-response sieves12–15 and polymers16 on the surface of gas-sensing materials has also been explored to enhance H2 sensitivity. These are new innovation, but the accurate identification for H2 needs to be improved. In addition, as one p-type metal oxide gas-sensing material, CoO has shown an affinity with oxygen and possesses multivalent characteristics.17 The pristine form of CoO is hardly used in the field of sensing due to its lower response than n-type semiconductors,18 though it has been reported that its gas-sensing can be significantly improved by introducing dopants,19 or by proper control of the morphology.20,21 However, the accurate identification of gases still needs to be improved.
The idea of combining different metal oxide materials to form a heterojunction was recently proposed in order to further improve the important sensing characteristics of resistive-type gas sensors.22,23 Generally, n-type materials have a higher Fermi energy level than that of p-type materials.24 Hence, when n-type and p-type heterojunctions are fabricated, the electrons of n-type metal oxides with a higher energy will pass through the interface to the vacant lower-energy states until the Fermi energy level achieves a stable condition. The potential barrier, which is attributed to the difference in Fermi energy levels, will show an obvious change when a heterojunction is surrounded by different oxidizing or reducing gases. Furthermore, the changes will be observed as a high response in composite heterojunction gas sensors.25 The synthesis of a heterojunction provides a significant method to incorporate different physical and chemical properties into one system.26
A large number of studies have applied first-principle calculations on the various properties of heterojunctions,27–30 and extensive research studies on the formation of heterojunctions have been published. Katoch et al.31 researched an n-p ZnO/CuO heterojunction as s nanofiber sensor to strengthen the primary ZnO nanofiber sensor sensing in H2S. Similarly, Choi et al.32 reported the gas-sensing of H2S was boosted in a CuO/SnO2 heterojunction more than in the bulk of SnO2 owing to the formation and disruption of the heterojunction. Ju et al.33 proved that a NiO/SnO2 heterojunction had a more rapid response to trimethylamine than pure SnO2. Other research into metal oxide p–n heterojunction NiO/ZnO,34 NiO/Fe2O3,35 and NiO/SnO236 have experimentally proved that the formation of a metal oxide heterojunction would obviously strengthen a sensor's gas sensitivity. The performances of metal oxide of Sn and Co can also be improved greatly by forming a p–n heterojunction.37,38 Heterojunctions have been applied to strengthen the sensing properties of sensors based on SnO2 through altering the influence at the synthesis interface.
However, explanations about the microstructure and theories about the mechanism of heterojunctions are obscure. Not only does this show a misfit, but also the structure and adhesion strength of the interface affect the effectiveness of promoting heterogeneous nucleation.39,40 It is very difficult to observe structures and the adhesion strength of an interface or the adsorption of H2 on a heterojunction surface through experimental methods. In this respect, an atomic level understanding about the H2-sensing mechanism on SnO2–CoO materials is crucial. Meanwhile, the gas-sensing characteristics, such as adsorption performance of metal oxide sensors, should be visualized for practical application.41 First-principles calculations have been employed to reveal the structures and adhesion strength of the interface at the atomic scale,42–45 and the adsorption of gases on a single metal oxide calculated,46–51 including the adsorption energy, adsorption distance, DOS, and electronic structure.
In this work, we studied the microstructure of SnO2/CoO and CoO/SnO2 heterojunctions by DFT, analyzing the transformation of the resistance, electronic structure, and other properties of H2 adsorption on different sites of the heterojunction surface. Moreover, this work can provide theoretical guidance for the fabrication of heterojunction gas sensors in order to accurately identify H2.
2. Methods
2.1 Calculation details
The Cambridge Serial Total Energy Package (CASTEP) Code was used for all the calculations based on density functional theory (DFT).52–54 The Generalized Gradient Approximation (GGA) of the Perdew–Burke–Ernzerhof (PBE) scheme was employed to describe the exchange–correlation functional. Considering the stability of the system and for optimization of the calculation speed, the plane wave cut-of energy was selected as 400 eV. The Brillouin zone was sampled by 3 × 3 × 1 k-points using the Monkhorst–Pack scheme. The convergence tolerance for the energy was selected as 2.0 × 10−6 eV per atom. The force, stress, and displacement tolerance of the convergence tolerance were set as 0.05 eV Å−1, 0.1 GPa, and 0.002 Å, respectively. To avoid the interaction between surface atoms, a vacuum layer of 15 Å was selected for each heterojunction surface system.2.2 CoO–SnO2 heterojunction models
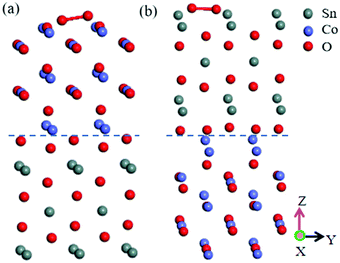
Chemiresistive gas sensors employing a p–n heterojunction offer a compelling high sensitivity and specific response.55 The interfacial free energy between the precipitations and the substrates control the process of particle coarsening. Being based on the lattice mismatch theory,56 the interface with the lowest lattice mismatch between the second particle and the pre-existing particle will be the preferred growth direction of the second particle on the pre-exiting particle.57 The primitive cells made from the CoO(110) surface and SnO2(100) surface possessed lattice constants of aCoO(110) = 4.2667 Å, bCoO(110) = 3.017013 Å, aSnO2(100) = 4.73727 Å, bSnO2(100) = 3.186383 Å. The mismatch between CoO(110) and SnO2(100) was less than 6%. Therefore, SnO2(100) and CoO(110) were selected to structure the heterojunction. As shown in Fig. 1(a and b), the surface termination of CoO(110) has two forms:58–60 CoO(110)(I) (1O and 1/4Co as termination) and CoO(110)(II) (1O and 1/2Co as termination). In addition, the surface termination of SnO2(100) has four different surface terminations: SnO2(100)(I) (1/4Sn-termination), SnO2(100)(II) (1/2O-termination), SnO2(100)(III) (1Sn-termination), and SnO2(100)(IV) (1O-termination), as shown in Fig. 1(c–f). There are sixteen kinds of heterojunction due to the four terminations of SnO2(100) and two terminations of CoO(110), which are shown in Fig. 2(a) and (b). In order to reduce the effect of lattice interactions on the adsorption, (1 × 2) supercellular SnO2(100)–CoO(110) heterojunctions were fabricated.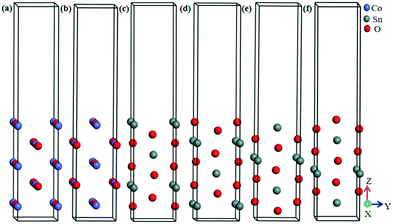 | ||
| Fig. 1 Surface structure of CoO(110) and SnO2(100). (a) CoO(110)(I), (b) CoO(110)(II), (c) SnO2(100)(I), (d) SnO2(100)(II), (e) SnO2(100)(III), (f) SnO2(100)(IV). | ||
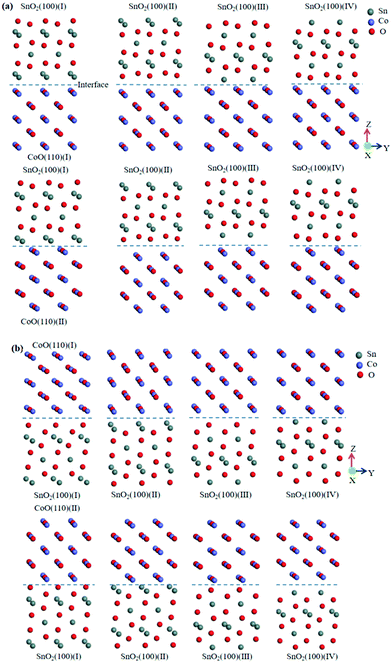 | ||
| Fig. 2 Different models of CoO/SnO2 heterojunction and SnO2/CoO heterojunction. (a) SnO2(100) was put on CoO(110); (b) CoO(110) was put on SnO2(100). | ||
3. Results and discussion
3.1 Adsorption properties of H2 on the heterojunction surface
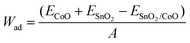 | (1) |
The interfaces of the SnO2(100)/CoO(110) system and CoO(110)/SnO2(100) system showed the same distances (2 Å), which were optimized by energy calculation for evaluating the interface properties of the heterojunction. The interface structures were optimized with in a fixed cell volume, and all the atoms at the interface models were allowed to relax in three directions.64 The Wad values of the heterojunction are shown in Table 1.
| Heterojunction | W ad (J m−2) |
|---|---|
| SnO2(100)(I)/CoO(110)(I) | −0.5179 |
| SnO2(100)(II)/CoO(110)(I) | −0.5187 |
| SnO2(100)(III)/CoO(110)(I) | −0.5203 |
| SnO2(100)(IV)/CoO(110)(I) | −0.5194 |
| SnO2(100)(I)/CoO(110)(II) | −0.5175 |
| SnO2(100)(II)/CoO(110)(II) | −0.5191 |
| SnO2(100)(III)/CoO(110)(II) | −0.5177 |
| SnO2(100)(IV)/CoO(110)(II) | −0.5191 |
| CoO(110)(I)/SnO2(100)(I) | −0.5189 |
| CoO(110)(I)/SnO2(100)(II) | −0.5185 |
| CoO(110)(I)/SnO2(100)(III) | −0.5195 |
| CoO(110)(I)/SnO2(100)(IV) | −0.5176 |
| CoO(110)(II)/SnO2(100)(I) | −0.5176 |
| CoO(110)(II)/SnO2(100)(II) | −0.5175 |
| CoO(110)(II)/SnO2(100)(III) | −0.5195 |
| CoO(110)(II)/SnO2(100)(IV) | −0.5182 |
A larger Wad is associated with a stronger binding force of the interfacial atoms, which suggests the stability of the SnO2(100)/CoO(110) and CoO(110)/SnO2(100) heterojunctions interfaces.65
Table 1 shows the adhesion work of the SnO2(100)/CoO(110) heterojunctions, where it can be seen that the adhesion work of the SnO2(100)(I)/CoO(110)(I) and SnO2(100)(I)/CoO(110)(II) heterojunctions were larger than that of the other heterojunctions. Therefore, they were more stable than the other SnO2(100)/CoO(110) heterojunctions. However, SnO2(100)(I)/CoO(110)(II) heterojunction had a larger Wad than that of the SnO2(100)(I)/CoO(110)(I) heterojunction. Therefore, the SnO2(100)(I)/CoO(110)(II) heterojunction was selected as the adsorption carrier of H2, which was attributed to its larger adhesion work.
Table 1 shows the adhesion work of the CoO(110)/SnO2(100) heterojunctions. It shows that the Wad values of the CoO(110)(II)/SnO2(100)(I), CoO(110)(II)/SnO2(100)(II), and CoO(110)(I)/SnO2(100)(IV) heterojunctions were larger than those of the other CoO(110)/SnO2(100) heterojunctions. Therefore, they were more stable than the other CoO(110)/SnO2(100) heterojunctions. Although three heterojunctions had approximately the same Wad, the CoO(110)(II)/SnO2(100)(II) heterojunction had the larger Wad. Consequently, the CoO(110)(II)/SnO2(100)(II) heterojunction system was chosen as the adsorption carrier of H2.
There were nine different types of adsorption conditions of H2 on SnO2(100)(I)/CoO(110)(II) and CoO(110)(II)/SnO2(100)(II) heterojunctions in an oxygen atmosphere (including the adsorption on the O2 molecule and different sites of the heterojunction surface), which are shown in Fig. 4(a–e) and (f–i).67
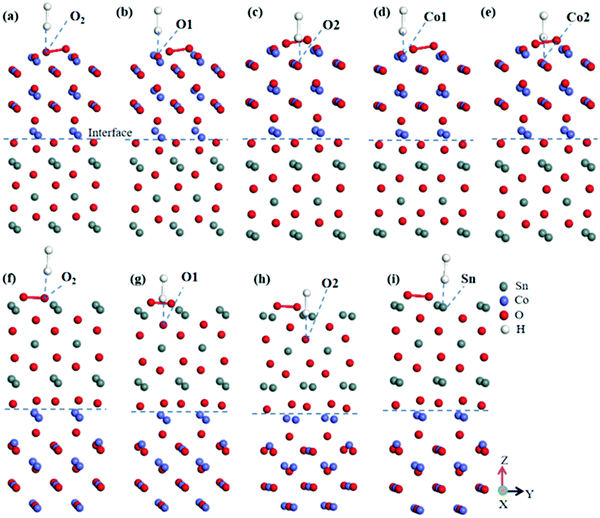 | ||
| Fig. 4 H2 adsorbed on different sites of heterojunction surfaces. (a–e) H2 on the SnO2(100)(I)/CoO(110)(II) heterojunction, (f–i) H2 on the CoO(110)(II)/SnO2(100)(II) heterojunction. | ||
The adsorption energy (Eads) is a key standard to estimate the adsorption property of H2 molecules on the heterojunction surface. It can be adopted to judge the adsorption strength of H2 molecules on a carrier, which is defined as follows:68,69
| Eads = Etotal − (Eheterojunction + Egas) | (2) |
The Eads and the adsorbed distance of H2 adsorption on the SnO2(100)(I)/CoO(110)(II) and CoO(110)(II)/SnO2(100)(II) heterojunctions are shown in Table 2. In addition, the parameters about Eads and the adsorbed distance of H2 on single material oxide CoO and SnO2 are shown in Tables S3 and S4 (ESI†).
| Heterojunction | Site | E ads (eV) | Distance (Å) |
|---|---|---|---|
| SnO2(100)(I)/CoO(110)(II) | O2 | −0.2286 | 2.4615 |
| O1 | −0.6962 | 3.3761 | |
| O2 | −0.3900 | 4.1691 | |
| Co1 | −0.0898 | 3.1388 | |
| Co2 | 1.6229 | 4.3275 | |
| CoO(110)(II)/SnO2(100)(II) | O2 | −0.2957 | 3.0844 |
| Sn | −0.3855 | 3.4432 | |
| O1 | −0.2551 | 3.2475 | |
| O2 | −0.2496 | 3.8159 |
Table 2 shows the Eads and adsorption distance when H2 was adsorbed on different sites of the SnO2(100)(I)/CoO(110)(II) heterojunction surface. There were five forms where H2 was adsorbed on the heterojunction surface, including the O site of an O2 molecule, and O1 site, O2 site, Co1 site, Co2 site of the heterojunction surface. The adsorption distance and Eads varied greatly. When H2 was adsorbed on the O site of an O2 molecule, the distance of H–O bonding was less than 3 Å, indicating the formation of a chemical bond. Except at the Co2 site, the Eads values were all negative when H2 was adsorbed on other sites of the heterojunction surface. This reveals the preferable stabilization of adsorption, especially the adsorption on an O site of an O2 molecule, and O1 site and O2 site of the heterojunction surface. Therefore, according to the Wads and adsorption distance, it was easy for H2 to be adsorbed on an O site of an O2 molecule. Compared with pure metal oxides CoO, the SnO2(100)(I)/CoO(110)(II) heterojunction improves the adsorption and bonding of H2 on an O2 molecule. Also, the adsorption of H2 on the heterojunction was more stable.
Table 2 shows the Eads and adsorbed distance of H2 adsorption on O site of an O2 molecule, Sn (Sn is equivalent because of supercell) site, and O1 site and O2 site of the CoO(110)(II)/SnO2(100)(II) heterojunction surface. The adsorption distance and Eads varied greatly. The adsorbed distances were all more than 3 Å when H2 was adsorbed on different sites of the heterojunction surface. This implies the existence of weak chemical bonding between H atoms and the adsorbed sites. In addition, the Eads values were obviously different when H2 was adsorbed on different surface sites. The Eads values were all negative when H2 was adsorbed on the heterojunction surface, revealing that the adsorption of H2 on the heterojunction was thermodynamically stable. Consequently, it was easy for H2 to be adsorbed on the CoO(110)(II)/SnO2(100)(II) heterojunction surface by analyzing the adsorbed distance and Eads. However, there was no formation of stronger chemical bonding between H2 molecules and the adsorbed sites. Compared with pure metal oxides SnO2, the existence of the CoO(110)(II)/SnO2(100)(II) heterojunction made the bonding effect between H2 and adsorption sites worse, but made the adsorption more stable, especially the adsorption of H2 on the O1 site.
In summary, based on the Eads and adsorption distance, H2 readily adsorbs on an O2 molecule of the SnO2(100)(I)/CoO(110)(II) heterojunction surface. In addition, H2 tends to be adsorbed on different sites of the CoO(110)(II)/SnO2(100)(II) heterojunction surface; however, stronger chemical bonds did not exist.
3.2 Influence of gas adsorption on the electronic conductivity and electronic structure
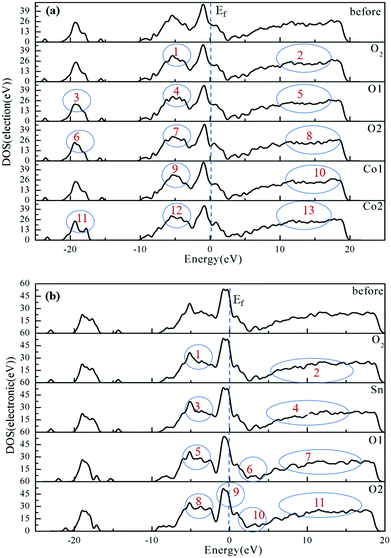
The adsorption of H2 in the oxygen atmosphere will affect the heterojunction resistance, which could be reflected in DOS. Therefore, the change in the resistance can be studied by analyzing the DOS. The generation of more peaks and increase in the DOS values were found over the tested energy range, which were attributed to the gas adsorption. The variations in DOS would further account for the increase in the electron energy and the better conductivity.72–76
The DOS values for H2 on the different sites of the SnO2(100)(I)/CoO(110)(II) and CoO(110)(II)/SnO2(100)(II) heterojunction surfaces are shown in Fig. 5(a) and (b). In addition, the DOS for H2 adsorbed on different sites of single metal oxides CoO and SnO2 are shown in Fig. S5(a) and (b) (ESI†).
Fig. 5(a) shows the DOS for H2 adsorption on different sites of the SnO2(100)(I)/CoO(110)(II) heterojunction surface. The DOS value was reduced when H2 was adsorbed on an O site of the O2 molecule, labeled as 1 and 2, revealing the heterojunction resistance had increased. The DOS value showed obvious decreases (labeled as 3, 4, and 5) when H2 was adsorbed on the O1 site, indicating the heterojunction resistance increased along with the adsorption of H2. When H2 was adsorbed on the O2 site, a decrease and disappearance of DOS peaks (labeled as 6, 7, and 8) were observed. All these results prove that the resistance increased when H2 was adsorbed on the O2 site of the heterojunction surface. The DOS value was lower and some peaks disappeared (labeled as 9 and 10) with H2 adsorbed on the Co1 site, reflecting that the heterojunction resistance had increased. When H2 was adsorbed on the Co2 site, the changes in the DOS occurred at the Fermi level (labeled as 11, 12, and 13). The DOS value was then reduced and some peaks disappeared, revealing that the heterojunction resistance had increased. On the whole, when H2 was adsorbed on different sites of the heterojunction surface, the heterojunction resistance increased. Compared with the DOS of the metal oxide CoO (Fig. S5(a), ESI†), the DOS value of the SnO2(100)(I)/CoO(110)(II) heterojunction decreases more obviously. Therefore, the heterojunction changed the gas adsorption performance for H2.
Fig. 5(b) shows the DOS of H2 adsorption on different sites of the CoO(110)(II)/SnO2(100)(II) heterojunction surface. With the adsorption of H2 on an O2 molecule, the DOS value was reduced distinctly (labeled as 1 and 2), implying the heterojunction resistance had improved. The DOS showed a slight alteration when H2 was adsorbed on the Sn site, indicated that the resistance of the heterojunction had slightly changed, together with the disappearance of peaks in the conduction band (labeled as 3 and 4), indicating that the heterojunction resistance was enlarged. In the DOS, the number of peaks disappeared or reduced (labeled as 5, 6, and 7) when H2 was adsorbed on the O1 site, indicating that the heterojunction resistance had increased. DOS showed a slight reduction and some peaks disappeared (labeled as 8, 9, 10, and 11) when H2 was adsorbed on the O2 site, indicating that the heterojunction resistance had increased. These results proved the heterojunction resistance increased with the adsorption of H2. The DOS changes of H2 on the heterojunction surface was opposite to that for H2 on the SnO2 surface (Fig. S5(b), ESI†). This shows that the presence of a heterojunction will change the response trend of H2 on the SnO2 surface.
In summary, compared with single metal oxides CoO and SnO2, the existence of the SnO2(100)(I)/CoO(110)(II) heterojunction and CoO(110)(II)/SnO2(100)(II) heterojunction changed the adsorption performance to H2. The response trend of the SnO2(100)(I)/CoO(110)(II) heterojunction to H2 was the same as that with CoO, but the response trend of the CoO(110)(II)/SnO2(100)(II) heterojunction to H2 was the opposite as that with SnO2. This will provide theoretical guidance for the accurate identification of H2 and the manufacture of sensors.
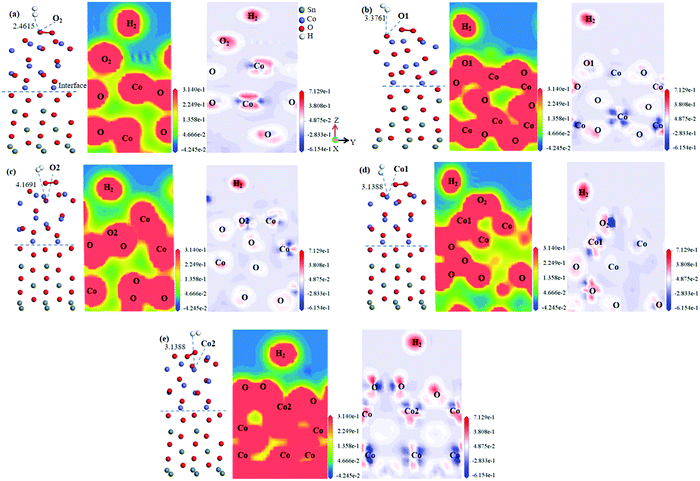
Fig. 6 displays the optimized structure, electronic density, and electronic density difference of H2 adsorption on different sites (including O2 molecule, O1 site, O2 site, Co1 site and Co2 site) of the SnO2(100)(I)/CoO(110)(II) heterojunction surface. The optimized structure of H2 adsorption on an O2 molecule of the SnO2(100)(I)/CoO(110)(II) heterojunction surface is shown in Fig. 6(a). The adsorbed distance was 2.4615 Å and Eads was −0.2286 eV, as shown in Table 2. The adsorbed distance was smaller than that for H2 on other sites of the heterojunction surface.
The blue ranges represent the depletion areas of the electrons, and the red areas show the accumulation region of the electrons. As displayed in Fig. 6(a), H2 was adsorbed on the O2 molecule of the heterojunction surface. The adsorbed distance was less than 3 Å and Eads was negative, and the electronic sharing occurred between H atoms and the O2 molecule. Therefore, the H atoms share an extensive amount of electrons with the O2 molecule, which indicates that H–O bonding has stronger covalence. In addition, the O2 molecule shares extensive electrons with atoms of the heterojunction surface, illustrating the stable adsorption of the O2 molecule on the heterojunction surface and the formation of a covalent bond with atoms of the heterojunction surface. As shown in Fig. 6(a), the H2 molecule and O2 molecule, which are adsorbed on the heterojunction surface, get an amount of electrons simultaneously. This implies the existence of an electronic transformation and chemical reaction between H atoms and O atoms of O2.
The electron sharing hardly occurred and the adsorption distance was greater than 3 Å when H2 was adsorbed on other sites of the heterojunction surface, and although there was slight electron transfer between the sites, there was no bonding. Therefore, O2 molecule was the best adsorption site of H2 on the heterojunction surface and the heterojunction was the best carrier of H2. In the air environment, the adsorbed O2 molecules extract electrons bands of the heterojunction and convert to oxygen species, such as O2−, O−, and O2−, covering the surface of the heterojunction.77,78 The subsequent reaction (H2 + Ox− → H2O + xe−) releases electrons back into the heterojunction surface.54 An atomic level understanding of the H2-sensing mechanism on the heterojunction was thus proved.
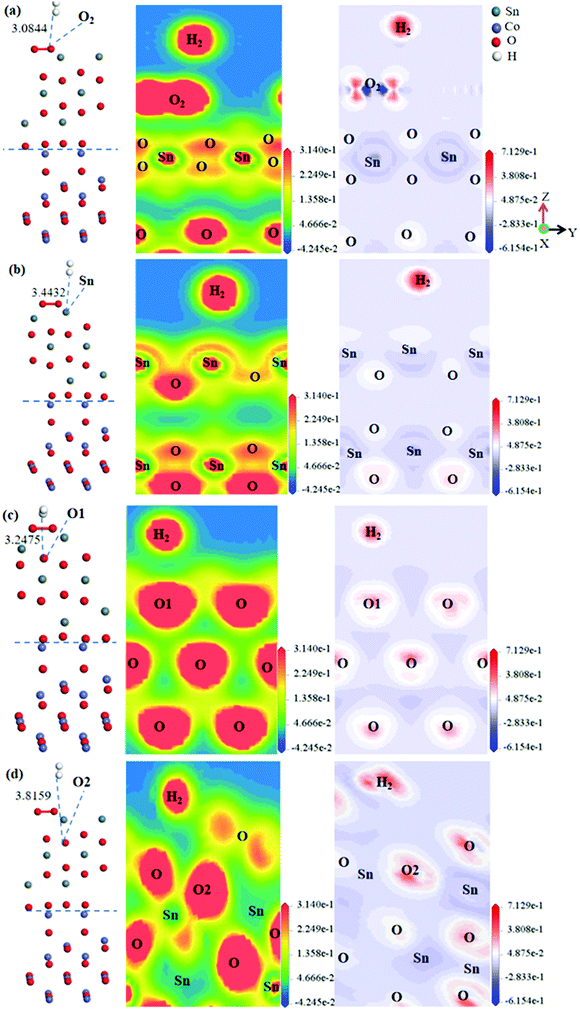
Fig. 7(a) reveals the electronic density when H2 was adsorbed on the O2 molecule. As shown, there was little electronic accumulation between the H atoms and O2 molecule when H2 was adsorbed on the O2 molecule. The H atoms shared a bit of electronic density with the O2 molecule on the heterojunction surface and the adsorbed distance was more than 3 Å, indicating the existence of a slight H–O covalent bond. Fig. 7(a) shows the electronic density difference when H2 was adsorbed on the O2 molecule of the heterojunction surface, whereby the H atoms acquired extensive electrons, and at the same time, a gain and loss of electrons occurred on the O2 molecule. Consequently, the transformation of the electronic density and the chemical reaction between H atoms and the O2 molecule were proved. Fig. 7(b) reveals the electronic density when H2 was adsorbed on the O1 atom of the CoO(110)(II)/SnO2(100)(II) heterojunction surface. There was clear electronic accumulation between H atoms and the O1 atom when H2 was adsorbed on the O1 atom. The H atoms shared some electrons with the O1 atom on the SnO2 side and the adsorbed distance was more than 3 Å, which suggests the existence of H–O bonding. Fig. 7(b) shows the electronic density difference when H2 was adsorbed on the O1 site of the heterojunction surface; whereby the H atoms acquired some electrons and the O1 atom of the heterojunction surface obtained some electrons too. Consequently, the slight transformation of the electronic density and a slight reaction between the H atoms and O1 atom were proved.
Fig. 7(c) and (d) show the electronic structure when H2 was adsorbed on the O2 site and Sn site of the heterojunction surface. Barely any electrons existed between H atoms and the O2 atom and Sn atom of the heterojunction surface. This indicated the formation of weaker H–O bonding and H-Sn bonding for H atoms sharing a few electrons with the O2 atom of the heterojunction surface. This means a small transformation in the electronic density and a slight reaction between H2 and the O1 atom and Sn atom. In summary, O2 molecule on the heterojunction was the best site for reacting with H2, and the O1 site was the best adsorption site for H2.
4. Conclusion
In this study, DFT was used to calculate the adsorption properties of H2 on CoO/SnO2 and SnO2/CoO heterojunction surfaces under air conditions. First, the optimal heterojunction structure was determined based on the Wad. Second, the most stable adsorption configuration of H2 in the air was identified based on the results of the Eads calculation. Finally, the mechanism of the gas adsorption process was revealed by DOS and by the electronic structure, and the following conclusions were obtained:(1) A smaller lattice mismatch and larger Wad are associated with a more stable interface. The CoO(110) surface and SnO2(100) surface were chosen for forming the structure of the heterojunction. SnO2(100)(I)/CoO(110)(II) and CoO(110)(II)/SnO2(100)(II) were more stable than other SnO2(100)/CoO(110) and CoO(110)/SnO2(100) heterojunctions. Therefore, the stability, resistance, and electronic structure of SnO2(100)(I)/CoO(110)(II) and CoO(110)(II)/SnO2(100)(II) heterojunctions were mainly studied as carriers of H2 in oxygen atmosphere.
(2) The heterojunction resistance increased when H2 was adsorbed on different sites of the SnO2(100)(I)/CoO(110)(II) surface, which, followed the same trend as for H2 adsorption on the CoO surface. Moreover, its trend of change was greater according to DOS. This shows that the heterojunction can change the gas adsorption performance of H2. The O2 molecule on the heterojunction surface is the best adsorption site for H2, because of the larger electron density distribution between them. In addition, the heterojunction is the best carrier of H2 for the existence of the reaction between H2 and surface oxygen ions. An atomic level understanding of the H2 sensing mechanism on the heterojunction was proved.
(3) The heterojunction resistance increased when H2 was adsorbed on different sites of the CoO(110)(II)/SnO2(100)(II) surface. Also, the heterojunction resistance showed the opposite trend to H2 adsorption on the SnO2 surface. When the SnO2/CoO heterojunction was exposed to H2 gas, H2 reacted with the oxygen ions on the SnO2 surface and the electrons returned to the conduction band, which led to energy band bending. Nonetheless, H2 hardly reacted with the oxygen ions on the CoO surface. As a result, the potential barrier of SnO2/CoO increased, leading to an increase in resistance and a p-type response to H2. Therefore, the presence of the heterojunction will change the trend of the response to H2. Meanwhile, according to the electronic density, the O1 site of the heterojunction surface was the best adsorption site for H2, because of the larger electron density distribution among them. Finally, as judged by Eads, the heterojunction improved the stability of H2 adsorption on the surface.
Conflicts of interest
There are no conflicts declare.Acknowledgements
We are very grateful for the support of the National Natural Science Foundation of China (Fund number: 51974157, 51874169, 51774180 and 51634004).References
- C. B. Lim and S. Oh, Microstructure evolution and gas sensitivities of Pd-doped SnO2-based sensor prepared by three different catalyst-addition processes, Sens. Actuators, B, 1996, 30, 223–231 CrossRef CAS.
- F. Hartmann, Semiconductor sensors, Nucl. Instrum. Methods Phys. Res, 2011, 628, 40–49 CrossRef CAS.
- A. T. Güntner and N. J. Pineau, et al., Sniffing Entrapped Humans with Sensor Arrays, Anal. Chem., 2018, 90, 4940–4945 CrossRef PubMed.
- M. Righettoni, A. Amann and E. S. Pratsinis, Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors, Mater. Today, 2015, 18, 163–171 CrossRef CAS.
- P. Reimann, A. Dausend and A. Schutze, A self-monitoring and self-diagnosis strategy for semiconductor gas sensor systems, SENSORS, 2008 IEEE, 2008, 192–195 Search PubMed.
- M. Schüler, T. Sauerwald and A. Schütze, A novel approach for detecting HMDSO poisoning of metal oxide gas sensors and improving their stability by temperature cycled operation, J. Sens. Sens. Syst., 2015, 4, 305–311 CrossRef.
- L. P. Huo, Y. Xi and Z. Liu, et al., Modulation of Potential Barrier Heights in Co3O4/SnO2 Heterojunction for Highly H2-Selective Sensors, Sens. Actuators, B, 2017, 244, 694–700 CrossRef CAS.
- N. V. Toan and N. V. Duy, et al., Fabrication of highly sensitive and selective H2 gas sensor based on SnO2 thin film sensitized with microsized Pd islands, J. Hazard. Mater., 2015, 301, 433–442 CrossRef PubMed.
- C. Liewhiran, N. Tamaekong and A. Wisitsoraat, et al., Ultra-sensitive H2 sensors based on flame-spray-made Pd-loaded SnO2 sensing films, Sens. Actuators, B, 2013, 176, 893–905 CrossRef CAS.
- C. B. Lim and S. Oh, Microstructure evolution and gas sensitivities of Pd-doped SnO2-based sensor prepared by three different catalyst-addition processes, Sens. Actuators, B, 1996, 30, 223–231 CrossRef CAS.
- F. D. Manchester, A. San-Martin and J. M. Pitre, The H-Pd (hydrogen-palladium) System, J. Phase Equilib., 1994, 15, 62–83 CrossRef CAS.
- G. Lu and J. T. Hupp, Metal–organic frameworks as sensors: a ZIF-8 based Fabry-Pérot device as a selective sensor for chemical vapors and gases, J. Am. Chem. Soc., 2010, 132, 7832–7833 CrossRef CAS PubMed.
- M. Drobek, J. H. Kim and M. Bechelany, et al., MOF-Based Membrane Encapsulated ZnO Nanowires for Enhanced Gas Sensor Selectivity, ACS Appl. Mater. Interfaces, 2016, 8, 8323–8328 CrossRef CAS.
- H. Tian, H. Fan and M. Li, et al., Zeolitic Imidazolate Framework Coated ZnO Nanorods as Molecular Sieving to Improve Selectivity of Formaldehyde Gas Sensor, ACS Sens., 2016, 1, 243–250 CrossRef CAS.
- M. S. Yao and W. Xiang, et al., MOF Thin Film-Coated Metal Oxide Nanowire Array: Significantly Improved Chemiresistor Sensor Performance, Adv. Mater., 2016, 28, 5229–5234 CrossRef CAS PubMed.
- J. Hong, S. Lee, J. Seo, S. Pyo, J. Kim and T. Lee, A highly sensitive hydrogen sensor with gas selectivity using a PMMA membrane-coated Pd nanoparticle/single-layer graphene hybrid, ACS Appl. Mater. Interfaces, 2015, 7, 3554–3561 CrossRef CAS PubMed.
- O. Lupan, V. Postica and J. Ttrup, et al., Hybridization of Zinc Oxide Tetrapods for Selective Gas Sensing Applications, ACS Appl. Mater. Interfaces, 2017, 9, 4084–4099 CrossRef CAS PubMed.
- V. Musat, E. Fortunato and A. M. Botelho do Rego, et al., Sol-gel cobalt oxide-silica nanocomposite thin films for gas sensing applications, Thin Solid Films, 2008, 516, 1499–1502 CrossRef CAS.
- M. Matsumiya, F. Qiu and W. Shin, et al., Thermoelectric CO Gas Sensor Using Thin-Film Catalyst of Au and Co3O4, J. Electrochem. Soc., 2003, 151, H7 CrossRef.
- Q. Jiao, M. Fu and C. You, et al., Preparation of hollow Co3O4 microspheres and their ethanol sensing properties, Inorg. Chem., 2012, 51, 11513–11520 CrossRef CAS PubMed.
- X. Yu, X. Liu and M. Wu, et al., Hierarchical radial Co3O4 microcrystal and application in gas sensor, Chin. J. Chem. Phys., 2014, 27, 99–102 CrossRef CAS.
- D. Barreca, E. Comini and A. P. Ferrucci, et al., First Example of ZnO-TiO2 Nanocomposites by Chemical Vapor Deposition: Structure, Morphology, Composition, and Gas Sensing Performances, Chem. Mater., 2007, 19, 5642–5649 CrossRef CAS.
- W. J. Moon, H. J. Yu and G. M. Choi, The CO and H2 gas selectivity of CuO-doped SnO2–ZnO composite gas sensors, Sens. Actuators, B, 2002, 87, 464–470 CrossRef CAS.
- J. H. Kim, J. H. Lee and A. Mirzaei, et al., SnO2 (n)-NiO (p) composite nanowebs: gas sensing properties and sensing mechanisms, Sens. Actuators, B, 2017, 258, 204–214 CrossRef.
- D. R. Miller, S. A. Akbar and P. A. Morris, Nanoscale metal oxide-based heterojunction for gas sensing: a review, Sens. Actuators, B, 2014, 204, 250–272 CrossRef CAS.
- Y. Liu, G. Li and R. Mi, et al., An environment-benign method for the synthesis of p-NiO/n-ZnO heterostructure with excellent performance for gas sensing and photocatalysis, Sens. Actuators, B, 2014, 191, 537–544 CrossRef CAS.
- J. Martinez, S. B. Sinnott and S. R. Phillpot, Adhesion and diffusion at TiN/TiO2 interfaces: a first principles study, Comput. Mater. Sci., 2017, 130, 249–256 CrossRef CAS.
- J. H. Kim, J. H. Lee and A. Mirzaei, et al., SnO2(n)-NiO(p) composite nanowebs: gas sensing properties and sensing mechanisms, Sens. Actuators, B, 2017, 258, 204–214 CrossRef.
- B. Xu, C. Zhu and X. He, et al., First-Principles Calculations on Atomic and Electronic Properties of Ge/4H-SiC Heterojunction, Adv. Conden. Matter Phys., 2018, 3, 1–9 Search PubMed.
- X. Fan, B. Chen and M. Zhang, et al., First-principles calculations on bonding characteristic and electronic property of TiC(111)/TiN(111) interface, Mater. Des., 2016, 112, 282–289 CrossRef CAS.
- A. Katoch, S. W. Choi and J. H. Kim, et al., Importance of the nanograin size on the H2S-sensing properties of ZnO–CuO composite nanofibers, Sens. Actuators, B, 2015, 214, 111–116 CrossRef CAS.
- S. W. Choi, Z. Jin and K. Akash, et al., H2S sensing performance of electrospun CuO-loaded SnO2 nanofibers, Sens. Actuators, B, 2012, 169, 54–60 CrossRef CAS.
- D. Ju, H. Xu and Q. Xu, et al., High triethylamine-sensing properties of NiO/SnO2 hollow sphere P–N heterojunction sensors, Sens. Actuators, B, 2015, 215, 39–44 CrossRef CAS.
- Q. Zhi, Y. Fu and B. Yu, et al., High and fast H2S response of NiO/ZnO nanowire nanogenerator as a self-powered gas sensor, Sens. Actuators, B, 2016, 222, 78–86 CrossRef.
- Y. T. Kwon and W. H. Yeo, Flexible, Wearable, and Stretchable Electronics, Flexible, Wearable, and Stretchable Electronics, 2020, 1–30.
- J. J. Kim, S. J. Chung and J. W. Choi, et al., Redox Reaction between Nitric Oxide and Carbon Monoxide over Perovskite-type Catalysts, J. Korean Inst. Chem. Eng., 1982, 20, 245 CAS.
- L. Wang, J. Deng and Z. Lou, et al., Cross-linked p-type Co3O4 octahedral nanoparticles in 1D n-type TiO2 nanofibers for high-performance sensing devices, J. Mater. Chem. A, 2014, 2, 10022–10028 RSC.
- X. T. Yin, J. Li and D. Dastan, et al., Ultra-high selectivity of H2 over CO with a p–n nanojunction based gas sensors and its mechanism, Sens. Actuators, B, 2020, 319, 128330 CrossRef CAS.
- B. L. Bramfitt, The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron, Metall. Trans., 1970, 1, 1987–1995 CrossRef CAS.
- H. Zhang and H. Xiong, First-principles study of NbC heterogeneous nucleation on TiC vs. TiN in microalloy steel, Ironmaking Steelmaking, 2018, 47, 77–83 CrossRef.
- A. Mirzaei, S. G. Leonardi and G. Neri, Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: a review, Ceram. Int., 2016, 42, 15119–15141 CrossRef CAS.
- Y. Jian, J. Huang and D. Fan, et al., First-principles investigation on the electronic property and bonding configuration of NbC(111)/NbN(111) interface, J. Alloys Compd., 2016, 689, 874–884 CrossRef.
- X. Fan, B. Chen and M. Zhang, et al., First-principles calculations on bonding characteristic and electronic property of TiC(111)/TiN(111) interface, Mater. Des., 2016, 112, 282–289 CrossRef CAS.
- L. Shi, H. Yan and Z. H. Lu, et al., Surface Property of the Cu Doped γ-Al2O3: A Density Functional Theory Study, Appl. Surf. Sci., 2020, 535, 147651 CrossRef.
- G. Feng, Q. Xiao and D. S. Wang, et al., Acid properties of Ni-modified ZSM-12: a first-principles Study, J. Fuel Chem. Technol., 2020, 48, 704–712 CrossRef CAS.
- G. Feng, J. Yang and C. Wang, et al., A first-principles study on Pd modified ZSM-12 zeolites, Microporous Mesoporous Mater., 2018, 260, 227–234 CrossRef CAS.
- G. Feng, M. V. Ganduglia-Pirovano and C. F. Huo, et al., Hydrogen spillover to copper clusters on hydroxylated γ-Al2O3, J. Phys. Chem. C, 2018, 122, 18445–18455 CrossRef CAS.
- X. Yu, X. Zhang and H. Wang, et al., High-coverage H2 adsorption on the reconstructed Cu2O(111) surface, J. Phys. Chem. C, 2017, 121, 22081–22091 CrossRef CAS.
- L. Shi, S. Meng and S. Jungsuttiwong, et al., High coverage H2O adsorption on CuAl2O4 surface: a DFT study, Appl. Surf. Sci., 2020, 507, 145162 CrossRef CAS.
- L. Ou, Y. Fan and Y. Liu, et al., First-Principle Study of the Adsorption and Dissociation of O2 on Pt(111) in Acidic Media, J. Phys. Chem. C, 2009, 113, 20657–20665 CrossRef CAS.
- J. Chen and C. Ye, A first-principle study of the effect of vacancy defects and impurities on the adsorption of O2 on sphalerite surfaces – Science Direct, Colloids Surf., A, 2010, 363, 56–63 CrossRef CAS.
- H. Xiong and Z. Liu, et al., First principles calculation of interfacial stability, energy and electronic properties of SiC/ZrB2 interface-Science Direct, J. Phys. Chem. Solids, 2017, 107, 162–169 CrossRef CAS.
- Y. Jian, J. Huang and D. Fan, et al., First-principles investigation on the electronic property and bonding configuration of NbC(111)/NbN(111) interface, J. Alloys Compd., 2016, 689, 874–884 CrossRef.
- W. H. Lee and X. H. Yao, First principle investigation of phase transition and thermodynamic properties of SiC, Comput. Mater. Sci., 2015, 106, 76–82 CrossRef CAS.
- L. Huo, Y. Xi and Z. Liu, et al., Modulation of Potential Barrier Heights in Co3O4/SnO2 Heterojunction for Highly H2-Selective Sensors, Sens. Actuators, B, 2017, 244, 694–700 CrossRef CAS.
- B. L. Bramfitt, The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron, Metall. Trans., 1970, 1, 1987–1995 CrossRef CAS.
- H. Zhang and H. Xiong, First-principles study of NbC heterogeneous nucleation on TiC vs., TiN in microalloy steel, Ironmaking Steelmaking, 2018, 47, 77–83 CrossRef.
- R. Liu, X. Yin and K. Feng, et al., First-principles calculations on Mg/TiB2 interfaces, Comput. Mater. Sci., 2018, 149, 373–378 CrossRef CAS.
- L. Xiao, Q. Hui and D. Shao, et al., First-principles study on the stability and electronic structure of Mg/ZrB2 interfaces, Sci. China: Mater., 2016, 59, 28–37 Search PubMed.
- H. H. Xiong, H. N. Zhang and J. H. Dong, Adhesion strength and stability of TiB2/TiC interface in composite coatings by first principles calculation, Comput. Mater. Sci., 2017, 127, 244–250 CrossRef CAS.
- L. Jian, Y. Yang and G. Feng, et al., First-principles study of stability and properties on β-SiC/TiC(111) interface, J. Appl. Phys., 2013, 114, 54–59 CrossRef.
- D. Yin, X. Peng and Y. Qin, et al., Electronic property and bonding configuration at the TiN(111)/VN(111) interface, J. Appl. Phys., 2010, 108, 033714 CrossRef.
- D. Y. Dang, L. Y. Shi and J. L. Fan, et al., First-principles study of W-TiC interface cohesion, Surf. Coat. Technol., 2015, 276, 602–605 CrossRef CAS.
- N. Chandran, M. Sall and J. Arvanitidis, et al., On the formation of graphene by Ge intercalation of a 4H-SiC surface, Mater. Sci. Forum, 2015, 821, 961–964 Search PubMed.
- H. Zhang and H. Xiong, First-principles study of NbC heterogeneous nucleation on TiC vs. TiN in microalloy steel, Ironmaking Steelmaking, 2018, 47, 77–83 CrossRef.
- H. J. Kim and J. H. Lee, Highly sensitive and selective gas sensors using p-type oxide semiconductors: overview, Sens. Actuators, B, 2014, 192, 607–627 CrossRef CAS.
- S. Chu and A. Majumdar, Opportunities and challenges for a sustainable energy future, Nature, 2012, 488, 294–303 CrossRef CAS PubMed.
- L. Tao, J. Huang and D. Dastan, et al., New insight into absorption characteristics of CO2 on the surface of calcite, dolomite, and magnesite, Appl. Surf. Sci., 2021, 540, 148320 CrossRef CAS.
- L. Tao, J. Huang and D. Dastan, et al., CO2 capture and separation on charge-modulated calcite, Appl. Surf. Sci., 2020, 530, 147265 CrossRef CAS.
- M. Viitala, et al., Conformation and energetics of benzene adsorbate on SnO2(110) surfaces: a first principles study, Surf. Sci., 2011, 605, 1563–1567 CrossRef CAS.
- Z. Wen, T. M. Liu and D. J. Liu, Formaldehyde gas sensing property and mechanism of TiO2–Ag nanocomposite, Phys. B, 2010, 405, 4235–4239 CrossRef.
- M. H. Li, H. C. Zhu and G. F. Wei, et al., DFT calculation and analysis of the gas sensing mechanism of methoxy propanol on Ag decorated SnO2(110) surface, RSC Adv., 2019, 9, 35862–35871 RSC.
- W. Zeng, T. M. Liu and D. J. Liu, et al., Hydrogen sensing and mechanism of M-doped SnO2 (M = Cr3+, Cu2+ and Pd2+) nanocomposite, Sens. Actuators, B, 2011, 160, 455–462 CrossRef CAS.
- W. Zeng, T. M. Liu and X. F. Lei, Hydrogen sensing properties of low-index surfaces of SnO2 from first-principles, Phys. B, 2010, 405, 3458–3462 CrossRef CAS.
- S. Yang, Z. Lan and H. Xu, et al., A First-Principles Study on Hydrogen Sensing Properties of Pristine and Mo-Doped Graphene, J. Nanotechnol., 2018, 2018, 1–5 CrossRef.
- Y. H. Zhang, L. F. Han and Y. H. Xiao, et al., Understanding dopant and defect effect on H2S sensing performances of graphene: a first-principles study, Comput. Mater. Sci., 2013, 69, 222–228 CrossRef CAS.
- A. Gurlo and R. Riedel, In situ and operando spectroscopy for assessing mechanisms of gas sensing, Angew. Chem., Int. Ed., 2007, 46, 3826–3848 CrossRef CAS PubMed.
- A. Gurlo, Interplay between O2 and SnO2: oxygen ionosorption and spectroscopic evidence for adsorbed oxygen, Phys. Chem. Chem. Phys., 2006, 7, 2041–2052 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cp04539c |
| This journal is © the Owner Societies 2022 |