Unconventional direct synthesis of Ni3N/Ni with N-vacancies for efficient and stable hydrogen evolution†
Doudou
Zhang
 a,
Haobo
Li
*b,
Asim
Riaz
a,
Haobo
Li
*b,
Asim
Riaz
 c,
Astha
Sharma
c,
Astha
Sharma
 a,
Wensheng
Liang
a,
Yuan
Wang
a,
Wensheng
Liang
a,
Yuan
Wang
 d,
Hongjun
Chen
e,
Kaushal
Vora
c,
Di
Yan
a,
Zhen
Su
d,
Antonio
Tricoli
d,
Hongjun
Chen
e,
Kaushal
Vora
c,
Di
Yan
a,
Zhen
Su
d,
Antonio
Tricoli
 e,
Chuan
Zhao
e,
Chuan
Zhao
 d,
Fiona J.
Beck
d,
Fiona J.
Beck
 a,
Karsten
Reuter
bf,
Kylie
Catchpole
*a and
Siva
Karuturi
a,
Karsten
Reuter
bf,
Kylie
Catchpole
*a and
Siva
Karuturi
 *ac
*ac
aSchool of Engineering, The Australian National University Canberra, ACT 2601, Australia. E-mail: kylie.catchpole@anu.edu.au; siva.karuturi@anu.edu.au
bTheoretical Chemistry and Catalysis Research Center, Technische Universität München, Lichtenbergstr. 4, 85747 Garching, Germany. E-mail: haobo.li@tum.de
cDepartment of Electronic Materials Engineering, Research School of Physics, The Australian National University Canberra, ACT 2601, Australia
dSchool of Chemistry, Faculty of Science, The University of New South Wales, Sydney, NSW 2052, Australia
eNanotechnology Research Laboratory, Faculty of Engineering, University of Sydney, NSW 2006, Australia
fFritz-Haber-Institut der Max-Planck-Gesellschaft, Faradayweg 4-6, 14195 Berlin, Germany
First published on 26th October 2021
Abstract
Transition metal nitrides are a fascinating class of catalyst materials due to their superior catalytic activity, low electrical resistance, good corrosion resistance and earth abundance; however, their conventional synthesis relies on high-temperature nitridation processes in hazardous environments. Here, we report a direct synthesis of Ni3N/Ni enriched with N-vacancies using one-step magnetron sputtering. The surface state of Ni3N(001) with 75% N-vacancies is hydrogen-terminated and exhibits four inequivalent Ni3-hollow sites. This leads to stronger H* binding compared to Ni(111), and is affirmed as the most stable surface termination under the electrochemical working conditions (pH ≈ 13.8 and E = −0.1 V) from the Pourbaix diagram. The Ni3N/Ni catalyst shows low crystallinity and good wettability and exhibits a low overpotential of 89 mV vs. RHE at 10 mA cm−2 in 1.0 M KOH with excellent stability over 3 days. This performance closely matches that of the Pt catalyst synthesized under the same conditions and surpasses that of other reported earth-abundant catalysts on planar substrates. The application of Ni3N/Ni as a cocatalyst on Si photocathodes produces an excellent ABPE of 9.3% and over 50 h stability. Moreover, its feasibility for practical application was confirmed with excellent performance on porous substrates and robustness at high operating currents in zero-gap alkaline electrolysis cells. Our work demonstrates a general approach for the feasible synthesis of other transition metal nitride catalysts for electrochemical and photoelectrochemical energy conversion applications.
Broader contextHigh purity hydrogen promises to be a zero-carbon fuel that can be widely adopted as a renewable energy carrier in the near future. The development of high performance catalysts that are non-precious, scalable, and robust is a crucial step for achieving a low levelized cost of hydrogen. Earth-abundant Ni exhibits hydrogen adsorption energies and exchange current densities close to that of Pt for the hydrogen evolution reaction. Among the derivatives of Ni, Ni-nitrides and their heterostructures are particularly promising due to their high binding capabilities for the adsorbates and relatively low electrical resistance. This work reports the direct synthesis of Ni3N enriched with N-vacancies using one-step reactive magnetron sputtering from earth-abundant Ni, which differs from the high-temperature nitridation process in the presence of hazardous chemicals. The important role of the N-vacancies for achieving high activity and stability from the low surface free energy (γ) and positive Bader charge values, and surface Pourbaix diagram under working conditions is rationalized by density functional theory calculations. Moreover, the Ni3N/Ni catalyst deposited on different (porous and planar) substrates produces remarkable performance and stability even at high current densities in zero-gap electrolysers, demonstrating its feasibility for practical application. |
Introduction
Converting intermittent renewable energy to value-added chemicals (e.g., hydrogen, ethanol, and methanol) is a feasible strategy to address the issues of sustainable energy storage, transportation, and consumption.1 In particular, research on the efficient production of green hydrogen through electrochemical water splitting using renewable electricity or photoelectrochemical (PEC) water splitting using sunlight has received tremendous attention in recent years. Central to these efforts is the development of high-performing earth-abundant catalysts with good operational stability for hydrogen evolution reactions (HERs) and oxygen evolution reactions (OERs) using scalable synthesis methods, which could allow the replacement of noble-metal catalysts such as Pt and Ir/Ru-based compounds in practical systems.2,3Ni-based compounds including oxides, sulphides, carbides, selenides and nitrides are highly attractive as HER catalysts due to their unique d-orbital electron configuration, earth-abundance and potential for low-cost synthesis.4–8 Computations by Trasatti and Nørskov et al. have verified that the hydrogen adsorption energies and exchange current densities of metallic Ni are close to those of Pt for HERs.9,10 Among the derivatives of Ni, Ni-nitrides and their heterostructures are particularly promising due to their high binding capabilities for adsorbates (atomic hydrogen, protons, or water molecules) and relatively low electrical resistance.11–13 Nevertheless, nitrides are usually synthesized by self-propagating high-temperature synthesis with nitridation at >400 °C in the presence of hazardous chemicals (such as azides, hydrazine, cyanamide, and ammonia), as the high strength of the N–N bonds hinders their direct synthesis.12,14,15
Reactive magnetron sputtering has been widely used as an industrial technique in preparing large-area uniform thin films with high adhesion to the underlying substrates.16 Monometallic and multimetallic electrocatalysts can be directly deposited onto target substrates with controlled loading amounts and desirable composition.17,18 Similarly, cocatalyst layers can be deposited onto semiconductor photoelectrodes in a clean vacuum environment without the risk of corrosion of semiconductor materials as in solution-based synthesis. The structural properties of sputter-deposited films can be controlled by adjusting the process parameters with good reproducibility. Moreover, catalysts with structural disorders and defects are shown to exhibit superior catalytic performance compared to their highly crystalline counterparts due to their high intrinsic activity from the abundant supply of active sites.3,19 For example, magnetron sputtering has been shown to generate oxygen vacancies due to the bombardment of energetic particles, and the concentration of such defects plays an important role in achieving high activity.20 Despite these advantages, there are no reports on the preparation of sputtered Ni-nitrides for electrochemical or PEC water splitting.
Crystalline-Si has revolutionized the photovoltaic industry due to its suitably narrow bandgap (∼1.1 eV), high earth-abundance and availability of mature manufacturing technologies, and it has become a subject of intense research for PEC water splitting.21 Various photoelectrodes have been successfully fabricated achieving high saturation photocurrent densities of up to 40 mA cm−2.21–23 Cocatalyst on Si photoelectrodes play a crucial role in achieving high photovoltage as well as good operational stability. Given the chemical sensitivity of Si in alkaline solutions, compact metal-based cocatalyst could be employed both as catalyst and protection layers. These cocatalyst layers could effectively isolate the Si surface from corrosive electrolytes and avoid it being etched away by the solution. Therefore, it is of significance to develop sputtering deposition of cocatalyst for Si photocathodes, as it produces compact films, which could protect Si from corrosion and is performed via a vacuum process compatible with Si fabrication.
Herein, we report for the first time the one-step synthesis of Ni3N/Ni with triangular pyramid nanostructures using magnetron sputtering for the electrochemical and PEC HER in alkaline solutions. In particular, the sputtered catalyst shows low crystallinity and N-vacancies with rich inequivalent N3-hollow active sites, strong H* binding strength and superior electrochemical surface area. As a result, the Ni3N/Ni electrode presents an HER activity comparable to that of Pt electrodes with an overpotential of 89 mV at 10 mA cm−2. Density functional theory (DFT) calculations rationalize the important role of N-vacancies for this high activity and stability from the low surface free energy (γ) and positive Bader charge values, and surface Pourbaix diagram under working conditions. A Si photocathode with Ni3N/Ni deposited as cocatalyst produces a current density of 38.8 mA cm−2 at 0 V vs. reversible hydrogen electrode (RHE) with 0.59 V onset potential, an applied bias photon-to-current efficiency (ABPE) of 9.3%, and with excellent durability of over 50 h in strong alkaline media. In addition, the Ni3N/Ni catalyst deposited on porous substrates produces remarkable performance and stability even at high current densities in zero-gap electrolyser cells demonstrating its feasibility for practical applications.
Results and discussion
Sputtering deposition of Ni3N/Ni and Ni3N/Ni/Si photocathode preparation
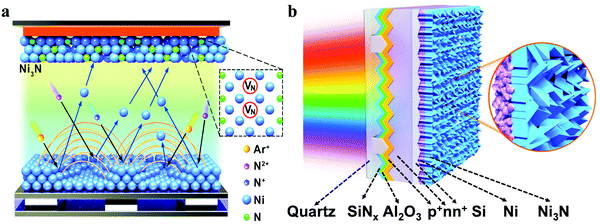
To verify the synthesis of Ni3N by magnetron sputtering and further optimize its HER activity, we first deposited Ni3N films on fluorine-doped tin oxide (FTO) glass substrates using a Ni sputter target and nitrogen plasma. Nitrogen plasma includes molecular, atomic, and ionic nitrogen (N1+and N2+) species, which lead to the formation of N-vacancies.24–26 Therefore, the formation of Ni3N can be described using the following reactions:| Nitrogen dissociation: e− + N2 → N + N + e− | (1) |
| Nitrogen ionization: e− + N2 → N2+ + 2e− | (2) |
| Nitrogen dissociative ionization: e− + N2 → N+ + N + 2e− | (3) |
| Ni3N formation: xNi + yN2 → NixN2y | (4) |
Considering that Ni3N is an interstitial compound, nitrogen atoms occupy the octahedral interstitial sites in the Ni lattice in order to minimize the repulsive N–N interactions.11,27 The nitrogen species from dissociation and ionization of nitrogen atoms permeate into the Ni lattice, resulting in the formation of Ni3N with N-vacancies as illustrated in Fig. 1a. To evaluate the PEC performance of Ni3N/Ni films as cocatalyst, Si photocathodes were fabricated with p+nn+ buried-junction structure, as illustrated in Fig. 1b. Surface texturing was introduced with micropyramids on the front side and microgrooves on the rear side to minimize reflection losses and enhance the catalytic active area, respectively. The front surface of the photocathodes was optimized for light-harvesting and hole collection by depositing a pinhole-free Al2O3 (∼10 nm) passivation layer, Si3N4 anti-reflection coating and Cr/Pd/Ag electrode grid. The rear surface was coated with the Ni3N/Ni cocatalyst film for hydrogen evolution in direct contact with an alkaline electrolyte. More details of the fabrication process are provided in the experimental section in the ESI.†
Synthesis and characterization of Ni3N/Ni films
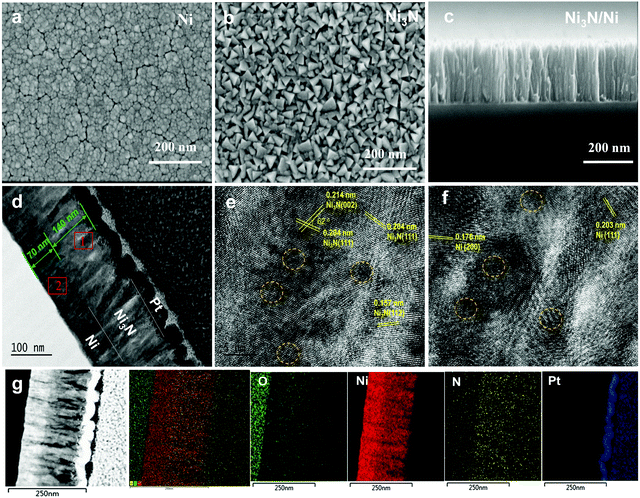
Following a systematic study (described in the next section) of various Ni, Ni3N and Ni3N/Ni films on FTO glass substrates, the bilayer film consisting of ∼140 nm of Ni3N and ∼70 nm of Ni deposited at 150 W direct current (DC) power presented the best HER activity and was chosen for in-depth material characterizations. Because the surface roughness of the FTO glass is relatively high, thin films were deposited on mirror polished Si wafers coated with thick SiOx for the purpose of material characterizations. The individual Ni film shows a nanoparticle morphology with a feature size of ∼20 nm with visible grain boundaries and cracks (Fig. 2a). The Ni3N film shows a triangular pyramid structure morphology (Fig. 2b), which is different from the Ni film and similar to that of the bilayer film (Fig. S1, ESI†). No obvious cracks appeared in the Ni3N film, which suggests that the film is stress-free. The cross-sectional images (Fig. 2c and d) of the bilayer film verify that it has a hierarchical structure with ∼140 nm-thick Ni3N and ∼70 nm-thick Ni.The high-resolution transmission electron microscopy (HRTEM) image of block 1 (Fig. 2e) shows disordered lattice fringes with inter-planar spacings of 0.204 and 0.214 nm, which are assigned to the (111) and (002) crystal planes of hexagonal Ni3N with an intersection angle of 62°, respectively.28,29 The related selected area electron diffraction (SAED) pattern (Fig. S2a, ESI†) with broad halos and sporadically located diffraction dots indicate low crystallinity of Ni3N. The HRTEM and SAED of block 2 in Fig. 2f and Fig. S2b (ESI†) confirm the presence of (111) and (200) crystal planes of cubic Ni with inter-planar spacings of 0.203 and 0.178 nm, respectively, with low crystallinity indicated by the fuzzy and random lattice fringes.30 In addition, the elemental mapping images of Ni3N/Ni (Fig. 2g) show that Ni is homogeneously distributed in both the layers, while N is uniformly located in the Ni3N layer. The energy dispersive X-ray (EDX) spectra in Fig. S3 (ESI†) confirm the presence of Ni and N as the major constituents.
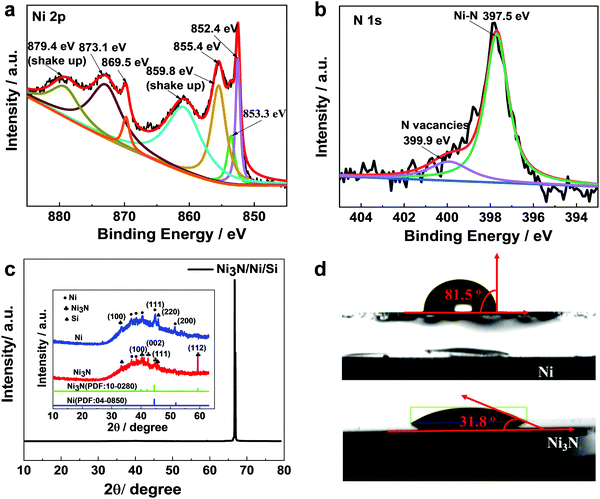
X-Ray photoelectron spectroscopy (XPS) was performed to examine the composition of the Ni3N film. From Ni 2p XPS spectra (Fig. 3a), two peaks at 852.4 and 853.3 eV are observed, which are assigned to metallic Ni and Ni(I) species of Ni3N, respectively.31–34 The peak at 855.4 eV is attributed to Ni2+ from surface oxidation, and the peak at 873.1 eV is assigned to metallic Ni.30 The peak at 869.5 eV belongs to 2p1/2 of Ni+, which is attributed to the presence of valence Ni1+ (Ni < 1+).11 The predominance of Ni < 1+ in Ni3N suggests that the electron density of a considerable fraction of Ni atoms is affected due to the existence of N-vacancies. The “shake up” satellites at the binding energies of 859.8 eV and 879.4 eV are also seen as significant Ni 2p peaks.18 Furthermore, the N 1s XPS spectrum in Fig. 3b presents peaks at 397.5 and 399.9 eV, which are ascribed to N species of Ni3N and N-vacancies, respectively. The latter peak at 399.9 eV agrees with a similar observation made on oxide nanomaterials with oxygen vacancies.35 In addition, detailed compositional analysis of XPS spectra reveals that the atomic ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N in Ni3N is ∼3.5
N in Ni3N is ∼3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is slightly nonstoichiometric due to the presence of N-vacancies in Ni3N.
1, which is slightly nonstoichiometric due to the presence of N-vacancies in Ni3N.
The X-ray diffraction (XRD) pattern for Ni3N/Ni/Si in the 2θ scan range of (10–80°) shows no discernible peaks of Ni and Ni3N (Fig. 3c). Upon magnification in the range 10–60°, the Ni3N spectrum shows weak and broad diffraction peaks attributable to hexagonal Ni3N (JCPDS: 10-0280) accompanied by some noisy peaks, indicating poor crystallinity or defective structure of the material prepared by magnetron sputtering. There also exist peaks corresponding to Si (ICSD#96-901-1057).36 Meanwhile, Ni prepared under the same conditions shows a cubic structure with low crystallinity. These results agree well with HRTEM observations (Fig. 2e and f) and suggest that Ni3N/Ni prepared by magnetron sputtering exhibits low crystallinity with disordered structure and N-vacancies.
We further measured the water contact angles on Ni/FTO and Ni3N/FTO samples. The surface wettability of the electrocatalyst indicates surface adsorption of water molecules and the interaction of the catalyst with electrolyte, thereby playing an important role in the electrocatalytic activity. The much smaller contact angle on Ni3N/FTO as illustrated in Fig. 3d suggests a higher wettability for Ni3N/Ni/FTO due to hydrophilic Ni–N bonds on the surface, which could be beneficial for enhanced HER kinetics.37
Electrochemical performance of Ni3N/Ni films
The electrocatalytic performance of Ni3N-based electrodes towards the HER was evaluated in a 1.0 M KOH electrolyte (pH = 14) saturated with N2 gas. The sputtering power and the thickness of Ni3N were first optimized based on the HER overpotential of the deposited films on FTO glass substrates at 10 mA cm−2 current. As shown in Fig. S4 in the ESI,† Ni3N/Ni synthesized at 150 W presents the best activity with the lowest overpotential. Similarly, Ni3N presents the lowest overpotential when the sputtering duration was fixed at 1000 s (Fig. S5, ESI†), which suggests that a further increase in Ni3N film thickness hinders its catalytic kinetics.To understand the relationship between the synthesis conditions and N-vacancies, we varied the N2: Ar gas ratio in the sputtering chamber while keeping the flow rate of Ar carrier gas constant. We also assessed their respective performances for the HER using linear sweep voltammetry (LSV) measurements as shown in Fig. 4a. The LSV data confirms that the Ni3N sputtered under 50% N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar gas ratio outperforms the other electrodes. As confirmed by the overpotentials at 10 mA cm−2 current as shown in the inset in Fig. 4a, the HER performance worsened when the ratio is either increased or decreased. Fig. S6 (ESI†) shows XPS spectra of N 1s of Ni3N catalysts synthesized under different gas ratios. When the N2
Ar gas ratio outperforms the other electrodes. As confirmed by the overpotentials at 10 mA cm−2 current as shown in the inset in Fig. 4a, the HER performance worsened when the ratio is either increased or decreased. Fig. S6 (ESI†) shows XPS spectra of N 1s of Ni3N catalysts synthesized under different gas ratios. When the N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar ratio was 70%, stoichiometric Ni3N was obtained with an atomic ratio of 3
Ar ratio was 70%, stoichiometric Ni3N was obtained with an atomic ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. With the decreasing gas ratio, the atomic ratio departed gradually from the stoichiometric ratio. When the ratio was below 10%, no N signal was detected, indicating the deposition of pure Ni with Ni-termination. However, N-vacancies first increased with the decreasing gas ratio until 40% as indicated by the area of the peak at 399.9 eV,11 and then decreased with a further decrease in the gas ratio due to the appearance of metallic Ni. Accordingly, the Ni–N peak position shifted to a lower position first and then to a higher position due to the change in the position of the d-band centre of Ni3N in the presence of N-vacancies.38–40 The superior performance of Ni3N deposited under 50% N2
1. With the decreasing gas ratio, the atomic ratio departed gradually from the stoichiometric ratio. When the ratio was below 10%, no N signal was detected, indicating the deposition of pure Ni with Ni-termination. However, N-vacancies first increased with the decreasing gas ratio until 40% as indicated by the area of the peak at 399.9 eV,11 and then decreased with a further decrease in the gas ratio due to the appearance of metallic Ni. Accordingly, the Ni–N peak position shifted to a lower position first and then to a higher position due to the change in the position of the d-band centre of Ni3N in the presence of N-vacancies.38–40 The superior performance of Ni3N deposited under 50% N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : Ar ratio is likely due to an optimal synthesis condition, which could, in turn, result in an optimal N-vacancy concentration as further explained in the later section using DFT calculations.
: Ar ratio is likely due to an optimal synthesis condition, which could, in turn, result in an optimal N-vacancy concentration as further explained in the later section using DFT calculations.
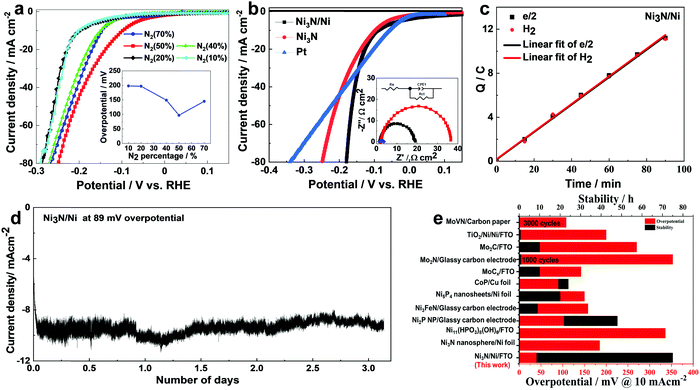 | ||
| Fig. 4 (a) LSV curves (with 90% iR-compensation) of Ni3N/FTO electrodes sputtered under different N2:Ar flows. The flow of Ar carrier gas was fixed at 20 sccm. The inset summarizes the overpotential at 10 mA cm−2 for different N2:Ar flows. (b) LSV curves (with 90% iR-compensation) of Ni3N/Ni, Ni3N, and Pt on FTO substrates and the corresponding Nyquist plots (inset in Fig. 4b). (c) H2 (red dots) evolved from the Ni3N/Ni cathode detected by gas chromatography and the calculated amounts of H2 (black dots). The measurement was performed at jHER = 10 mA cm−2. (d) Chronoamperometry measurement of the Ni3N/Ni for the HER at an overpotential of 89 mV in 1.0 M KOH. (e) Comparison of the overpotential and stability of Ni3N/Ni for the HER with other reported earth-abundant catalysts on planar substrates at a current density of 10 mA cm−2. | ||
As Ni is often used as a substrate material to prepare high-performance heterostructured catalysts, we also investigated the effect of Ni inclusion by preparing Ni3N/Ni bilayer electrodes. Pt, known as the best HER catalyst, was also sputtered on FTO for comparison. The LSV curves obtained for different electrodes for the HER in 1.0 M KOH are shown in Fig. 4b. The Ni3N/Ni/FTO electrode exhibits a commendable HER performance presenting low overpotentials of only 89 and 155 mV at 10 and 40 mA cm−2, respectively. In comparison, the Pt/FTO electrode requires corresponding overpotentials of 57 and 169 mV to deliver the same HER current densities. It is noted that low overpotential at 40 mA cm−2 current density is particularly beneficial for PEC hydrogen generation on Si photocathodes. Interestingly, the addition of Ni film significantly enhances the performance of sputtered Ni3N films. Compared to the Ni3N/FTO electrode, the Ni3N/Ni/FTO electrode shows a reduction in overpotential at 40 mA cm−2 by ∼30 mV (see Fig. S7, ESI†). This drastic improvement in the HER activity could be attributed to the synergistic effect of Ni3N and Ni, which could enhance the electronic conductivity and relieve the film stress, thereby improving the HER performance. For example, a similar observation was reported for hematite deposited on FTO substrates, which resulted in lattice strain between hematite and the substrate. The lattice strain was addressed by adding a thin layer of other materials at the interface.41
The Tafel plots of the electrodes (see Fig. S8, ESI†) derived from the polarization curves in Fig. 4b confirm that Ni3N/Ni/FTO (38 mV dec−1) has a much lower slope compared to Ni3N/FTO (60 mV dec−1), but only slightly higher than that of Pt/FTO (28 mV dec−1). Since the Tafel slope of an electrocatalyst is an indication of its HER reaction kinetics,42,43 the lower Tafel slope of Ni3N/Ni/FTO illustrates that the bilayer catalyst possesses faster kinetics and superior catalytic activity.
To further analyze the HER performance of different electrodes, electrochemical impedance spectroscopy (EIS) was conducted. As shown by the Nyquist plots inset in Fig. 4b, Ni3N/Ni/FTO presents a much smaller charge transfer resistance (Rct) at the electrode–electrolyte interface compared to Ni3N/FTO (16.19 vs. 33.47 Ω cm−2, Table S1, ESI†), illustrating an enhanced charge transport upon the addition of the Ni layer. In order to assess the specific intrinsic activities of Ni3N/Ni/FTO and Ni3N/FTO, their electrocatalytic activities were normalized by the electrochemically active surface area (ECSA) measured using the double layer capacitance method on the basis of cyclic voltammetry in 1.0 M KOH electrolyte (Fig. S9, ESI†). The higher double-layer capacitance (Cdl) of Ni3N/Ni/FTO (1.10 mF cm−2) compared to that of Ni3N/FTO (0.65 mF cm−2) indicates that Ni3N/Ni/FTO possesses a higher ECSA.3,44,45 This further confirms that the inclusion of the Ni layer plays a key role in boosting the catalytic activity of Ni3N films.
The faradaic efficiency measurements (Fig. 4c) of the Ni3N/Ni cathode based on the measured and calculated amounts of the evolved H2 gas prove that there were no undesirable side reactions. To further examine the durability of Ni3N/Ni/FTO as a cathode, a fixed overpotential of 89 mV was applied. As shown in Fig. 4d, a stable cathodic current density of ∼10 mA cm−2 was maintained for over 3 days with negligible degradation, demonstrating its remarkable stability in an alkaline medium. In addition, as summarized in Fig. 4e and Table S2 (ESI†), the hierarchical Ni3N/Ni/FTO exhibits the smallest overpotential and superior stability at 10 mA cm−2 among other earth-abundant alkaline catalysts reported thus far on planar substrates. These results confirm that the sputtered Ni3N/Ni films exhibit not only exceptionally high HER catalytic activity, which closely matches that of Pt but also robust electrochemical stability for practical applications.
Density functional theory calculations
DFT calculations were performed to understand the surface structure and the origin of the high activity of Ni3N/Ni. Ni3N(001) is a flat metallic surface and can be either N- or Ni-terminated. The Ni3N(001) facet is chosen for the calculation, which is the same surface as the (002) facet observed in both TEM images (Fig. 2e) and XRD patterns (Fig. 3c). To simulate the effect of surface N-vacancies, the N-terminated and Ni-terminated surfaces are defined as 0% and 100% N-vacancies, respectively, while the surface also depleted of sub-surface N is defined as 200% N-vacancy (see also Fig. S10, ESI†). Based on this, a series of surface structures with gradual concentrations of N-vacancies from 0% to 200% were constructed, which also include NH-terminated surfaces in the aqueous conditions. The corresponding surface free energies (γ) were calculated to identify the most stable surface structures of Ni3N(001) under both the synthesis and operating conditions. As shown in Fig. 5a, γ is related to the chemical potential for N (μN). With a decrease in μN to be more negative, the concentration of N-vacancies of the most stable surface structure with lowest γ (marked in colored regions) increases gradually from 0% to 200%. In the N2 atmosphere as representative of the synthesis process, the surface with 75% N-vacancies is the most stable. To further relate the surface structure to the experimental HER working condition, a Pourbaix diagram of the most stable surface terminations among the whole range of 0–200% N-vacancy concentrations with possible *N or *NH surface terminations as a function of the working potential (E) and the pH value was calculated as shown in Fig. 5b. As marked by black dashed square, at pH ≈ 13.8 and E = −0.1 V in aqueous solution, the surface with 75% N-vacancies is the most stable, but it is hydrogen-terminated. N-vacancies are thus found to be strongly enriched at the surface, which agrees with the XPS characterization results in Fig. 3.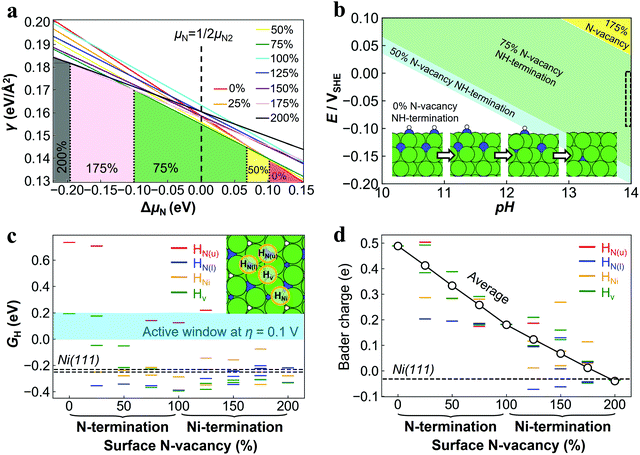 | ||
| Fig. 5 (a) Surface free energy (γ) of Ni3N(001) with varying amounts of N-vacancies as a function of surface chemical potential difference to gaseous N2 (ΔμN). The N-vacancy concentration of 0–100% corresponds to the vacancies of the surface layer, and 100–200% to the sub-surface layer. The coloured areas show the region of most stable structures under the lines with the lowest γ. Black, pink, green, yellow and red lines correspond to combined N-vacancy concentrations in the surface and sub-surface layer of 200% (Ni), 175%, 75%, 50% and 0% (Ni3N), respectively (atomic structures are shown in Fig. S10, ESI†). 75% N-vacancies concentration is most stable at gaseous N2 conditions (horizontal black dashed line). (b) Surface Pourbaix diagram of NixNyHz with N with reference to dissolved N2. The HER working condition is 1.0 M KOH (pH ≈ 13.8) and E = −0.1 eV, as approximately marked in the black dashed box. (c) Adsorption free energies of H* (GH) on 4 inequivalent Ni3-hollow sites exhibited by a Ni3N(001) surface with varying degrees of surface N-vacancies. Multiple data points of the same colour correspond to the non-equivalent sites with different distances to surface N. The GH on the inequivalent 2 Ni3-hollow sites offered by Ni(111) are marked as horizontal dashed lines for comparison. The high HER activity region (GH within 0–0.2 eV) is marked in light blue, see the text. (d) Bader charge of 4 inequivalent Ni3-hollow sites exhibited by a Ni3N(001) surface with varying degrees of surface N-vacancies. Positive Bader charge values represent atoms with a positive charge. The corresponding values for the Ni3-hollow sites at Ni(111) are shown as dashed lines for comparison (the two inequivalent sites share the same value). The average values of the surface Ni atoms for each termination are marked as circles. | ||
The Ni3N(001) surface with or without N-vacancies is metallic, as evidenced by the finite projected density of states (pDOS) around the Fermi level as shown in Fig. S11 (ESI†). As judged from the pDOS shape, the 75% N-vacancy Ni3N(001) surface resembles a regular Ni(111) surface. This shows that the surface is electrically conducting and the standard descriptor to assess the HER activity on transition metal surfaces, the H adsorption free energy (GH), could also be employed here. From a thermodynamic point of view, the optimum HER activity is achieved at GH ∼ 0 eV.46,47 According to a recently proposed model of overpotential influence on the volcano line,48 the working overpotential (η) of +0.1 V shifts the high HER activity region to ∼0–0.2 eV. DFT calculations were conducted to determine GH on a variety of adsorption sites of Ni3N(001). Especially, Ni3 sites (hollow sites formed by three surface Ni atoms) offered by the Ni3N(001) surface is found to be similar to those on Ni(111) and are thus suggested as potentially active sites for the HER. There are 4 such inequivalent Ni3-hollow sites (see inset in Fig. 5c): one above a lower-layer N (HN(l)), one above an upper-layer N(HN(u)), one above a Ni atom (HNi) and one on the pore without sub-surface atoms (Hv). Each site type can have two or three different configurations when N-vacancies are present at the surface.
In order to avoid the risk from the insufficient representation of the selected site structure, a systematic computational screening of all active-site motives was performed to achieve a general trend to present the performance of the catalyst.49 As shown in Fig. 5c, the adsorption free energies of the intermediate H*(GH) exhibit a general decreasing trend with increasing N-vacancy concentration, indicating an increase in the H* binding strength at the Ni3N(001) surface. In the limit of a pure Ni-termination (200% vacancies), the binding then essentially resembles one of the regular Ni(111) surface with GH around −0.25 eV. Intriguingly, at around 75–100% N-vacancies concentration, i.e., at the surface termination identified as most stable under HER working conditions, significantly weaker binding is possible at the HN(u) site that then falls exactly into the high HER activity window. On this basis, the higher HER activity than that of regular Ni(111) would be expected, consistent with the measured higher HER performance as shown in Fig. 4b.
In order to further understand the origin of the different binding strength of H* on surfaces with N-vacancies, the d-band center position (Fig. S12, ESI†) and Bader charge (Fig. 5d) of the four inequivalent Ni3-hollow sites were calculated. While there is a considerable spread over the four sites for each termination, there are trends in the average values of the d-band center position and Bader charge of the surface Ni atoms. With an increase in N-vacancy concentration, the d-band center position first increases and then decreases, whereas the Bader charge decreases monotonously. Therefore, compared to the d-band center, the Bader charge value is more appropriate to explain the influence of the surface N-vacancy concentration on the catalytic performance of the surface Ni atoms. For the most stable 75–100% N-vacancies termination with optimum HER activity, the d-band position is Ni-like, while the average Bader charge at the active sites is still positive at about 0.2 e, i.e., the Ni atoms transfer charge to N atoms to keep the surface positively charged. Compared with the Ni(111) surface, where Ni atoms have almost zero charges, the electron-deficient sites on Ni3N allow a weakened H bonding with higher HER activity. Instructively, for Ni-based catalysts, due to the strong binding between Ni and H atoms, adjusting the content of non-metallic atoms appropriately to make the surface atoms have an average positive charge of about 0.2 e that could achieve an optimal HER catalytic activity. This could be employed as a general strategy to design Ni-based catalysts with high activity.
Photoelectrochemical characterization of Ni3N/Ni/Si photocathodes
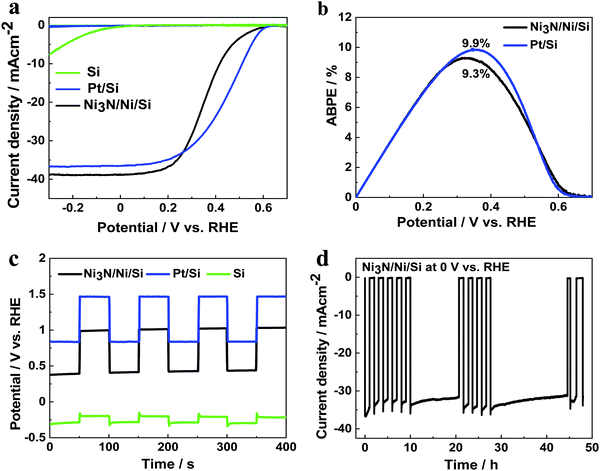
To evaluate the performance of sputter-deposited Ni3N/Ni as a cocatalyst for PEC water splitting, LSV measurements of Si, Pt/Si and Ni3N/Ni/Si photocathodes were conducted in a three-electrode system, illuminated from the front p+ side. As shown in Fig. 6a, the Si photocathode without any cocatalyst presents a low onset potential below the thermodynamic HER potential of 0 V vs. RHE, rendering it ineffective for water splitting. In contrast, the photocathodes modified with Pt and Ni3N/Ni catalysts show much-improved performance. The Ni3N/Ni/Si photocathode exhibits an onset potential at 0.59 V and a photocurrent density of 38.8 mA cm−2 at 0 V vs. RHE. In comparison, Pt/Si delivers a slightly lower photocurrent density of 36.5 mA cm−2 with a similar onset potential. Fig. 6b shows that Ni3N/Ni/Si displays an ABPE of 9.3%, which is comparable to that of the Pt/Si photocathode (9.9%), suggesting that Ni3N/Ni co-catalyst is a superior choice to construct low-cost photocathodes.To further understand the positive shift of onset potential for the as-prepared electrodes, open circuit potential (OCP) were measured in the dark and under illumination as shown in Fig. 6c. The OCPs for the samples move towards higher potentials upon exposure to the incident light, which is caused by the formation of a built-in electric field. The OCP differences before and after illumination are 0.1, 0.60, and 0.64 and for Si, Ni3N/Ni/Si and Pt/Si photocathodes, respectively, which corresponds well with their onset potential values. Evidently, the integration of the Ni3N/Ni and Pt cocatalyst greatly enhances the transfer of photogenerated holes toward the substrate, thereby delivering high photovoltage. The wavelength-dependent incident photon-to-current conversion efficiency (IPCE) for Ni3N/Ni/Si photocathode (Fig. S13, ESI†) measured at 0 V vs. RHE in the wavelength range of 300–1200 nm reveals good light-harvesting ability of the photocathode at energies above the bandgap of Si. The stability measurement (Fig. 6d) over 50 h under simulated one sun illumination indicates that the Ni3N/Ni catalyst film effectively stabilizes Si photocathodes in alkaline solutions. On the other hand, the Si photocathode without any cocatalysts was etched by the solution immediately (Fig. S14, ESI†). These results confirm that the sputtered Ni3N/Ni film serves not only as a high-performance cocatalyst for Si photocathodes but also as an excellent protection layer in alkaline media.
Ni3N/Ni on porous substrates and application in zero-gap cells
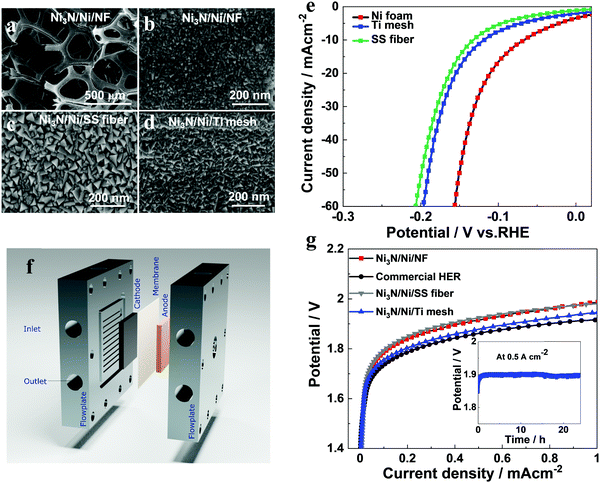
In order to evaluate the performance of the Ni3N/Ni catalyst on porous substrates and at high current densities relevant to practical applications, we fabricated Ni3N/Ni catalysts on different porous substrates, including Ni foam (NF), Ti mesh and stainless steel fiber (SS fiber), and evaluated their performance in zero-gap alkaline water electrolysis cells. The substrates were cleaned following the procedure as described in the experimental section in (ESI†). Ni3N/Ni was sputtered on different porous substrates using the synthesis conditions optimized on FTO substrates. This is expected to yield a similar thickness, except for small changes due to the variation in the surface structure of different porous substrates. From SEM images of the blank substrates (Fig. S15a–c, ESI†), it is clear that different porous substrates present different surface characteristics. Among them, SS fiber presents a flatter and smoother surface except for the rubbed surface, which is comparable to that of planar FTO substrate. On the other hand, Ti mesh and NF present rough and uneven surfaces. Upon the catalyst coating, there is no obvious change in the surface morphology observed in SEM images collected at low magnification (Fig. 7a and Fig. S15d, e, ESI†). This indicates that sputter deposition results in a compact coating of catalyst layers on porous substrates with no aggregation. From SEM images collected at higher magnification (Fig. 7b–d), obvious surface features can be noticed. Ni3N/Ni shows a similar triangular pyramid structure on all porous substrates. However, the size of the structures varies among different substrates, which is due to the difference in their surface properties. Noticeably, the morphology of Ni3N/Ni on SS fiber is almost identical to that on FTO substrates due to its flatter and smoother surface.The electrochemical performance of different porous electrodes was examined using LSV measurements in a three-electrode system in 1.0 M KOH. As shown in Fig. 7e, the Ni3N/Ni/NF electrode outperforms the other porous electrodes presenting ∼66 mV overpotential at 10 mA cm−2. In comparison, the Ni3N/Ni on SS fiber and Ti mesh presented 132 and 118 mV overpotentials, respectively. It is noted that the overpotential of NF is even lower than that observed on FTO, which is due to its large interfacial surface area. In addition, Ni3N/Ni/NF shows excellent electrochemical stability, as indicated by nearly constant overpotential at 15 mA cm−2 for 139 hours (Fig. S16a, ESI†). The LSV data of Ni3N/Ni/NF collected before and after the stability test shows negligible change (Fig. S16b, ESI†). These results confirm that our Ni3N/Ni catalyst with N-vacancies directly synthesized using reactive magnetron sputtering presents promising HER performance even on porous substrates, which also compare favorably among other porous Ni nitrides reported in the literature (Table S3, ESI†). The Nyquist plots from the EIS measurements (Fig. S17 and Table S4, ESI†) confirm that the Ni3N/Ni SS fiber electrode presents the largest semicircle and the highest charge transfer resistance (26.28 Ω cm2) among all electrodes, which results in a low HER performance.
To observe the performance of Ni3N/Ni at high current densities for overall water splitting, we designed zero-gap cells as illustrated by the schematic in Fig. 7f. Further details on the zero-gap cell configuration are provided in the experimental section. Commercially-available NiCoFe on Ni fiber (cathode) and NiFe2O4 on stainless steel fiber (anode) were obtained from Dioxide Materials Inc. and used as a ref. 50. The performances of the commercial electrodes for the HER and OER in the three-electrode configuration are presented in Fig. S18 (ESI†). In all zero-gap cell measurements, the NiFe2O4 electrode was used as an anode and the cell was stabilized at 0.25 A cm−2 for 1 h, prior to recording the response.
It can be seen that water electrolysis commences at a cell voltage >1.65 V and very high current densities are achieved with increasing cell voltages. The cell voltages for current densities of 0.5 A cm−2 for Ni3N/Ni on Ti mesh, SS fiber and NF are ∼1.88 V, 1.90 V and 1.92 V, respectively, which is approximate to the performance of commercial NiCoFe electrode with 1.85 V. It is interesting to note that Ni3N/Ni on NF does not show any superiority in the performance in the zero-gap cell different to its performance in three-electrode measurements at low current densities. We attribute this to the relatively low through-plane conductivity of the NF substrate compared to the other porous substrates resulting in voltage losses at high current densities as confirmed from the EIS data (Fig. S19 and Table S5, ESI†). On the other hand, the Ni3N/Ni on Ti mesh shows comparable performance with that of the commercial HER electrode. Ti mesh presents high through-plane conductivity and supports efficient bubble dissipation through its 2D porous structure, thereby outperforming the other porous electrodes in zero-gap cells. The performance also compares favorably to the other reported Ni-based electrodes in a single-cell water electrolysis cell (Table S6, ESI†). The long-term performance of the Ti mesh electrode in the zero-gap cell is shown as an inset in Fig. 7g, confirming that it maintains stable activity even at very high operating currents. In summary, the performances on various porous substrates and in zero-gap alkaline water electrolysis cells confirm the feasibility of sputtered Ni3N/Ni with N-vacancies as a highly versatile HER electrocatalyst with potential for practical applications.
Conclusions
A hierarchical catalyst comprising pyramidal structured Ni3N/Ni with N-vacancies was directly synthesized by reactive magnetron sputtering for alkaline hydrogen evolution reaction. Due to a synergistic enhancement of N-vacancies, high wettability, and high electrochemical surface area, the Ni3N/Ni bilayer catalyst presented an excellent activity together with remarkable stability. The DFT calculations demonstrated that 75% N-vacancies concentration on the surface leading to superior activity and stability under strong alkaline media. Different from Ni(111), such Ni3N(001) surfaces with N-vacancies exhibit metallic properties, lower surface free energy, positive charge sites and stronger H* binding strength under electrochemical working conditions. The Ni3N/Ni decorated as a cocatalyst on Si photocathodes enhances its onset potential to 0.59 V, achieving 9.3% applied bias photon-to-current efficiency with high operational stability. Moreover, commendable performances and robustness were observed on porous substrates and at high current densities in the zero-gap alkaline electrolysis cell. The demonstration of earth-abundant Ni3N/Ni catalysts with superior HER performance and one-step synthesized using reactive magnetron sputtering, i.e., without the need of a high-temperature nitridation process, provides a general approach for the fabrication of transition metal nitrides for electrochemical and photoelectrochemical hydrogen production.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support from the Australian government through the Australian Research Council (ARC) and Australian Renewable Energy Agency (ARENA) is gratefully acknowledged. Access to fabrication and characterization facilities of the Australian National Fabrication Facility (ANFF) and Centre for Advanced Microscopy is also gratefully acknowledged. H. L. gratefully acknowledges funding from the Alexander-von-Humboldt foundation. K. R. acknowledges funding by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy – EXC 2089/1 – 390776260. The authors gratefully acknowledge the Gauss Centre for Supercomputing e.V. (http://www.gauss-centre.eu) for funding this project by providing computing time through the John von Neumann Institute for Computing (NIC) on the GCS Supercomputer JUWELS at Jülich Supercomputing Centre (JSC).References
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed
.
- I. Roger, M. A. Shipman and M. D. Symes, Nat. Rev. Chem., 2017, 1, 1–13 CrossRef
.
- D. Zhang, J. Z. Soo, H. H. Tan, C. Jagadish, K. Catchpole and S. K. Karuturi, Advanced Energy and Sustainability Research, 2021, 2, 2000071 CrossRef
.
- V. Vij, S. Sultan, A. M. Harzandi, A. Meena, J. N. Tiwari, W.-G. Lee, T. Yoon and K. S. Kim, ACS Catal., 2017, 7, 7196–7225 CrossRef CAS
.
- Y. Li, X. Bao, D. Chen, Z. Wang, N. Dewangan, M. Li, Z. Xu, J. Wang, S. Kawi and Q. Zhong, ChemCatChem, 2019, 11, 5913–5928 CrossRef CAS
.
- Z. Wang, J. Fan, B. Cheng, J. Yu and J. Xu, Mater. Today Phys., 2020, 100279 CrossRef
.
- X. Zheng, X. Han, Y. Zhang, J. Wang, C. Zhong, Y. Deng and W. Hu, Nanoscale, 2019, 11, 5646–5654 RSC
.
- Y. Yan, B. Y. Xia, B. Zhao and X. Wang, J. Mater. Chem. A, 2016, 4, 17587–17603 RSC
.
- S. Trasatti, J. Electroanal. Chem. Interfacial Electrochem., 1972, 39, 163–184 CrossRef CAS
.
- J. Greeley, J. K. Nørskov, L. A. Kibler, A. M. El-Aziz and D. M. Kolb, Chem. Phys. Chem., 2006, 7, 1032–1035 CrossRef CAS PubMed
.
- B. Liu, B. He, H. Q. Peng, Y. Zhao, J. Cheng, J. Xia, J. Shen, T. W. Ng, X. Meng and C. S. Lee, Adv. Sci., 2018, 5, 1800406 CrossRef
.
- F. Song, W. Li, J. Yang, G. Han, P. Liao and Y. Sun, Nat. Commun., 2018, 9, 1–10 CrossRef PubMed
.
- W. Hua, H. Sun, H. Liu, Y. Li and J.-G. Wang, Appl. Surf. Sci., 2021, 540, 148407 CrossRef CAS
.
- W. F. Chen, K. Sasaki, C. Ma, A. I. Frenkel, N. Marinkovic, J. T. Muckerman, Y. Zhu and R. R. Adzic, Angew. Chem., Int. Ed., 2012, 51, 6131–6135 CrossRef CAS PubMed
.
- M. Shalom, D. Ressnig, X. Yang, G. Clavel, T. P. Fellinger and M. Antonietti, J. Mater. Chem. A, 2015, 3, 8171–8177 RSC
.
- P. J. Kelly and R. D. Arnell, Vacuum, 2000, 56, 159–172 CrossRef CAS
.
- J. Liang, Q. Liu, T. Li, Y. Luo, S. Lu, X. Shi, F. Zhang, A. M. Asiri and X. Sun, Green Chem., 2021, 23, 2834–2867 RSC
.
- D. Zhang, L. Meng, J. Shi, N. Wang, S. Liu and C. Li, Electrochim. Acta, 2015, 169, 402–408 CrossRef CAS
.
- L. Chen and Q. Xu, Science, 2020, 367, 737 CrossRef CAS
.
- J. Li, C. Shu, C. Liu, X. Chen, A. Hu and J. Long, Small, 2020, 16, 2001812 CrossRef CAS PubMed
.
- Z. Luo, T. Wang and J. Gong, Chem. Soc. Rev., 2019, 48, 2158–2181 RSC
.
- D. Zhang, J. Shi, W. Zi, P. Wang and S. Liu, ChemSusChem, 2017, 10, 4324–4341 CrossRef CAS PubMed
.
- S. K. Karuturi, H. Shen, A. Sharma, F. J. Beck, P. Varadhan, T. Duong, P. R. Narangari, D. Zhang, Y. Wan and J. H. He, Adv. Energy Mater., 2020, 10, 2000772 CrossRef CAS
.
- B. Ouyang, Y. Zhang, Z. Zhang, H. J. Fan and R. S. Rawat, Small, 2017, 13, 1604265 CrossRef PubMed
.
- J. S. Kang, M.-A. Park, J.-Y. Kim, S. H. Park, D. Y. Chung, S.-H. Yu, J. Kim, J. Park, J.-W. Choi and K. J. Lee, Sci. Rep., 2015, 5, 1–11 Search PubMed
.
- D. Kuznetsov, G. Ugodnikov and I. Filatov, Tech. Phys. Lett., 2008, 34, 87–89 CrossRef CAS
.
-
N. E. Brese and M. O'Keeffe, Complexes, Clusters and Crystal Chemistry, 1992, pp. 307–378 Search PubMed
.
- K. Xu, P. Chen, X. Li, Y. Tong, H. Ding, X. Wu, W. Chu, Z. Peng, C. Wu and Y. Xie, J. Am. Chem. Soc., 2015, 137, 4119–4125 CrossRef CAS
.
- M.-S. Balogun, Y. Zeng, W. Qiu, Y. Luo, A. Onasanya, T. K. Olaniyi and Y. Tong, J. Mater. Chem. A, 2016, 4, 9844–9849 RSC
.
- D. Zhang, J. Shi, Y. Qi, X. Wang, H. Wang, M. Li, S. Liu and C. Li, Adv. Sci., 2018, 5, 1801216 CrossRef PubMed
.
- T. Liu, M. Li, C. Jiao, M. Hassan, X. Bo, M. Zhou and H.-L. Wang, J. Mater. Chem. A, 2017, 5, 9377–9390 RSC
.
- D. Gao, J. Zhang, T. Wang, W. Xiao, K. Tao, D. Xue and J. Ding, J. Mater. Chem. A, 2016, 4, 17363–17369 RSC
.
- S. H. Park, Y.-H. Cho, M. Choi, H. Choi, J. S. Kang, J. H. Um, J.-W. Choi, H. Choe and Y.-E. Sung, Surf. Coat. Technol., 2014, 259, 560–569 CrossRef CAS
.
- Y. Wang, Z.-W. Fu, X.-L. Yue and Q.-Z. Qin, J. Electrochem. Soc., 2004, 151, E162 CrossRef CAS
.
- L. Xu, Q. Jiang, Z. Xiao, X. Li, J. Huo, S. Wang and L. Dai, Angew. Chem., 2016, 128, 5363–5367 CrossRef
.
- E. Rohaeti and I. Hikmawati, J. Mater. Sci. Technol., 2010, 265–272 Search PubMed
.
- H. Ang, H. T. Tan, Z. M. Luo, Y. Zhang, Y. Y. Guo, G. Guo, H. Zhang and Q. Yan, Small, 2015, 11, 6278–6284 CrossRef CAS
.
- S. Geng, F. Tian, M. Li, X. Guo, Y. Yu, W. Yang and Y. Hou, J. Mater. Chem. A, 2021, 9, 8561–8567 RSC
.
- B. S. Mun, M. Watanabe, M. Rossi, V. Stamenkovic, N. M. Markovic and P. N. Ross Jr, J. Chem. Phys., 2005, 123, 204717 CrossRef PubMed
.
- Y. Pan, K. Sun, Y. Lin, X. Cao, Y. Cheng, S. Liu, L. Zeng, W.-C. Cheong, D. Zhao and K. Wu, Nano Energy, 2019, 56, 411–419 CrossRef CAS
.
- F. Le Formal, M. Grätzel and K. Sivula, Adv. Funct. Mater., 2010, 20, 1099–1107 CrossRef CAS
.
- Y. Zheng, Y. Jiao, Y. Zhu, L. H. Li, Y. Han, Y. Chen, A. Du, M. Jaroniec and S. Z. Qiao, Nat. Commun., 2014, 5, 1–8 Search PubMed
.
- N. Krstajić, M. Popović, B. Grgur, M. Vojnović and D. Šepa, J. Electroanal. Chem., 2001, 512, 16–26 CrossRef
.
- W.-F. Chen, J. T. Muckerman and E. Fujita, Chem. Commun., 2013, 49, 8896–8909 RSC
.
- Y. Yoon, B. Yan and Y. Surendranath, J. Am. Chem. Soc., 2018, 140, 2397–2400 CrossRef CAS
.
- J. K. Nørskov, T. Bligaard, A. Logadottir, J. Kitchin, J. G. Chen, S. Pandelov and U. Stimming, J. Electrochem. Soc., 2005, 152, J23 CrossRef
.
- J. Greeley, T. F. Jaramillo, J. Bonde, I. Chorkendorff and J. K. Nørskov, Nat. Mater., 2006, 5, 909–913 CrossRef CAS
.
- K. S. Exner, Angew. Chem., Int. Ed., 2020, 59, 10236–10240 CrossRef CAS
.
- H. Li and K. Reuter, ACS Catal., 2020, 10, 11814–11821 CrossRef CAS
.
- Z. Liu, S. D. Sajjad, Y. Gao, H. Yang, J. J. Kaczur and R. I. Masel, Int. J. Hydrogen Energy, 2017, 42, 29661–29665 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ee02013g |
| This journal is © The Royal Society of Chemistry 2022 |