Disinfection of constructed wetland effluent by in situ electrochemical chlorine production for water reuse†
Suanny
Mosquera-Romero
 abc,
Antonin
Prévoteau
abc,
Antonin
Prévoteau
 ad,
Jan B. A.
Arends
ad,
Jan B. A.
Arends
 ad,
Diederik P. L.
Rousseau
ad,
Diederik P. L.
Rousseau
 c,
Luis
Dominguez-Granda
c,
Luis
Dominguez-Granda
 b and
Korneel
Rabaey
b and
Korneel
Rabaey
 *ad
*ad
aCenter for Microbial Ecology & Technology (CMET), Ghent University, Coupure Links 653, B-9000 Gent, Belgium. E-mail: korneel.rabaey@ugent.be
bESPOL Polytechnic University, Escuela Superior Politécnica del Litoral, ESPOL, Centro del Agua y Desarrollo Sustentable, Facultad de Ciencias Naturales y Matemáticas, FCNM, P.O. Box 09-01-5863, Guayaquil, Ecuador
cDepartment of Green Chemistry and Technology, Ghent University, Graaf Karel de Goedelaan 5, B-8500 Kortrijk, Belgium
dCenter for Advanced Process Technology for Urban Resource Recovery (CAPTURE), Frieda Saeysstraat 1, 9052 Ghent, Belgium Web: www.capture-resources.be
First published on 9th November 2021
Abstract
Constructed wetlands (CWs) are globally used for the treatment of wastewater. Due to various causes, often the water is not fully treated in terms of pathogen removal, requiring additional treatment. Here we evaluated the electrochemical disinfection (ED) of CW effluents to guarantee safe wastewater reclamation in decentralized settings. We used a two-chamber electrochemical cell to produce chlorine at a Ti/RuO2 anode with a synthetic electrolyte containing 18.3 mol Cl− m−3 and subsequently tested it with CW effluents from two locations (Ecuador and Belgium). The effluents ran first to the anode for disinfection by chlorine and then to the cathode for recovering a circumneutral pH. Different flow rate, current density, and membrane type combinations were tested with the synthetic electrolyte to optimize chlorine production and later to disinfect CW effluents. The system produced about twice as much free chlorine when an anion exchange membrane was selected rather than a cation exchange membrane because of chloride electromigration to the anolyte. A 5-log removal of fecal indicators was observed without pathogen regrowth within 7 days after treatment when residual chlorine remained, allowing for non-potable water reuse. Lower residence times (15 s) and current densities (50 A m−2) induced the most energy-efficient operation with a charge density of 10.4 A h m−3 and an energy consumption of <0.1 kW h m−3. These results encourage CW + ED use, especially in low-income countries.
Water impactWe successfully disinfected effluents from two constructed wetlands for water reclamation by in situ chlorine electrogeneration. The system does not consume chemicals and requires an acceptable amount of energy (<0.5 kW h m−3). This opens up opportunities as a sustainable route for decentralized sanitation and water supply, especially in remote locations. |
1. Introduction
Water reclamation from decentralized water systems has been proposed to tackle water scarcity in urban areas of developed countries.1 A similar approach can be applied in rural communities in less-developed countries, where poor sanitation and inadequate water provision threaten human health, being the reality of mostly rural communities.2 In decentralized settings, the potential application of constructed wetlands (CWs) for domestic wastewater treatment has become a popular solution due to low maintenance and energy requirements, although it has the drawback of variable removal efficiencies for enteric pathogens.3 Thus, efficient disinfection of CW effluents appears necessary to allow water reclamation for non-potable water reuse.Chlorination is the most frequently applied chemical-based method for wastewater disinfection, providing both direct disinfection and residual protection.4 Electrochemical disinfection (ED) via in situ oxidant production has gained importance in the last few decades as an alternative to conventional chemical dosing.4–6 ED, particularly by production of free chlorine, is thought to be practical, especially for remote installations, by circumventing the burden of transport and storage of concentrated hypochlorite solutions or chlorine gas during on-site disinfection at a somewhat larger scale.7 The process consists of the oxidation of chloride ions already present in the polluted water to active chlorine species, which can then inactivate pathogens.8,9 The chlorine produced at the anode (eqn (1))10 undergoes hydrolysis (eqn (2)) and ionization (eqn (3)) depending on the solution's pH.
| 2Cl− → Cl2 + 2e− E° = 1.26 V vs. SHE | (1) |
| Cl2 + H2O → HOCl + H+ + Cl− pKa = 2 | (2) |
| HOCl → H+ + OCl− pKa = 7.5 | (3) |
Two types of electrochemical configurations have been studied for in situ chlorine production to treat contaminated water: the direct electrolysis (in-line systems) of a polluted stream and the production of a concentrated chlorinated solution to be later dosed into contaminated water.4,12 The latter is applied in bigger water treatment plants by using brines to produce strong oxidants,17 in contrast to the former, which is mainly proposed for small-scale applications.14,18 For direct electrolysis of contaminated water, most studies have focused on a one-chamber electrochemical cell operating in batch mode.4,7,15,19,20 However, an in-line system could continuously disinfect effluents for medium–low-sized (wastewater production <10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 person equivalents21) applications in a divided chamber by considering that water oxidation promotes acidic conditions in the anolyte with enhanced disinfection.12,18 Despite the progress in understanding the ED system, the technology has not yet reached widespread application,16,22 probably due to a lack of knowledge dissemination and few experts in the area of ED technologies.18 Thus, there is a need to demonstrate the application of the system in real case studies to identify how reactor design and operational parameters may affect the disinfection of contaminated water.
000 person equivalents21) applications in a divided chamber by considering that water oxidation promotes acidic conditions in the anolyte with enhanced disinfection.12,18 Despite the progress in understanding the ED system, the technology has not yet reached widespread application,16,22 probably due to a lack of knowledge dissemination and few experts in the area of ED technologies.18 Thus, there is a need to demonstrate the application of the system in real case studies to identify how reactor design and operational parameters may affect the disinfection of contaminated water.
Therefore, the coupling of CW systems with ED (CW + ED) in decentralized wastewater treatment23 has been proposed. In this work, we first investigated how the membrane selection and operational parameters impacted the chlorine production and associated energy cost with a synthetic electrolyte. We then demonstrated and studied the electrochemical disinfection of municipal wastewater pre-treated using a CW system at two geographical locations: Ecuador (18.3 ± 5.5 mol Cl− m−3) and Belgium (5.7± 2.4 mol Cl− m−3) for water reclamation.
2. Material and methods
2.1. Selection of membranes and optimal operational settings
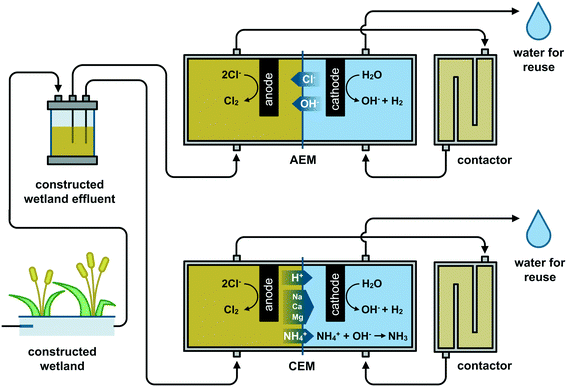
The first set of experiments was conducted with synthetic solutions of NaCl (Carl Roth, Germany) to assess the influence of the operational parameters.8,15 The concentration of the NaCl solutions (18.3 mol Cl− m−3) was selected, similar to the average chloride concentration and conductivity of a CW effluent from a case study in Ecuador (see 2.2). Data collected on the Ecuadorian CW operation from January until July 2019 showed an effluent conductivity of 2.50 ± 0.48 mS cm−1, with chloride as the principal anion (Fig. S1†).The reactor design is illustrated in Fig. 1. The two-chamber electrochemical cell consisted of two endplates on each side and two parallel Perspex frames (internal dimensions 5 × 20 × 2 cm) in the middle. The anodic and cathodic compartments were divided by either a 100 cm2 cation exchange membrane (CEM F-1010, Fumatech, Germany) or an anion exchange membrane (AEM International, USA). Membranes were pretreated according to the supplier before use, i.e., soaking in 0.4 M NaCl solution for at least 24 h. A power supply (30 V capacity, Velleman, Belgium and Sunshine, China) was used to set a fixed current during the experiments. The synthetic solution was first pumped to the anodic compartment (0.2 L), passed through a contactor (0.2 L), and finally to the cathodic compartment (0.2 L), after which the treated water can be collected (Fig. 1). The anode was a 5 × 20 cm Ti/RuO2 electrode (MMO Magneto, Netherlands), and the cathode was a 5 × 20 cm stainless-steel mesh (AISI 316 L mesh size 495 μm, wire thickness 200 μm, Solana, Belgium). Two Ag/AgCl (3 M KCl, +0.21 V vs. SHE) reference electrodes (RE-1B, ALS Japan) were installed, one located at ∼0.3 cm from the anode and the other at ∼0.3 cm from the cathode. The reference electrodes were checked vs. the standard calomel electrode to verify their stability. The system ran continuously by setting the same flow rate with a peristaltic pump (Masterflex® L/S®, Cole-Parmer, USA) for both compartments.
To study the chlorine production, combinations of five different flow rates (Q: 0.3, 1.3, 3.3, 8.3, 13.3 × 10−6 m−3 s−1) and three current densities (j: 50, 100, 200 A m−2) were applied with each type of membrane (AEM and CEM) as detailed in Table 1. The overall duration of each experimental trial was 120 min, and the chlorine production was averaged from 3 samplings of anolyte effluent collected after 60, 90, and 120 min of operation. Duplicate experiments were performed for all treatments.
| Q, 10−6 m3 s−1 | HRT anode, s | j, A m−2 | Charge density, A h m−3 | Experimental label | |
|---|---|---|---|---|---|
| AEM | CEM | ||||
| 0.3 | 600 | 50 | 416.7 | Q 0.3 j 50 A | Q 0.3 j 50 C |
| 100 | 833.3 | Q 0.3 j 100 A | Q 0.3 j 100 C | ||
| 200 | 1666.7 | Q 0.3 j 200 A | Q 0.3 j 200 C | ||
| 1.3 | 150 | 50 | 104.2 | Q 1.3 j 50 A | Q 1.3 j 50 C |
| 100 | 208.3 | Q 1.3 j 100 A | Q 1.3 j 100 C | ||
| 200 | 416.7 | Q 1.3 j 200 A | Q 1.3 j 200 C | ||
| 3.3 | 60 | 50 | 41.7 | Q 3.3 j 50 A | Q 3.3 j 50 C |
| 100 | 83.3 | Q 3.3 j 100 A | Q 3.3 j 100 C | ||
| 200 | 166.7 | Q 3.3 j 200 A | Q 3.3 j 200 C | ||
| 8.3 | 24 | 50 | 16.7 | Q 8.3 j 50 A | Q 8.3 j 50 C |
| 100 | 33.3 | Q 8.3 j 100 A | Q 8.3 j 100 C | ||
| 200 | 66.7 | Q 8.3 j 200 A | Q 8.3 j 200 C | ||
| 13.3 | 15 | 50 | 10.4 | Q 13.3 j 50 A | Q 13.3 j 50 C |
| 100 | 20.8 | Q 13.3 j 100 A | Q 13.3 j 100 C | ||
| 200 | 41.7 | Q 13.3 j 200 A | Q 13.3 j 200 C | ||
The coulombic efficiency (CE, %) was determined to evaluate the fraction of the current dedicated to chlorine production:
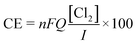 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1).
485 C mol−1).
The specific energy consumption per treated volume (SEC, kW h m−3) was used to evaluate the energy efficiency of the selected system.
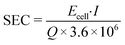 | (5) |
2.2. Disinfection experiments with real CW effluents
CW effluents were collected from two locations, one with a higher chloride content (18.3 ± 5.5 mol Cl− m−3) in Guayaquil, Ecuador and one with a lower chloride content (5.7 ± 2.4 mol Cl− m−3) in De Pinte, Belgium. The system in Ecuador treated domestic wastewater (0.24 m3 s−1) using a two-stage vertical-flow CW system planted with Echinochloa polystachya with a total area of 3 ha. The system in Belgium was a two-stage system consisting of two vertical-flow and two horizontal subsurface-flow CWs, both planted with common reed (Phragmites australis) in an extension of 0.3 ha and treating domestic wastewater with an average inflow of 0.001 m3 s−1. The characterization of the wastewater influent and effluent from the two sites is presented in Table S1† and is based on three monthly sampling campaigns. In every location, CW effluent was extracted from the nearest well and stored in 20 L closed plastic containers at 4 °C before use.Based on the results in section 2.1, combinations of flow rate (Q) and current density (j) were selected aiming to produce a sufficient amount of chlorine (dose of 5–20 mg Cl2 L−1 or 0.07 to 0.28 mol Cl2 m−3) for wastewater disinfection.24
In the presence of ammonia in the effluent (Table S1†), the produced free chlorine could react to form combined chlorine.17,25 The observed total residual chlorine (free chlorine and combined chlorine) after the anodic chamber and after the cathodic chamber was obtained by the addition of KI (Carl Roth, Germany) into the DPD reaction.26
2.3. Analytical and microbial analysis
The free chlorine (aqueous chlorine, Cl2; hypochlorous acid, HOCl; and hypochlorite, OCl−) production was measured at the anolyte by the DPD colorimetric method based on the standard method 4500.17,26 For readability purposes, free chlorine concentrations will be designated as their equivalent in mol Cl2 m−3 throughout this manuscript.Two variations of the standard procedure were implemented to measure free chlorine due to practicalities in both locations (Ecuador and Belgium). First, a calibration curve was constructed based on the absorbance at 326 nm wavelength using a spectrophotometer (Biochrome WPA1100nm II, Germany). For the second variation, a standard calibration curve from the 8021 HACH program, accepted by the EPA (HACH, 2014), was adopted for other locations by using a photometer (DR6000/3600 HACH, USA).26 Both methods were corroborated to give similar results (Fig. S2†) using the chlorine standard PourRite Ampules (NIST) (Hach, USA).
Ion chromatography (IC, Metrohm Switzerland) was used to determine the concentrations of anions (Metrohm 930 equipped with a Metrosep A Supp 5–150/4.0 column and conductivity detector, eluent: 1.0 mM NaHCO3 and 3.2 mM Na2CO3) and cations (Metrohm 761 equipped with a Metrosep C6-250/4.0 column and conductivity detector, eluent: 1.7 mM HNO3 and 1.7 mM dipicolinic acid). The pH (Metrohm 744, Switzerland) was analyzed during all the experiments.
Disinfection effectiveness was determined by the spread-plate and membrane-filter (0.45 μm, Merck, USA) standard methods 9215.26 The effluent after passing by the electrochemical chambers was sampled at intervals of 30 minutes. After sampling, sodium sulfite (Carl Roth, Germany) was added to mitigate the effect of residual free chlorine, and then the ED was analyzed at the sampled time point. Two types of agar were used: R2A agar (Carl Roth, Germany) to determine the total heterotrophic plate counts (HPCs) after 5 days of incubation at 28 °C, and MacConkey agar (Carl Roth, Germany) to determine coliform bacteria and E. coli (red colonies) after 24 h of incubation at 37 °C. HPC is a more conservative measurement as it can count a vast range of organisms, including those more resistant to disinfection than the total coliforms.17,27 The enumeration of colonies was expressed as CFU (colony-forming units) per 100 mL of sample in each experiment. To calculate the inactivation of pathogens, the logarithmic values of the population (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N) at each examined point were subtracted from the logarithmic value of the initial population expressed as CFU per 100 mL.
N) at each examined point were subtracted from the logarithmic value of the initial population expressed as CFU per 100 mL.
To assess if the treated water remained disinfected upon storage, glass-serum bottles of 0.2 L were half-filled with CW + ED effluents under an air atmosphere. The bottles were closed and stored in the dark in a temperature-controlled room at 30 °C. Each day, for a total of 7 days, two bottles previously not opened were collected and tested for HPC and fecal indicators as described before. The incubation experiments were run twice using CW effluents of 2 sampling campaigns.
3. Results and discussion
3.1. Chlorine production as a function of operational parameters and reactor configuration
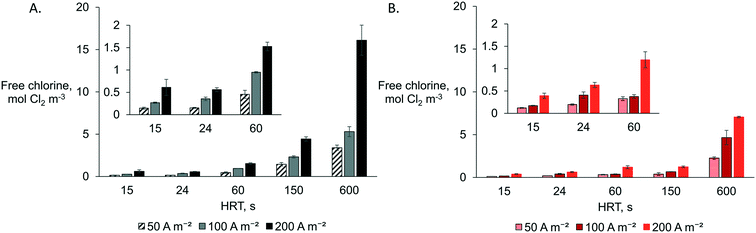
A combination of the highest anodic HRT (600 s) and the highest current density (200 A m−2) produced the highest free chlorine concentration as measured in the anolyte effluent (AEM: 16.1 ± 1.8 mol Cl2 m−3 and CEM: 7.1 ± 0.1 mol Cl2 m−3) (Fig. 2). This observation was expected as more charge (A h) is provided per cubic meter of synthetic electrolyte passing through the anode. The reactors equipped with an AEM produced higher free chlorine than those with a CEM for all specific operational combinations (Q and j). The latter can be explained due to chloride migration from the catholyte to the anolyte through the AEM, which mitigates chloride mass transport limitation to the anode by increasing the effective concentration in the anode compartment. For example, for Q0.3j200A, 8.7 ± 0.1 mol Cl− m−3 (Fig. S3†) was removed from the catholyte and transported to the anolyte for chlorine production. Higher chloride concentrations in the anolyte can reduce the time and energy required for disinfection via chlorine evolution.27 By setting the lowest HRT (15 s), the system produced the lowest amount of chlorine (<0.6 mol Cl2 m−3). However, even at the lowest applied current density of 50 A m−2, the reactor still produced enough chlorine (AEM: 0.15 ± 0.02 mol Cl2 m−3 and CEM: 0.12 ± 0.01 mol Cl2 m−3) for theoretical in-line disinfection as a minimum of 0.07 mol Cl2 m−3 is recommended according to the EPA (dose of 0.07 to 0.28 mol Cl2 m−3 for wastewater24). The current density and flow rate can easily be adjusted to control chlorine production as a function of the nature and contamination level of the CW effluent while considering local regulations.8Acidic pH (below 3) is preferable to maximize the selectivity for chlorine production instead of oxygen and chlorate evolution.13 Acidity build-up was observed in the unbuffered anolyte of all the configurations tested. However, at the highest HRT of 600 s, the pH of the anolyte when an AEM was used tended to be slightly higher (2.8–2.3) than that with a CEM (2.5–2.2). The latter can be attributed to slight OH− electromigration from the catholyte to the anolyte through the AEM, mainly observed at the highest current density (j200). The transport of OH− through the AEM towards the anode shall be considered if a very high HRT (>600 s) and current is applied as it can interfere with the preferable acidic conditions close to the anode and hence decrease the selectivity towards chlorine evolution.13
The respective CEs for each reactor configuration were relatively stable for all tested operational parameters (Fig. S4†). However, the coulombic efficiencies were higher when an AEM (CE ∼ 58%) was used rather than a CEM (∼39%). Although CE increases with current density for chlorine evolution in industrial chlor-alkali processes (electrolyte of >856 mol Cl− m−3),13 this was not the case in our more diluted solution (Fig. S4†). The latter agrees with previous observations on the electrolysis of dilute chloride solutions (below 0.07 mol m−3) where an increase in the current density did not necessarily lead to a higher CE.13,15 However, mainly when using a CEM, the CE was slightly lower at a higher HRT (lower flow rate) than at a lower HRT, while it seems not to have this tendency for all cases with the AEM configuration. For the CEM configuration, the lower CE at a lower flow rate and higher current can be explained by a substantial chloride mass transport limitation to the anode electrode surface. In contrast, migration replenishes Cl− to the anolyte for the AEM configuration. Thus, a higher flow rate mitigating mass transport limitations (by increasing both convection and Cl− feeding rate) will impact the CEM system more than the configuration with the AEM.
The lowest CEs (16–23%) were observed at a high HRT (150–600 s) and a high current density (200 A m−2), when using a CEM. Similarly, when using an AEM, lower CEs (34–52%) were observed at high current density (100–200 A m−2) and at the lowest HRT (600 s) than those at the highest HRT (CE 69–78%) (Fig. S4†). The low CE implies that other anodic side-products are favored at high charge densities, mainly oxygen,10,18 rather than chlorine in dilute chloride solutions. Moreover, other undesired chlorinated by-products, such as perchlorate, will be produced when BDD electrodes are used under these conditions28 rather than MMOs at the anode potentials observed (Fig. S5†). In-line systems would thus be more appropriate at a lower HRT, e.g., 10 s tested here, to meet the recommended chlorine dose for CW effluent disinfection. However, a lower HRT (higher flow rate) in the in-line system could face possible long-term issues as organic and inorganic (e.g., Ca and Mg) contaminants can accumulate faster in the chamber, in the membrane, or at the cathode, inducing organic fouling or inorganic precipitation with subsequent system failure.8,14 In this approach, a lower current density (50–100 A m−2) could mitigate precipitation at the cathode by limiting alkaline conditions (pH > 8) and electromigration of cations such as Ca and Mg towards the CEM. A current density of ∼50 A m−2 has been preferred for chlorine evolution in disinfection systems with MMOs by other authors.12,29 Thus, it has to be evaluated if a combination of a lower current density and a low HRT will allow optimal use of the system as an in-line disinfection unit.
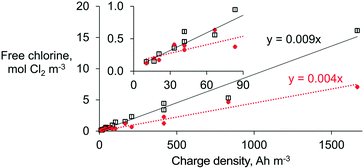
The free chlorine concentration increased quasi-proportionally with the charge density (from 10.4 and 1666 A h m−3) for each respective type of membrane (Fig. 3). At least twice the free chlorine production was achieved when an AEM (∼0.009 mol Cl2 A h−1) was used instead of a CEM (∼0.004 mol Cl2 A h−1) under similar operational conditions. However, the difference in efficiency between membranes is much less pronounced at the lowest charge densities (≤70 A h m−3) because the chloride mass transport limitation—and its mitigation by using an AEM—is then negligible.
A chlorine dose between 0.07 and 0.28 mol Cl2 m−3 is desired for in-line disinfection to achieve microbial pathogen inactivation in wastewater effluents.24 The latter implies charge densities as low as 10 to a maximum of 66.7 A h m−3, and at this range, the selection of membranes becomes indistinctive (Fig. 3). Thus, for lower chlorine concentrations necessary for in-line disinfection of effluents, the type of membrane is unlikely to influence the ED performance. However, the presence of interferences (e.g., competing ions, and direct and indirect oxidation of organics) and a lower chloride concentration could lead to investing more charge density than predicted here.
AEMs can thus provide more free chlorine than CEMs at higher charge densities (≥83 A h m−3) but AEMs are not resistant to oxidants during long-term operation (Fumatech, Germany and International membranes, USA), the opposite of CEMs, which can be made of perfluorosulfonic acid polymers resistant to chlorine. For instance, Nafion (CEM of Dupont, USA) is widely used in industrial applications for chlorine production13 and has a similar chemical structure to the one used in this study. The CEM in a membrane cell chlor-alkali process has been reported to last for at least a year,13 which represents harsher conditions than the ones studied here. However, only a few reports showed the operation of membrane cells for chlorine production in water treatment with an AEM, lasting at least a month.30,31 In our test, in the span of 10 h of experiments with the AEM at an anodic HRT of 600 s and applied current density from 100 to 200 A m−2, the membrane changed color, and NH4+—the main functional group of this AEM—leached into the electrolyte (Fig. S6A and B†). Furthermore, the area specific resistance of the AEM decreased by 28.3 ± 2.7% after exposure to ∼14 mol Cl2 m−3 during the 10 h operation (see the ESI† and Materials and methods). The latter could indicate the deterioration of the membrane porosity that allows perm-selective anion exchange. The observations in this study could motivate more researchers in materials science for the synthesis of ceramic AEMs that are more resistant to chlorine.
To understand the energy efficiency of the different operational combinations, the specific energy consumption (SEC) was determined. Electrochemical production of chlorine at the recommended dose for in-line wastewater disinfection (0.07 to 0.28 mol Cl2 m−3) was achieved at anodic HRTs from 15 to 60 s and current densities ranging from 50 to 100 A m−2, independent of the type of membrane and with a SEC below 0.5 kW h m−3 (Fig. 4).
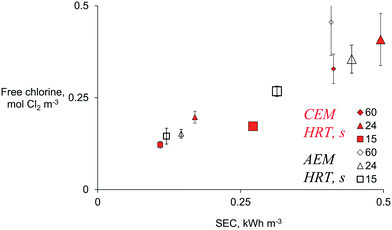 | ||
| Fig. 4 The production of free chlorine vs. specific energy consumption (SEC) of the electrochemical system using a diluted chloride solution of 18.3 mol Cl− m−3 in a two-chamber system divided by an AEM (empty black symbols) or a CEM (full red symbols), at 50 A m−2 (small symbols) and 100 A m−2 (large) and different HRTs. Data corresponding to SEC > 0.5 kW h m−3 (higher HRT and current densities) are included in Fig. S7.† Error bars represent standard deviations of n = 5 replicates. | ||
The SEC ranged from 0.1 to 31 kW h m−3 for all the combinations of parameters tested (Fig. S7†). As expected, the SEC increased with charge density. In simple terms, at the same current density, SEC increases with HRT; and at the same HRT, the SEC increases with current density (Fig. 4). A SEC of less than 0.3 kW h m−3 is acceptable for small and medium-sized disinfection systems,16 and that SEC can be achieved with an anodic HRT between 15 and 24 s. A SEC of 0.4 kW h m−3 was obtained when an HRT of 60 s and the lowest tested current density (50 A m−2) were employed to produce free chlorine (AEM: 0.46 ± 0.09 mol m−3, CEM: 0.32 ± 0.04 mol m−3). The latter is a lower SEC than the one required to produce similar free chlorine (AEM: 0.61 ± 0.18 mol m−3, CEM: 0.40 ± 0.06 mol m−3) at the lowest HRT of 15 s and the highest current density of 200 A m−2 (∼1 kW h m−3). This insight could indicate that reducing the flow rate (increasing HRT) in the ED system could be more energy efficient than increasing the current density for providing a required chlorine dose. However, the practicalities in a real-scale plant need to be further investigated, as usually, the amount of wastewater to be treated depends on other factors, such as water consumption in the community, which could hinder controlling Q for efficient ED. Moreover, an in-line system at low current densities (50 A m−2) will demand a large electrode surface area to provide the charge density necessary to disinfect all CW flux (e.g., 3 m2 MMO electrodes are required to treat the 0.001 m3 s−1 flow of the CW in Belgium). There is still a need to evaluate if the SEC and the investment cost can compete with current disinfection technologies, as up-to-date ED methods are considered expensive solutions.18
Considering the other scenario to produce concentrated solutions of chlorine at higher HRTs and current densities for later disinfection of the main flow, the SEC in Fig. S7† needs to be recalculated since the total effluent to be treated is larger than the small volume passing through the ED system. This means that if the combination of Q0.3j200A that produced the highest chlorine (16.1 ± 1.8 mol Cl2 m−3) is diluted approximately 57 times in the final effluent to reach the recommended maximum chlorine dosage of 0.28 mol m−3, the recalculated SEC will be 0.42 kW h m−3. The latter SEC could still be interesting to explore for optimal energy investment in ED systems. Q0.3j200A proposes a solution for less surface of the anode to be implemented for disinfection in place. However, our SEC analysis does not consider the extra units to perform the mixing operation, the safety risks for operators, and the faster degradation of the ED system at higher chlorine concentrations. Further studies should focus on the different aspects of a practical application to reach a final conclusion if the in-line systems are more favorable for disinfecting effluents with diluted chloride concentrations.
In-line systems can allow a pH difference between the cathodic and anodic chambers that can provide additional disinfection as a pH greater than 11 or lower than 3 can be categorized as toxic for most microorganisms.17 The anolyte pH was below 3.5 for all configurations with the unbuffered synthetic solution tested. After flowing through the cathode, the pH obtained was from neutral to alkaline (7.57–9.61). However, during ED of real CW effluents with a natural buffering capacity, the toxic/germicidal effects can be mainly attributed to the chlorine production in the anode as the pH was maximum decreasing from 7.5 to 6.2 in the tests with the highest charge density (83.3 A h m−3) applied. Thus, an acidic pH could contribute to the disinfection only in the absence of buffer components in the water or at higher charge densities than those required for chlorine production for disinfection purposes. Therefore, for simplicity, the disinfection mechanisms in the following section will be mainly attributed to the predicted chlorine production in the anolyte.
3.2. Disinfection of constructed wetland effluents
Based on the previous analysis, various operational parameters (Table 2) were tested for ED of real CW effluents by predicting the chlorine production at the anode at the dosage level for disinfection (e.g., 0.07–0.28 mol Cl2 m−3 for wastewater24). The microbial counting of the indicators used in this study showed similar results for the anolyte and the catholyte samples (Fig. S8†), stressing that disinfection mainly occurred in the anodic compartment. Thus, an ideal contactor design shall be considered to comply with the current recommendations for contact time and residual chlorine (i.e., CT concept) to effectively remove other pathogens, such as Giardia lamblia.17,24,25 Furthermore, the pH difference between anolyte (pH 6.9 ± 0.5) and catholyte (7.6 ± 0.12) with the selected operational parameters (e.g., the highest tested HRT of 60 s and current density of 100 A m−2) were close to circumneutral pH. Thus, additional disinfection after the passage through the cathode can be discarted. Therefore, the presented pathogen inactivation results focus on the anodic HRT and the current applied.| Location | Configuration | Total heterotrophic bacteria CFU 100 mL−1 | Total coliforms CFU 100 mL−1 | ||||
|---|---|---|---|---|---|---|---|
| Initial | After disinfection | log removal | Initial | After disinfection | log removal | ||
| a Absence of colonies in a 100 mL sample by the membrane filtration method. n.t. not tested. | |||||||
| Ecuador | Q 13.3 j 50 A | 2.7 × 105 | 0a | 5.4 | 9.6 × 104 | 0a | 5.0 |
| Q 13.3 j 50 C | n.t. | n.t. | n.t. | 2.7 × 104 | 4 | 3.8 | |
| Belgium | Q 13.3 j 100 A | 8.2 × 105 | 3 × 105 | 0.4 | 5.5 × 104 | 13 | 3.7 |
| Q 13.3 j 100 C | 3.5 × 105 | 0.4 | 10 | 3.7 | |||
| Q 3.3 j 100 A | 3.0 × 103 | 2.4 | 1 | 4.7 | |||
| Q 3.3 j 100 C | 3.0 × 103 | 2.4 | 14 | 3.6 | |||
In the case study in Ecuador, a single combination of flow rate (13.3 × 10−6 m−3 s−1) and current density (50 A m−2) for the AEM and CEM configurations was tested for ED (Table 2). The operational parameters were selected based on the predicted chlorine production, given the Ecuadorian CW effluent's natural chloride content (8.34 ± 5.5 mol Cl− m−3). Considering the cell voltage (8.2 V) of the system and the electricity cost in Ecuador (0.1 $ per kW h−1), the disinfection unit will have a very low cost of 0.0086 $ per m3. It has to be considered that this calculation only gives an idea on energy consumption per volume of treated water, without considering capital costs, and at first view, is competitive with other disinfection processes (Table S3†). Q13.3j50A allowed a 5.4-log removal of HPC and 5-log removal of fecal indicators with an applied 10.4 A h m−3 at a SEC of 0.09 kW h m−3. The Q13.3j50C configuration also showed an efficient 3.8-log removal of fecal indicators with the same charge density and SEC. Thus, both disinfection systems comply with the Ecuadorian national legislation that requires at least a 3-log removal (99.9% inactivation) of fecal indicators in the effluent before discharge.32 Similarly, the final CW + ED effluent (Tables 2 and S1†) complies with most of the international water reuse guidelines summarized in Table S2.†
In Belgium, the lower chloride content (5.7 ± 2.4 mol Cl− m−3) in the CW effluent impacted the selection of operational parameters for ED. By applying the conditions above (Q13.3j50), less chlorine production than that needed for disinfection (<0.07 mol m−3) was observed when the electrolyte contained 5.7 ± 2.4 mol Cl− m−3 (Fig. S9†). The latter was expected, as other authors showed a decline in chlorine production with the decrease in chloride concentration.8,15 Thus, to increase chlorine production, the strategy applied was to increase the current density or to decrease the flow rate. Therefore, one current density (100 A m−2) and two flow rates (13.3 × 10−6 m−3 s−1 and 3.3 × 10−6 m−3 s−1) were tested, using either an AEM or a CEM. Conversely, a high cell voltage (27 V) was observed due to the lower conductivity of the CW effluent. By considering the electricity cost in Belgium (0.3 $ per kW h−1), the ED unit showed a cost of 0.17 $ per m3 at the highest flux (13.3 × 10−6 m3 s−1) and 0.7 $ per m3 at 3.3 × 10−6 m3 s−1. Decreasing the flow rate improved the disinfection from 3.7-log removal of fecal indicators (Q13.3j100A) to 4.7-log removal when using an AEM (Q3.3j100A). However, similar inactivation of fecal indicators of 3.7-log and 3.6-log was observed when using a CEM (Q13.3j100A and Q3.3j100C). For both AEM and CEM configurations, the removal of HPC was negligible at a higher flow rate (Q13.3j100) and improved to 2.4-log removal at a lower flow rate (Q3.3j100). All Belgian CW + ED effluents are within the European non-potable water reuse guidelines (Table S2†). However, the reclaimed water would be rejected in countries with stricter guidelines for total coliforms (e.g., China and California <3 CFU 100 mL−1).
A higher chlorine production by increasing chloride in the bulk could be the reason for why Q3.3j100A could disinfect 1-log removal more than Q3.3j100C for the fecal indicators at 83.3 A h m−3. A 0.68 mol m−3 Cl− surplus was measured at the anolyte in the Q3.3j100A configuration with only a 0.14 mol m−3 Cl− surplus in Q13.3j100A. In contrast, the ED tested with a CEM (Q3.3j100C and Q13.3j100C) did not show an increase in Cl− (<0.02 mol m−3) at the anolyte. Since HPC could account for viable bacteria more resistant to chlorine,17,27 the improved disinfection at lower flow rates could also be aided due to other strong oxidants produced in the ED system. For instance, minute concentrations of H2O2 (0.7 mg L−1 in 5 min electrolysis at 1000 A m−2) were reported for this type of Ti/RuO2 electrode by Jeong et al. (2009). Thus, this indicated that an increase in chlorine production with an AEM, due to chloride electromigration, does not necessarily show a better HPC inactivation than when a CEM was applied under the same conditions (Q3.3j100). Our results agree with a previous report, where under the same conditions of contact time and chlorine concentrations, the electrochemical disinfection with titanium-oxide-based electrodes was at least three times more effective than direct sodium hypochlorite dosing (Kerwick et al., 2005). During the ED, the enhanced disinfection was attributed to the generation of other possible oxidizing agents,4,11 and to a minor extent, to the acidic pH close to the anode when the effluent flows through the electrode.16
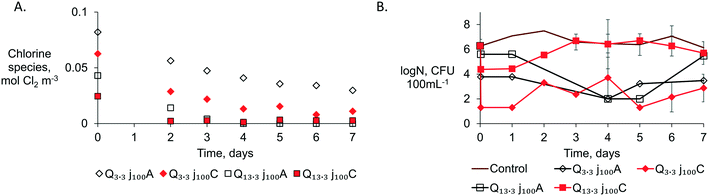
The presence of ammonia in the tested Belgian CW effluent (1.4 ± 0.1 mol m−3), in addition to its lower chloride content, could explain the lower disinfection efficiency compared to the ED of the Ecuadorian effluent where no ammonia was detected. Ammonia can react with the produced chlorine to form chloramines which have a 1000 times lower disinfection action than free-chlorine.17 Thus, using a CEM in ED may improve the disinfection efficiency by partially removing the NH4+ from the anolyte through the CEM by electromigration (Fig. 1).33 A 28% removal of initial NH4+ from the anolyte and accumulation in the catholyte was observed at the lower flow rates with the CEM configuration (Q3.3j100C). In contrast, in Q13.3j100C, Q13.3j100A, and Q3.3j100A, the changes in NH4+ in the anolyte were limited to a 0.2% maximum. Despite the fact that the NH4+ removal with a CEM led to an increase of non-combined chlorine of ∼27% compared to the results obtained with an AEM,33 the total chlorine species produced with the AEM configuration were consistently higher than those with a CEM (Fig. 5A). The latter implies that at charge densities ≥83.3 A h m−3 and with a molar relation Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N of 4
N of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the AEM configuration presents an advantage over the CEM one for practical ED.
1, the AEM configuration presents an advantage over the CEM one for practical ED.
On the other hand, using a CEM, with the flow pattern proposed here, might tend to up-concentrate various cations, due to electromigration (Fig. 1), in the catholyte, making the system more prone to precipitation issues under the alkaline conditions in the cathode vicinity. For instance, the Q3.3j100C configuration showed an accumulation of 12.6% Ca in the catholyte. Thus, the passage through the cathode to set the neutral pH for water reclamation could be done intermittently between acid cleaning to remove precipitation.27
The anodic effluent of all configurations tested in Belgium was stored in glass bottles and tested daily for coliform counting and HPC for 7 days (Fig. S10†). All tested bottles from the 4 configurations (Q13.3j100A, Q13.3j100C, Q3.3j100A, Q3.3j100C) in Table 2 showed a higher fecal indicator removal after 24 h of incubation after ED (Fig. S11†). These observations revealed the importance of an appropriate contactor design to allow a higher pathogen inactivation after the anode and to also ensure that chlorine removal is maximal prior to discharge. The fecal indicators were absent, i.e., no presence of E. coli colonies on the agar plates during the membrane-filtration tests for 7 days, except for Q13.3j100C from day 5 (Fig. S11†). The total residual chlorine (Fig. 5A) is the most indicative reason for the progressive disinfection and maintenance of the pathogen-free effluent over time. No regrowth of fecal indicators was observed in the CW + ED effluents as long as chlorine species above the detection limit (0.003 mol Cl2 m−3) were measured. The regrowth of presumably E. coli (red colonies) was observed on day 5 in the Q13.3j100C configuration when the residual chlorine decreased below 0.003 mol Cl2 m−3 (Fig. 5A). Once the total residual chlorine is not detectable, a higher probability of pathogen regrowth can be expected due to the presence of organics, as organic mineralization with MMOs is not likely.11 Also, higher organic removal will typically demand higher specific charge densities than those for disinfection.27 Therefore, residual chlorine proved to be crucial to avoid pathogen regrowth to reclaim water from CW + ED effluents. Evidently, if the water is straight after treatment discharged, then only disinfection is needed and not ensuring the absence of regrowth.
In contrast, the HPC did not consistently decrease over the days for all the configurations tested, showing the possible presence of microorganisms resistant to chlorine. An average of 3-log removal of HPC was maintained over days 2 to 7 with the configurations tested at Q3.3j100 (HRT 60 s) with both membranes (Fig. 5B). For the configurations with the highest flow rate, Q13.3j100, an increase in the HPC was observed on day 3 for the system assembled with a CEM and after day 5 when an AEM was used. Further research could determine the fraction of HPC that could count for other pathogens of concern that chlorine did not rule out. The chlorine resistance pathogens, that could be identified in the HPC indicator, are suggested to be considered in the non-potable reuse standards to assure safe water reuse (Table S2†).
Despite the oxidation of organics with chlorine being able to produce disinfection by-products (DBPs), it has been demonstrated that these DBPs are a matter of concern at charge densities above 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 A h m−3 by treating latrine water.34 The present study used considerably lower charge densities from 10.4 to 83.3 A h m−3 to disinfect constructed wetland effluents (at a lower organic load) effectively. Although the presence of monochloramines is controversial in drinking water due to the risk of carcinogenic effects, the reclaimed water here is aimed for non-potable use (e.g., flushing toilets). Furthermore, other exposure routes such as inhalation can be discarded, as the volatile trichloramine is formed at higher Cl2
000 A h m−3 by treating latrine water.34 The present study used considerably lower charge densities from 10.4 to 83.3 A h m−3 to disinfect constructed wetland effluents (at a lower organic load) effectively. Although the presence of monochloramines is controversial in drinking water due to the risk of carcinogenic effects, the reclaimed water here is aimed for non-potable use (e.g., flushing toilets). Furthermore, other exposure routes such as inhalation can be discarded, as the volatile trichloramine is formed at higher Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N (ref. 17) than the ones that were needed for disinfection (e.g., max molar ratio Cl2
N (ref. 17) than the ones that were needed for disinfection (e.g., max molar ratio Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N 0.4
N 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in this research. Therefore, it can be assumed that DBPs should not be problematic in the disinfected CW effluents. It is suggested to further corroborate the absence and presence of DBPs during a long-term pilot trial of the in-line ED system designed here.
1) in this research. Therefore, it can be assumed that DBPs should not be problematic in the disinfected CW effluents. It is suggested to further corroborate the absence and presence of DBPs during a long-term pilot trial of the in-line ED system designed here.
The electrode and membrane longevity shall be evaluated in the ED system for practical implementation of the system by continuously feeding CW effluent. Considering the operational costs due to electricity consumption (here revised), the replacement of materials, initial capital cost, and life cycle analysis, a more realistic analysis, can be drafted to evaluate the techno-economic feasibility of the proposed technology. The criteria used in this study can be tested in different constructed wetland systems even at lower chloride concentrations where chlorine can still be produced (reported chlorine electrogeneration with a minimum chloride content of 1 mol m−3)7 for effluent disinfection and safe water storage for reuse.
4. Conclusions
The type of membrane dividing the chamber and the nature of CW effluent impacted the production of chlorine. The use of AEM configurations in ED increased the chlorine production because of substantial chloride electromigration from the catholyte to the anolyte when the applied charge density was higher than 83.3 A h m−3. However, we showed that the lower stability of the AEM against oxidants does not warrant its usage for this particular purpose.CW effluents were successfully disinfected to comply with legislations for both discharge at a lower specific energy cost (0.1 kW h m−3) and fitting non-potable water reuse.
Shorter contact times than the ones recommended for direct chlorination were required to inactivate fecal indicators during the electrochemical disinfection showing the added value of electrochemical approaches. An adequate contactor tank after the anodic compartment would allow compliance with the recommendation for residence times for removal of other pathogens not detected here. Residual disinfection was corroborated as the remaining chlorine species prevented regrowth of fecal indicators over 7 days. These results encourage the application of an electrochemical system in remote locations to allow the combination of CW + ED to reclaim water for non-potable usages.
Abbreviations and symbols
| AEM | Anion exchange membrane |
| CEM | Cation exchange membrane |
| CFU | Colony-forming unit |
| CW | Constructed wetland |
| ED | Electrochemical disinfection |
| HPC | Heterotrophic plate count |
| HRT | Hydraulic retention time |
| MMO | Mixed metal oxide |
| Q | Flow rate |
| j | Current density |
| SEC | Specific energy consumption |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Fund Ernest Solvay, managed by the King Baudouin Foundation, under grant 2020-B7120700-214824. SM is supported by the Bijzonder Onderzoeksfonds (BOF18/DOS/035) scholarship and ESPOL. We thank the students from ESPOL Maria Paula Valarezo, Leticia Tituaña, Andrés Andino, Armando Guagalanga, Ariana Gonzalez, and Alejandro Carrion for their efforts in collecting some of the experimental data. AP and KR are supported by a Ghent University Bijzonder Onderzoeksfonds GOA grant (BOF19/GOA/026). We thank the current and previous group members for the interesting discussions that led to this work.References
- K. Rabaey, T. Vandekerckhove, A. Van De Walle and D. L. Sedlak, Water Res., 2020, 116276 CrossRef CAS PubMed.
- JMP, 2015 Annual Report, WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation, 2015 Search PubMed.
- S. Wu, P. Carvalho, J. Muller, V. Manoj and R. Dong, Sci. Total Environ., 2016, 541, 8–22 CrossRef CAS PubMed.
- M. I. Kerwick, S. M. Reddy, A. H. L. Chamberlain and D. M. Holt, Electrochim. Acta, 2005, 50, 5270–5277 CrossRef CAS.
- Y. Wang, L. Claeys, D. Van Der Ha, W. Verstraete and N. Boon, Appl. Microbiol. Biotechnol., 2010, 87, 331–341 CrossRef CAS PubMed.
- J. B. A. Arends, S. Van Denhouwe, W. Verstraete, N. Boon and K. Rabaey, Bioresour. Technol., 2014, 155, 352–358 CrossRef CAS PubMed.
- L. Mezule, M. Reimanis, V. Krumplevska, J. Ozolins and T. Juhna, Water Sci. Technol.: Water Supply, 2014, 14, 158–164 CAS.
- A. Kraft, M. Stadelmann, M. Blaschke, D. Kreysig, B. Sandt, F. Schröder and J. Rennau, J. Appl. Electrochem., 1999, 29, 861–868 CAS.
- M. E. H. Bergmann and A. S. Koparal, J. Appl. Electrochem., 2005, 35, 1321–1329 CrossRef CAS.
- H. Dong, W. Yu and M. R. Hoffmann, J. Phys. Chem. C, 2021, 125, 20745–20761 CrossRef CAS.
- J. Jeong, C. Kim and J. Yoon, Water Res., 2009, 43, 895–901 CrossRef CAS PubMed.
- H. Bergmann, Disinfection of Water, Electrochemical, 2014 Search PubMed.
- R. K. B. Karlsson and A. Cornell, Chem. Rev., 2016, 116, 2982–3028 CrossRef CAS PubMed.
- A. Kraft, Platinum Met. Rev., 2008, 52, 177–185 CrossRef CAS.
- S. Neodo, D. Rosestolato, S. Ferro and A. De Battisti, Electrochim. Acta, 2012, 80, 282–291 CrossRef CAS.
- H. Bergmann, T. Iourtchouk, K. Schoöps and K. Bouzek, Chem. Eng. J., 2002, 85, 111–117 CrossRef CAS.
- F. L. Burton, G. Tchobanoglous, R. Tsuchihashi, H. D. Stensel and Metcalf & Eddy Inc., Wastewater Engineering: Treatment and Resource Recovery, McGraw-Hill Education, 2013 Search PubMed.
- H. Bergmann, Curr. Opin. Electrochem., 2021, 28, 100694 CrossRef CAS.
- C. Bruguera-Casamada, I. Sirés, E. Brillas and R. M. Araujo, Sep. Purif. Technol., 2017, 178, 224–231 CrossRef CAS.
- A. Cano, P. Cañizares, C. Barrera-Díaz, C. Sáez and M. A. Rodrigo, Chem. Eng. J., 2012, 211–212, 463–469 CrossRef CAS.
- G. Libralato, A. Volpi Ghirardini and F. Avezzù, J. Environ. Manage., 2012, 94, 61–68 CrossRef CAS PubMed.
- H. Särkkä, A. Bhatnagar and M. Sillanpää, J. Electroanal. Chem., 2015, 754, 46–56 CrossRef.
- G. V. Talekar, P. Sharma, A. Yadav, P. Clauwaert, K. Rabaey and S. Mutnuti, npj Clean Water, 2018, 1, 1–9 CrossRef.
- EPA, Wastewater Technology Fact Sheet: Disinfection for Small Systems, Washington DC, 2003 Search PubMed.
- C. Wallis-Lage, White's Handbook of Chlorination and Alternative Disinfectants, John Wiley & Sons, Inc, Hoboken, 5th edn, 2010, vol. 1 Search PubMed.
- American Public Health Association, Standard Methods for the Examination of Water and Wastewater, American Water Works Association/Water Environment Federation, Washington DC, 20th edn, 1998 Search PubMed.
- C. E. Schaefer, G. M. Lavorgna, T. S. Webster, M. A. Deshusses, C. Andaya and A. Urtiaga, Water Supply, 2016, 17, 526–536 CrossRef.
- J. Llanos, I. Moraleda, C. Sáez, M. A. Rodrigo and P. Cañizares, Chemosphere, 2019, 220, 637–643 CrossRef CAS PubMed.
- S. K. Varigala, M. Hegarty-Craver, S. Krishnaswamy, P. Madhavan, M. Basil, P. Rosario, A. Raj, V. Barani, C. A. Cid, S. Grego and M. Luettgen, Environ. Sci.: Water Res. Technol., 2020, 6, 1400–1411 RSC.
- Y. Yang, L. Lin, L. K. Tse, H. Dong, S. Yu and M. R. Hoffmann, Environ. Sci.: Water Res. Technol., 2019, 5, 51–59 RSC.
- G. Puggioni, S. Milia, E. Dessì, V. Unali, N. Pous, M. D. Balaguer, S. Puig and A. Carucci, Water Res., 2021, 206 DOI:10.1016/j.watres.2021.117736.
- Texto Unificado de Legislación Secundaria de Medio Ambiente, Libro VI, Anexo 1, Norma de Calidad Ambiental y de Descarga de Efluentes: Recurso Agua, 2017 Search PubMed.
- Y. Yang, L. Lin, L. K. Tse, H. Dong, S. Yu and M. R. Hoffmann, Environ. Sci.: Water Res. Technol., 2019, 5, 51–59 RSC.
- J. T. Jasper, Y. Yang and M. R. Hoffmann, Environ. Sci. Technol., 2017, 51, 7111–7119 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ew00708d |
| This journal is © The Royal Society of Chemistry 2022 |