One-pot ethanol production under optimized pretreatment conditions using agave bagasse at high solids loading with low-cost biocompatible protic ionic liquid†
José A.
Pérez Pimienta
 *ab,
Gabriella
Papa
c,
Jian
Sun
*ab,
Gabriella
Papa
c,
Jian
Sun
 cd,
Vitalie
Stavila
cd,
Vitalie
Stavila
 e,
Arturo
Sanchez
e,
Arturo
Sanchez
 a,
John M.
Gladden
a,
John M.
Gladden
 cd and
Blake A.
Simmons
cd and
Blake A.
Simmons
 c
c
aLaboratorio de Futuros en Bioenergía, Unidad Guadalajara de Ingeniería Avanzada, Centro de Investigación y Estudios Avanzados (CINVESTAV), Zapopan, Mexico
bDepartment of Chemical Engineering, Universidad Autónoma de Nayarit, Tepic, Mexico. E-mail: japerez@uan.edu.mx
cJoint BioEnergy Institute, Biological Systems and Engineering Division, Lawrence Berkeley National Laboratory, Emeryville, CA, USA
dDepartment of Biomass Science and Conversion Technology, Sandia National Laboratories, Livermore, CA, USA
eEnergy Nanomaterials Department, Sandia National Laboratories, Livermore, CA, USA
First published on 30th November 2021
Abstract
Agave bagasse (AG) is a potential bioenergy feedstock due to its high biomass productivity, even in semiarid lands. In particular, ionic liquid (IL) pretreatment using aprotic ILs (AILs) has greatly reduced AG recalcitrance towards downstream processing by lowering lignin content and achieving high sugar yields. However, AIL's low biocompatibility towards enzymes and bacteria combined with the high initial cost has limited further development of this technology. In a wash-free one-pot (OP) ethanol conversion process, the evaluation of AG pretreatment with a biocompatible low-cost protic IL (PIL), 2-hydroxyethylammonium acetate ([2-HEA][OAc]) was achieved, where PIL pretreatment was followed by enzymatic saccharification, then ethanol fermentation in a single vessel. The pretreatment conditions were optimized using a central composite design to enable high sugar conversion at low PIL content. Under optimized pretreatment conditions (160 °C, 60% IL loading and 1.5 h), a yield of 132 kg of ethanol per Ton of untreated biomass was estimated using high solids loading (30% solids loading) under a PIL-OP scheme. High lignin removal (>50%), a decreased cellulose crystallinity, and high glucan conversion (>85%) were achieved with PIL-pretreated AG comparable to yields obtained in an AIL-AG pretreated sample using 1-ethyl-3-methyl-imidazolium acetate ([C2C1Im][OAc]). These results using [2-HEA][OAc] demonstrate the potential of AG in an OP scheme with improved total ethanol yields paving the way towards a more feasible IL-based biorefinery.
Introduction
Agave bagasse (AG) has proven to be an attractive bioenergy feedstock due to its high biomass productivity (up to 44 Ton per ha per year), even in semiarid lands and high-temperature stress.1 However, biomass recalcitrance hinders the efficient conversion of the lignocellulosic fraction into biofuels and/or value-added products. Therefore, a pretreatment stage must be incorporated for downstream biofuels and/or bioproducts generation to achieve profitable yields.2 Different biomass pretreatment methods with their unique mode of action and chemistry have been applied to AG using either alkali,3 ammonia fiber expansion (AFEX),4 dilute acid,5 extrusion,6 hydrothermal,7 ionic liquid (IL)8 and organosolv.9 Among them, IL pretreatment in AG has demonstrated to be an attractive and promising method to achieve a high glucan to glucose and xylan to xylose conversion (above 90%) during enzymatic saccharifications.7,10Another feature of IL pretreatment compared to other processes is the non-degradation of the carbohydrates into fermentation inhibitors such as acetic acid or furfural under mild process conditions while enabling solvent recovery and high recyclability.11 Most of IL pretreatment research in AG have been carried out using 1-ethyl-3-methylimidazolium acetate ([C2C1Im][OAc]) an aprotic IL (AIL), capable of decreasing cellulose crystallinity and lignin content (up to 48%) while obtaining ∼82% ethanol yield with an ethanologenic Escherichia coli strain.12 In the last decade, AILs have been studied in a broad range of feedstocks (grass, agricultural and woody biomass)13,14 with encouraging results including high delignification and sugar production either by enzymes or by an acidolysis procedure.15 The correlation between the hydrogen bond basicity of the anion and the solvation ability of aprotic ILs to swell and/or dissolve biomass promoting cellulose dissolution, lignin depolymerization and sugar yields has been widely studied in the literature.16,17 However, specific challenges are associated with using AILs, such as an initial high IL price and toxicity issues in the downstream processing, including multiple water-wash steps. A promising approach to overcome these obstacles is the use of protic ILs (PILs), whose production is easier and less expensive than AILs ($0.7–1.4 kg−1vs. ∼$50 kg−1).18,19 The main difference between AILs and PILs is the permanency of the positive cation charge after its synthesis in AILs and no equilibrium between neutral and ion species, while for PILs, the charged and neutral species are in equilibrium.20
Due to the weaker hydrogen-bonding basicity interactions of PILs formed by reversible proton transfer (both proton-donor and proton-acceptor centres in their molecules), the biomass dissolution has not been widely demonstrated in a PIL.21 Despite this, in literature, PILs has been extensively discussed as to their ability to dissolve lignin.18 Some works have shown that PILs produced by acetic acid and amines such as the 2-hydroxyethylammonium acetate ([2-HEA][OAc]) can extract lignin, demonstrating improved biocompatibility with enzymes and yeast.18,20,22 Moreover, to exploit protic ionic liquids as amphiphile self-assembly media inducing micelle formation and the solvophobic effect, recent studies highlighted the importance of further studies at the molecular level that considers the solubility of aromatic species in PILs and the interaction with either the polar and apolar phases.23 The employment of integrated systems approaches such as “one-pot” process configurations that integrate IL pretreatment, saccharification, and fermentation followed by direct extraction of sugar and recovery of lignin is key for future commercialization and scale-up this IL-based process. Within the IL pretreatment technology, the one-pot process can be achieved by either using an IL tolerant cellulases cocktail (such as JTherm)24 or with a PIL that include the benefits of reducing water consumption without the need of a water-wash step as previously required by the AIL which benefits the overall pretreatment costs. Recently, a comparison between PIL vs. AIL as pretreatment agents was performed in woody biomass blends for ethanol production, exhibiting the potential of PILs to achieve high sugar streams.25 Another advantage that [2-HEA][OAc] has in a one-pot configuration for biofuel production when compared to per example, cholinium lysinate [Ch][Lys], is that it does not require to adjust the pH before the sequential simultaneous enzymatic saccharification and fermentation (S-SSF) as this step will impact significantly during IL recycle and reuse.26,27 A recently demonstrated scalability in 680 L pilot-scale fermentation provides comprehensive data to define the overall efficiency of IL pretreatment as a function of parameters such as biomass tissue type and solid loading at pilot scale level using [Ch][Lys] in a one-pot IL pretreatment and saccharification.28 No reports are found in the scientific literature describing either AG pretreatment using PILs or in a consolidated biofuel production process. This work presents for the first time the use of a biocompatible PIL ([2-HEA][OAc]) in a one-pot process in AG conducting biomass pretreatment, saccharification, and fermentation in a single vessel without any solid/liquid separation and/or pH adjustment at high solids loading. While applying different characterization methods (compositional analysis, FTIR, XRD, pyrolysis-GC/MS) to evaluate the pretreatment effectiveness. The optimization of the pretreatment conditions was conducted to reduce PIL content while maintaining high sugar generation. A qualitative comparison between the performance of PIL [2-HEA][OAc] and an AIL ([C2C1Im][OAc]) was performed by assessing their delignification capacity and sugar generation obtained by enzymatic saccharification. In addition, lignin extracted from AG using the enzymatic mild acidolysis lignin (EMAL) protocol was prepared to compare it with the high lignin sample obtained after the one-pot process.
Experimental
Biomass samples and preparation
Agave bagasse (AG) (A. tequilana Weber variety Blue) was donated by Destilería Rubio that employed an autoclave during the cooking process of Tequila production from western Mexico and prepared as described elsewhere.7 AG was ground by a Wiley Mill through a 20-mesh screen and separated by a vibratory sieve system. Compositional analyses of untreated, IL pretreated, and one-pot AG were performed using the standard analytical procedures of the National Renewable Energy Laboratory (NREL) by the two-step sulfuric acid hydrolysis method (NREL/TP-510-42618).29 In addition, another agave bagasse sample was subjected to PIL pretreatment named as AG-diffuser coming from Destileria Leyros located in western Mexico that employs a diffuser during the cooking process of Tequila production.Chemicals
All of the chemicals were reagent grade and purchased from Sigma-Aldrich (St Louis, MO) if not specified otherwise. The [2-HEA][OAc] used in this study was prepared according to literature,20,30 and its structure was compared with research data. The commercial enzyme products cellulase (Celic CTec2 and Cellic CTec3) and hemicellulase (Cellic HTec 2 and Cellic HTec3) were gifts from Novozymes, North America (Franklinton, NC).Optimization of IL pretreatment using response surface methodology
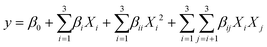 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) Cellic® Ctec3 (107.7 ± 2.1 mg mL−1) and HTec3 (80.4 ± 5.4 mg mL−1), respectively, were used at a concentration of 20 mg protein per g of biomass.13 Untreated AG was used as a control. After IL pretreatment of some specific runs, the solids were water-wash as described elsewhere,31 cooled down to room temperature and stored at 4 °C for compositional analysis. The glucan to glucose and xylan to xylose conversion (%) were calculated as previously described.7 Point validation at the optimized process conditions obtained from the CCD was performed at 10 and 30% solids loading.
1 (v/v) Cellic® Ctec3 (107.7 ± 2.1 mg mL−1) and HTec3 (80.4 ± 5.4 mg mL−1), respectively, were used at a concentration of 20 mg protein per g of biomass.13 Untreated AG was used as a control. After IL pretreatment of some specific runs, the solids were water-wash as described elsewhere,31 cooled down to room temperature and stored at 4 °C for compositional analysis. The glucan to glucose and xylan to xylose conversion (%) were calculated as previously described.7 Point validation at the optimized process conditions obtained from the CCD was performed at 10 and 30% solids loading.
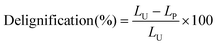 | (2) |
Upon completing the saccharification reaction, two sequential centrifugation steps at 4000 rpm for 10 min were served to achieve the solid–liquid separation of the resulting slurry. The liquid fraction was placed in serum bottles, diluted to concentrations of 2.5, 5 and 10% IL, and carried out the S-SSF during 24 as previously described.18 Ethanol yield was evaluated and taken into consideration along with IL tolerance to select the most appropriate strain for the bench-scale OP scheme.12 The OP scheme was carried out in a 1L Parr reactor under the optimal PIL-OP pretreatment variables evaluated in the CCD to achieve high sugar conversion while lowering IL dosage. The reactor loading was 30 g (dry basis) of untreated AG at 30 wt% solids loading with stirring at 60 rpm powered by a Heidolph RZR 2052 mechanical stirrer (Heidolph Instruments GmbH & Co KG, Schwabach, Germany) using a PTFE paddle-type impeller. After pretreatment, a cooling control was activated to maintain standard saccharification parameters for 24 h in order to obtain a sugars rich stream for ethanol fermentation. Then, the previously selected strain with high fermentative performances was evaluated in a PIL-OP scheme without pH adjustment or nutriments additions during 48 h. All reactions were monitored by removing 100 μL of the supernatant filtered through 0.45 μm membranes and measuring the sugars and ethanol concentration with an HPLC. All assays were performed in duplicate, and the data are reported as the mean ± standard deviation.
Analysis
All samples were centrifuged and filtered through 0.45 μm filters and diluted with water before analyses. Theoretical ethanol yield was calculated as previously reported taking into consideration that the S. cerevisiae BY4741 consumes only C6 sugars.12
The operation was repeated three times, discharging the hydrolysate each time, adding fresh enzyme and buffer solution, followed by washing five times with 400 mL g−1 biomass DI water. The washed unhydrolyzed solid was lyophilized. The obtained lignin from the mild enzymatic treatment was placed in 20 mL plastic vials and ball-milled two times for 5 min to fine powder, using the Tissue Lyser with 10 mm stainless steel balls at speeds of 30 cycles per minute and 5 min intervals between cycles. The solid recovered (i.e. crude lignin) was treated in acidified (HCl) dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (85
water (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 v
15 v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) mixture under a hot reflux condenser for 2 h. The mixtures were filtered and washed with dioxane
v) mixture under a hot reflux condenser for 2 h. The mixtures were filtered and washed with dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (85
water (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 v
15 v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) and fresh dioxane. The combined filtrates solution was neutralized with sodium bicarbonate, concentrated under vacuum using a rotary evaporator, and then dissolved in acidified DI water (pH 2, HCl) to precipitate lignin. Finally, the precipitated lignin was recovered by centrifugation, washed and freeze-dried. EMAL was chosen as representative of the “native” lignin initially present in the biomass feedstocks studied.
v) and fresh dioxane. The combined filtrates solution was neutralized with sodium bicarbonate, concentrated under vacuum using a rotary evaporator, and then dissolved in acidified DI water (pH 2, HCl) to precipitate lignin. Finally, the precipitated lignin was recovered by centrifugation, washed and freeze-dried. EMAL was chosen as representative of the “native” lignin initially present in the biomass feedstocks studied.
Crystallinity measurement
XRD diffractograms of untreated and pilot-scale pretreated biomass were acquired with a PANalytical Empyrean diffractometer equipped with a PIXcel3D detector with Cu Kα radiation.Samples were scanned in the range of 5–50° (2θ) with a step size of 0.026° at 45 kV and 40 mA under ambient temperature. The crystallinity index (CrI) was calculated by using the following equation:
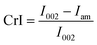 | (3) |
FTIR-ATR spectroscopy analysis
Fourier transform infrared (FTIR) spectroscopy was conducted using a Bruker Optics Vertex system (Billerica, MA, USA) with a built-in diamond-germanium ATR (attenuated total reflection) single reflection crystal. Untreated, IL pretreated, PIL-OP residue and EMAL samples were pressed uniformly against the diamond surface using a spring-loaded anvil.Sample spectra were obtained in triplicates using an average of 32 scans in the NIR range (800–2000 cm−1) with a spectral resolution of 4 cm−1. Air and water were used as background for untreated and pretreated biomass samples, respectively. Baseline correction was conducted and vector-normalization using OPUS software from Bruker Optics.
Pyrolysis-GC/MS
The ratio between syringyl and guaiacyl lignin monomers was determined in untreated and pretreated samples by pyrolysis coupled with gas chromatography–mass spectrometry. Samples of 0.5 mg were pyrolyzed at 550 °C using the pyroprobe 5200 (CDS Analytical, Inc., Oxford, PA, USA) connected to a gas chromatography–mass spectrometry (GC/MS) system (Agilent 6890) composed of a Trace GC Ultra and a Polaris-Q MS (Thermo Electron Corporation, Waltham, MA, USA) equipped with a TR-SMS column (60 m 0.25 mm ID 0.25 lm) and operated in split mode (40 mL min−1) using He as carrier. The chromatograph program was set as follows: 5 min at 50 °C, followed by an increase of 5 °C min−1 to 300 °C, finally maintained at 300 °C for 5 min. Pyrolysis products were identified based on their mass spectra using the NIST08 mass spectrum library. Compounds of syringyl (S), guaiacyl (G) and p-hydroxyphenyl (H) origin were quantified from the pyrogram using the peak area. The S/G ratio was calculated as the sum of all peaks of S molecules divided by the sum of all peak areas of G molecules.Results and discussion
Optimization of IL pretreatment conditions in a PIL-OP scheme
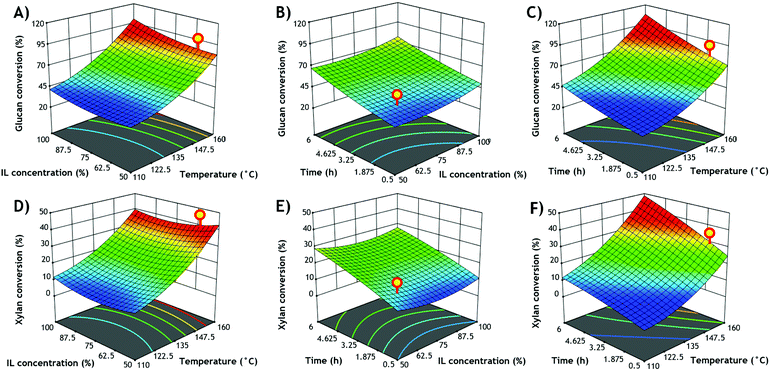
Utilizing a PIL-OP scheme could potentially reduce operation cost within a biorefinery scheme as its specific process design could decrease energy input required while preventing losses during mass transfer between stages in a typical SHF (separate hydrolysis and fermentation) route for biofuels and value-added products generation.24,33 Hence, the use of more biocompatible ILs to enzymes and microbes along with the optimization of the pretreatment conditions could determine a viable outcome and possible near-future industrial applications.Although there are some studies of biomass pretreatment using low-cost PIL among different feedstocks (capable of extracting more than 70% of lignin from corn stover34) and/or using an OP scheme, no data is currently available on AG. Based on previous reports around the response of AIL-pretreated AG with [C2C1Im][OAc] on sugar conversion,10,11 it was selected a broad range of process conditions temperatures (110–160 °C), [2-HEA][OAc] loadings (60–100%) and retention times (0.5–6.0 h) for the CCD analysis. Glucan and xylan conversion responses generated from the different CCD conditions are presented in Table 2.
| Factor 1 | Factor 2 | Factor 3 | Glucan conversion (%) | Xylan conversion (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | IL (%) | Time (h) | Observeda | Predictedb | Residualc | Observeda | Predictedd | Residualc |
| a Glucan and xylan conversion experimentally determined. b Calculated by using the multiple regression model: glucan conversion (%) = 365.91173 − 5.04206 × temperature − 1.82364 × IL concentration + 0.658486 × time + 0.002135 × temperature × IL concentration + 0.054046 × temperature × time − 0.009197 × IL concentration × time + 0.021504 × temperature2 + 0.012018 × IL concentration2 − 0.26773 × time2. c Difference between observed and predicted values. d Xylan conversion (%) = 129.03587 − 2.24613 × temperature − 0.139742 × IL concentration − 2.70466 × time − 0.003018 × temperature × IL concentration + 0.057846 × temperature × time − 0.013698 × IL concentration × time + 0.010842 × temperature2 + 0.004111 × IL concentration2 − 0.131845 × time2. | ||||||||
| 110 | 75 | 3.3 | 32.9 | 36.3 | −3.4 | 7.6 | 8.1 | −0.5 |
| 120 | 60 | 1.6 | 36.3 | 29.7 | 6.6 | 8.4 | 5.5 | 2.9 |
| 120 | 60 | 4.9 | 46.6 | 45.6 | 1.0 | 14.5 | 13.9 | 0.6 |
| 120 | 90 | 4.9 | 49.2 | 51.3 | −2.1 | 13.3 | 15.3 | −2.0 |
| 120 | 90 | 1.6 | 36.1 | 36.3 | −0.2 | 7.2 | 8.3 | −1.0 |
| 135 | 75 | 0.5 | 29.0 | 33.2 | −4.2 | 5.9 | 7.5 | −1.6 |
| 135 | 50 | 3.3 | 46.3 | 51.9 | −5.6 | 15.7 | 19.3 | −3.6 |
| 135 | 75 | 3.3 | 52.1 | 50.6 | 1.4 | 19.4 | 17.5 | 1.8 |
| 135 | 75 | 3.3 | 50.2 | 50.6 | −0.4 | 16.7 | 17.5 | −0.8 |
| 135 | 75 | 3.3 | 48.3 | 50.6 | −2.4 | 16.3 | 17.5 | −1.3 |
| 135 | 75 | 3.3 | 52.6 | 50.6 | 2.0 | 18.2 | 17.5 | 0.6 |
| 135 | 75 | 3.3 | 48.6 | 50.6 | −2.1 | 16.4 | 17.5 | −1.2 |
| 135 | 75 | 3.3 | 50.4 | 50.6 | −0.3 | 17.2 | 17.5 | −0.3 |
| 135 | 75 | 6 | 68.2 | 63.5 | 4.7 | 27.5 | 25.2 | 2.3 |
| 135 | 100 | 3.3 | 69.9 | 63.8 | 6.1 | 25.3 | 20.5 | 4.8 |
| 150 | 60 | 1.6 | 60.6 | 58.8 | 1.7 | 24.8 | 23.1 | 1.6 |
| 150 | 60 | 4.9 | 79.8 | 80.0 | −0.2 | 37.8 | 37.2 | 0.6 |
| 150 | 90 | 1.6 | 65.9 | 67.3 | −1.4 | 22.2 | 23.2 | −1.0 |
| 150 | 90 | 4.9 | 80.6 | 87.5 | −6.9 | 32.6 | 35.9 | −3.3 |
| 160 | 75 | 3.3 | 95.2 | 91.3 | 3.9 | 41.3 | 40.2 | 1.1 |
Interestingly, relatively low sugars conversion was achieved at the lower temperatures (below 135 °C). This was unexcepted. When AG was compared alongside switchgrass (SWG) at the same process conditions (120–160 °C, 3 h) with [C2C1Im][OAc], the higher sugar yields were obtained in AG at 120 °C while SWG resulted in 160 °C. Similarly, when SWG was evaluated with [2-HEA][OAc], the higher sugar yields were achieved at 160 °C.18 Also, when two different experimental designs (2K factorial and CCD) were used in AG (from an autoclave cooking process during Tequila production) for the optimization of AIL pretreatment conditions using [C2C1Im][OAc] data indicated 119 °C and 142 min as optimal process conditions.11
A clear trend was observed as higher temperatures during IL pretreatment lead to a higher glucan/xylan conversion. Among the three independent variables in the CCD, the temperature had the most significant impact on glucan conversion, while temperature and time were similarly relevant to xylan conversion (Fig. 1). This is in agreement with previously reported results obtained via a CCD design series of similar experiments on multiple feedstocks.11,35
The highest glucan and xylan conversion yields were 95.2% and 41.3%, respectively achieved at 160 °C, 75% IL loading and 3.3 h. The responses of the CCD were analyzed using ANOVA and fitted to a response surface quadratic model (ESI Table S1†). The coefficients of determination (R2) were 0.9553 and 0.9579 for glucan and xylan conversion, respectively.
Using these data, a new model to calculate % glucan and xylan conversion was developed, and equations have been suggested and reported in Table 2. The model proposed indicates that the pretreatment temperature is the principal responsible for the glucan and xylan conversion. A comparison of the values obtained with the experimental results indicated that the model was satisfactory. Moreover, the adjusted R2 (0.9150 for glucan conversion and 0.9200 for xylan conversion) confirm the adequacy of the model. In order to improve the biorefinery economics, it is necessary to employ aqueous solutions of ILs or decrease IL usage, hence lowering viscosity and making handling easier besides enhancing mass transfer.36,37 For these reasons, the optimal pretreatment parametric combination was directed to achieve the highest sugar conversion while decreasing PIL dosages. Thus, the optimum pretreatment condition was: 160 °C, 60 wt% IL loading and 1.5 h using 10% solids loading. It can be observed that this process conditions decrease IL consumption and time when compared to the 160 °C experimental runs in the CCD (Table 2). The PIL-OP pretreatment variables were verified, and the experimental values at the optimum conditions were 79.1% for glucan conversion and 36.7% for xylan conversion.
The observed sugars conversion values were similar, confirming the precision of the model with a variation below 4%. Nevertheless, it is acknowledged the need to operate the pretreatment reactor using a high biomass loading (>20 wt%) in order to reduce capital cost and operational expenditures while at the same time increasing final sugar concentration and biofuel titer.38,39 Hence, the solid loading was increased to 30 wt% during IL pretreatment at the previously defined optimum process conditions. The interactions of pretreatment conditions (temperature, IL loading and residence time) at a high solids loading were able to maintain relatively high glucan and xylan conversions rates (i.e. 72.9% and 36.1%, respectively).
Also, the addition of water diminished viscosity while maintaining a high sugar conversion, as observed. Finally, in the PIL-OP scheme, these pretreatment conditions (160 °C for 1.5 h, using 60 wt% [2-HEA][OAc]/water and 30 wt% solids loading) were applied during the S-SSF stage for ethanol production.
Effects of the type of feedstock and IL in solids composition
To assess the effects of IL pretreatment on different AG samples, the main components of the plant cell wall (glucan, xylan and lignin) were monitored and quantified (Fig. 2). Untreated AG presented a typical solids composition similar to previous reports with 35% glucan, 19.0% xylan and 16.1% lignin.1 After IL pretreatment of AG at optimized conditions with [2-HEA][OAc] at both 10% and 30% solid loadings, a delignification of 54.7% and 23.0%., respectively, were achieved. While at the same time increasing their relative glucan per gram of biomass.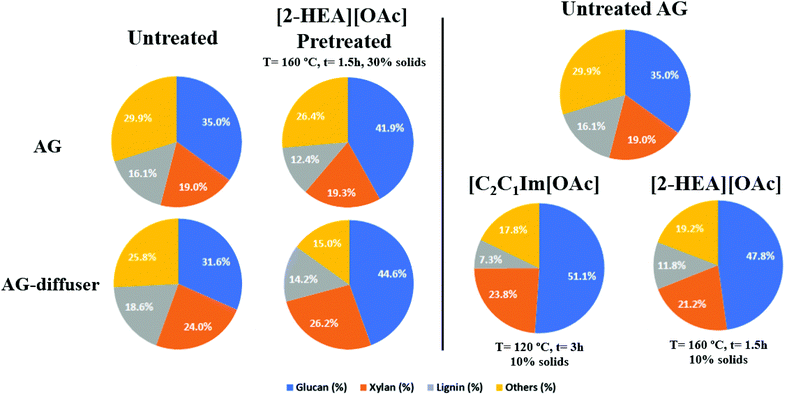 | ||
| Fig. 2 Compositional analysis of the major components of selected untreated and IL pretreated Agave bagasse samples. | ||
This extent of delignification (>50%) was also observed in other feedstocks (switchgrass and sugarcane straw) using [2-HEA][OAC] as a pretreatment agent with a solids loading of 6 and 10% w/w%.18,37
For comparison purposes, untreated AG pretreated using [C2C1Im][OAc] at 120 °C for 3 h at 10 wt% solid loading showed a delignification of 26.7%, which is in the range of previous reports.7,8,12 Some notable differences between AG pretreated with [2-HEA][OAc] and [C2C1Im][OAc] can be clearly shown in the solid recovery (56.5% vs. 78.4%) due to pretreatment severity (160 °C vs. 120 °C). Unlike other bioenergy feedstocks such as corn stover or sugarcane bagasse, AG is subjected to an industrial transformation process in Tequila production where a cooking stage using primarily either autoclaves or diffusers is employed. However, this thermal process modifies the structure and recalcitrance of AG, as previously shown.40
Therefore, another raw Agave sample (AG-diffuser) was evaluated to compare the effectiveness of the optimized pretreatment conditions using [2-HEA][OAc] in a more recalcitrant sample as previously demonstrated.40 When compared to AG, untreated AG-diffuser has lower initial glucan (35.0% vs. 31.6%) and higher lignin (16.1% vs. 18.6%) content. These differences could be attributed to Agave cooking during Tequila manufacturing. More severe process conditions were applied in AG (autoclave with 105 °C and 18 h) than AG-diffuser (70 °C and 6 h). When AG diffuser was pretreated with [2-HEA][OAc] at 30% solids loading, a similar delignification value was achieved compared to AG (23.7% vs. 26.7). Even with its relatively higher lignin content, pretreatment with [2-HEA][OAc] was capable of maintaining its effectiveness. Therefore, no significant differences between substrates in the delignification values were found at optimal conditions to achieve high sugar conversion in AG and AG-diffuser using [2-HEA][OAc] at 160 °C, unlike a previous report where differences in delignification were found using [C2C1Im][OAc] at the previous proven conditions at 120 °C.40 This lack of difference between delignification values can be attributed to numerous factors specific to the specific environmental conditions from the biomass origin, particle size, extraction, and post-harvest procedures, as well as IL and Agave cooking type.
Structural changes on selected Agave samples
After pretreatment, numerous simultaneous changes occurred in biomass recalcitrance, including changes in the lignin and hemicellulose content, lignin–carbohydrate complexes, accessibility, and crystallinity.41 The cellulose crystalline structure, due to its highly ordered and water-insoluble nature, is challenging for enzymes to achieve an efficient hydrolysis reaction. Thus, decreasing the cellulose crystallinity while simultaneously having a high delignification will increase the availability of active enzymes and rest in an efficient saccharification reaction.42As in previous findings, untreated AG (Fig. 3) presented distinctive and defined peaks at 2θ = 14.8°, 24.5°, 30.0° and 38.0° corresponding to monohydrate calcium oxalate (CaOX), which is characteristics of succulent plants that incorporate the crassulacean acid metabolism (per example, Opuntia ficus indica).12,43,44 Interestingly, the pretreatment performed with [2-HEA][OAc] at two different biomass loadings (10 and 30 wt%) showed two different effects on CrI. In particular, an increase in CrI up to 42.7% was obtained in the [2-HEA][OAc]-pretreated sample at 10% solids loading that includes a sharp peak at 22.1°. In comparison, a decrease of CrI to 32.0% was observed at 30% solids loading during pretreatment compared to the untreated AG (36.9%). These results could be attributed to removing amorphous cell wall components in Agave, including calcium oxalate, lignin and hemicellulose.26 Besides, the intensity of the CaOX peaks was reduced after pretreatment, while a sharp cellulose peak at 22.5° and a lower and broader peak at ∼15.9° are now more defined. Besides, the solid residue recovered after the PIL-OP process was analyzed to obtain its XRD pattern.
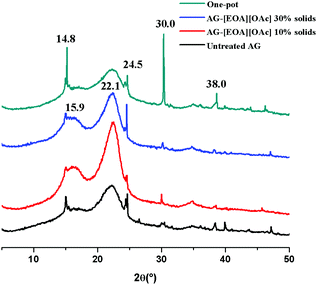 | ||
| Fig. 3 XRD spectra of untreated, IL pretreated at 10 and 30% solids loading at optimized conditions and one-pot agave bagasse. | ||
Limited information exists on the crystallinity of PIL pretreated and PIL-OP samples when compared to AIL. After pretreatment with two different PILs (trie-thylammonium hydrogen sulfate [TEA][HSO4] and 1-butylimidazolium hydrogen sulfate [HBIM][HSO4]), the cellulose crystallinity increased after pretreatment in the ratio of 2 to 10% when compared to the untreated biomass, hornbeam (Carpinus betulus L.).45
On the other hand, Sun et al.18 showed that CrI in switchgrass pretreated with [2-HEA][OAc] decreased when compared to the untreated biomass (68% vs. 49%) while still retaining the cellulose I structure (as in this study). The XRD spectra from the PIL-OP samples indicated a 34.5% CrI with more intense CaOX peaks as a result of the carbohydrates consumption and fermentation to ethanol during S-SSF and a corresponding concentration of the other constituents (CaOX, lignin, non-consumed carbohydrates, and ashes). To further confirm biomass structural changes near FTIR spectra of untreated vs. pretreated AG with [C2C1Im][OAc] and [2-HEA][OAc] as well as untreated AG vs. OP and EMAL samples were recorded using different carbohydrate and lignin bands, including two bands associated to CaOX (Fig. 4).
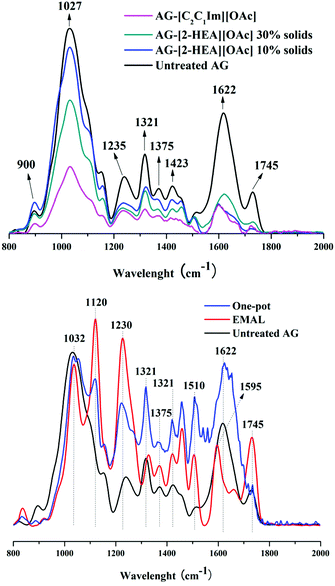 | ||
| Fig. 4 FTIR spectra of agave bagasse solids. Untreated vs. pretreated with [C2C1Im][OAc] and [2-HEA][OAc] (top); untreated vs. OP and EMAL samples (bottom). | ||
The peak at 900 cm−1 is observed to decrease in both the 30% solids [2-HEA][OAc] and [C2C1Im][OAc] pretreated samples while increasing in the 10% solids [2-HEA][OAc] when compared to the untreated sample, which is consistent with the XRD pattern, indicating a modification of cellulose crystallinity. Reduced peaks intensities of the CaOX peaks at 1321 and 1622 cm−1 occurred in all pretreated samples while the opposite was observed in the OP and EMAL samples. In addition, the reduced presence of specific functional groups in the pretreated biomass (more evident on the [C2C1Im][OAc] sample) on C–O stretching in cellulose and hemicellulose (1027 cm−1) and C–O stretching in lignin and hemicellulose (1235 cm−1) was ascribed to characteristic IL pretreatment response on cell wall component reconfigurations after lignin removal and cellulose dissolution.46
Besides, a C–H deformation/bending at 1375 and 1423 cm−1 associated with condensed syringyl/guaiacyl unit and aromatic ring, respectively, can be observed at similar values for the [2-HEA][OAc] samples.47,48 When the untreated is compared against the OP and EMAL samples, the difference between lignin-related peaks intensity on the latter is easily noticeable. The most important functional groups of lignin include carbonyls, phenolic hydroxyls, aromatic rings and methoxyls at 1595 and 1220 cm−1 (CO stretching in aryl ester), including sharp peaks characteristic of aromatic compounds derived from lignin can be easily correlated. These high-intensity peaks can also be found in sugarcane bagasse pretreated with [2-HEA][OAc] for 3.5 h at 150 °C; however, they are covered beneath the CaOX peaks in AG.22 Interestingly, the band at 1120 cm−1 associated with the aromatic C–H in-plane deformation typical for syringyl units is observed in high intensity for the EMAL sample.48
Py-GC/MS and lignin S/G ratios in AG samples
As shown in ESI Fig. S1 and Table S2,† syringol, vanillic acid, isoeugenol, 3-tert-butyl-4-hydroxyanisole, and 4-allyl-2,6-dimethoxy phenol were the most predominant aromatic compounds in untreated AG, EMAL and OP samples identified by Py-GC/MS.The most available compounds in terms of relative abundance (>25%) were the 4-allyl-2,6-dimethoxyphenol (S-lignin), representing up to 33% of the detected compounds in the OP sample. The syringol was also detected in a recent report in Agave tequilana and also present in other Agave species (Agave sisalana).49,50 Although S and G lignin-derived monomers were predominantly found in AG, one compound recognized as H-lignin (4-methylphenol) was noticed in the OP samples in a small proportion (>1.5%). Previous studies have also described the lignin S/G ratio as an essential factor to gauge biomass recalcitrance that can influence cross-linking between the cell wall components and modification of the biomass saccharification yields cellulases.41,51
The S/G ratios observed for the untreated AG was 1.07, which is relatively lower than previous reports with Agave tequilana bagasse (1.57).40 The S/G ratios of EMAL and OP samples were 0.94 and 1.59, respectively.
One-pot ethanol production from AG pretreated at high solids loading using optimized pretreatment conditions
For the first time using in an OP scheme, AG was assessed as a biofuel feedstock using the conditions evaluated in the CCD to achieve high sugar production while lowering IL content during biomass pretreatment resulting in 160 °C, 60% IL loading and 1.5 h using 30% solids loading. Interestingly, the data obtained from preliminary lab-scale experiments using three different S. cerevisiae strains (BY4741, CEN.PK and W303) indicated that [EAO][OAc] did not have an inhibitory effect on either of the yeast strains evaluated. In a PIL-OP, it is preferred to use a higher concentration of IL during the fermentation phase to avoid further dilution of sugars which would prevent the inhibition that would occur at a higher concentration (15%), as shown above.18 In the presence of [2-HEA][OAc] at 10 wt%, the three employed strains had a similar ethanol yield after 24 h of fermentation as follows: BY4741 (99.0 ± 0.6), W303 (96.2 ± 4.7), and Cen.pk (99.4 ± 0.1). However, the relatively faster glucose consumption was achieved by the B4741 strain; therefore, this strain was selected for the experimental runs in the 1 L reactor (bench-scale). One-pot ethanol production in the 1 L Parr reactors was achieved using a single vessel for the three sequential stages (pretreatment, saccharification and fermentation), as presented in Fig. 5. No pH adjustment or additional nutriments were used during the fermentation stage of the OP experiments, and no washing step or solids removal were employed in the pretreatment and saccharification stages.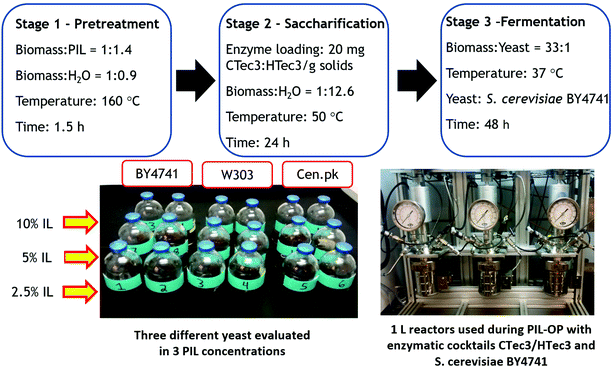 | ||
| Fig. 5 General flow chart of the one-pot scheme constituted from three stages (pretreatment, saccharification and fermentation). | ||
After yeast inoculation with B4741, glucose to ethanol yield of ∼97% was obtained after 48 h of fermentation in the 1 L reactors. Results showed that 266 kg of glucose and 76 kg of xylose were generated during the enzymatic saccharification stage, taking 1 Ton of untreated biomass as a basis. The solid residue recovered after the PIL-OP process still contains usable carbohydrates, whereas the lignin content was concentrated (∼22%). Using a 30 wt% solids loading reduced IL usage as low as 1.4 kg per kg of biomass; this high solid loading is necessary to achieve a higher yield in a simplified OP conversion process. The final ethanol yields of the PIL-OP scheme are a direct consequence of the pretreatment nature. Hence, a total of 132 kg of ethanol was obtained per Ton of untreated biomass. It was demonstrated an ethanol production higher than previous findings that produced 121 kg of ethanol/ton of untreated biomass using [C2C1Im][OAc] pretreated AG than employed an ethanologenic Escherichia coli strain that even consumed C5 and C6.12
These results reaffirm the benefits of biocompatible PIL, enabling biorefinery schemes that can reduce cost bottlenecks that typically diminish IL pretreatment's economic performance, such as IL cost and rheological properties (i.e. high viscosity), solid/liquid separation and pH adjustment. Moreover, different strategies and research need to be integrated to improve ethanol yield and overall process economics.
In this regard, the advances in recent years to identify and synthesize low-cost and biocompatible ILs that avoid pH adjustment and water-wash steps have contributed to its consideration as an economically viable pretreatment technology within a biorefinery. In order to improve essential cost segments that limit further development of the PIL-OP-scheme (i.e., IL cost, enzyme loading and its relatively low final ethanol concentration), further research efforts should be made on developing engineered yeast or bacteria capable to tolerate higher PIL concentrations and/or consuming C5 and C6 sugars during S-SSF. Other bioreactor configurations for high solids can be considered to achieve a higher tier ethanol production loading (i.e., peg-mixer). Finally, research intensification to develop cellulases cocktails capable of tolerating high IL loadings (such as the JTherm developed at JBEI), as well as IL tolerant yeast strain capable of achieving a sustained growth at IL loadings above 15 wt% (or higher), could lead to a more cost-efficient IL-based biorefinery scheme.
Conclusions
A low-cost and biocompatible PIL ([2-HEA][OAc]) at 160 °C and 1.5 h showed pretreatment performance comparable to a well-known AIL ([C2C1Im][OAc]) at 120 °C and 3 h in AG at 30% solids loading, achieving high delignification (>50% lignin), low cellulose crystallinity and the disruption of important cell wall linkages. No significant difference in delignification between two different AG samples (AG and AG-diffuser) were found after PIL pretreatment. Lowering PIL usage up to 60% while maintaining high glucan conversion was carried out within an OP scheme at high solids loading (30%), which has been estimated to produce 132 kg ethanol per Ton of untreated biomass at optimized conditions (160 °C and 1.5 h). Finally, the current PIL-OP scheme in AG presents an attractive approach to eliminate costly process steps (pH adjustment and water-wash) while reducing total processing time and increasing sugars and ethanol yields which could be improved even more with further research.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Energy Sustainability Fund from the Mexican Secretary of Energy, Grant no. 249564 (Mexican Bioenergy Innovation Centre, Bioalcohols Cluster). This work was part of the DOE Joint BioEnergy Institute (http://www.jbei.org) supported by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the U.S. Department of Energy.References
- J. A. Pérez-Pimienta, M. G. López-Ortega and A. Sanchez, Biofuels, Bioprod. Biorefin., 2017, 11, 732–748 CrossRef.
- S. P. S. Chundawat, G. T. Beckham, M. E. Himmel and B. E. Dale, Annu. Rev. Chem. Biomol. Eng., 2011, 2, 121–145 CrossRef CAS PubMed.
- K. L. Galindo-Hernández, A. Tapia-Rodríguez, F. Alatriste-Mondragón, L. B. Celis, J. Arreola-Vargas and E. Razo-Flores, Int. J. Hydrogen Energy, 2018, 43, 22116–22125 CrossRef.
- C. A. Flores-Gómez, E. M. Escamilla Silva, C. Zhong, B. E. Dale, L. da Costa Sousa and V. Balan, Biotechnol. Biofuels, 2018, 11, 1–18 CrossRef.
- D. I. Díaz-Blanco, J. R. de la Cruz, J. C. López-Linares, T. K. Morales-Martínez, E. Ruiz, L. J. Ríos-González, I. Romero and E. Castro, Ind. Crops Prod., 2018, 114, 154–163 CrossRef.
- C. Montiel, O. Hernández-Meléndez, E. Vivaldo-Lima, M. Hernández-Luna and E. Bárzana, BioEnergy Res., 2016, 1005–1014 CrossRef CAS.
- J. A. Pérez-Pimienta, C. A. Flores-Gómez, H. A. Ruiz, N. Sathitsuksanoh, V. Balan, L. da Costa Sousa, B. E. Dale, S. Singh and B. A. Simmons, Bioresour. Technol., 2016, 211, 216–223 CrossRef.
- J. A. Perez-Pimienta, M. G. Lopez-Ortega, P. Varanasi, V. Stavila, G. Cheng, S. Singh and B. A. Simmons, Bioresour. Technol., 2013, 127, 18–24 CrossRef CAS PubMed.
- L. Caspeta, M. A. Caro-Bermúdez, T. Ponce-Noyola and A. Martinez, Appl. Energy, 2014, 113, 277–286 CrossRef CAS.
- J. A. Pérez-Pimienta, N. Sathitsuksanoh, V. S. Thompson, K. Tran, T. Ponce-Noyola, V. Stavila, S. Singh and B. A. Simmons, Biotechnol. Biofuels, 2017, 10, 72 CrossRef PubMed.
- J. A. Pérez-Pimienta, J. P. A. Icaza-Herrera, J. A. Méndoza-Pérez, V. González-Álvarez, H. O. Méndez-Acosta and J. Arreola-Vargas, Bioresour. Technol., 2019, 275, 78–85 CrossRef.
- J. A. Pérez-Pimienta, A. Vargas-Tah, K. M. López-Ortega, Y. N. Medina-López, J. A. Mendoza-Pérez, S. Avila, S. Singh, B. A. Simmons, I. Loaces and A. Martinez, Bioresour. Technol., 2017, 225, 191–198 CrossRef PubMed.
- B. Satari, K. Karimi and R. Kumar, Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: A review, Royal Society of Chemistry, 2019, vol. 3 Search PubMed.
- S. K. Bhatia, S. S. Jagtap, A. A. Bedekar, R. K. Bhatia, A. K. Patel, D. Pant, J. Rajesh Banu, C. V. Rao, Y. G. Kim and Y. H. Yang, Bioresour. Technol., 2020, 300, 122724 CrossRef CAS PubMed.
- N. Sun, F. Xu, N. Sathitsuksanoh, V. S. Thompson, K. Cafferty, C. Li, D. Tanjore, A. Narani, T. R. Pray, B. A. Simmons and S. Singh, Bioresour. Technol., 2015, 186, 200–206 CrossRef CAS PubMed.
- N. Sun, R. Parthasarathi, A. M. Socha, J. Shi, S. Zhang, V. Stavila, K. L. Sale, B. A. Simmons and S. Singh, Green Chem., 2014, 16, 2546–2557 RSC.
- C. G. Yoo, Y. Pu and A. J. Ragauskas, Curr. Opin. Green Sustainable Chem., 2017, 5, 5–11 CrossRef.
- J. Sun, N. V. S. N. M. Konda, R. Parthasarathi, T. Dutta, M. Valiev, F. Xu, B. A. Simmons and S. Singh, Green Chem., 2017, 19, 3152–3163 RSC.
- G. Papa, S. Rodriguez, A. George, A. Schievano, V. Orzi, K. L. Sale, S. Singh, F. Adani and B. A. Simmons, Bioresour. Technol., 2015, 183, 101–110 CrossRef CAS PubMed.
- R. M. Dias, F. H. B. Sosa and M. C. da Costa, Polym. Bull., 2020, 3637–3656 CrossRef CAS.
- M. M. Hossain, A. Rawal and L. Aldous, ACS Sustainable Chem. Eng., 2019, 7, 11928–11936 CAS.
- E. G. A. Rocha, T. C. Pin, S. C. Rabelo and A. C. Costa, Fuel, 2017, 206, 145–154 CrossRef CAS.
- H. J. Jiang, S. Imberti, B. A. Simmons, R. Atkin and G. G. Warr, ChemSusChem, 2019, 12, 270–274 CrossRef CAS PubMed.
- J. Shi, J. M. Gladden, N. Sathitsuksanoh, P. Kambam, L. Sandoval, D. Mitra, S. Zhang, A. George, S. W. Singer, B. A. Simmons and S. Singh, Green Chem., 2013, 15, 2579–2589 RSC.
- L. Das, E. C. Achinivu, C. A. Barcelos, E. Sundstrom, B. Amer, E. E. K. Baidoo, B. A. Simmons, N. Sun and J. M. Gladden, ACS Sustainable Chem. Eng., 2021, 9, 4422–4432 CrossRef CAS.
- T. Dutta, G. Papa, E. Wang, J. Sun, N. G. Isern, J. R. Cort, B. A. Simmons and S. Singh, ACS Sustainable Chem. Eng., 2018, 6, 3079–3090 CrossRef CAS.
- P. Halder, S. Kundu, S. Patel, A. Setiawan, R. Atkin, R. Parthasarthy, J. Paz-Ferreiro, A. Surapaneni and K. Shah, Renewable Sustainable Energy Rev., 2019, 105, 268–292 CrossRef CAS.
- C. A. Barcelos, A. M. Oka, J. Yan, L. Das, E. C. Achinivu, H. Magurudeniya, J. Dong, S. Akdemir, N. R. Baral, C. Yan, C. D. Scown, D. Tanjore, N. Sun, B. A. Simmons, J. Gladden and E. Sundstrom, ACS Sustainable Chem. Eng., 2021, 4042–4053 CrossRef CAS.
- A. Sluiter, B. Hames, R. Ruiz and C. Scarlata, Lab. Anal. Proced. Natl. Renew. Energy Lab. Golden, CO, NREL/TP-510-42618.
- M. Iglesias, R. Gonzalez-Olmos, I. Cota and F. Medina, Chem. Eng. J., 2010, 162, 802–808 CrossRef CAS.
- J. A. Perez-Pimienta, M. G. Lopez-Ortega, J. A. Chavez-Carvayar, P. Varanasi, V. Stavila, G. Cheng, S. Singh and B. A. Simmons, Biomass Bioenergy, 2015, 75, 180–188 CrossRef CAS.
- S. Wu and D. S. Argyropoulos, J. Pulp Pap. Sci., 2003, 29, 235–240 CAS.
- F. Xu, J. Sun, N. V. S. N. M. Konda, J. Shi, T. Dutta, C. D. Scown, B. A. Simmons and S. Singh, Energy Environ. Sci., 2016, 9, 1042–1049 RSC.
- E. C. Achinivu, R. M. Howard, G. Li, H. Gracz and W. A. Henderson, Green Chem., 2014, 16, 1114–1119 RSC.
- D. Fu and G. Mazza, Bioresour. Technol., 2011, 102, 8003–8010 CrossRef CAS PubMed.
- J. A. Pérez-Pimienta, N. Sathitsuksanoh, V. S. Thompson, K. Tran, T. P. Noyola, V. Stavila, S. Singh and B. A. Simmons, Biotechnol. Biofuels, 2017, 1–15 Search PubMed.
- F. A. Ferrari, J. F. B. Pereira, G.-J. Witkamp and M. B. S. Forte, ACS Sustainable Chem. Eng., 2019, 7, 12779–12788 CrossRef CAS.
- R. Agrawal, B. Bhadana, A. S. Mathur, R. Kumar, R. P. Gupta and A. Satlewal, Front. Energy Res., 2018, 6, 1–12 CrossRef.
- J. Zhang, W. Hou and J. Bao, Adv. Biochem. Eng./Biotechnol., 2015, 75–90 CrossRef PubMed.
- J. A. Pérez-Pimienta, R. M. Mojica-Álvarez, L. M. Sánchez-Herrera and A. Mittal, BioEnergy Res., 2018, 11, 551–561 CrossRef.
- K. Karimi and M. J. Taherzadeh, Bioresour. Technol., 2016, 203, 348–356 CrossRef CAS PubMed.
- X. Meng, C. G. Yoo, M. Li and A. J. Ragauskas, J. Appl. Biotechnol. Bioeng., 2016, 1, 87–94 Search PubMed.
- J. A. Pérez-Pimienta, M. G. Lopez-Ortega, J. A. Chavez-Carvayar, P. Varanasi, V. Stavila, G. Cheng, S. Singh and B. A. Simmons, Biomass Bioenergy, 2015, 75, 180–188 CrossRef.
- L. Yang, M. Lu, S. Carl, J. A. Mayer, J. C. Cushman, E. Tian and H. Lin, Biomass Bioenergy, 2015, 76, 43–53 CrossRef CAS.
- I. Semerci and G. Ersan, Ind. Crops Prod., 2021, 159, 113021 CrossRef CAS.
- C. Li, G. Cheng, V. Balan, M. S. Kent, M. Ong, S. P. S. Chundawat, L. daCosta Sousa, Y. B. Melnichenko, B. E. Dale, B. A. Simmons and S. Singh, Bioresour. Technol., 2011, 102, 6928–6936 CrossRef CAS PubMed.
- X. H. Li and S. Bin Wu, BioResources, 2014, 9, 6277–6289 Search PubMed.
- A. M. Cãpraru, V. I. Popa, T. Mãlutan and G. Lisa, Cellul. Chem. Technol., 2009, 43, 409–418 Search PubMed.
- J. A. Pérez Pimienta, G. Papa, A. Rodriguez, C. A. Barcelos, L. Liang, V. Stavila, A. Sanchez, J. M. Gladden and B. A. Simmons, Green Chem., 2019, 21, 3152–3164 RSC.
- J. Rencoret, G. Marques, A. Gutiérrez, L. Nieto, J. I. Santos, J. Jiménez-Barbero, Á. T. Martínez and J. C. del Río, Holzforschung, 2009, 63, 691–698 CAS.
- J. S. Lupoi, S. Singh, R. Parthasarathi, B. A. Simmons and R. J. Henry, Renewable Sustainable Energy Rev., 2015, 49, 871–906 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc03774a |
| This journal is © The Royal Society of Chemistry 2022 |