Open Access Article
Open Access ArticleBio-based copolyesters involving 1,4:3,6-dianhydrohexitols and sebacic acid: 2,6-pyridinedicarboxylic acid as platforms for high gas barrier food packaging†
Xuelian
Liu
 a,
Laurent
Lebrun
a,
Laurent
Lebrun
 *b,
Nadège
Follain
*b,
Nadège
Follain
 b and
Nicolas
Desilles
b and
Nicolas
Desilles
 *a
*a
aNormandie Université, INSA Rouen Normandie, CNRS UMR 6270, 685 Avenue de l’Université, 76800 Saint Etienne du Rouvray, France. E-mail: xuelian.liu@insa-rouen.fr; nicolas.desilles@insa-rouen.fr
bNormandie Université, Université Rouen Normandie, CNRS UMR 6270, Faculté des Sciences, 76821 Mont Saint Aignan, France. E-mail: laurent.lebrun@univ-rouen.fr; nadege.follain@univ-rouen.fr
First published on 9th November 2021
Abstract
Proposing renewable structures for food packaging applications is necessary for contributing to a low-carbon and sustainable world. Thus, bio-based copolyesters, involving isosorbide (or isomannide), sebacoyl dichloride, and a second rigid acid structure (succinyl dichloride, isophthaloyl chloride or 2,6-pyridinedicarbonyl dichloride), were synthesized via polycondensation reactions. The 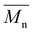 values ranged from 10
values ranged from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 to 18
600 to 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. 1H NMR spectroscopy established the random copolymerization and enabled the calculation of the respective monomer ratios in the copolymers. All copolyesters were thermally stable with Td5% higher than 328 °C and Tg ranging from 30 to 64 °C. The tensile test revealed plastic fractures for aliphatic copolyesters and brittle fractures for aromatic ones. The highest Young's modulus and tensile strength were obtained for aromatic copolyesters, whereas the largest elongation at break was observed for aliphatic ones. All copolyesters showed good gas barrier properties, and the best result was comparable to the widely used semi-crystalline PET.
000 g mol−1. 1H NMR spectroscopy established the random copolymerization and enabled the calculation of the respective monomer ratios in the copolymers. All copolyesters were thermally stable with Td5% higher than 328 °C and Tg ranging from 30 to 64 °C. The tensile test revealed plastic fractures for aliphatic copolyesters and brittle fractures for aromatic ones. The highest Young's modulus and tensile strength were obtained for aromatic copolyesters, whereas the largest elongation at break was observed for aliphatic ones. All copolyesters showed good gas barrier properties, and the best result was comparable to the widely used semi-crystalline PET.
1. Introduction
In 2015, at the Paris Climate Conference (COP21), 196 parties came to an agreement to adopt the first-ever, legally binding global climate convention that decided to develop a low carbon society.1 This convention came into force on 4th November 2016,2 and a subsequent low-carbon world conference is planned by the France government to be conducted in June 2021 to further clarify national strategies.3 Thus, our world is being highly promoted to move towards a low carbon society. To achieve this goal, a large number of research studies have been devoted to the development of clean energy and bio-based products. However, the current global food packaging market is still dominated by traditional polymeric materials, which conflicts the concept of low carbon and sustainable development. According to 2018 data, 45% of the global fossil fuel supplies not intended for energy purposes were used to produce plastics, which is expected to increase to 60% by 2050,4 and the volume of food packaging plastics is estimated to be around 40% of the whole plastics consumption.5 The extensive use of petroleum-based polymers is threatening our planet by depleting crude oil and accelerating global warming. Thus, more efforts are needed to develop polymers from bio-based feedstocks.Among the various bio-based feedstocks, 1,4:3,6-dianhydrohexitols attracted much research attention in recent years. Besides, their molecular rigidity and chirality made them popular as platforms for developing new bio-based polymers with high glass transition temperature and/or special optical properties in the past decades.6–8 Also, their non-toxicity allows them to be used in food packaging and bio-medical devices.7,8 Particularly, the large scale production of high purity isosorbide (POLYSORB®) by Roquette further facilitates the development of low carbon footprint polymers based on this molecule such as polyesters, polycarbonates and thermoplastic polyurethanes (TPUs).9,10 Furthermore, according to Okada et al., the aliphatic polyesters based on 1,4:3,6-dianhydrohexitols are biodegradable, which further enhances their environmentally friendly competitiveness.11
Our previous work suggested that the aliphatic polyesters based on isosorbide (IS), or isomannide (IM), and sebacoyl chloride (C10), named ISC10 and IMC10, respectively, have the potential for food packaging application with satisfactory CO2/O2 selectivity (αCO2/O2).12 However, their low glass transition temperature (Tg) might be a limitation in terms of mechanical, and water and gas barrier properties. Copolymerization with rigid (cyclic or aromatic) moieties and/or shorter aliphatic segments is usually considered as an effective strategy to obtain polyesters with enhanced Tg.13 In addition, the properties can be readily tuned by varying the monomer type and the sequence of incorporation.6 Succinic acid (SA) is one of the most popular short-chain aliphatic bio-based diacid, and its derivative succinyl dichloride (C4) has already shown potential for the preparation of high Tg polyesters with IS or IM.7,8 Besides, some rigid cyclic monomers, such as 2,5-furandicarboxylic acid (FDCA25) and vanillin, have also been used as bio-based cyclic building blocks.14–16 Recently, another promising rigid structure containing a pyridine ring, named 2,6-pyridinedicarboxylic acid (PDA26) or dipicolinic acid, which could also be obtained from biomass, attracted our attention for the preparation of fully bio-based polyesters.17,18
PDA26 has a chemical structure similar to isophthalic acid (IPA), but its polar pyridine ring instead of a non-polar benzene ring may not only bring chain rigidity but also pyridine functionality.17,18 As reported by Burgess et al., poly(ethylene 2,5-furandicarboxylate) (PEF) showed 19 × CO2, 11 × O2 and 2 × H2O barrier compared to poly(ethylene terephthalate) (PET).19 These excellent barrier properties of PEF compared to those of PET were attributed to its asymmetric polar furan ring.19,20 The structural similarity and polarity of PDA26 to FDCA25 motivated us to investigate its influence on the properties of ISC10 and IMC10. Given the chelation of the pyridine ring with metal catalysts18 and the spontaneous melting/decomposition (248–250 °C) of PDA26, the chlorine derivative, 2,6-pyridinedicarbonyl dichloride (PDD26), which is more active and has a much lower melting temperature (Tm = 56–58 °C), was chosen as a better option for catalyst-free polymerization and evaluating the potential of the same structures as those that would be obtained with carboxylic acids from biomass.
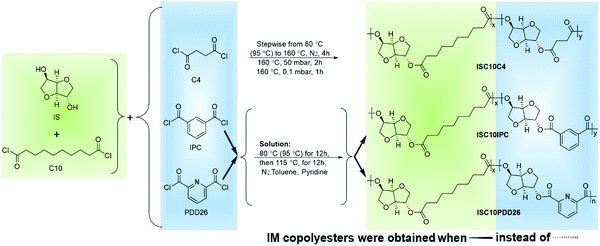
Thus, this article will focus on the preparation of fully bio-based copolyesters, ISC10 and IMC10, by incorporating PDD26 or C4. For comparison, the copolyesters involving petroleum-based IPA will also be prepared. Comprehensive discussions on the thermal, mechanical and barrier properties will be done not only between aliphatic and aromatic structures, but also within aromatic structures (between polar pyridine and non-polar benzene rings).
2. Experimental section
2.1 Materials
Isosorbide (IS, 98%), isomannide (IM, 95%), succinyl chloride (C4, 95%), sebacoyl chloride (C10, ≥95%), 2,6-pyridinedicarbonyl dichloride (PDD26, 97%), ethylene glycol (>99%) and decane (>99%) were purchased from Sigma-Aldrich. Isophthaloyl chloride (IPC, 98%) was provided by Alfa Aesar. Dry toluene (99.85%) and dry pyridine (99.5%) were obtained from ACROS Organics. Dichloromethane (CH2Cl2, reagent grade) was purchased from Fisher Scientific. Methanol (CH3OH, reagent grade) was supplied by VWR. Gases (N2 (>99.99%), CO2 (>99.99%), and O2 (>99.99%)) used for gas and water vapor permeation were purchased from Messer France S.A.S. All reagents were used as received without further purification.2.2 Synthesis of copolyesters
The copolyesters were synthesized according to Scheme 1. The preparation of ISC10C4 and IMC10C4 followed a previously published procedure.12 ISC10PDD26, ISC10IPC, IMC10PDD26 and IMC10IPC were prepared according to the following steps: IS (or IM, 10 mmol), C10 (5 mmol) and PDD26 (or IPC, 5 mmol) were weighed in a 100 mL three-necked round-bottomed flask equipped with a mechanical stirrer and a condenser. With the introduction of a continuous N2 flow, 15 mL of toluene was added and the flask was dipped into an oil bath (80 and 95 °C for IS and IM, respectively). When the solid particles (IS and PDD26) disappeared, 5 mL of pyridine was added dropwise and the temperature was kept at 80 °C (or 95 °C) for 12 h, then increased to 115 °C and maintained for 12 h under N2 atmosphere. After cooling to room temperature, the media were precipitated in methanol and purified 3 times by repeated dissolution/precipitation (CH2Cl2/CH3OH). Finally, they were dried for 2 days at 40 °C under reduced pressure (15 mbar).2.3 Preparation of polymer films
The polymer films were prepared by compression molding using a hot press machine from Scamex (Press 20 ton 300 × 300). Film thickness was controlled to be around 150 μm using the thickness of an aluminum window. Each sample was pressed for 5 min under 50 bars according to the measured thermal properties (30–60 °C above Tg or just above Tm) and then stored in a desiccator at room temperature under vacuum with P2O5 protection before measurements. IMC10C4 was isothermally crystallized at 55 °C for 3 days before storage. The fully transparent films obtained are shown in Fig. 1.2.4 Size exclusion chromatography (SEC)
The copolyester molar masses were measured by size exclusion chromatography (PL-GPC50 from Varian) at 25 °C using two PLgel MIXED-C 5 μm (300 × 7.5 mm) columns and a RI detector. The samples were prepared by dissolving polymers (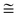 10 mg) in dichloromethane (1.7 mL, HLPC grade), and PMMA standards were used for calibration.
10 mg) in dichloromethane (1.7 mL, HLPC grade), and PMMA standards were used for calibration.
2.5 Fourier transform infrared (FTIR)
FTIR measurements were performed using a Spectrum Two instrument from PerkinElmer, in the ATR mode (diamond cell) at 25 °C. Each sample was scanned 10 times from 4000 to 650 cm−1.2.6 1H nuclear magnetic resonance (1H NMR)
Chemical structures were determined by 1H NMR (Bruker, 300 MHz) with a TopSpin acquisition system at room temperature (20 °C) in CDCl3. The chemical shifts (δ) were expressed in parts per million (ppm) and referenced to the non-deuterated residual solvent peak (δ = 7.26 ppm).2.7 Thermogravimetric analysis (TGA)
The thermal stability of the polymers was investigated by TGA (Q500 from TA Instruments) under nitrogen atmosphere. Two aluminum layers were used to protect the platinum pan from corrosion by the potentially released chlorine. Around 10 mg of the sample was loaded in the pan and heated from 30 to 600 °C at a heating rate of 10 °C min−1.2.8 Differential scanning calorimetry (DSC)
The thermal properties of the polymers were investigated by DSC (Q2000 from TA Instruments). Around 10 mg of the sample was enclosed in an aluminum pan, and heated under a N2 atmosphere from −30 to 200 °C at 10 °C min−1, followed by cooling and a second heating.2.9 Tensile tests
The mechanical properties of the polymer films were established by tensile tests at room temperature (23 °C) using a ZwickRoell Z010 apparatus with testXpert II software. A 500 N load cell and 10 mm min−1 cross-head speed were operated. The dumbbell-shaped specimens were 1BB type according to ISO 527-2. More than 8 tests were performed on each sample to determine the final average value.2.10 Surface energy
The water contact angle θw (°) and surface energy γt (mN m−1) with polar (γp) and dispersive (γd) parts were determined on films at room temperature (23 °C) with water (MilliQ Millipore® Water system), ethylene glycol, and decane. The size of the droplet was controlled to 3 μL and the photo time within 500 ms. Each result was averaged from at least five measurements. The surface energy was calculated using Windrop++ Carrousel software according to the Owens & Wendt method.2.11 Liquid water sorption
The liquid water sorption measurements were performed at 25 °C. The measurement was performed in triplicate for each sample (A = 1 cm2) using a balance (precision 0.1 mg): the initially dry film (md) was immersed into pure water (MilliQ Millipore Water system) and the film mass (mt) was periodically weighed by carefully removing the surface water. Then, the variation of the mass gain (Mt) vs. time (t) was calculated according to eqn (1).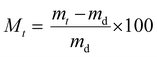 | (1) |
2.12 Water vapor sorption
The water vapor sorption behavior of the film was evaluated using the DVS1 Advantage apparatus (Surface Measurement Systems Ltd) at 25 °C as described before.21 Different water vapor activity steps (aw = 0–0.95) were applied to the initially dry film (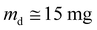 ). Each step was completed when an equilibrium (meq) was attained. The water vapor sorption isotherms were plotted according to the mass gain at equilibrium Meq (eqn (2)) vs. aw.
). Each step was completed when an equilibrium (meq) was attained. The water vapor sorption isotherms were plotted according to the mass gain at equilibrium Meq (eqn (2)) vs. aw.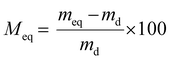 | (2) |
2.13 Water vapor permeation
The water vapor permeation measurements were performed at 25 °C using a previously described permeation cell.22 The water vapor flux J through the film (A = 3.6 cm2) as a function of time t was measured at different aw. The permeability coefficient P was calculated at the steady-state (Jst) according to eqn (3), where Δaw is the difference in water activity across the film (thickness L).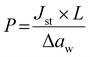 | (3) |
2.14 Gas permeation
The gas (CO2, O2 and N2) permeation measurements were performed at 25 °C according to the previously described time-lag method (upstream pressure p1 = 4 bar and downstream compartment initially under vacuum).23 The permeability coefficient P (expressed in barrer, 1 barrer = 10−10 cm3 (STP) cm cm−2 s−1 cmHg−1) was calculated according to eqn (4):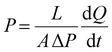 | (4) |
The gas diffusion coefficient D was calculated according to eqn (5) (tL is the time-lag value obtained from the extrapolation of the steady-state asymptote to the time axis):
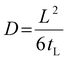 | (5) |
3. Results and discussion
3.1 Molar masses
ISC10C4 (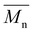 = 18
= 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) showed a higher molar mass than IMC10C4 (
000 g mol−1) showed a higher molar mass than IMC10C4 (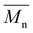 = 13
= 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1). This was probably due to the decreased reactivity already observed in our previous work between IM and C4 which resulted in lower molar masses for IMC4. The copolymerization involving aromatic moieties was much more difficult to achieve in bulk since their rigidity reduced chain-end mobility.24 Thus, the synthesis in toluene with pyridine (method established by Storbeck et al.25) was tried. The molar masses are presented in Table 1. The higher molar masses obtained with IS compared to IM were probably due to the higher reactivity of IS.8 Unfortunately, a general decrease in molar masses was observed when incorporating aromatic moieties, such as IPC and PDD26. This confirmed the difficulty already encountered in producing high molar mass polyesters with 1,4:3,6-dianhydrohexitols and aromatic compounds.8 However, satisfactory molar masses above 10
000 g mol−1). This was probably due to the decreased reactivity already observed in our previous work between IM and C4 which resulted in lower molar masses for IMC4. The copolymerization involving aromatic moieties was much more difficult to achieve in bulk since their rigidity reduced chain-end mobility.24 Thus, the synthesis in toluene with pyridine (method established by Storbeck et al.25) was tried. The molar masses are presented in Table 1. The higher molar masses obtained with IS compared to IM were probably due to the higher reactivity of IS.8 Unfortunately, a general decrease in molar masses was observed when incorporating aromatic moieties, such as IPC and PDD26. This confirmed the difficulty already encountered in producing high molar mass polyesters with 1,4:3,6-dianhydrohexitols and aromatic compounds.8 However, satisfactory molar masses above 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 were still obtained, with high yields around 90%.
000 g mol−1 were still obtained, with high yields around 90%.
| Copolyester | ISC10C4 | ISC10IPC | ISC10PDD26 |
|---|---|---|---|
| a SEC conducted in CH2Cl2 with PMMA standards. b Dispersity. c In precipitated polymer. d Calculated from 1H NMR; X is the diol (IS or IM); Y is the acyl dichloride. e DSC second heating. f DSC first heating. g Multiple peaks. | |||
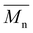 (g mol−1)
(g mol−1) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
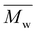 (g mol−1)
(g mol−1) |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
| Đ | 1.9 | 2.9 | 2.3 |
| Yieldc (%) | 91 | 90 | 85 |
X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C10 C10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y molar ratio in polymerd Y molar ratio in polymerd |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| T g (°C) | 30 | 61 | 40 |
| T m (°C) | — | — | — |
| ΔHmf (J g−1) | — | — | — |
3.2 Structures
The FTIR spectra (Fig. S1, ESI†) showed the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O of ester groups stretching at 1730 and 1340–1130 cm−1, respectively, which firstly confirmed the successful esterification. Then, the successful copolymerization was confirmed by the signals of the functional groups of the different components: CH2 stretching at 3050–2820 cm−1, aromatic C
O and C–O of ester groups stretching at 1730 and 1340–1130 cm−1, respectively, which firstly confirmed the successful esterification. Then, the successful copolymerization was confirmed by the signals of the functional groups of the different components: CH2 stretching at 3050–2820 cm−1, aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching at 1584 cm−1, ester C–O stretching for aromatics at 1309, 1295, 1316, 1298 cm−1, and for aliphatics at 1235, 1230, 1157, 1234, 1228 and 1154 cm−1.
C stretching at 1584 cm−1, ester C–O stretching for aromatics at 1309, 1295, 1316, 1298 cm−1, and for aliphatics at 1235, 1230, 1157, 1234, 1228 and 1154 cm−1.
All proton signals found at the expected chemical shifts in 1H NMR spectra (Fig. S2, ESI†) further confirmed the chemical structures. The integrations globally corresponded to the different comonomers. The more split spectrum of ISC10C4 was due to the exo–endo stereoscopic effect of IS, which induced an irregular structure.26 Besides, the more complex and split spectra of aromatic copolyesters compared to aliphatic ones were probably due to the structural difference between aliphatics and aromatics which induced distinct environments for hydrogens in IS and IM. The molar ratio of each component in the polymer was then calculated by considering the proton integrations of each comonomer (the equation is listed in Fig. S2, ESI†), shown in Table 1.
In general, the molar ratio of the polymer was almost the feeding molar ratio. The slight differences could be explained by the small-scale used for the synthesis: a small difference in monomer weighing cannot be ruled out, and controlling the accuracy of the feeding ratio to ensure the targeted stoichiometric ratio is a challenging factor. However, some bigger differences were observed when using PDD26, especially with IS, which may be linked to its lower reactivity.
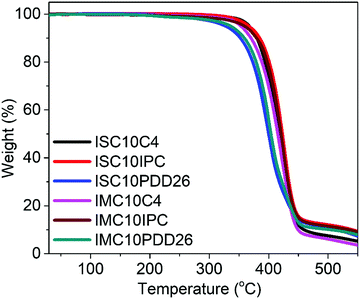
3.3 Thermal degradation
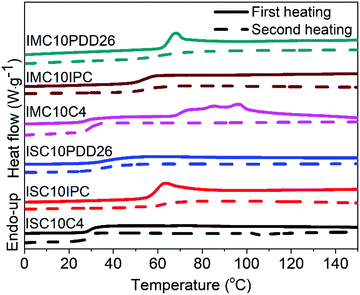
Fig. 2 shows the thermal stability of copolyesters analyzed by TGA and the corresponding data are provided in Table S1 (ESI†). All copolyesters appeared to be thermally stable up to 328 °C with less than 5% weight loss. The copolyesters containing C4 and IPC showed higher thermal degradation temperatures compared to those containing PDD26, which could be linked with the higher C–C bond energy (83 kcal mol−1) than the C–N bond energy (73 kcal mol−1). No significant difference could be noticed between the use of IS and IM.3.4 Differential scanning calorimetry (DSC)
The DSC thermograms of copolyesters are presented in Fig. 3 and the corresponding values are provided in Table 1.As stated previously, the stereoscopic difference between IS and IM had little influence on Tg; nevertheless, some differences were observed between these copolyesters.12,26 Considering ISC10IPC and IMC10IPC had the same molar composition in comonomers, the higher Tg of ISC10IPC could be linked to its higher molar mass. On the contrary, ISC10PDD26 and IMC10PDD26 had similar molar masses, but IMC10PDD26 incorporated a much higher amount of PDD26, thus leading to a higher Tg. Finally, the same Tg measured for ISC10C4 and IMC10C4 was a cooperation result of molar mass and monomer ratio. On the other hand, a higher Tg was systematically obtained by incorporating aromatic moieties. Excluding ISC10PDD26 which had a low content of PDD26, the pyridine structure (Tg = 64 °C for IMC10PDD26) was more efficient in improving Tg than the benzene structure (Tg = 55 °C for IMC10IPC). All copolyesters showed increased Tg compared to ISC10 (Tg = 2 °C) and IMC10 (Tg = 0 °C).12
Unfortunately, the incorporation of comonomers prevented the polymers from crystallization, except IMC10C4 which exhibited a melting with ΔHm = 12 J g−1. Its multiple melting peaks (Tm = 74/84/96 °C) with a wide melting range were probably due to a crystallization polymorphism coupling with a melting/crystallization/re-melting process, which is a common trait of linear polyesters comprising both semi-rigid and flexible chains.14,26 However, the absence of melting during the DSC second heating indicated the difficulty encountered in reorganizing the molecular chain orderly from the melt.27
To provide preliminary information for IMC10C4 film preparation, the crystallization behavior of this polymer was further investigated by isothermal crystallization, after the first melting at 150 °C, at Ti = 55 °C for 0 to 5 days (ti) (Fig. S3, ESI†). After a broadening of the endothermic peak and an increase in the melting enthalpy when the crystallization time increased, the crystallization seemed to reach its final state after three days at 55 °C. Hence, these annealing conditions were used before IMC10C4 film preparation.
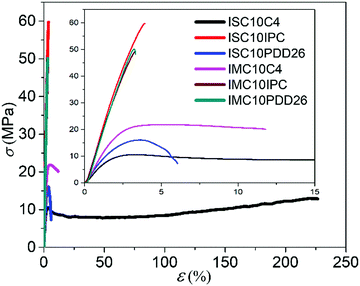
3.5 Mechanical properties
The representative tensile curves of polymer films are shown in Fig. 4 and the corresponding tensile parameters are recorded in Table 2.| Polyester film | E (MPa) | σ b (MPa) | ε b (%) |
|---|---|---|---|
| E, Young's modulus; σb, stress at break; εb, strain at break. | |||
| ISC10C4 | 770 ± 90 | 11.8 ± 1.1 | 220 ± 20 |
| ISC10IPC | 2100 ± 50 | 59.0 ± 1.7 | 3.6 ± 0.4 |
| ISC10PDD26 | 850 ± 80 | 8.0 ± 2.6 | 6.0 ± 1.5 |
| IMC10C4 | 1180 ± 80 | 21.0 ± 2.7 | 12.0 ± 1.0 |
| IMC10IPC | 1820 ± 30 | 50.0 ± 1.7 | 3.0 ± 0.3 |
| IMC10PDD26 | 1900 ± 40 | 50.0 ± 1.2 | 3.0 ± 0.4 |
Fig. 4 discloses the brittle fractures of semi-aromatic copolyesters and the plastic fractures of aliphatic ones. The incorporation of rigid comonomers, such as C4, IPC and PDD26, naturally increased the stiffness (E increased at least 6 times) and the tensile strength (σb increased at least 3 times) of ISC10 (E = 127 ± 20 MPa, σb = 4.2 ± 0.8 MPa, εb = 19 ± 5%) and IMC10 (E = 125 ± 10 MPa, σb = 1.0 ± 0.2 MPa, εb = 16 ± 3%), but decreased the elongation at break (except ISC10C4 which will be discussed later).12 This phenomenon was even more observed for IPC and PDD26 (but to a lesser extent for ISC10PDD26 due to its lower PDD26 content) compared to C4: the aromatic moieties brought more chain stiffness, strength, and brittleness. Furthermore, despite a lower molar mass, IMC10C4 showed higher E (1180 MPa) and σb (21.0 MPa) and restricted elongation at break (εb = 12%) compared to ISC10C4, certainly due to its crystallinity.
It should be stressed that ISC10C4 presented a very particular behavior. Indeed, the incorporation of C4 in ISC10C4 not only brought much higher E (770 MPa vs. 127 MPa) and σb (11.8 MPa vs. 4.2 MPa) compared to ISC10, but also largely increased εb (220% vs. 19%).12 The increase in E and σb was probably due to the increase of Tg, above room temperature, while the increase in εb may be related to its high molar mass coupled with a Tg close to the ambient temperature, thus even slight localized heating due to the tensile stress could favor chain disentanglement.28 Another interesting phenomenon was observed for ISC10C4: the stretched sample, even after being broken at its maximum elongation (220%), recovered almost its original dimensions and retained only a 10% strain (5.5 cm vs. 5 cm) after being brought to the external temperature of the human body (35–37 °C) (Fig. S4 (ESI†) and attached video).
3.6 Contact angle
The copolyesters showed no obvious difference in surface properties (Table 3). Only a slightly lower θw (or higher γt) was observed for ISC10PDD26 and IMC10PDD26 compared to other copolyesters, probably due to the presence of the polar pyridine sites. All copolyesters showed similar surface energies and water contact angles compared to ISC10 (γt = 31.0 mN m−1, θw = 85 ± 1.0°) and IMC10 (γt = 29.6 mN m−1, θw = 83 ± 0.5°).12| Polyester films | γ t (mN m−1) | γ d (mN m−1) | γ p (mN m−1) | θ w (°) |
|---|---|---|---|---|
| γ t, total surface energy with dispersive (γd) and polar (γp) parts; θw, water contact angle. | ||||
| ISC10C4 | 28.8 | 23.4 | 5.4 | 86 ± 1.7 |
| ISC10IPC | 28.6 | 23.2 | 5.4 | 87 ± 1.2 |
| ISC10PDD26 | 30.5 | 24.2 | 6.3 | 84 ± 1.5 |
| IMC10C4 | 28.8 | 23.3 | 5.5 | 86 ± 0.8 |
| IMC10IPC | 28.3 | 23.1 | 5.2 | 87 ± 1.0 |
| IMC10PDD26 | 31.2 | 24.3 | 6.9 | 83 ± 1.3 |
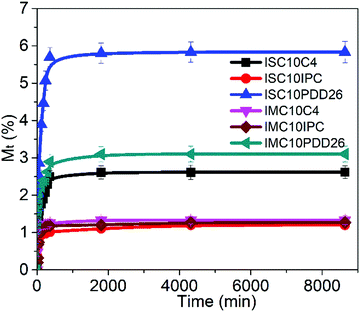
3.7 Liquid water sorption
The liquid water sorption results are presented Fig. 5.All copolyesters showed increased liquid water sorption compared to ISC10 (Meq = 0.55 ± 0.05%) and IMC10 (Meq = 1.03 ± 0.09%), which is probably because their large amorphous domain allows a larger water uptake.29 ISC10PDD26 (Meq = 5.8 ± 0.5%) and IMC10PDD26 (Meq = 3.1 ± 0.3%) showed a higher liquid water sorption, which could be due to the presence of hydrophilic pyridine sites in the polymer structure. However, the higher water uptake of ISC10PDD26 compared to IMC10PDD26 was unexpected since ISC10PDD26 contained less pyridine moieties (30 mol% for ISC10PDD26; 45 mol% for IMC10PDD26). It should be mentioned that water sorption in polyesters is complex since water molecules show great interactions with the polymer matrix.30 Thereby, the sorption of water molecules in polymers can lead to dimensional changes through the swelling of the polymer matrix and the decrease of the glass transition temperature (Tg), which will, in turn, result in larger water sorption.30–32 Thus, the effect of liquid water sorption at Tg for ISC10PDD26 and IMC10PDD26 was checked by DSC measurements (Fig. 6). Tg after water sorption (Tg,H2O) for ISC10PDD26 (Tg,H2O = 20 °C) was below the experimental temperature (T = 25 °C), while for IMC10PDD26 (Tg,H2O = 43 °C) it remained above 25 °C. Even if Tg,H2O for both ISC10PDD26 and IMC10PDD26 was decreased by 20 °C, the high initial Tg of IMC10PDD26 (64 °C) allowed its Tg,H2O to stay above 25 °C, in contrast to ISC10PDD26. The lower Tg,H2O of ISC10PDD26 than 25 °C indicated that the sample was in the rubber state with a larger free volume, which allowed higher water uptake.33,34
 | ||
| Fig. 6 The DSC thermograms of copolyester films before (solid) and after (dash) liquid water sorption. | ||
More generally, we noticed that all the samples showed a decreased Tg after water sorption, which is known as water-induced plasticization.35,36 For ISC10IPC, IMC10IPC and IMC10PDD26, their high initial Tg allowed preserving their glassy state because their final Tg,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O stayed above 25 °C, in contrast to ISC10C4, ISC10PDD26 and IMC10C4. IMC10C4, IMC10IPC and ISC10IPC showed better resistance to water plasticization with a lower decrease of Tg. Consequently, the liquid water sorption was less pronounced for them. The nonpolar aromatic benzene ring in IPC and the semi-crystalline nature of IMC10C4 can explain this behavior. Combining the changes in Tg with the water sorption behavior, we can generally conclude that the ability to resist water plasticization is an important factor determining the water sorption of these glassy state copolyesters.
H2O stayed above 25 °C, in contrast to ISC10C4, ISC10PDD26 and IMC10C4. IMC10C4, IMC10IPC and ISC10IPC showed better resistance to water plasticization with a lower decrease of Tg. Consequently, the liquid water sorption was less pronounced for them. The nonpolar aromatic benzene ring in IPC and the semi-crystalline nature of IMC10C4 can explain this behavior. Combining the changes in Tg with the water sorption behavior, we can generally conclude that the ability to resist water plasticization is an important factor determining the water sorption of these glassy state copolyesters.
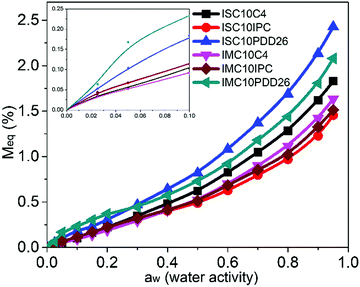
3.8 Dynamic vapor sorption
The water vapor sorption isotherms of copolyester films are presented in Fig. 7.All copolyesters exhibited an increase in water vapor sorption compared to ISC10 and IMC10 (e.g. Meq = 0.58% and Meq = 1.08% at water activity aw = 0.95, respectively).12 The values at aw = 0.95 followed the same order as observed for liquid water sorption with the highest sorption value for ISC10PDD26 and the lowest for ISC10IPC. All isotherms displayed sigmoid profiles and obeyed the three steps of Park's model with a first Langmuir, then a Henry sorption and finally the aggregation of water molecules.37 The corresponding Park's model parameters are provided in Table 4. At aw < 0.1, typical Langmuir-type sorption is related to the adsorption of water molecules onto the specific hydrophilic sites, such as polar groups or micro-voids on the surface of films.38 At this aw, ISC10PDD26 and IMC10PDD26 exhibited higher sorption with larger bL values compared to other copolyesters due to their hydrophilic pyridine sites. The higher Langmuir constants of ISC10IPC compared to ISC10C4 are related to the Langmuir sites of the former (AL = 1.20 × 10−3 (g g−1) vs. AL = 0.55 × 10−3 (g g−1)). A possible explanation could be found in their structure: even though the whole benzene ring is a non-polar structure, the IPC ring has a dipolar moment greater than the C4 unit. The lowest Langmuir-type sorption of IMC10C4 was probably due to the presence of crystallites that reduced the value of AL.30,39,40 When the preferential sites were occupied, the isotherms became linear owing to random dissolution/diffusion (Henry's type sorption) of water molecules in the polymer matrix.41 The solubility coefficient S (analogous to kH) can be then calculated from the slope of the linear part (aw = 0.2–0.7) of the isotherms. The large S values for ISC10PDD26 (S = 10.6 × 10−3 gH2O gpol−1) and IMC10PDD26 (S = 8.1 × 10−3 gH2O gpol−1) could be explained by their hydrophilic pyridine sites. ISC10IPC (S = 6.3 × 10−3 gH2O gpol−1) and IMC10IPC (S = 5.8 × 10−3 gH2O gpol−1) showed lower solubility compared to ISC10C4 (S = 8.3 × 10−3 gH2O gpol−1) and IMC10C4 (S = 7.1 × 10−3 gH2O gpol−1), which was contrary to their Langmuir-type sorption. This was probably due to the larger water-induced polymer swelling of the latter,30,32 which has been previously discussed for the liquid water sorption. At aw > 0.7, an exponential increase of sorbed water concentration was observed due to the formation of water aggregates. Ka values were larger for ISC10PDD26 and IMC10PDD26 indicating more water aggregates but with similar sizes regardless of the sample. This reveals that ISC10PDD26 and IMC10PDD26 were more hydrophilic than other copolyesters for the whole range of water vapor activity. It was not surprising that the polar pyridine sites showed strong affinities to water molecules.
| Polyester film | A L × 103 (g g−1) | b L | k H × 103 (g g−1) | K a × 103 (g g−1) | n a |
|---|---|---|---|---|---|
| A L, Langmuir's capacity constant; bL, Langmuir affinity constant; kH, Henry's type solubility coefficient; Ka, equilibrium constant for clustering reaction; na, average number of water molecules per cluster. | |||||
| ISC10C4 | 0.55 ± 0.03 | 0.42 ± 0.01 | 10.6 ± 1.1 | 0.92 ± 0.05 | 4.0 ± 0.1 |
| ISC10IPC | 1.20 ± 0.07 | 0.47 ± 0.04 | 3.9 ± 0.2 | 0.85 ± 0.03 | 4.0 ± 0.2 |
| ISC10PDD26 | 0.55 ± 0.05 | 3.00 ± 0.20 | 15.5 ± 1.3 | 1.32 ± 0.13 | 3.0 ± 0.1 |
| IMC10C4 | 0.35 ± 0.02 | 0.40 ± 0.01 | 9.0 ± 0.5 | 0.87 ± 0.04 | 4.0 ± 0.1 |
| IMC10IPC | 1.30 ± 0.09 | 0.50 ± 0.03 | 3.7 ± 0.2 | 0.80 ± 0.03 | 5.0 ± 0.2 |
| IMC10PDD26 | 0.12 ± 0.01 | 38.4 ± 2.0 | 10.4 ± 0.9 | 1.00 ± 0.07 | 4.0 ± 0.1 |
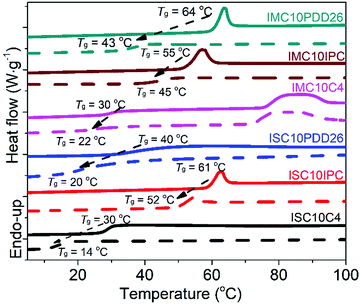
3.9 Water vapor permeation
The water barrier properties were evaluated using water vapor permeation measurements as reported in Fig. 8.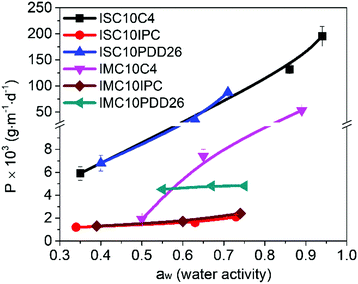 | ||
| Fig. 8 Water vapor permeation coefficient (PH2O) of copolyester films at different water activities (aw). | ||
The high PH2O values over the entire water activity range for both ISC10C4 and ISC10PDD26 could be attributed to the polymer swelling. It is surprising to obtain a high PH2O value for IMC10C4 because this polyester is semi-crystalline. This could be explained by its low Tg that decreased in the presence of water and consequently induced large swelling at high aw values. The PH2O values of ISC10IPC, IMC10IPC, and IMC10PDD26 were generally lowest and constant, which validated the ability of these polymers to resist swelling. The higher PH2O values of IMC10PDD26 compared to those of ISC10IPC and IMC10IPC could be due to the higher water solubility brought by the polar pyridine sites. Then the lowest PH2O values for ISC10IPC and IMC10IPC seemed reasonable, due to their resistance to swelling and low polarity. All copolyesters showed reduced PH2O values compared to ISC10 and IMC10,12 which was probably due to the increased Tg. Thus, we can generally conclude that the Tg and polarity of these copolyesters were the two dominant factors that influence their water vapor permeation behaviors.
3.10 Gas permeation
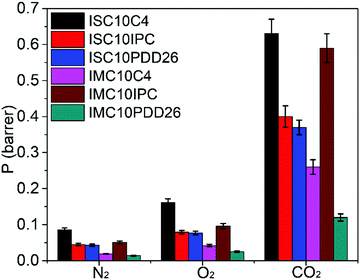
As described in the experimental part, the gas permeation was conducted under differential pressure. However, IMC10IPC and IMC10PDD26 were not resistant enough to the experimental pressure. Consequently, a PDMS membrane was used as a support to assist their gas permeation measurements. In this case, diffusion coefficient (D) values cannot be accurately calculated using the time-lag method, thus only permeation coefficients (P) were discussed for these two samples. Their P values were calculated according to the law of resistors in series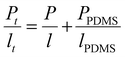 , where Pt and lt are the total permeability and thickness, PPDMS and lPDMS are the permeability and thickness of the PDMS membrane, and P and l are the permeability and thickness of the tested film. The gas permeation coefficients (P) for N2, O2 and CO2 are presented in Fig. 9 (main data are provided in Table S2, ESI†).
, where Pt and lt are the total permeability and thickness, PPDMS and lPDMS are the permeability and thickness of the PDMS membrane, and P and l are the permeability and thickness of the tested film. The gas permeation coefficients (P) for N2, O2 and CO2 are presented in Fig. 9 (main data are provided in Table S2, ESI†).
Even with the loss of crystallinity, all copolyesters showed enhanced gas barrier properties (decreased P values) compared to ISC10 (PN2 = 0.09 barrer, PO2 = 0.26 barrer, PCO2 = 1.32 barrer) and IMC10 (PN2 = 0.18 barrer, PO2 = 0.46 barrer, PCO2 = 2.04 barrer).12 This was due to the increase in the Tg values for all copolyesters: their glassy state at 25 °C (experimental temperature) allowed limited chain movements for gas penetration. All copolyesters showed P values following the well-known order reported by Van Krevelen:41PN2 < PO2 < PCO2. This ranking is a result of the double dependence of permeability on diffusivity, mainly on the kinetic diameter of the permeants (dN2 (0.364 nm) > dO2 (0.346 nm) > dCO2 (0.33 nm)), and solubility, mainly on the critical temperature (Tc) of the permeants (Tc(CO2) (31 °C) > Tc(O2) (−118 °C) > Tc(N2) (−147 °C)).34
It is known that the large sorption of CO2 molecules will plasticize a glassy polymer matrix.42 However, CO2 (Tc(CO2) = 31 °C) is much less condensable than water (Tc(H2O) = 374 °C); thus, the plasticization by CO2 usually needs a high feeding pressure (depending on the polymer, but usually >10 bar).43 Considering the low CO2 feeding pressure (3 bar) in our case, the CO2 plasticization effect will be ignored in our discussion.
It should always be kept in mind that P is a combined result of D (obtained by the time-lag method) and S (deduced from P and D coefficients); any factor that affects D and S can consequently influence P. To support our discussion, the S and D values of ISC10C4, ISC10IPC, ISC10PDD26, and IMC10C4 are provided in Table 5.
| D × 1010 (cm2 s−1) | S × 103 (cm3(STP) cm−3 cmHg−1) | |||||
|---|---|---|---|---|---|---|
| N2 | O2 | CO2 | N2 | O2 | CO2 | |
| ISC10C4 | 9.4 ± 0.6 | 16 ± 1 | 76 ± 5 | 9.0 ± 0.6 | 10.1 ± 0.6 | 8.3 ± 0.7 |
| ISC10IPC | 4.9 ± 0.3 | 8.2 ± 0.6 | 48 ± 3 | 9.2 ± 0.7 | 9.6 ± 0.6 | 8.3 ± 0.6 |
| ISC10PDD26 | 4.5 ± 0.3 | 6.8 ± 0.5 | 28 ± 2 | 9.5 ± 0.7 | 11.3 ± 0.8 | 13.3 ± 0.9 |
| IMC10C4 | 2.5 ± 0.2 | 5.4 ± 0.4 | 35 ± 2 | 7.7 ± 0.5 | 7.8 ± 0.6 | 7.4 ± 0.5 |
Thus, the influence of several factors on the gas permeation properties was discussed. Firstly, the main chain type (aliphatic vs. aromatic) was investigated. The incorporation of aromatic moieties (ISC10IPC and ISC10PDD26) is generally more efficient in increasing gas barrier properties than the incorporation of the aliphatic one (ISC10C4). This was probably due to the higher chain stiffness which caused lower mobility of chain segments, thus creating diffusion restriction.44 Concerning the aromatic moieties (m-phenylene ring vs. 2,6-pyridine ring), the lower P values of ISC10PDD26 and IMC10PDD26 compared to ISC10IPC and IMC10IPC indicated that the 2,6-pyridine ring structure brought more barrier properties. Considering that the copolyesters were amorphous, the lower gas permeability of ISC10PDD26 and IMC10PDD26 may be due to the polar pyridine ring which induced chain–chain (inter/intrachain) attractions, thus allowing a close chain–chain packing and restricting chain segmental motions.45 Similarly, the higher PDD26 incorporation ratio could explain the higher gas barrier properties of IMC10PDD26 compared to ISC10PDD26. Besides, the semi-crystalline IMC10C4 was more barrier (PN2 = 0.019 barrer, PO2 = 0.04 barrer, PCO2 = 0.26 barrer) than amorphous ISC10C4 (PN2 = 0.084 barrer, PO2 = 0.12 barrer, PCO2 = 0.59 barrer), which was expected since crystallites are generally impermeable to gases.46,47 This explanation was verified by both lower S and D values for IMC10C4. Finally, even if ISC10IPC showed slightly enhanced gas barrier properties than IMC10IPC, we cannot generally conclude that the exo/endo stereoscopic structure of IS was more barrier to gases than the endo–endo stereoscopic structure of IM, because the higher chain entanglement caused by the larger molar masses of ISC10IPC also decreases gas permeability.
To sum up, the enhanced Tg effectively improved the gas barrier properties of ISC10 and IMC10 polyesters. Besides, retaining crystallinity is a supplemental positive factor for gas barrier properties. Regardless of the semi-crystalline nature of IMC10C4, the chemical structures of the introduced rigid moieties showed the abilities (Ap) to decrease P values in the following order: Ap(PDD26) > Ap(IPC) > Ap(C4). All copolyesters showed better gas barrier than PLA containing 98% L-lactide (PN2 = 4.99 barrer, PO2 = 0.11–0.56 barrer and PCO2 = 1.88 barrer). Although the barrier properties of IMC10PDD26 were much lower than those of PEF (PO2 = 0.004 barrer and PCO2 = 0.012 barrer),29 they were comparable to those of semi-crystalline PET (PO2 = 0.018–0.030 barrer and PCO2 = 0.12–0.16 barrer).44 These efficient gas barrier properties of IMC10PDD26 may be attributed to its asymmetrical polar 2,6-pyridine ring.
4. Conclusions
This work was focused on the preparation of bio-based ISC10 and IMC10 copolyesters with enhanced properties. The general idea was to incorporate a third rigid monomer via copolycondensation. The third rigid monomer was either aliphatic (C4) or aromatic (IPC and PDD26). High molar masses were achieved for aliphatic copolyesters, while generally lower molar masses were obtained for aromatic ones. All copolyesters showed good thermal stability with Td5% higher than 328 °C and Tg ranging from 30 to 64 °C. The third rigid monomer, and more generally the aromatic skeleton, especially the pyridine skeleton, significantly improved the Tg of ISC10 and IMC10. All copolyesters showed improved barrier properties (both to water and gases) compared to ISC10 and IMC10. IMC10PDD26 showed the best gas barrier properties, which was linked to its pyridine structure. These high gas barrier properties were comparable to those of the widely used semi-crystalline PET.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the China Scholarship Council [grant number 201701810120] for financial support. They also thank Dr K. Fatyeyeva and Dr C. Chappey for permeation measurements.References
- COP21-Paris Climate Conference 2015, https://www.gouvernement.fr/en/paris-climate-conference, accessed 2015-12-12.
- The Paris Agreement, https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement, accessed 2016-11-04.
- Low-Carbon World, https://www.novabuild.fr/rendez-vous/low-carbon-world-23-24-juin-2021, accessed 2021-05-09.
- Transition to low-carbon world needs to be faster, says DNV GL report – News – The Chemical Engineer, https://www.thechemicalengineer.com/news/transition-to-low-carbon-world-needs-to-be-faster-says-dnv-gl-report/, accessed 2020-09-17.
- The companies leading the charge against food packaging waste, https://www.forbes.com/sites/katejacksonk/2021/03/27/the-companies-leading-the-charge-against-food-packaging-waste/?sh=5d3925565be1, accessed 2021-03-27.
- S. Legrand, N. Jacquel, H. Amedro, R. Saint-Loup, M. Colella, J. P. Pascault, F. Fenouillot and A. Rousseau, ACS Sustainable Chem. Eng., 2020, 8, 15199–15208 CrossRef CAS.
- D. J. Saxon, A. M. Luke, H. Sajjad, W. B. Tolman and T. M. Reineke, Prog. Polym. Sci., 2020, 101, 101196 CrossRef CAS.
- F. Fenouillot, A. Rousseau, G. Colomines, R. Saint-Loup and J. P. Pascault, Prog. Polym. Sci., 2010, 35, 578–622 CrossRef CAS.
- POLYSORB®: a pure isosorbide from sorbitol, a starch derivative, https://www.roquette.com/industries/selected-products/industry-performance-materials-isosorbide, accessed 2021-05-10.
- Polyester resin: plant-based POLYSORB® isosorbide for polyester resin, https://www.roquette.com/industries/performance-materials/polyesters, accessed 2021-05-13.
- M. Okada, Y. Okada and K. Aoi, J. Polym. Sci., Part A: Polym. Chem., 1995, 33, 2813–2820 CrossRef CAS.
- X. L. Liu, N. Desilles and L. Lebrun, Eur. Polym. J., 2020, 134, 109846 CrossRef CAS.
- C. Lavilla and S. Muñoz-Guerra, Green Chem., 2013, 15, 144–151 RSC.
- Q. F. Sousa, C. Vilela, A. C. Fonseca, M. Matos, C. S. R. Freire, G. J. M. Gruter, J. F. J. Coelho and A. J. D. Silvestre, Polym. Chem., 2015, 6, 5961–5983 RSC.
- H. Y. Zhang, M. Jiang, Y. P. Wu, L. Li, Z. P. Wang, R. Wang and G. Y. Zhou, ACS Sustainable Chem. Eng., 2021, 9, 6799–6809 CrossRef CAS.
- S. V. Mankar, M. N. G. Gonzalez, N. Warlin, N. G. Valsange, N. Rehnberg, S. Lundmark, P. Jannasch and B. Z. Zhang, ACS Sustainable Chem. Eng., 2019, 7, 19090–19103 CrossRef CAS.
- M. K. McClintock, G. W. Fahnhorst, T. R. Hoye and K. Zhang, Metab. Eng., 2018, 48, 208–217 CrossRef CAS PubMed.
- A. Pellis, J. W. Comerford, S. Weinberger, G. M. Guebitz, J. H. Clark and T. J. Farmer, Nat. Commun., 2019, 10, 1–9 CrossRef CAS.
- S. K. Burgess, R. M. Kriegel and W. J. Koros, Macromolecules, 2015, 48, 2184–2193 CrossRef CAS.
- S. K. Burgess, J. E. Leisen, B. Kraftschik, C. R. Mubarak, R. M. Kriegel and W. J. Koros, Macromolecules, 2014, 47, 1383–1391 CrossRef CAS.
- N. Follain, S. Roualdes, S. Marais, J. Frugier, M. Reinholdt and J. Durand, J. Phys. Chem. C, 2012, 116, 8510–8522 CrossRef CAS.
- S. Marais, M. Metayer and M. Labbe, J. Appl. Polym. Sci., 1999, 74, 3380–3395 CrossRef CAS.
- K. Fatyeyeva, A. Dahi, C. Chappey, D. Langevin, J. M. Valleton, F. Poncin-Epaillard and S. Marais, RSC Adv., 2014, 4, 31036–31046 RSC.
- S. N. Vouyiouka, E. K. Karakatsani and C. D. Papaspyrides, Prog. Polym. Sci., 2005, 30, 10–37 CrossRef CAS.
- R. Storbeck, M. Rehahn and M. Ballauff, Die Makromol. Chemie Macromol. Chem. Phys., 1993, 194, 53–64 CrossRef CAS.
- H. Marubayashi, T. Ushio and S. Nojima, Polym. Degrad. Stab., 2017, 146, 174–183 CrossRef CAS.
- Q. Lu, Z. Yang, X. Li and S. Jin, J. Appl. Polym. Sci., 2010, 116, 2060–2070 Search PubMed.
- D. C. Prevorsek and B. T. De Bona, J. Polym. Sci., Part B: Polym. Phys., 1981, 19, 605–622 Search PubMed.
- S. K. Burgess, G. B. Wenz, R. M. Kriegel and W. J. Koros, Polymer, 2016, 98, 305–310 CrossRef CAS.
- S. K. Burgess, D. S. Mikkilineni, D. B. Yu, D. J. Kim, C. R. Mubarak, R. M. Kriegel and W. J. Koros, Polymer, 2014, 55, 6861–6869 CrossRef CAS.
- D. Langevin, J. Grenet and J. M. Saiter, Eur. Polym. J., 1994, 30, 339–345 CrossRef CAS.
- K. Galić and N. Ciković, Polym. Test., 2001, 20, 599–606 CrossRef.
- B. Ben Doudou, E. Dargent, S. Marais, J. Grenet and Y. Hirata, J. Polym. Sci., Part B: Polym. Phys., 2005, 43, 2604–2616 CrossRef.
- T. Messin, N. Follain, A. Guinault, G. Miquelard-Garnier, C. Sollogoub, N. Delpouve, V. Gaucher and S. Marais, J. Membr. Sci., 2017, 525, 135–145 CrossRef CAS.
- R. M. Hodge, T. J. Bastow, G. H. Edward, G. P. Simon and A. J. Hill, Macromolecules, 1996, 29, 8137–8143 CrossRef CAS.
- C. L. Buquet, B. Ben Doudou, C. Chappey, E. Dargent and S. Marais, J. Phys. Chem. B, 2009, 113, 3445–3452 CrossRef.
- G. S. Park, Synthetic Membranes: Science, Engineering and Applications, Springer, Netherlands, Dordrecht, 1986, pp. 57–107 Search PubMed.
- J. Grank and G. S. Park, Diffusion in Polymers, CRC Press, London and New-York, 1996 Search PubMed.
- I. Erukhimovich and M. O. de la Cruz, J. Polym. Sci., Part B: Polym. Phys., 2004, 45, 3003–3009 CrossRef.
- A. S. Michaels, W. R. Vieth and J. A. Barrie, J. Appl. Phys., 1963, 34, 1–12 CrossRef CAS.
- D. W. Van Krevelen and K. Te Nijenhuis, Properties of polymers, Elsevier, 4th edn, 2009 Search PubMed.
- R. Swaidan, B. Ghanem, E. Litwiller and I. Pinnau, Macromolecules, 2015, 48, 6553–6561 CrossRef CAS.
- A. F. Ismail and W. Lorna, Sep. Purif. Technol., 2002, 27, 173–194 CrossRef CAS.
- G. L. Robertson, Food packaging-principles and practice, Taylor & Francis, 3rd edn, 2013 Search PubMed.
- L. Jia and J. Xu, Polym. J., 1991, 23, 417–425 CrossRef CAS.
- J. Trifol, D. Plackett, P. Szabo, A. E. Daugaard and M. Giacinti Baschetti, ACS Omega, 2020, 5, 15362–15369 CrossRef CAS.
- H. Hu, R. Zhang, W. B. Ying, L. Shi, C. K. Yao, Z. Y. Kong, K. Wang, J. G. Wang and J. Zhu, Polym. Chem., 2019, 10, 1812–1822 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: FTIR and 1H NMR spectra and some supplemental figures (DSC, tensile test), tables (TGA, gas permeation coefficients) and the tensile test video. See DOI: 10.1039/d1ma00797a |
| This journal is © The Royal Society of Chemistry 2022 |