Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fabrication of flower-like bismuth vanadate hierarchical spheres for an improved supercapacitor efficiency
Subramanian
Balachandran
 abc,
Rajan
Karthikeyan
abc,
Rajan
Karthikeyan
 de,
Kumaravel Jeeva
Jothi
f,
Veerappan
Manimuthu
g,
Natarajan
Prakash
h,
Zheming
Chen
a,
Tongxiang
Liang
de,
Kumaravel Jeeva
Jothi
f,
Veerappan
Manimuthu
g,
Natarajan
Prakash
h,
Zheming
Chen
a,
Tongxiang
Liang
 d,
Chengzhi
Hu
d,
Chengzhi
Hu
 *c,
Feng
Wang
*c,
Feng
Wang
 *a and
Mingshu
Yang
*a
*a and
Mingshu
Yang
*a
aBeijing National Laboratory for Molecular Sciences, Key Laboratory of Engineering Plastics, Institute of Chemistry, Chinese Academy of Sciences, Zhongguancun North First Street 2, Beijing 100190, P. R. China. E-mail: yms@iccas.ac.cn; wangfeng0822@iccas.ac.cn; hucz@sustech.edu.cn; Fax: +86-10-62561945; Tel: +86-10-62561945
bCenter for Nanoscience and Technology, Chennai Institute of Technology, Sarathy Nagar, Kundrathur, Chennai 600069, Tami Nadu, India
cDepartment of Mechanical and Energy Engineering, Southern University of Science and Technology, Shenzhen, Guangdong, China
dEngineering Research Centre for Hydrogen Energy Materials and Devices, College of Rare Earths (CORE), Jiangxi University of Science and Technology, Ganzhou 341000, Jiangxi, China
eDivision of Physical Sciences, Department of Science and Humanities, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Thanadalam 600056, Tamil Nadu, India
fCentral Institute of Plastics Engineering and Technology, Plastics Technology Guindy Chennai, Chennai 600032, Tami Nadu, India
gDepartment of Electrical and Electronic Engineering Southern University of Science and Technology Nanshan District, Shenzhen, Guangdong 518055, P. R. China
hDivision of Chemistry, Department of Science and Humanities, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Thanadalam 600056, Tamil Nadu, India
First published on 29th September 2021
Abstract
A cost-effective and simple method has been developed for the preparation of flower-like hierarchical spheres of semiconductor oxide materials. Efficient self-assembly of BiVO4 flower-like hierarchical spheres was performed via a simple hydrothermal method, followed by calcination at 500 °C. X-ray diffraction (XRD) studies distinguished diffraction planes of the monoclinic BiVO4 phase, which was further confirmed by field emission scanning electron microscopy (FESEM) analysis. The electronic and optical properties of BiVO4 were studied via X-ray photoelectron spectroscopy (XPS) and UV-visible spectroscopy. XPS analysis confirmed the binding energy relations and the formation of BiVO4, and also an increase in the Bi–O, V–O bond strengths in BiVO4. The remarkable efficiency of pseudocapacitor electrode materials depends on the consistent fabrication of nano architectures. The carbon-free BiVO4 electrodes showed a higher specific capacitance of 1203 F g−1 at 2 A g−1, without detectable degradation after 2000 cycles and a beneficial cycling stability was achieved because of the electrochemical activity of the distinctive porous hierarchical architecture. Our synthetic method suggests a simple procedure for the design and fabrication of bismuth vanadate hierarchical nano architectures, encouraging electrochemical energy storage. These nanocomposites could be utilized as anode materials in lithium-ion batteries.
Introduction
The fabrication of 3D micro/nanostructures with hierarchical or complex structures in recent years have gained considerable recognition for their popularity in numerous fields,1–3 such as medicine,4–8 microfluidics,9,10 electronics,11–13 optics,14–16 and chemistry.17–19 Encouraged by the great multiplicity of fascinating properties, researchers have carried out experiments to find out new functionalities and used various methods for preparing multiscale hierarchical surfaces or three-dimensional (3D) structures. The inorganic nanomaterials show large numbers of size and shape-dependent properties. Therefore, synthesizing nanomaterials with controlled shape, size, dimension and structure has unique implications.20,21 Extensive examination of hierarchical nanostructures made from nanocrystals, such as nanoparticles,22 nanowires/belts/rods23,24 and nanoplates/sheets/disks,25,26 showing exclusive physical and chemical properties different from that of the nanocrystals. Numerous hierarchical nanostructures with metals,27 oxides,28,29 sulfides,30–34 hydrates,35etc.,36–38 have been fabricated for multiple applications. Therefore, discovering and exploring new nanoarchitectures or hierarchical nanostructures are promising for next-generation devices. Besides their abilities and properties, addressing fundamental science questions and their utilization in technical applications is much needed.The supercapacitors play a important role in next-generation energy storage systems, because of its attractiveness as a source of energy. In contrast to batteries, they are high-power storage devices, maintenance-free, thus providing a long life-cycle with rapid charge–discharge. They require a simple charging system without a memory effect, which is more secure than the conventional storage devices. Physical energy storage is advantageous over chemical energy storage for operational safety with more extended periods. Thus, supercapacitors satisfy longer lifecycles because of their high charging and discharging rates. Supercapacitors have a better reputation mainly to electric vehicles and their remarkable potential to facilitate future journeys. In the recent times, there have been extensive efforts to develop novel electrode materials with higher capacity, unique and more compatible miniature designs. More advances in optimizing electrode materials such as surface morphology, crystallinity, porosity, surface chemical reactivity, capacitance, and storage efficiency play an important role in deciding the supercapacitor quality, which may help to maximize supercapacitors’ widespread potential as power storage devices.39,40 Pseudo-capacitors are excellent candidates to solve the efficiency difference between batteries and supercapacitors.
To overcome the above-mentioned issues, the BiVO4 ternary oxide semiconductor is drawing attention due to its superior attributes such as nontoxicity, solar light utilization for water splitting,41–44 toxic chemical degradation,45–50 and high stability and low bandgap (2.4 eV) in nature. However, the photo-induced BiVO4 charge carriers (electron and holes) can easily recombine, leading to poor photocatalytic efficiency.51–53 BiVO4 has three stable crystalline forms: namely the monoclinic scheelite structure (ms-BiVO4), tetragonal zircon (tz-BiVO4), and tetragonal scheelite (ts-BiVO4). Among them, ms-BiVO4 exhibits better electron conductivity54 and light-induced catalytic activity relative to other forms. The band structure of BiVO4 with conduction and valence bands comprising of V 3d, Bi 6s, and O 2p orbitals are characteristic nature of BiVO4. Hence, numerous researchers focus on developing visible light-active photocatalysts using BiVO455–57 compared to their energy storage-related applications. The list of previous BiVO4-based energy storage works is limited, and their specific capacitance results are summarized in Table 1.
| No. | Source materials | Synthesized product | Method, temperature & time | Specific capacitance | Structure & morphology | Ref. |
|---|---|---|---|---|---|---|
| 1 | Graphene oxide, Bi(NO3)3·5H2O, NH4VO3 | Gr/BiVO4 | Hydrothermal 180 °C | 479 F g−1 at 5 A g−1 | Free standing composites | Deng et al., 201860 |
| 2 | Bi (NO3)3·5H2O, NH4VO3, Graphene oxide, | rGO/BiVO4 | Hydrothermal 180 °C – 24 h | 94.83 F g−1 at 1.4 A g−1 | Rod-like structures | Patil et al., 201662 |
| 3 | Bi (NO3)3·5H2O, Na2VO3, NaOH, Sodium dodecyl benzene sulfonate (SDS), GO | rGO/BiVO4 | Hydrothermal 200 °C – 1.5 h | 400 F g−1 at 5 mV s−1 | m-BiVO4 nanoparticles on/rGO | Dutta et al., 201859 |
| 4 | Bi (NO3)3·5H2O, NH4VO3, Sodium dodecyl benzene sulfonate (SDS) | SWCNT/BiVO4 | Hydrothermal 160 °C – 18 h | 395 F g−1 | m-BiVO4 nanoparticles on/SWCNT | Khan et al., 201461 |
| 5 | Bismuth oxyiodide electrodeposited on FTO, annealed at 450 °C in vanadyl acetylacetonate | rGO/BiVO4 | Annealed at 450 °C | 141.8 F g−1 at 0.2 A g−1 | m-BiVO4/rGO | Roy et al., 202063 |
| 6 | Bi (NO3)3·5H2O, NH4VO3, Graphene oxide, | rGO/BiVO4 | Hydrothermal 180 °C – 24 h | 484 F g−1 at 5 mV s−1 | BiVO4/rGO nanocomposites | Sengottayan et al., 201964 |
| 7 | Bi (NO3)3·5H2O, NH4VO3, Polymerization: aniline, H2SO4, potassium peroxoxdisulphate | BiVO4/PANI | Solution synthesis at 120 °C – 12 h annealed at 400 °C – 3 h | 701 F g−1 at 1 A g−1 | BiVO4/PANI nanocomposites | Srinivasan et al., 202065 |
| 8 | Bi (NO3)3·5H2O, NH4VO3, AgNO3, NaBH4 | BiVO4: Ag | Solution synthesis | 109 F g−1 at 1 A g−1 | BiVO4 nanosheets/Ag nanoparticles hybrid | Zhang et al., 201766 |
| 9 | Bi (NO3)3·5H2O, NH4VO3, Graphene oxide, sodium dodecyl benzene sulfonate (SDS) | PRGO/BiVO4 for Li-Ion batteries | Hydrothermal 180 °C – 12 h | 160 F g−1 at 0.9 A g−1 | Nanorods of BiVO4/PRGO | Dubai et al., 201858 |
| 10 | Bi (NO3)3·5H2O, NH4VO3 | BiVO4 | Hydrothermal 220 °C – 24 h | 1203 F g−1 at 2 A g−1 | Nanosheets composed hierarchical m-BiVO4 | This work |
According to our literature survey, in almost all of the BiVO4-based energy storage devices,58–66 the materials were fabricated by a hydro/solvothermal process, and the temperature ranged from 120 °C to 220 °C. The source materials of bismuth nitrate pentahydrate and ammonium vanadate are common in the presence of nitric acid solvent. However, the use of graphene/reduced graphene oxide or other conductive materials in support of BiVO4 is dominant in improving the conductivity of the electrodes for supercapacitor applications. Up until this point, only few studies have been accounted for BiVO4 as an anode material for supercapacitors. In recent times, it has been proven that BiVO4 can improve the performance of supercapacitors as an electrode material due to its admirable physicochemical behavior and high stability. This study focuses on flower-shaped BiVO4 for supercapacitor application without addition of any noble metals (such as Au and Ag) or carbon materials (such as RGO, SWCNT, and graphene) etc. The addition of noble metals, graphene, reduced graphene oxides are more expensive. Controlling only the shape of BIVO4 may influence the higher activity. The overall approach result with the nanocomposites of different morphologies ranging from particles to nanorods. The morphological and surface area of the BiVO4 material is essential in addition to its structural control (m-BiVO4). The highest specific capacitance of 479 F g−1 was achieved by Deng et al. with the current density of 5 A g−1. Here, graphene was used as the conducting support and the effect of the structural and morphological features of BiVO4 was not explicated towards its specific capacitance.60
Thus, we present a facile and robust one-step67–69 carbon-free synthesis of BiVO4 hierarchical structures via a hydrothermal method and achieved the highest specific capacitance for supercapacitor applications. This surfactant-free process approach is energy-efficient and reliable for structure and morphological control. The detailed results and discussions are as follows.
Experimental section
Materials and methods
Bismuth nitrate pentahydrate [Bi(NO3)3·5H2O, purity of 98%] and ammonium metavanadate (NH4VO3, purity of 99.0%) were purchased from Sigma-Aldrich. Solvents of 1 M HNO3 (70% pure), sodium hydroxide and ethanol were purchased from Merck. All the chemical products were of analytical grade and used without further purification. All the tests were done using deionized (DI) water.The hydrothermal method was used to synthesize BiVO4. First, 30 mL of NH4VO3 (5 mmol) was preheated to 75 °C and then 30 mL of Bi(NO3)3·5H2O (5 mmol) aqueous suspension was added and stirred for 15 min until it turns into light-yellow solution. Then pH of the solution mixture was adjusted to 7 with dilute NaOH. Further, stirring was carried out for 30 min before transferring into a 100 mL volume Teflon-lined stainless autoclave and kept at 220 °C for 24 h. After the reaction, the products were washed with DI water, absolute ethanol, acetone and centrifuged. This process was repeated five times to obtain pure precipitate without any impurities and unreacted products. The dried sample was calcined at 500 °C in an ambient air atmosphere for 5 h to get the BiVO4 nanomaterial.
Characterizations
A variety of analytical measurement techniques were utilized to characterize the as-prepared BiVO4 material. A field emission scanning electron microscope (FESEM, Hitachi S-4100) was used to visualize the morphology of the material. Before the measurement, the sample was prepared on a carbon tape platform and coated with platinum by a magnetron sputter for 5 min. Morphological and microstructural behaviors of BiVO4 nanosheets were studied via high-resolution transmission electron microscopy (TEM, JEM-2100, JEOL, Japan), X-ray diffraction (XRD, Japan, Rigaku D/max-2500) and UV-visible DRS (TU-1901, Pgener al) spectrometer. The X-ray photoelectron spectra (XPS) were collected on an ESCA Lab 220i-XL, VG Scientific X-ray photoelectron spectrometer, using MgKα X-ray as an excitation source. The base pressure was about 3 × 10−9 mbar.Electrode preparation
The electrochemical behaviors of the electrodes were analyzed by utilizing a three-electrode system. Here, the three electrodes used were a Pt wire (as the counter electrode), Ag/AgCl electrode (as the reference electrode) and the as-synthesized BiVO4 hierarchical spheres (1 mg) (as the working electrode). First, BiVO4 was dispersed in distilled water (1 mL) under sonication for ∼60 min, and 5 μL of the dispersion solution was drop-cast onto the surface of the glassy carbon (GCE) working electrode before CV and GCD measurements. The GCE was pre-treated with acetone, absolute ethanol and deionized water for 15 min to get the clean surface. 2 M KOH was used as the electrolyte. Working electrodes were prepared separately by blending 95% of the as-synthesized BiVO4, along with a 5% Nafion®perfluorinated resin solution (0.5% w/v, 5 μL) as a binder in ethanol solvent. After coating the as-prepared slurry on GCE, it was dried at 80 °C for 30 min and then the electrode weight was measured to figure out the quantity of as-synthesized materials on the GCE.Results and discussion
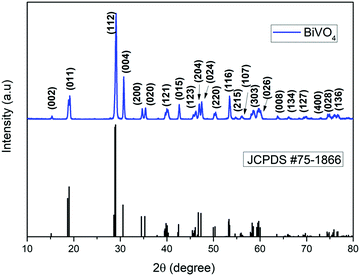
The structural behavior of the as-synthesized sample was studied on a powder X-ray diffractometer, and the results are shown in Fig. 1. The diffraction planes from XRD patterns well-matched with the corresponding monoclinic BiVO4 (JCPDS card no. 75-1866).70 The peak positions of 15.13°, 19°, 28.97°, 30.55°, 34.51°, 35.24°, 40.28°, 42.49°, 46.09°, 46.75°, 47.31°, 50.33°, 53.32°, 55.93°, 57.93°, 58.31°, 59.71°, 63.58°, 66.23°, 69.72°, 72.78°, 74.83° and 76.72° are belong to (002), (011), (112), (004), (200), (020), (121), (015), (123), (204), (024), (220), (116), (215), (107), (303), (026), (008), (134), (127), (400), (028) and (136) reflection planes, respectively. The clear matching of planes without any peak position shift suggests that the as-synthesized BiVO4 phase is of high purity and free from any other trace intermediates.71 The intense sharp peaks reveal the highly crystalline nature of BiVO4 (Fig. 1).The surface morphology of the as-prepared BiVO4 was investigated via FESEM, as shown in Fig. 2. The FESEM image (Fig. 2a) shows that the samples possessed monodispersed BiVO4 hierarchical sheet composed of architectures. These 3D nanosheets composed of architectures expose high active surfaces at the inner core of the ball-like structure. The hydrothermal method under closed pressure for the high concentration source materials has influenced the hierarchical structural growth. It can be seen that the material is composed of a large number of nanosheets with the diameter of hierarchical spheres ranging from 2–2.5 μm. To validate the above range of size, we have calculated the hierarchical size distribution related histogram and the mean size was found to be 2.3 μm, as shown in Fig. 2c. The shape of the nanosheets was uniform, and thickness was found to be ∼25 nm from Fig. 2b. To elucidate the size confirmation of nanosheets, we performed the image analysis and the mean thickness of nanosheets was measured by comparing roughly 200 different nanosheets (Fig. 2d). Several tiny pores existing on the flat structures could be due to the discharge of water molecules and the structural contraction during the calcination process.72 For the hierarchical structure formation, a mechanism was proposed involving the growth of intercrossed nanosheets in the oriented attachment, followed by “Ostwald ripening.” In addition, there were pore structures observed on the hierarchical spheres of BiVO4; this could be due to the results of cross-linked sheets with the exposed mesopores to microporous structures.34,73,74
 | ||
| Fig. 2 FESEM images of BiVO4 (a) wide view and (b) narrow view (c) histogram of the size of hierarchical structures (d) histogram of the nanoheet thickness. | ||
The hierarchical structure formation was further studied via transmission electron microscopy (TEM). Since the particles size was in the micrometer order, during the sample preparation process smaller sized particles were deposited on the TEM sample grid, which was then analysed. Fig. 3 shows the respective TEM and high-resolution TEM images of the BiVO4 nanostructure. From Fig. 3a, it is clear that these floral hierarchical spheres are actually madeup of several nanosheets linked with each other. The interlinking of nanosheets formed 3D hierarchical structures, which mostly diffracted the electrons in TEM. However, the high-resolution TEM image in Fig. 3c reveals lattice fringes with different grain boundaries. It could be due to the composition of multiple sheets. The corresponding Fast Fourier Transform (FFT) patterns were used to measure the interplanar distances of 0.314 nm and 0.293 nm. These lattice fringes were related to the (112) and (004) reflection planes in the XRD pattern of monoclinic scheelite BiVO4 (m-BiVO4).57 The above crystal planes were the dominant peak position of m-BiVO4, which again confirmed the presence of the highly crystalline phase of pure BiVO4. The selected area electron diffraction (SAED) pattern implies the polycrystalline nature of the BiVO4 material (inset of Fig. 3c). The corresponding SAED pattern highlights the bright patterns of first order circles. Due to the polycrystalline nature of the BiVO4 material, multiple circular ring patterns of bright fringes were observed.
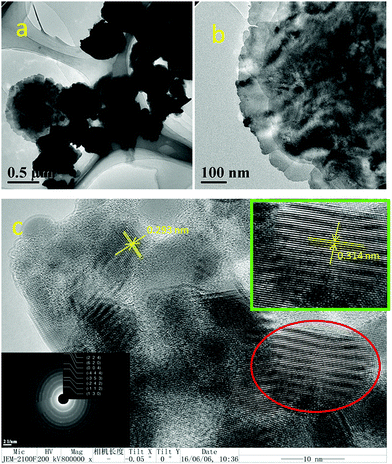 | ||
| Fig. 3 TEM images of BiVO4 at (a) 500 nm, (b) 100 nm scale magnifications, (c) high resolution TEM images and the corresponding lattice fringes at 10 nm scale with the SAED pattern. | ||
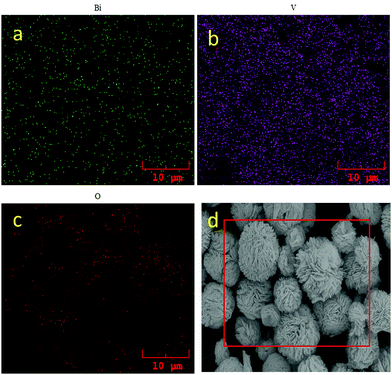
To confirm Bi, V, O elemental distribution on BiVO4, EDS elemental mapping was carried out via the FESEM analysis. Fig. 4 shows the elemental mapping of bismuth, vanadium, oxygen and the corresponding FESEM image. There is a homogenous distribution of Bi, V and O observed with the uniform patterns on Fig. 4a–c. Thus, the EDS mapping revealed that the material was composed of Bi, V and O without any form of trace impurities.
Fig. 5 shows the XPS spectra of BiVO4. The C–C peak position of 284.8 eV clearly indicates that the wide spectrum of BiVO4 is a charge corrected spectrum. Also, small amounts of C–O–C and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O are present at 288.9 and 286.5 eV, respectively. Fig. 5a is observed because of the carbon used for calibration during the measurements. Fig. 5b, c, d and f correspond to the spectra of O 1s, Bi 4f, V 2p and survey spectrum, respectively. The O 1s peak at 531.77 eV is due to the adsorbed H2O or surface hydroxyl group from the atmospheric moisture. This broad peak can be fitted into two peaks at about 530.04 eV and 533.28 eV, which is related to the existence of non-equivalent lattice oxygens (Fig. 5b). It is assigned to the lattice oxygen (O2−) of Bi2O22+ on the composite surface and for the surface adsorbed oxygen, respectively. The binding energy peaks at 164.4 eV and 159.1 eV (Fig. 5c) are related to Bi 4f5/2 and Bi 4f7/2, respectively, showing the presence of Bi3+ in the lattice of Bi2O22+. The metallic Bi binding energy values of 4 f5/2 and Bi 4f7/2 are 164.4 eV and 159.1 eV, respectively.75 The symmetric peaks of Bi 4f are well matched with the compounds of bismuth oxides and its spin–orbit component has a well seprated Δ value of 5.3 eV equivalent to bismuth oxides.
O are present at 288.9 and 286.5 eV, respectively. Fig. 5a is observed because of the carbon used for calibration during the measurements. Fig. 5b, c, d and f correspond to the spectra of O 1s, Bi 4f, V 2p and survey spectrum, respectively. The O 1s peak at 531.77 eV is due to the adsorbed H2O or surface hydroxyl group from the atmospheric moisture. This broad peak can be fitted into two peaks at about 530.04 eV and 533.28 eV, which is related to the existence of non-equivalent lattice oxygens (Fig. 5b). It is assigned to the lattice oxygen (O2−) of Bi2O22+ on the composite surface and for the surface adsorbed oxygen, respectively. The binding energy peaks at 164.4 eV and 159.1 eV (Fig. 5c) are related to Bi 4f5/2 and Bi 4f7/2, respectively, showing the presence of Bi3+ in the lattice of Bi2O22+. The metallic Bi binding energy values of 4 f5/2 and Bi 4f7/2 are 164.4 eV and 159.1 eV, respectively.75 The symmetric peaks of Bi 4f are well matched with the compounds of bismuth oxides and its spin–orbit component has a well seprated Δ value of 5.3 eV equivalent to bismuth oxides.
 | ||
| Fig. 5 High resolution XPS spectra of BiVO4 (a) C 1s, (b) O 1s, (c) Bi 4f, (d) V 2p and (f) survey spectra. | ||
The binding energy peaks at 524.4 eV and 517.2 eV belong to V 2p1/2 and V 2p3/2, respectively, which is related to the +5 oxidation state of vanadium, as shown in Fig. 5d.57,76 The deconvolution of vanadium spectrum showed a peak around 518.9 eV, which corresponded to the +4 oxidation state. Thus, although the vanadium was present in the lattice as V+5, the reduction of V+5 to V+4 could occur due to the presence of surface defects. It does influence the electronic structure of monoclinic scheelite BiVO4.77 Besides the structural confirmation from XRD, the XPS analysis provides the required binding energies for BiVO4 formation. The XPS spectra clearly confirm the formation of BiVO4 from the elemental analysis.
UV-Diffuse reflectance spectroscopy (UV-DRS) is a suitable analysis to disclose the band structure of a semiconductor. The optical behavior of a semiconductor is determined by the photo-absorption properties also by diffusion related to its band energy structure of photo-induced electrons and holes. As displayed in Fig. 6, a pure BiVO4 sample shows high absorption till 560 nm.78,79 The transition from a valence band formed by Bi 6s to a CB of V 3d is reflected by the strong Ultraviolet to visible light absorption.80 In particular, the edge at ∼560 nm is related to the characteristic peak of BiVO4 (Fig. 6a). It shows the outstanding narrow bandgap with a high absorption coefficient. Visible light-induced photo absorption abilities are helpful to elucidate the electronic transition and excitation of more active species. The material bandgap (Eg) can be defined using eqn (1):71
| ahν = A(hν − Eg)n | (1) |
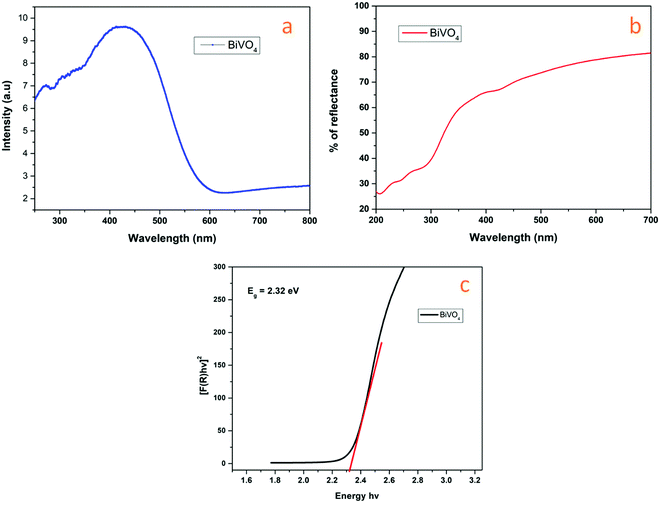 | ||
| Fig. 6 UV-DRS of BiVO4 (a) absorbance and (b) reflectance spectra (c) relationship between Kubelka–Munk function vs. band gap. | ||
The valence band (VB) and conduction band (CB) edges of hierarchical BiVO4 semiconductor can be determined using eqn (2) and (3).
| ECB = X − Ee − 0.5Eg | (2) |
| EVB = Eg + ECB | (3) |
 | (4) |
Fig. 7 shows the BiVO4 electro-chemical properties obtained using a three-electrode system in a 2.0 M aqueous KOH solution. The as-prepared BiVO4 showed a near-perfect rectangular CV curve that showed good capacitive behavior (Fig. 7a). The BiVO4 material showed pseudocapacitive nature with a well-resolved redox peak at 2 mV s−1. Even at a rate of 100 mV s−1, the CV curve indicated its rectangular shape, and this symmetric peak behavior represents a more significant reversibility of redox reactions. As shown in Fig. 7a, the anodic (at −0.33, −0.49 and −0.65 V) and cathodic peaks (at −0.515, 0.62 and 0.78 V) were observed at different potentials.83,84 The well-organized redox peaks in the CV curve confirm the influence of faradaic processes in the energy storage.85 The observed redox behavior was also similar to that of the Bi2O3 material, i.e., the reduction of Bi3+ to metallic Bi (−0.515 V and 0.62 V) during the negative sweep and oxidation of metallic Bi to Bi3+ (−0.35 V at 2 mV) during the positive sweep.86 It is valuable to note that the peak current value increased with the scan rate. Also, small shifts were observed in the redox peak position in the positive potential directions (0.35 V to 0.11 V (100 mV)) with no deterioration (Fig. 7b).
EIS was measured at a frequency range of 0.01 Hz to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz at the open-circuit configuration. BiVO4 showed a semicircle shape in the observed EIS spectrum at a higher frequency with a spike in the lower frequency (Fig. 7c). The slope in the low-frequency area related to the Warburg impedance (W) reflected the diffusion of electrolytes in host materials through pore electrodes and protons. An intersecting curve on the real part at a high frequency indicates the solution resistance (Rs), and the semicircle diameter represents the charge transfer resistance (Rct).83,84
000 Hz at the open-circuit configuration. BiVO4 showed a semicircle shape in the observed EIS spectrum at a higher frequency with a spike in the lower frequency (Fig. 7c). The slope in the low-frequency area related to the Warburg impedance (W) reflected the diffusion of electrolytes in host materials through pore electrodes and protons. An intersecting curve on the real part at a high frequency indicates the solution resistance (Rs), and the semicircle diameter represents the charge transfer resistance (Rct).83,84
The capacitive performance was investigated through a charge–discharge behavior of BiVO4 surfaces, and also at the same region of 0.3 cm2 diameter, the applied currents were measured (Fig. 7b). BiVO4 electrodes were measured by separate, narrow potential windows between −1.0 to −0.4 V and −0.3 to 0.1 V. Due to the synergy between two components, the hybrid supercapacitor-electrode was working within the window of −1.0–0.1 V. Bismuth vanadate materials had significant increase in the voltage at levels above a certain point leading to significant variations of the linearity in their galvanostatic charge–discharge curves. The charge–discharge curves of BiVO4 were similar in shape to the equilateral triangles under high current conditions.89 During the charging and discharging process, linear curves show strong reversibility of the material. Furthermore, the charge–discharge curves of the hybrid supercapacitor at different currents kept a similar shape, indicating broad current range sustainability. The specific capacitance of modified electrodes was shown to be a more reliable candidate for supercapacitor devices, especially in the case of devices with minimal active materials.86,87
Current increase or decrease affects the specific capacity, but the flat plateau of the hybrid electrode compared to the BiVO4 electrode offered achievable rate improvements. The hybrid BiVO4 supercapacitor electrode's spatial capacitance increased until a saturation limit was achieved.
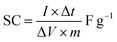 | (5) |
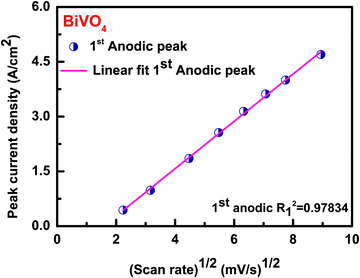
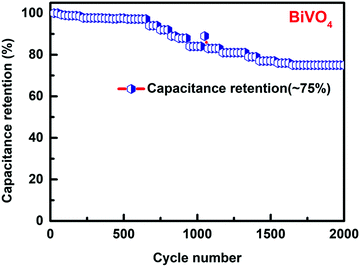
Fig. 8 shows the relationship between peak current density vs. square root of the scan rate plot of BiVO4. The calculated plot visualized as linear with the correlation coefficient (R2) of 0.97834 for the 1st anodic peak implies that the whole electrode reaction is controlled by the diffusion controlled-process.90–94Fig. 9 shows the recycling stability test results under the current density of 2 Ag−1. The specific capacitance values decreased during consecutive cycle runs. At around 2000 cycles, 75% of the specific capacitance value was retained. This value is higher than the typical BiVO4 material reported in the literature.64 The decrease in the cyclic stability of 75% could be due to the absence of the carbon additive. Among the known reported studies, the as-synthesized BiVO4 stands out as a better material.
Conclusion
Hierarchical BiVO4 was fabricated by a simple and one-step hydrothermal procedure. Such architectures have been designed with nanosheets that are highly orientated. The Ostwald's ripening mechanism played a key role in the growth design of 3D sheet composed architectures. The as-prepared hierarchical m-BiVO4 (1203 Fg−1) showed excellent electrochemical properties because of its smaller particle size and structure. This was ascribed to the full utilization of m-BiVO4 and increased electrical conductivity and ionict ransfer. Notably, stable cycle capability was also achieved. Hence, BiVO4 is a promising candidate for superior supercapacitor fabrications. We foresee that our synthesis method to hierarchical m-BiVO4 nanocomposites will be very beneficial for the development of solar cells and other energy storage devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Dr Balachandran Subramanian is grateful for the financial support of the CAS President's International Fellowship Initiative (2016PM045) of China, the National Natural Science Foundation of China (51650110503 & 51133009), and the National Basic Research Program of China (2012CB720304). Dr Karthikeyan Rajan is thankful for the National Natural Science Foundation of China (51871114).References
- L. Feng, S. Li, Y. Li, H. Li, L. Zhang, J. Zhai, Y. Song, B. Liu, L. Jiang and D. Zhu, Adv. Mater., 2002, 14, 1857–1860 CrossRef CAS.
- M. Choi, K. Na, J. Kim, Y. Sakamoto, O. Terasaki and R. Ryoo, Nature, 2009, 461, 246–249 CrossRef CAS.
- Y. Xiu, L. Zhu, D. W. Hess and C. P. Wong, Nano Lett., 2007, 7, 3388–3393 CrossRef CAS PubMed.
- R. Murugan and S. Ramakrishna, Compos. Sci. Technol., 2005, 65, 2385–2406 CrossRef CAS.
- S. Wu, X. Liu, T. Hu, P. K. Chu, J. P. Y. Ho, Y. L. Chan, K. W. K. Yeung, C. L. Chu, T. F. Hung, K. F. Huo, C. Y. Chung, W. W. Lu, K. M. C. Cheung and K. D. K. Luk, Nano Lett., 2008, 8, 3803–3808 CrossRef CAS PubMed.
- Y. L. Jung and H. J. Donahue, Tissue Eng., 2007, 13, 1879–1891 CrossRef.
- V. L. Colvin, Nat. Biotechnol., 2003, 21, 1166–1171 CrossRef CAS PubMed.
- T. Dvir, B. P. Timko, D. S. Kohane and R. Langer, Nat. Nanotechnol., 2011, 6, 13–22 CrossRef CAS.
- G. Chen, G. T. McCandless, R. L. McCarley and S. A. Soper, Lab Chip, 2007, 7, 1424–1427 CAS.
- S. Url, T. J. Archive and T. Archive, Science, 2007, 288, 1026–1029 Search PubMed.
- H. Cao, J. O. Tegenfeldt, R. H. Austin and S. Y. Chou, Appl. Phys. Lett., 2002, 81, 3058–3060 CrossRef CAS.
- C. Ye, L. Zhang, X. Fang, Y. Wang, P. Yan and J. Zhao, Adv. Mater., 2004, 16, 1019–1023 CrossRef CAS.
- Y. T. Tseng, W. H. Tseng, C. H. Lin and R. M. Ho, Adv. Mater., 2007, 19, 3584–3588 CrossRef.
- J. A. Rogers, M. Meier, A. Dodabalapur, E. J. Laskowski and M. A. Cappuzzo, Appl. Phys. Lett., 1999, 74, 3257–3259 CrossRef CAS.
- A. R. Parker and H. E. Townley, Nat. Nanotechnol., 2007, 2, 347–353 CrossRef CAS.
- J. Henzie, M. H. Lee and T. W. Odom, Nat. Nanotechnol., 2007, 2, 549–554 CrossRef CAS.
- Z. Li, Y. Ding, Y. Xiong, Q. Yang and Y. Xie, Chem. Commun., 2005, 918–920 RSC.
- R. Srivastava, M. Choi and R. Ryoo, Chem. Commun., 2006, 4489–4491 RSC.
- W. G. Bae, H. N. Kim, D. Kim, S. H. Park, H. E. Jeong and K. Y. Suh, Adv. Mater., 2014, 26, 675–700 CrossRef CAS.
- R. F. Service, Science, 2005, 309, 95 CrossRef CAS.
- W. Fischle, Y. Wang, C. D. Allis, C. Opin, C. Biol, J. A. Martens, F. Winston, C. Opin, G. Dev, M. M. Smith, C. Opin and C. Biol, Science, 2004, 303, 348–352 CrossRef.
- T. D. Ewers, A. K. Sra, B. C. Norris, R. E. Cable, C. H. Cheng, D. F. Shantz and R. E. Schaak, Chem. Mater., 2005, 17, 514–520 CrossRef CAS.
- Y. Mao, M. Kanungo, T. Hemraj-benny and S. S. Wong, J. Phys. Chem. B, 2006, 110, 702–710 CrossRef CAS PubMed.
- X. Chen, X. Wang, Z. Wang, X. Yang and Y. Qian, Cryst. Growth Des., 2005, 5, 347–350 CrossRef CAS.
- L. S. Zhong, J. S. Hu, H. P. Liang, A. M. Cao, W. G. Song and L. J. Wan, Adv. Mater., 2006, 18, 2426–2431 CrossRef CAS.
- M. Mo, J. C. Yu, L. Zhang and S. K. A. Li, Adv. Mater., 2005, 17, 756–760 CrossRef CAS.
- L. Qu and L. Dai, J. Phys. Chem. B, 2005, 109, 13985–13990 CrossRef CAS PubMed.
- Z. Li, Y. Ding, Y. Xiong and Y. Xie, Cryst. Growth Des., 2005, 5, 1953–1958 CrossRef CAS.
- C. Jiang, W. Zhang, G. Zou, W. Yu and Y. Qian, J. Phys. Chem. B, 2005, 109, 1361–1363 CrossRef CAS.
- X. Gou, F. Cheng, Y. Shi, L. Zhang, S. Peng and J. Chen, J. Am. Chem. Soc., 2006, 128, 7222–7229 CrossRef CAS PubMed.
- Z. He, S. H. Yu, X. Zhou, X. Li and J. Qu, Adv. Funct. Mater., 2006, 16, 1105–1111 CrossRef CAS.
- N. Zhao and L. Qi, Adv. Mater., 2006, 18, 359–362 CrossRef CAS.
- Y. Huang, X. Duan and C. M. Lieber, Small, 2005, 1, 142–147 CrossRef CAS.
- R. Karthikeyan, M. Navaneethan, J. Archana, D. Thangaraju, M. Arivanandhan and Y. Hayakawa, Dalton Trans., 2014, 43, 17445–17452 RSC.
- J. Zhang, S. Liu, J. Lin, H. Song, J. Luo, E. M. Elssfah, E. Ammar, Y. Huang, X. Ding, J. Gao, S. Qi and C. Tang, J. Phys. Chem. B, 2006, 110, 14249–14252 CrossRef CAS.
- C. Wang, D. Chen, X. Jiao and C. Chen, J. Phys. Chem. C, 2007, 111, 13398–13403 CrossRef CAS.
- H. Shi, L. Qi, J. Ma and N. Wu, Adv. Funct. Mater., 2005, 15, 442–450 CrossRef CAS.
- Q. Gong, X. Qian, X. Ma and Z. Zhu, Cryst. Growth Des., 2006, 6, 1821–1825 CrossRef CAS.
- M. Conte, Fuel Cells, 2010, 10, 806–818 CrossRef CAS.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- J. Su, L. Guo, S. Yoriya and C. A. Grimes, Cryst. Growth Des., 2010, 10, 856–861 CrossRef CAS.
- L. Zhang, D. Chen and X. Jiao, J. Phys. Chem. B, 2006, 110, 2668–2673 CrossRef CAS PubMed.
- W. Yin, W. Wang, L. Zhou, S. Sun and L. Zhang, J. Hazard. Mater., 2010, 173, 194–199 CrossRef CAS.
- H. Qing Jiang, H. Endo, H. Natori, M. Nagai and K. Kobayashi, J. Eur. Ceram. Soc., 2008, 28, 2955–2962 CrossRef.
- L. Zhou, W. Wang, S. Liu, L. Zhang, H. Xu and W. Zhu, J. Mol. Catal. A: Chem., 2006, 252, 120–124 CrossRef CAS.
- C. Yu, S. Dong, J. Feng, J. Sun, L. Hu, Y. Li and J. Sun, Environ. Sci. Pollut. Res., 2014, 21, 2837–2845 CrossRef CAS.
- A. Zhang and J. Zhang, Spectrochim. Acta, Part A, 2009, 73, 336–341 CrossRef PubMed.
- S. J. Hong, S. Lee, J. S. Jang and J. S. Lee, Energy Environ. Sci., 2011, 4, 1781–1787 RSC.
- W. Yao, H. Iwai and J. Ye, J. Chem. Soc., Dalton Trans., 2008, 1426–1430 RSC.
- M. Long, W. Cai and H. Kisch, J. Phys. Chem. C, 2008, 112, 548–554 CrossRef CAS.
- X. Zhang, Y. Gong, X. Dong, X. Zhang, C. Ma and F. Shi, Mater. Chem. Phys., 2012, 136, 472–476 CrossRef CAS.
- J. Su, L. Guo, N. Bao and C. A. Grimes, Nano Lett., 2011, 11, 1928–1933 CrossRef CAS PubMed.
- P. Ju, P. Wang, B. Li, H. Fan, S. Ai, D. Zhang and Y. Wang, Chem. Eng. J., 2014, 236, 430–437 CrossRef CAS.
- H. Seo, Y. Ping and G. Galli, Chem. Mater., 2018, 30, 7793–7802 CrossRef CAS.
- M. Han, X. Chen, T. Sun, O. K. Tan and M. S. Tse, CrystEngComm, 2011, 13, 6674–6679 RSC.
- A. Kudo, K. Omori and H. Kato, J. Am. Chem. Soc., 1999, 121, 11459–11467 CrossRef CAS.
- M. S. S. Balachandran, N. Prakash, K. Thirumalai, M. Muruganandham and M. Sillanpaa, Ind. Eng. Chem. Res., 2014, 53, 8346–8355 CrossRef.
- D. P. Dubal, K. Jayaramulu, R. Zboril, R. A. Fischer and P. Gomez-Romero, J. Mater. Chem. A, 2018, 6, 6096–6106 RSC.
- S. Dutta, S. Pal and S. De, New J. Chem., 2018, 42, 10161–10166 RSC.
- L. Deng, J. Liu, Z. Ma, G. Fan and Z. H. Liu, RSC Adv., 2018, 8, 24796–24804 RSC.
- Z. Khan, S. Bhattu, S. Haram and D. Khushalani, RSC Adv., 2014, 4, 17378–17381 RSC.
- S. S. Patil, D. P. Dubal, V. G. Deonikar, M. S. Tamboli, J. D. Ambekar, P. Gomez-Romero, S. S. Kolekar, B. B. Kale and D. R. Patil, ACS Appl. Mater. Interfaces, 2016, 8, 31602–31610 CrossRef CAS.
- A. Roy, P. Majumdar, P. Sengupta, S. Kundu, S. Shinde, A. Jha, K. Pramanik and H. Saha, Electrochim. Acta, 2020, 329, 135170 CrossRef CAS.
- C. Sengottaiyan, N. A. Kalam, R. Jayavel, R. G. Shrestha, T. Subramani, S. Sankar, J. P. Hill, L. K. Shrestha and K. Ariga, J. Solid State Chem., 2019, 269, 409–418 CrossRef CAS.
- R. Srinivasan, E. Elaiyappillai, S. Anandaraj, B. Kumar Duvaragan and P. M. Johnson, J. Electroanal. Chem., 2020, 861, 113972 CrossRef CAS.
- Z. J. Zhang, Q. C. Zheng and L. Sun, Ceram. Int., 2017, 43, 16217–16224 CrossRef CAS.
- C. Gai, F. Zhang, Q. Lang, T. Liu, N. Peng and Z. Liu, Appl. Catal., B, 2017, 204, 566–576 CrossRef CAS.
- M. Davis, C. Gümeci, C. Kiel and L. J. Hope-Weeks, J. Sol-Gel Sci. Technol., 2011, 58, 535–538 CrossRef CAS.
- Q. Fu, X. Wang, J. Zhou, J. Xia, Q. Zeng, D. Lv, C. Zhu, X. Wang, Y. Shen, X. Li, Y. Hua, F. Liu, Z. Shen, C. Jin and Z. Liu, Chem. Mater., 2018, 30, 4001–4007 CrossRef CAS.
- Y. Wen, Y. Zhao, M. Guo and Y. Xu, J. Mater. Sci., 2019, 54, 8236–8246 CrossRef CAS.
- L. Yan, W. Zhao and Z. Liu, Dalton Trans., 2016, 45, 11346–11352 RSC.
- J. H. Pan, X. Zhang, A. J. Du, H. Bai, J. Ng and D. Sun, Phys. Chem. Chem. Phys., 2012, 14, 7481–7489 RSC.
- Y. Qin, F. Zhang, Y. Chen, Y. Zhou, J. Li, A. Zhu, Y. Luo, Y. Tian and J. Yang, J. Phys. Chem. C, 2012, 116, 11994–12000 CrossRef CAS.
- J.-H. Lee, Sens. Actuators, B, 2009, 140, 319–336 CrossRef CAS.
- K. R. Tolod, S. Hernández, M. Castellino, F. A. Deorsola, E. Davarpanah and N. Russo, Int. J. Hydrogen Energy, 2020, 45, 605–618 CrossRef CAS.
- G. Silversmit, D. Depla, H. Poelman, G. B. Marin and R. De Gryse, J. Electron Spectrosc. Relat. Phenom., 2004, 135, 167–175 CrossRef CAS.
- M. D. Rossell, P. Agrawal, A. Borgschulte, C. Hébert, D. Passerone and R. Erni, Chem. Mater., 2015, 27, 3593–3600 CrossRef CAS.
- Y. Lu, Y. S. Luo, H. M. Xiao and S. Y. Fu, CrystEngComm, 2014, 16, 6059–6065 RSC.
- A. Kudo, I. Tsuji and H. Kato, Chem. Commun., 2002, 1958–1959 RSC.
- Z. He, Y. Shi, C. Gao, L. Wen, J. Chen and S. Song, J. Phys. Chem. C, 2014, 118, 389–398 CrossRef CAS.
- R. G. Pearson, Inorg. Chem., 1988, 27, 734–740 CrossRef CAS.
- W. Y. Changchang Maa, Jeongwoo Lee, Youjoong Kim, Won Cheol Seo and Hyun Jung, J. Colloid Interface Sci., 2020, 581, 514–523 Search PubMed.
- V. Vivier, A. Régis, G. Sagon, J. Y. Nedelec, L. T. Yu and C. Cachet-Vivier, Electrochim. Acta, 2001, 46, 907–914 CrossRef CAS.
- Y. C. Zhang, H. Yang, W. P. Wang, H. M. Zhang, R. S. Li, X. X. Wang and R. C. Yu, J. Alloys Compd., 2016, 684, 707–713 CrossRef CAS.
- V. D. Nithya, B. Hanitha, S. Surendran, D. Kalpana and R. Kalai, Selvan, Ultrason. Sonochem., 2015, 22, 300–310 CrossRef CAS.
- S. T. Senthilkumar, R. Kalai Selvan, M. Ulaganathan and J. S. Melo, Electrochim. Acta, 2014, 115, 518–524 CrossRef CAS.
- J. Liu, J. Jiang, M. Bosman and H. J. Fan, J. Mater. Chem., 2012, 22, 2419–2426 RSC.
- C. Zhou, Y. Zhang, Y. Li and J. Liu, Nano Lett., 2013, 13, 2078–2085 CrossRef CAS PubMed.
- S. Yoon, E. Kang, J. Kon Kim, C. Wee Lee and J. Lee, Chem. Commun., 2011, 47, 1021–1023 RSC.
- Y. Gogotsi and P. Simon, Science, 2011, 334, 917–918 CrossRef CAS PubMed.
- W. W. Liu, Y. Q. Feng, X. Bin Yan, J. T. Chen and Q. J. Xue, Adv. Funct. Mater., 2013, 23, 4111–4122 CrossRef CAS.
- S. X. Wang, C. C. Jin and W. J. Qian, J. Alloys Compd., 2014, 615, 12–17 CrossRef CAS.
- Y. Tian, S. Cong, W. Su, H. Chen, Q. Li, F. Geng and Z. Zhao, Nano Lett., 2014, 14, 2150–2156 CrossRef CAS PubMed.
- S. Jayasubramaniyan, S. Balasundari, P. A. Rayjada, R. A. Kumar, N. Satyanarayana and P. Muralidharan, J. Mater. Sci.: Mater. Electron., 2018, 29, 21194–21204 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |