Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High CO2 separation performance on a metal–organic framework composed of nano-cages lined with an ultra-high density of dual-side open metal sites†
Liangjun
Li‡
 *a,
Jiangxiu
He‡
ab,
Wenli
Xu
a,
Kuitong
Zhang
c,
Tao
Xing
c,
Zhi
Li
cd,
Dewen
Zhen
*e,
Bo
Xiong
e,
Zhixing
Ge
e,
Xi
Zhang
e,
Shanyu
Wang
e,
Fuzhao
Zhang
a,
Xin
Gu
*a,
Jiangxiu
He‡
ab,
Wenli
Xu
a,
Kuitong
Zhang
c,
Tao
Xing
c,
Zhi
Li
cd,
Dewen
Zhen
*e,
Bo
Xiong
e,
Zhixing
Ge
e,
Xi
Zhang
e,
Shanyu
Wang
e,
Fuzhao
Zhang
a,
Xin
Gu
 a,
Pengcheng
Dai
a,
Pengcheng
Dai
 a,
Dandan
Liu
a,
Lingzhi
Yang
a,
Dandan
Liu
a,
Lingzhi
Yang
 a and
Xuebo
Zhao
a and
Xuebo
Zhao
 *a
*a
aDepartment of Energy Storage Science and Engineering, College of New Energy, China University of Petroleum (East China), Qingdao, 266580, China. E-mail: lilj@upc.edu.cn; Zhaoxuebo@upc.edu.cn
bSinopec Qingdao Petrochemical Co., Ltd., Qingdao, 266580, China
cNew Energy Division, Shandong Energy Group Co., Ltd. Zoucheng, Jining, China
dSchool of Materials Science and Engineering, Xi’an Jiaotong University, Xi’an, 710049, China
eNew Energy Department, Research Institute of Petroleum Exploration & Development, China National Petroleum Corporation, Langfang, 065007, China. E-mail: zdw69@petrochina.com.cn
First published on 12th November 2021
Abstract
Developing efficient adsorbents for CO2 separation is the crucial step of CO2 sequestration. which has found many applications, including in carbon capture from flue gas and natural gas purification. Although many adsorbents have been reported in the past few decades, developing efficient adsorbents with high separation selectivity and excellent capacity is technically challenging due to the trade-off effect between the adsorption capacity and selectivity. This work reports the CO2 separation performance of a microporous MOF, which comprises nano-cages and an ultra-high density of dual-side open metal sites. The adsorption results reveal a prominent CO2 uptake and outstanding separation selectivity for CO2/N2 and CO2/CH4. Furthermore, this MOF also exhibits the preferable adsorption of C2–C3 light hydrocarbons over CH4. Simulation studies reveal that the dual-side open metal sites are the preferable adsorption sites for CO2, C2H4, and C3H6. Therefore, the outstanding separation performance can be attributed to the combined effects of nano-cages and the high density of open metal sites.
Introduction
With the increasing concern over global warming, the reduction of carbon emissions into the atmosphere has been a focus worldwide.1 Generally, there are two major routes of reducing carbon dioxide emissions. First, carbon capture and sequestration (CCS) from flue gas is the primary strategy to minimize human carbon emissions in the current stage.2,3 Second, replacing the high-carbon energy source (such as petroleum, coal, etc.) with a low-carbon energy source (such as CH4, H2, etc.) is the ultimate solution to carbon reduction.4,5 Regarding CO2 capture, CO2 separation from gas mixtures through pressure swing adsorption (PSA) has been regarded as an alternative technique of CO2 separation to the conventional chemical absorption process, which is energy-intensive and corrosive.6–9 The advantages, such as high energy efficiency, low plant investments, and easy mobility, render this technique with very promising potential for CO2 capture.10,11 Besides, the separation of CO2 from natural gas is central to natural gas production due to its incredible relevance to energy value and transportation safety.12,13 As an alternative technique to the conventional cryogenic distillation process that is energy and cost-intensive, the physisorption-based PSA process has been the most promising strategy for CO2/CH4 separation due to its low energy consumption and low investments.14,15The adsorbent is the core material for the PSA process that determines the separation efficiency and energy cost. Metal–organic frameworks (MOFs) represent an emerging family of crystalline adsorbents with a uniform structure, amenable pore environments, and tuneable functionalities among various adsorbents.16,17 Besides their encouraging prospects in a wide range of applications, they are also considered as ideal adsorbents for CO2 separation.18,19 In the past two decades, various MOFs with high CO2 separation selectivities have been reported. However, constructing MOFs with high separation CO2 selectivity and excellent capacity is still challenging and urgent. This work reports the separation performance of a MOF composed of nano-cages lined with a high density of dual-side open metal sites. The gas adsorption study reveals both high adsorption capacity and excellent separation selectivity for CO2. Theoretical studies and breakthrough experiments also validate the effectiveness of this strategy in promoting the separation efficiency for CO2 capture from flue gas and natural gas.
Results and discussion
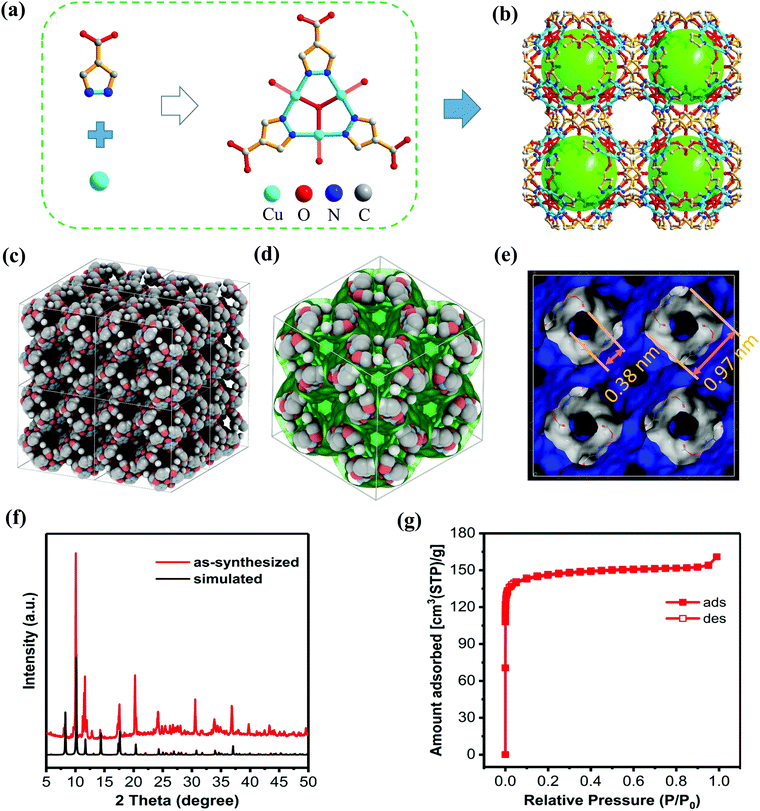
To construct a MOF with multiple functionalities, employing a multiple-functional ligand is the most direct method. 1H-pyrazole-4-carboxylic acid (H2PCA) is the simplest multiple-functional ligand composed of carboxylate and pyrazole groups. Through the solvothermal reaction of H2PCA and Cu2+ ions, a three-dimensional porous MOF with nano-cages was obtained (see Experimental details in the ESI,† S1). Deep blue block-like single crystals with a homogeneous morphology were obtained in a high yield. Single-crystal X-ray diffraction analysis reveals a three-dimensional network with a formula of [Cu3(μ3-OH)(PCA)3]. The structural retrieval shows that the crystal structure of this MOF has been reported in former literature.20 However, the gas adsorption properties of this MOF have not been reported yet.This MOF is crystallized in a high symmetrical cubic system, with a space group of F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3c. The coordination of three pyrazole groups with three Cu2+ ions and one bridging oxygen atom gives rise to triangle trinuclear [Cu3(μ3-OH)(PCA)3] secondary building units (SBUs) (see Fig. 1a and b). The [Cu3(μ3-OH)(Pz)3] (Pz represents pyrazole) group in this SBU is a commonly encountered coordination sphere created by pyrazole and transition metal ions. Other than forming the [Cu3(μ3-OH)(PCA)3] SBU, the Cu(II) atom is also coordinated to the carboxylate group on another [Cu3(μ3-OH)(PCA)3] group, giving rise to a planar square coordination environment for Cu(II). Notably, a high density of open metal sites on SBUs can be formed directly without evacuating the coordinated water molecules. Structural analysis reveals that nano-cages with a diameter of 0.97 nm and windows with a diameter of 0.38 nm are involved in this MOF, with a high porosity of 47% calculated from the PLANTON21 program (see Fig. 1c–e).
3c. The coordination of three pyrazole groups with three Cu2+ ions and one bridging oxygen atom gives rise to triangle trinuclear [Cu3(μ3-OH)(PCA)3] secondary building units (SBUs) (see Fig. 1a and b). The [Cu3(μ3-OH)(Pz)3] (Pz represents pyrazole) group in this SBU is a commonly encountered coordination sphere created by pyrazole and transition metal ions. Other than forming the [Cu3(μ3-OH)(PCA)3] SBU, the Cu(II) atom is also coordinated to the carboxylate group on another [Cu3(μ3-OH)(PCA)3] group, giving rise to a planar square coordination environment for Cu(II). Notably, a high density of open metal sites on SBUs can be formed directly without evacuating the coordinated water molecules. Structural analysis reveals that nano-cages with a diameter of 0.97 nm and windows with a diameter of 0.38 nm are involved in this MOF, with a high porosity of 47% calculated from the PLANTON21 program (see Fig. 1c–e).
The PXRD pattern of the sample prepared from a scale-up experiment is consistent well with the pattern simulated from the single-crystal data, suggesting that this sample possesses the same structure with [Cu3(μ3-OH)(PCA)3] and has a high degree of crystallinity (as shown in Fig. 1e). Thermal gravimetric analysis (TGA) reveals that this MOF is stable up to 270 °C (see TGA curves in the ESI,† S2). After thermal activation at 130 °C, N2 adsorption at 77 K was performed on this sample. As shown in Fig. 1f, the typical type-I isotherm indicates the microporous nature of this MOF, which is agreeable with the crystallographic data. The BET surface area derived from the N2 isotherm is 583 m2 g−1, with a pore volume of 0.249 cm3 g−1.
Owing to the square planar coordination geometry for Cu(II) and unique configuration, the dual sides of each Cu(II) center are exposed in the nano-cages, leading to a relatively high density of accessible open metal sites for this MOF. The density of accessible open metal sites is calculated to be 28 nm−3, much higher than the famous MOFs with a high density of open metal sites, such as HKUST-1 (10.5 nm−3) and MOF-74 (4.6 nm−3). Besides, the nitrogen atoms in the ligand and oxygen atoms in the carboxylate groups could act as additional active sites for gas adsorption. The combination of nano-cages and the high density of open metal sites render this MOF a promising material for CO2 capture.
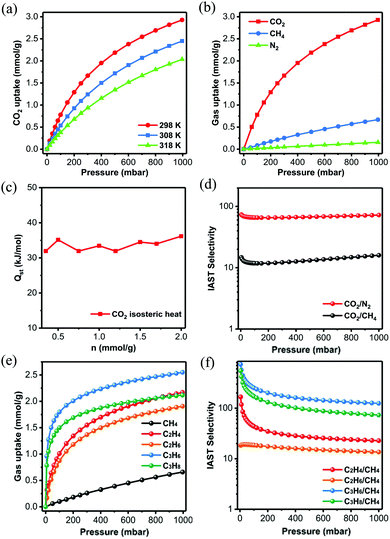
The isotherms of CO2, N2, and CH4 at ambient temperatures were measured on the activated sample of [Cu3(μ3-OH)(PCA)3] to investigate its gas adsorption properties. As shown in Fig. 2a, [Cu3(μ3-OH)(PCA)3] exhibits a CO2 uptake of 2.93 mmol g−1 (65.6 cm3 g−1) at 1 bar and 298 K. With increasing temperature, the CO2 uptake gradually decreases. In contrast to the high uptake of CO2, [Cu3(μ3-OH)(PCA)3] exhibits a near-linear N2 isotherm and displays a negligible uptake of N2 at 298 K (as shown in Fig. 2b). The significant difference between the isotherms of CO2 and N2 indicates the high adsorption selectivities for CO2/N2. Similar to N2, [Cu3(μ3-OH)(PCA)3] also displays a linear isotherm for CH4 at 298 K, exhibiting a CH4 uptake of 0.67 mmol g−1 at 298 K and 1 bar.
Based on CO2 isotherms at different temperatures, the CO2 adsorption enthalpy (Qst) was calculated by using the Clausius–Clapeyron relation (see the calculated procedure in the ESI,† S3). As shown in Fig. 2c, the Qst of CO2 adsorption is in the range of 31.5–36.1 kJ mol−1, with minor differences with increasing adsorption amounts. These Qst values are apparently larger than that of common MOFs, such as HKUST-1 (25.26 kJ mol−1)22 and NiDABCO (20 kJ mol−1).23 The large value of Qst suggests the high adsorption potential of [Cu3(μ3-OH)(PCA)3] to CO2 molecules. The high adsorption potentials to CO2 molecules could be attributed to the synergistic effect of nano-cages and the high density of open metal sites.
Based on adsorption isotherms, the equilibrium adsorption selectivity for the CO2/N2 mixture can be evaluated by the ideal adsorbed solution theory (IAST), of which several studies have widely validated the reliability.10,24 As shown in Fig. 2d, the IAST selectivity for the CO2/N2 mixture is calculated as 70 at 1 bar and 298 K (see calculation procedures in the ESI,† S4). This value is among the high ranks of adsorbents and is significantly higher than that of the common MOFs (see comparison Table in the ESI,† S5).18 Induced by the higher adsorption potential for CO2, [Cu3(μ3-OH)(PCA)3] shows a high CO2/CH4 selectivity. The equilibrium CO2/CH4 selectivity calculated from IAST is in the range of 11.8–15.9, which is also superior to the bench-marking MOFs, such as HKSUT-1 (5.5)18,22 and NiDABCO.23
The outstanding CO2 separation performance of [Cu3(μ3-OH)(PCA)3] also encourages us to investigate the separation performance for light hydrocarbons, which play crucial roles in petroleum chemistry. As shown in Fig. 2e, [Cu3(μ3-OH)(PCA)3] exhibits type-I isotherms for C2H4, C2H6, C3H6, and C3H8, which is in clear contrast with CH4. At 298 K and 1 bar, adsorption uptakes of 2.2, 1.9, 2.5, and 2.1 mmol g−1 are observed for C2H4, C2H6, C3H6, and C3H8, respectively. We can clearly see that the adsorption uptake increases with the chain length of light hydrocarbons. Furthermore, the adsorption uptakes of C2H4 and C3H6 are higher than those of C2H6 and C3H8, implying that alkenes are more preferentially adsorbed on [Cu3(μ3-OH)(PCA)3] than alkanes. Based on the isotherms at different temperatures, the Qst values of adsorption for C2H4, C2H6, C3H6, and C3H8 are calculated as 35.4, 33.8, 54.6, and 45.8 kJ mol−1, respectively. The higher Qst values for C3 molecules compared to C2 molecules indicate that [Cu3(μ3-OH)(PCA)3] displays a higher interaction strength with the hydrocarbons with longer chain length. Besides, [Cu3(μ3-OH)(PCA)3] shows a higher interaction strength with alkenes than alkanes. The preferential adsorption of alkenes to alkanes could be attributed to the π-electrons of alkenes, which could give back donations to open metal sites of MOFs. Due to the higher adsorption potentials, [Cu3(μ3-OH)(PCA)3] exhibits higher adsorption selectivity for C2/C3 hydrocarbons than CH4. Based on their adsorption isotherms, the equilibrium adsorption selectivities for C2H4/CH4, C2H6/CH4, C3H6/CH4, and C3H8/CH4 at 298 K are in the range of 22–167, 13–18, 124–737, and 73–550, respectively (as shown in Fig. 2f). Notably, at 298 K and 1 bar, high IAST selectivities of 124 and 73 have been reached for C3H6/CH4 and C3H8/CH4, outperforming common adsorbents and common MOFs.25,26 The excellent adsorption uptake and separation selectivity demonstrates the promising potential of [Cu3(μ3-OH)(PCA)3] in separating light hydrocarbons.
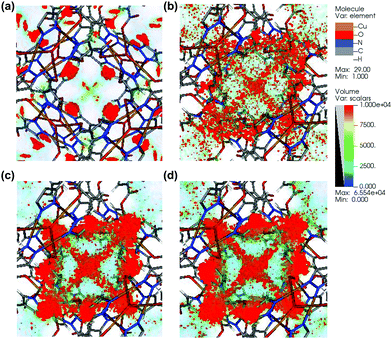
To investigate the separation mechanism, the Grand canonical Monte Carlo (GCMC)27 simulations for CO2, CH4, C2H4, and C2H6 adsorption on [Cu3(μ3-OH)(PCA)3] were performed (as shown in Fig. 3). It is clearly shown that CO2 molecules are concentrated near the tri-nuclear open metal sites, and the density of CO2 molecules near open metal sites is much higher than other sites. This phenomenon demonstrates that these open metal sites are the preferred adsorption sites for CO2. Compared to CO2, the CH4 molecules are more dispersed in the pore space, and the density near the open metal sites is much lower. This comparison result suggests the pronounced function of open metal sites in CO2 adsorption. Like CO2, the open metal sites exert high adsorption potentials to C2H4 and C2H6, which is agreeable with the experimental results. The density distributions of C2H4 and C2H6 molecules are much higher than CH4, indicating the preferable adsorption of C2H4 and C2H6 over CH4 molecules. The simulation results also confirm the effectiveness of incorporating a high density of open metal sites in enhancing the separation performance of MOFs.
To validate the actual separation performance of [Cu3(μ3-OH)(PCA)3], the breakthrough experiments were performed on the column packed with [Cu3(μ3-OH)(PCA)3] (as shown in Fig. 4). At a feed gas ratio of 50/50 and the pressure of 1 bar, N2 quickly breaks through from the packed column, while CO2 requires a rather long time to elute from the column (see Fig. 4a). The significant distinction in the breakthrough time of reaching the outlet of the packed column suggests that [Cu3(μ3-OH)(PCA)3] is capable of separating the mixed CO2/N2 (50/50) gas completely. Similarly, CO2 and CH4 also exhibit a remarkable difference in times of breaking through from the pack column at a feed gas ratio of 50/50, revealing a prominent separation performance for CO2/CH4 (see Fig. 4b). These results suggest that [Cu3(μ3-OH)(PCA)3] possesses great potential in capturing CO2 from flue gas and natural gas.
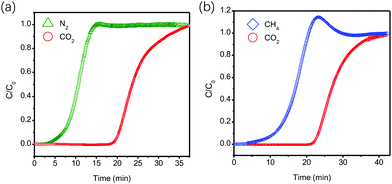 | ||
| Fig. 4 (a) Experimental column breakthrough curves for (a) CO2 and N2 mixtures (50/50, v/v) and (b) CO2 and CH4 mixtures (50/50, v/v) at a feed gas rate of 2 mL min−1. | ||
Conclusions
In summary, we herein revealed the outstanding CO2 separation performance on a metal–organic framework ([Cu3(μ3-OH)(PCA)3]) comprising nano-cages and a high density of dual-side open metal sites. Induced by the exquisite configuration of the network and tri-nuclear secondary building units, an ultra-high density of accessible open metal sites is generated in the pore channels of [Cu3(μ3-OH)(PCA)3]. As a result, this MOF exhibits significant differences in adsorption uptakes for CO2/N2 and CO2/CH4, making it a promising candidate for capturing CO2 from flue gas and natural gas. Furthermore, this MOF also shows outstanding separation performance in separating CH4 from C2–C3 light hydrocarbons. Theoretical investigation reveals that the open metal sites are preferable adsorption sites for CO2 and C2–C3 components. Breakthrough experiments also validate the outstanding separation performance for this MOF. The results of this work will open a new way to develop functional MOFs with high separation performance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the Major Scientific and Technological Innovation Project of Shandong Province (2020CXGC010402), the National Natural Science Foundation of China (No. 21973254 and 21401215), and the Fundamental Research Funds for the Central Universities (No. 18CX02068A).Notes and references
- R. S. Haszeldine, Science, 2009, 325, 1647–1652 CrossRef CAS PubMed.
- P. G. Boyd, A. Chidambaram, E. García-Díez, C. P. Ireland, T. D. Daff, R. Bounds, A. Gładysiak, P. Schouwink, S. M. Moosavi, M. M. Maroto-Valer, J. A. Reimer, J. A. R. Navarro, T. K. Woo, S. Garcia, K. C. Stylianou and B. Smit, Nature, 2019, 576, 253–256 CrossRef CAS PubMed.
- M. Oschatz and M. Antonietti, Energy Environ. Sci., 2018, 11, 57–70 RSC.
- C. A. Rowland, G. R. Lorzing, E. J. Gosselin, B. A. Trump, G. P. A. Yap, C. M. Brown and E. D. Bloch, J. Am. Chem. Soc., 2018, 140, 11153–11157 CrossRef CAS.
- L. Li, J. G. Bell, S. Tang, X. Lv, C. Wang, Y. Xing, X. Zhao and K. M. Thomas, Chem. Mater., 2014, 26, 4679–4695 CrossRef CAS.
- R. T. Yang, Adsorbents: Adsorbents: Fundamentals and Applications, John Wiley & Sons, Hoboken, 2003 Search PubMed.
- C. Liu, Y. Zhou, Y. Sun, W. Su and L. Zhou, AIChE J., 2011, 57, 645–654 CrossRef CAS.
- M. Luberti, D. Friedrich, S. Brandani and H. Ahn, Adsorption, 2014, 20, 511–524 CrossRef CAS.
- Y.-T. Wang, C. McHale, X. Wang, C.-K. Chang, Y.-C. Chuang, W. Kaveevivitchai, O. Š. Miljanić and T.-H. Chen, Angew. Chem., Int. Ed., 2021, 60, 14931–14937 CrossRef CAS PubMed.
- P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80–84 CrossRef CAS PubMed.
- F. G. Kerry, Industrial Gas Handbook: Gas separation and Purification, CRC Press, Boca Raton, FL, 2007 Search PubMed.
- O. T. Qazvini, R. Babarao and S. G. Telfer, Nat. Commun., 2021, 12, 197 CrossRef CAS PubMed.
- Y. Belmabkhout, P. M. Bhatt, K. Adil, R. S. Pillai, A. Cadiau, A. Shkurenko, G. Maurin, G. Liu, W. J. Koros and M. Eddaoudi, Nat. Energy, 2018, 3, 1059–1066 CrossRef CAS.
- M. K. Taylor, T. Runcevski, J. Oktawiec, J. E. Bachman, R. L. Siegelman, H. Jiang, J. A. Mason, J. D. Tarver and J. R. Long, J. Am. Chem. Soc., 2018, 140, 10324–10331 CrossRef CAS.
- Y.-S. Bae, K. L. Mulfort, H. Frost, P. Ryan, S. Punnathanam, L. J. Broadbelt, J. T. Hupp and R. Q. Snurr, Langmuir, 2008, 24, 8592–8598 CrossRef CAS.
- H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, Science, 2013, 341 Search PubMed.
- A. Cadiau, Y. Belmabkhout, K. Adil, P. M. Bhatt, R. S. Pillai, A. Shkurenko, C. Martineau-Corcos, G. Maurin and M. Eddaoudi, Science, 2017, 356, 731–735 CrossRef CAS PubMed.
- J.-R. Li, Y. Ma, M. C. McCarthy, J. Sculley, J. Yu, H.-K. Jeong, P. B. Balbuena and H.-C. Zhou, Coord. Chem. Rev., 2011, 255, 1791–1823 CrossRef CAS.
- X. Lv, L. Li, S. Tang, C. Wang and X. Zhao, Chem. Commun., 2014, 50, 6886–6889 RSC.
- S. Su, Y. Zhang, M. Zhu, X. Song, S. Wang, S. Zhao, S. Song, X. Yang and H. Zhang, Chem. Commun., 2012, 48, 11118–11120 RSC.
- A. Spek, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS.
- Q. Min Wang, D. Shen, M. Bülow, M. Ling Lau, S. Deng, F. R. Fitch, N. O. Lemcoff and J. Semanscin, Microporous Mesoporous Mater., 2002, 55, 217–230 CrossRef.
- P. Mishra, S. Edubilli, B. Mandal and S. Gumma, Microporous Mesoporous Mater., 2013, 169, 75–80 CrossRef CAS.
- A. L. Myers and J. M. Prausnitz, AIChE J., 1965, 11, 121–127 CrossRef CAS.
- M. Zhang, X. Xin, Z. Xiao, R. Wang, L. Zhang and D. Sun, J. Mater. Chem. A, 2017, 5, 1168–1175 RSC.
- K. Liu, B. Li, Y. Li, X. Li, F. Yang, G. Zeng, Y. Peng, Z. Zhang, G. Li, Z. Shi, S. Feng and D. Song, Chem. Commun., 2014, 50, 5031–5033 RSC.
- D. Dubbeldam, S. Calero, D. E. Ellis and R. Q. Snurr, Mol. Simul., 2016, 42, 81–101 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic details, crystal structural parameters, PXRD pattern, calculation procedures and additional kinetic profiles. See DOI: 10.1039/d1ma00919b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |