Open Access Article
Open Access ArticleRecent trends of contrast agents in ultrasound imaging: a review of the classifications and applications
Ali
Tarighatnia
abc,
Mohammad Reza
Fouladi
c,
Nader D.
Nader
d,
Ayuob
Aghanejad
 *a and
Hossein
Ghadiri
*bc
*a and
Hossein
Ghadiri
*bc
aResearch Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences, Tabriz, Iran. E-mail: aghanejaday@tbzmed.ac.ir; Tel: +98 41 33367914
bDepartment of Medical Physics and Biomedical Engineering, Tehran University of Medical Sciences, Tehran, Iran. E-mail: h-ghadiri@tums.ac.ir
cResearch Center for Molecular and Cellular Imaging, Advanced Medical Technologies and Equipment Institute, Tehran University of Medical Sciences, Tehran, Iran
dDepartment of Anesthesiology, University at Buffalo, Jacobs School of Medicine and Biomedical Sciences, Buffalo, New York, USA
First published on 31st March 2022
Abstract
Ultrasound (US) imaging, due to its capabilities of real-time imaging, portability, low cost and favorable safety, is frequently used as a diagnostic modality for the visualization of different diseases. US imaging is currently the first step in estimating the severity of oncological diseases, cardiovascular conditions, and for accurate assessment and diagnosis. Novel contrast agents have propelled US imaging into a new realm in the cellular and molecular fields and improved its sensitivity and specificity for detecting earlier stages of diseases. Selecting nanoparticles with appropriate structure and performance and a promising feature of binding to the target is a powerful strategy for the targeted imaging and early detection of disease. Here, we update the classification of the most attractive ultrasound contrast agents (USCAs), especially with regards to their advantages and disadvantages for application in US imaging. We also discuss how various technical detection modes of ultrasound imaging and quantitative analysis are affected by disease diagnosis. The clinical translations of US diagnostic strategies have prompted us to explore nanoparticle-based USCAs against various diseases. We also looked into the applications of USCAs in the diagnosis of cardiovascular disorders and oncological diseases based on anatomical section classification.
1. Introduction
Diagnostic imaging tasks are the first step in estimating the severity of a disease and for making an accurate assessment. The late diagnosis of malignant diseases and heart problems can lead to increased cost of cancer care and higher mortality rate.1 Different types of imaging modalities (e.g., positron emission tomography (PET), single-photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), computed tomography (CT), and US) have been used for the early detection of diseases in the clinical setting.2,3 In this regard, one of the frequently used imaging modalities that can evaluate both cardiovascular diseases and oncologic conditions is ultrasound (US) imaging.4–6 Among the different imaging modalities, US imaging has assumed a critical role compared to the other modalities due to its real-time and portable imaging capabilities. As a low-cost method with good safety (non-ionizing radiation) and due to its non-invasive and highly penetrating nature, it is more frequently used compared to the other modalities.7,8In US imaging, ultrasound waves propagate through different tissue interfaces. Depending on the acoustic impedance changes between interfaces, the intensity of the produced echoes from the boundaries of structures and from the underlying texture will change.9 However, while US imaging has a crucial role in assessing the extent of cancer lesions and cardiovascular pathologies, this technique cannot detect the diseases at early stages. In this regard, US imaging using nanoparticles (NPs) as contrast agents can provide early-stage diagnosis and improve imaging sensitivity and specificity. Moreover, US contrast imaging optimizes therapeutic strategies and reduces the mortality rate and the cost of care.10
Technically, ultrasound contrast agents (USCAs) increase the difference in acoustic impedance between tissues or within vascular/tissue interfaces, enhancing the reflected acoustic echoes. The acceptance criteria for USCAs are excellent acoustic impedance changes, appropriate stability, a proper size that can enable extravasation of the vascular space, good compatibility, and the necessary safety protections for live tissues. Various parameters can affect the acoustic echo intensity of USCAs, including the particle size, core material, substances, and shell thickness.11
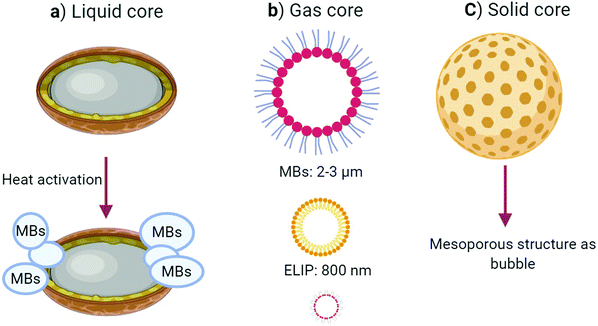
Although numerous NPs are utilized for many applications, including in diagnosis, targeted therapy, drug delivery,12–14 we focus here on the use of diagnostic micro/nanomaterials as USCAs, which can allow cardiovascular and oncologic detection. Generally USCAs are divided into three classes based on the type of their cores; gas, solid, and liquid (Fig. 1). In the following, some properties of the most recent USCAs in these classes are summarized.
2. Ultrasound contrast agents (USCAs)
2.1. USCAs with a gas core
USCAs with a gas core induce high acoustic impedance differences within tissue interfaces, and they can generate the highest acoustic intensity among the other classes. The utilization of gas-core USCAs for the induction of ultrasound contrast started in 1980, in which microbubbles (MBs) were produced by agitating normal saline serum using two syringes connected to a three-way stopcock. The designed MBs enabled a rigorous acoustic intensity when employing an echocardiogram device from a cardiovascular system. USCAs in this field are divided into microbubble (MB) or nano-bubble (NB) groups based on their size. MBs are often stabilized by lipid, synthetic polymer, or protein coatings.15Notwithstanding that the echo intensities of MBs are stronger than NBs, they suffer from poor stability in the bloodstream, a complex structure, and a shorter circulation lifetime due to their rapid detection and elimination by the reticular endothelial system (RES). Also, MB gas-core USCAs cannot be actively targeted for extravascular lesions because of their large sizes. These features have mainly restricted their applications to vascular space imaging, evaluating cardiac chambers, or targeting the intravascular markers of cancer.16 Even so, some targeted MBs have entered the primary clinical phases; some of the latest successes are presented in the applications section briefly.
It is worth noting that there is in fact a strong correlation between the particle size and acoustic echo reflectivity. On the other hand, NB gas-core USCAs can escape from the RES, penetrate the tumor tissue through the endothelial gap, and accumulate at the target site. Several microbubble USCAs, e.g., Definity, Echovist, and Sonovue, have been approved for clinical applications. USCAs with a gas core can be covered by lipid or polymer shells. These USCAs are either composed of gas or converted to gas by a unique reaction. They have some advantages like a high biocompatibility, favorable biodegradation, easy large-scale fabrication, simple surface modification, and satisfactory acoustic echogenicity.17 This type of USCAs includes nano-bubbles (NBs), echogenic liposomes (ELIPs), gas vesicles (GVs), and gas-producing nanoparticles (GPNPs).
The structures of NBs consist of a gas core with a coating layer, including lipid, protein, polymers (e.g., poly-lactic-co-glycolic acid (PLGA), poly-lactic acid (PLA), and polyethylene glycol (PEG)), to optimize the stability of the NBs’ structure. Furthermore, several strategies, such as employing a heavy gas core and conjugation with a targeting agent, have been implemented to maximize the stability of NBs.18,19 Due to their small size, they can be actively targeted in the extravascular space to detect cancer, though they have a relatively short half-life and lower stability. In one study, Yang et al. engineered nano-sized bubbles conjugated with affibody to detect human epidermal growth factor receptor 2 (HER2) overexpressing breast cancer cells using a conventional ultrasound scanner. The in vitro and in vivo images revealed that the NBs-affibody could be used as targeted USCAs in breast cancer diagnosis.20 Also, several targeting moieties have been conjugated to NBs, including prostate-specific membrane antigen (PSMA), anti-vascular endothelial growth factor (VEGFR) antibodies, and ovarian cancer antigen (CA-125), to create novel targeted ultrasound contrast agents.21–23 For example, anti-VEGFR-2 armed NBs were fabricated to selectively target the aorta atherosclerotic plaque in animal models (Fig. 2a–d).
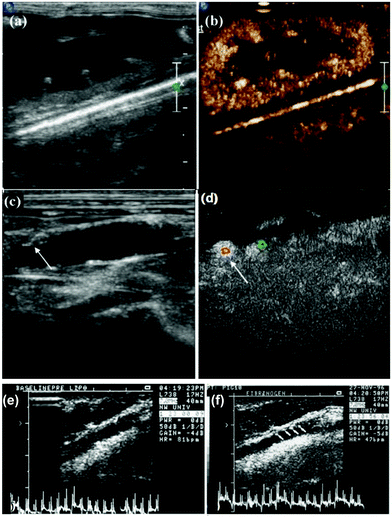 | ||
| Fig. 2 Biotinylated nano-bubbles for the in vivo US imaging of rabbit kidney (a) without NB injection and (b) after the injection of anti-VEGFR-2-conjugated NBs. Visualization of the abdominal aorta (c) without NB injection and (d) after the injection of the targeted NBs. Adapted with permission from ref. 22. (e) Comparison of the intravascular ultrasound (IVUS) images of the left carotid of swine after an injection of saline and (f) after an injection of targeted ELIPs. Adapted with permission from ref. 25. | ||
ELIPs are nanoscaled sacs with polar lipid bilayer shells in which air is entrapped inside their core or between phospholipid bilayers. They are used to enhance the ultrasound signal intensity. Due to their small size, liposomes can escape the vessel space and accumulate inside the tumor tissue. Furthermore, they can even be actively targeted by special binding moieties to connect to the surface, which subsequently enhances the acoustic signal. As mentioned above, the core between the liposome walls is filled with gas. ELIPs, as ultrasound contrast agents, enhance the ultrasound image contrast more than that of solid cores while their induced contrast is less than that of MBs. For bubble entrapment within the liposome structure, special engineering is required to obtain a better echo intensity from the liposomes24,25 (Fig. 2e and f).
Gas vesicles (GVs) are another type of novel gas-core ultrasound contrast agents. These vesicles are gas-filled protein shell enclosures with cylindrical shapes of a size in the range of 44–600 nm that have high gas permeability and are resistant to water penetration. These GVs may emanate from natural biological structures containing genetically encoded gas nanostructures formed by cyanobacterial and haloarchaeal host organisms or from Escherichia coli that can produce appropriate in vitro and in vivo acoustic echoes. These vesicles are often coated with gas vesicle protein A or B (GPA/B) or an external scaffold protein called gas vesicle protein C (VGC).26–29
GPNPs contain reactive compounds inside their base or shell, which release their compositions at temperatures higher than 42 °C. Following decomposition, they generate oxygen (O2) or carbon dioxide (CO2) gasses when reaching the target site. The released gas is used as an ultrasound contrast agent that enhances the acoustic intensity. Kang et al. developed a US contrast platform based on poly vanillin oxalate (PVO) NPs, which generate CO2 through hydrogen peroxide (H2O2)-triggered bubbles. These theranostic NPs significantly increased the ultrasound echo intensity due to accumulation at the liver target site.30 In another study, Min et al. offered a new strategy for CO2-producing bubbles as theranostic ultrasound contrast agents entitled gas-NPs with high performance for tumor investigation. These contrast agents have a higher echo intensity that requires employing a high-frequency ultrasound scanner with novel gas-generating NPs both in in vitro and in vivo settings.31 In another report, H2O2 was converted to oxygen (O2) bubbles by decorating magnetic nanoparticles (MNPs) on black phosphor sheets, which increased the acoustic intensity during US imaging. This acoustic enhancement appeared to be due to the overexpression of these reactive radicals at the tumor site rather than the normal tissues in the presence of magnetic nanoparticles (MNPs) through an active or inactive targeting mechanism. As a reminder, the intensity and efficiency of gas production depend on the temperature, hydrogen peroxide concentration, and acidic environment.32 Some of the most critical studies on USCAs containing a gas core are summarized in Table 1.
| Core type | Shell materials | Size (nm) | US mode | Freq. (MHZ) | Destructive threshold (MI) | Half-life in vivo | Lesion detection | Advantages/disadvantages | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Biotin–DSPE | PEG2000 lipid | 320 | D Mode | NA | NA | NA | Atherosclerotic plaque-aorta | Long-term stability, targeting VEGFR2 | 22 |
| DSPE–C3F8 | mPEG2000 lipid | 277 | CHI Mode | 12 | 0.1 | 30 min | Prostate cancer | High stability, improved sensitivity and specificity for PSMA | 33 |
| C3F8 | PEG-lipid | 533 | B & CE Mode | 13–24 | NA | 10 min | Breast cancer | Long-term stability targeting AS1411 | 34 |
| DPPE–DPPG | Lipid | 1400 | B Mode | 20 | NA | 120 min | — | High stability, high echo, no toxicity | 35 |
| Raw bovin milk HEPES | BSA | 110 | B & CHI Mode | 40 | NA | — | Synovial fluid knee | No toxicity | 36 |
| CaCO3 | PDA–BSA–RBC | 572 | B Mode | 40 | NA | 90 min | NA | High stability, high echo | 37 |
| CaCO3 | Polymer Pul-PCB | 380 | B-Mode | 40 | NA | 60 min | Liver Hpg2 | High stability | 38 |
| Bacteria–archaea | Protein | 45–600 | CHI Mode | 5–20 | NA | 20 min | IVC liver | High stability and biocompatible | 29 |
2.2. USCAs with a liquid core
Generally, in terms of echogenicity and stability, liquid-core USCAs have advantages over gas-core USCAs and disadvantages over solid-core USCAs. Liquid-core USCAs provide poor contrast enhancement because of their weak acoustic scattering inside the arteries due to their low impedance. In this regard, liquid-based materials, such as perfluorocarbon (PFC), including perfluoro-pentane (PFP), perfluorooctyl bromide (PFOB), and per-fluoro-hexane (PFH), if they accumulate in the target tissue/cells or change phase from liquid to a gas by applying thermal energy, either or both, have produced an impressive echo in preclinical experiments.39Nanodroplets (NDs) as USCAs consist of a liquid core with a low boiling point, and include perfluorocarbon (PFC), perfluorooctyl bromide (PFOB), and perfluoro-pentane (PFP) encapsulated with an organic shell. These can be converted from a liquid phase to the gas phase upon heating. They are also called phase-change droplets (PCDs).40
The NDs can be transformed based on activation or a radiation source trigger through an ultrasound beam with a frequency-dependent heat generation pulse duration and variable temperature, which is the basis for acoustic droplet vaporization (ADV). Also, micron-sized bubbles could be obtained from near-infrared (NIR) irradiation, which is the basis for optical droplet vaporization (ODV). There are other stimulation strategies for converting the liquid phase to gas, including the use of magnets or magnetic, microwave, and radiofrequency droplet vaporization (magnetic droplet vaporization (MDV), microwave droplet vaporization (MWDV), and radiofrequency droplet vaporization (RFDV)). In the MDV method, the magnetic NPs can potentially generate heat under a magnetic field induction and release the encapsulated materials inside the NPs, subsequently increasing the ultrasound image contrast. Due to NIR's lower penetration, the RFDV stimulation amount is reduced compared to in ADV stimulation by the US beam. ADV has a higher penetration depth, and it is consequently more efficient in phase transformation.41–43 Some of the USCAs containing solid cores are summarized in Table 2.
| Core type | Shell material | Size (nm) | US mode | Freq (MHZ) | Phase transition | Destructive threshold (MI) | Half-life in vivo | Lesion detection | Advantages/disadvantage | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| PFB | PEG5000 | 114 | B Mode | 4 | Liquid to gas (ADV) | 1.7 | 45 min | Fibrosarcoma | High echo, minimal side effects | 44 |
| PFP | PEG-FA | 47 | B & CE Mode | 5–12 | Liquid to gas (ADV) | 0.1 | 30 min | Prostate | PSMA targeting, long-term stability | 33 |
| PFH | RBC-IR780 | 261 | B & CE Mode | NA | Liquid to gas (ADV) | — | — | — | High stability | 45 |
| PFP | PLGA-PVA | 294 | B & CE Mode | 7.5 | Liquid to gas (ODV) | NA | 4 h | Tissue tumor | Dual modality (US-MRI), minimal side effects | 46 |
| PFH | PLGA-PVA | 435 | B Mode | 30 | Liquid to gas (ODV) | NA | 2 h | Lymph nodes | High sensitivity/stability, low toxicity | 47 |
| DFB | PEG-GLC-CYN | 400 | B Mode | 8 | Liquid to gas (ODV) | 1.2 | NA | — | Multimodal, high stability | 48 |
2.3. USCAs with a solid core
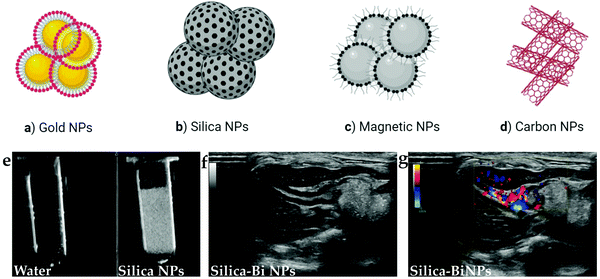
Solid-core USCAs have been developed as suitable surrogates to other USCAs for ultrasound contrast enhancement due to their unique properties. These properties include higher stability and desirable acoustic impedance changes between the solid-core materials and soft tissue. These features of solid-core USCAs lead to more robust reflectivity production, an improved signal to noise ratio (SNR), and more contrast enhancement. Solid-core USCAs have high echo intensities, both intravascular and within the soft tissues.49,50These NPs have various features and select properties, including small size, multi-functionality, favorable stability, facile fabrication process, highly targeted turnover, high abundance, and versatile structures. Typically, solid-based ultrasound contrast agents have a relatively lower acoustic intensity than organic types, e.g., MBs. Solid-core agents have bases made up of different materials, including silica, gold, carbon, magnetic, which are reviewed in the following.51Fig. 3 schematically illustrates the different types of solid NPs that are currently employed as USCAs.
Silica-based NPs have drawn significant attention as USCAs owing to their select properties, such as biocompatibility, unique porosity structure, large pore volume, high specific surface area, and controllable particle size, which has seen them proposed as enhanced USCAs with broad applications. Silica-based NPs are easy to surface modify, which allows them to be synthesized and designed in different ways and using different compositions, including solid silica, mesoporous silica nanospheres (MSNs), Stober silica nanospheres (SSNs), mesocellular foam (MCF), rattle-type, hollow mesoporous silica nanoparticles (HMSNs), exosome-like silica (ELS), and silica-based composites. A suitable echo can be achieved in the solid form due to this type's solid nature, which allows higher impedance differences. In HMSNs, a desirable echogenicity can be produced. Furthermore, with a unique rattle-type design, an increase in US echo over HMSNs has been observed.52–54
Carbon-based NPs in the form of multi-walled carbon nanotubes (MWCNTs) can effectively increase the US signal compared to clinical contrast media (SonoVue) and graphene oxide. This carbon design type has succeeded in extending the echo time to show internal organs, both ex vivo and in vivo, during US imaging. The excellent echo for such a design may be due to the entrapment of small bubbles in the nano complex walls, making a considerable impedance difference.55
Gold in certain forms and morphologies can have major diagnostic applications in US imaging, e.g., in echogenic enhancers. For example, gold nanoshells (NSs) have excellent echo intensity in both B and pulse inversion harmonic ultrasound imaging. Also, the integration of gold with other materials, such as PFH and PFOB, with unique light absorption properties enables laser or high-intensity focused ultrasound (HIFU) conversion to heat and MB production, facilitating US contrast enhancement.45,56
Moreover, magnetic NPs can be excited by an external magnetic field. This process produces heat and then diffuses the produced heat to the surrounding environment. The operation mechanism in the presence of magnetic NPs inside a target site in which a gas is trapped is such that when the area is exposed to a magnetic field while performing simultaneous US imaging, due to the release of gas inside the magnetic NPs under heating, a suitable echo can be obtained.57 Selected categories of USCAs containing solid cores are summarized in Table 3.
| Core type | Shell materials | Size (nm) | US mode | Freq (MHZ) | Destructive threshold | Half-life in vivo | Lesion detection | Advantages/disadvantages | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Silica | APTES-HER2 | NA | B Mode | 10 | — | — | Breast | High stability, low toxicity, biocompatible | 58 |
| Silica | APTES-CYN | 384 | B & D Mode | 5 | 1.3 | 60–120 min | Myocardium | Biocompatible, biodegradable | 59 |
| Gold | BSA-ICG | 51 | B Mode | 33 | — | — | — | High stability | 60 |
| Carbon | Protein | 279 | B & CE Mode | NA | — | 20 min | Breast | Easy modification | 54 |
| Carbon | Silica | 20–30 | B & THI Mode | 5–12.5 | 0.9 | — | — | No toxicity | 61 |
| Iron | PEG-PLGA | 9–18 | CE | NA | — | 72 h | Ovarian | Biocompatible, high stability, dual modal (US-MR) | 62 |
3. Ultrasound detection methods for the qualitative and quantitative assessment of contrast-enhanced images
Both targeted and non-targeted USCAs can be detected with various US imaging methods, including B-mode, Doppler (power or color), harmonic imaging, pulse inversion and multi-pulse imaging, and the contrast mode technique.63Typically the B-mode and power or color Doppler imaging modalities are primary used in clinical practice. The B-mode is the most common US imaging method, which shows the organs in gray levels corresponding to the amplitude of the returned acoustic intensity. Still, the Doppler mode mainly demonstrates tissue perfusion, including allowing assessing stenosis or a lack of flow of large arteries up to 200 μm in diameter.64 In harmonic imaging, also called non-linear scattering response, which employs a specific frequency (e.g., 1F), the microbubbles contrasts will have non-linear contraction and expansion. In contrast, the surrounding tissues have regular oscillation, resulting in receiving waves 2F in frequency. The harmonic signal can be amplified and detected using a proper filtering technique (such as amplitude modulation and pulse inversion). It has been shown that harmonic imaging, contrast mode, and power Doppler imaging provide higher resolution, a stronger SNR, and greater details of blood flow, respectively.65
However, these modes may not detect specific conditions, including microcirculation within the vessels and tiny changes of the tissues in the early stages. Using USCAs, ultrasound sensitivity has improved its utility in diagnosing lesions, quantifying microvascular blood volumes, and assessing he blood flow to vital organs in humans.66 In this regard, several techniques have been suggested; in the first method, there is no need for continuous US imaging, and obtaining an image of the target site at certain delayed phases is sufficient to prevent signal interference between the target tissue's circulatory system. Also, ideally USCAs will have a desirable opportunity to accumulate in the target tissue. The delay time depends on the contrast agents' core, shell, and target tissue structure. Contrary to the above-mentioned method, continuous US imaging of the target site should be performed by another approach. However, the curves' increase and decrease in signal intensity can be investigated. Ultimately, the images are subtracted from each other. There are several significant points in contrast-enhanced ultrasound (CEUS) imaging, and in particular, choosing the best imaging protocol is always an essential part of clinical imaging tasks, such as the optimal choice of using plane waves or focused imaging, the values of the focal depth, the F-number, mechanical index, dynamic range, bandwidth, and number of angles, which all play drastically important roles. Moreover, optimizing the structure of the transducer, selecting the appropriate detection mode, and choosing the proper qualitative and quantitative analysis methods are recommended. Furthermore, early and delayed phases should be evaluated in in vivo ultrasound experiments using targeted USCAs.67
Several models and methods have been developed for the quantitative analysis of US images to improve their diagnostic power. One of the most used quantification methods is the analysis of the time-intensity curve (TIC). TIC is the curve that shows changes in the echo amplitude over time and is extracted from the administered USCA route. It can be used to evaluate the wash-in and wash-out or increase and decrease in intensity over time due to the transfer of USCAs through the elective tissue. Generally, this method is known as temporal analysis. On the other hand, other parameters, such as the blood flow, contrast dose, area under the curve (AUC) for peak enhancement (PE), and volume, may be extracted by using this method.67
Furthermore, CEUS imaging for probing tissue perfusion, TIC, as well as statistics-based time-Nakagami curve (TNC) approaches can be used for tissue perfusion quantification. As the most recent development in this era, window-modulated compounding (WMC) Nakagami parameter ratio imaging has been used to overcome the inefficiencies of TNC in tissue clutters and in the presence of sub-resolvable effects.68
There is another quantitative analysis method named spatial analysis. In this analysis method, informative parameters, like the regional density uniformity and type of intravascular plaque lesions, are extracted from the perfusion and distribution of USCAs. The regional density uniformity can be used for detecting whether the cancer is malignant or not. It is worth mentioning that spatial analysis can be used with abnormal and very small vessels, in which temporal analyses are inefficient.68 By integrating temporal and spatial analyses, another methodology known as spatiotemporal analysis can be seen. In this method, more comprehensive information, including on the vascular architecture, detailed perfusion, fractal dimensions, and nano contrast dispersion, is obtained from an arbitrary region of interest (ROI).68
Furthermore, in a latest attempt, due to the need to assess several parameters simultaneously, the multiparametric image analysis method using machine learning has been developed.69 A significant development in the quantitative analysis of CEUS images has been obtained using radiomics methods to classify and evaluate some malignancies. Also, machine learning was applied in one study through a random forest classification algorithm, using the labels extracted from histopathology data from radical prostatectomy specimens as a reference to draw benign and malignant ROIs.70
4. Application trends of USCAs in the diagnosis of diseases
In recent years, with the improvement of USCAs, more information is available on pathological and physiological conditions. Therefore, the US modality has found broad applications in visualizing the body structure, including vessel evaluation and assessments of pathologic conditions. Several studies have focused on specific ultrasound contrast agents or a particular disease, and have illustrated the improvement of contrast-enhanced ultrasound imaging techniques for detecting particular cancer.71,724.1. USCAs in cardiovascular imaging
USCAs have been developed for both clinical and preclinical cardiovascular applications, with the major clinical applications focused on the cardiovascular field using USCAs based on MBs. Applications have been established for the delineation of valvular, atrial, and ventricular cavities malfunctions, as well as for demonstration of endocardia boundaries in myocardial perfusion, and for the visualization of inflammation, thrombosis, and ischemic lesions. Some USCAs have been commercialized and used in the clinic, including development as a myocardial contrast to display tissue perfusion or for red blood cell (RBC) tracking. Injected MBs represent the volume of blood entering the myocardium through the microcirculation; thereby increasing the echo signal.73–75Inflammation is a pathophysiological manifestation of vascular disease, in which leukocytes are activated and transported to the extravascular space and accumulated in inflamed tissues. Receptor mediators with integrin, P-selectin, and E-selectin integrated MBs are used to display myocardial inflammation, signs of ischemic lesion, or the presence of an atheroma plaque. Furthermore, endothelial inflammation occurs in the following conditions: atherosclerosis, ischemia, and implant rejection, which can be detected by antibody attached intercellular adhesion molecule-1 (ICAM1) and P-selectins conjugated with MBs.76,77Fig. 4 presents the ultrasound imaging of left anterior descending coronary artery (LAD) ischemia in a canine heart using TS-07-009 (MBs conjugated with MFG01035 recombinant protein) and P-selectin conjugated MBs (MB-PSGL-1). The results reveal that MB-PSGL-1, with more selectivity to P-selectin than E-selectin, exhibited a higher uptake at the ischemic sites than TS-07-009 with a similar selectivity to P- and E-selectin (Fig. 4F and G).78
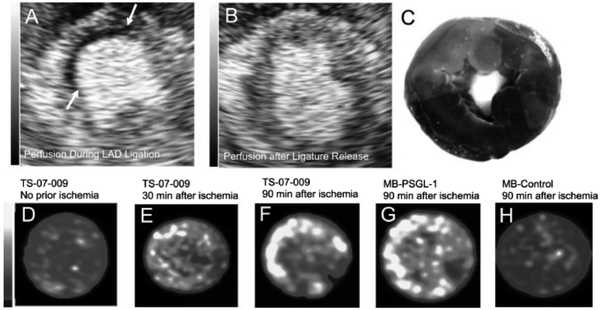 | ||
| Fig. 4 US images of LAD ischemia in canine heart. Short axis myocardial perfusion images (A) during ligation of the LAD suture and (B) after release of the LAD suture (arrows indicate the location of the ischemic zone. (C) Phthalocyanine staining image of the perfused zones (dark) and ischemic regions (bright red). TS-07-009 accumulation images in (D) the baseline heart, (E) at 30 min and (F) 90 min post LAD ischemia cessation. (G) Uptake of P-selectin-conjugated MB (MB-PSGL-1) and (H) unarmed MBs (control group) 90 min post cessation of ischemia. Adapted with permission from ref. 78. | ||
Angiogenesis describes the formation of new capillaries from pre-existing vessels and is prone to bleeding into the atherosclerotic plaque, which can activate the platelets and cause thrombosis. Briefly, hypoxia occurs when an atheroma plaque grows up in the arterial wall, which is considered a triggering factor for the development of angiogenesis, also known as vasa-vasorum. Angiogenesis plays a vital role in assessing atheroma plaque progression deposited between the vascular walls; there is also a correlation between atherosclerosis and angiogenesis.79
In this regard, several markers activated on endothelial cells, including integrin (αvβ3) and vascular endothelial growth factor (VEGFR-2) conjugated with MBs as contrast-enhanced ultrasound agents, have been developed. It has been shown that the mean pixel intensity (MPI) of VEFR-2@ MBs as CEUS is higher than targeted αvβ3 in the detection of angiogenesis.80
The targeted ultrasound imaging of atherosclerosis has two specific features compared to other endothelial lesions: first, the loss of signal demonstration in the atheroma plaque regions and second, the inability to identify atherosclerosis using a low-frequency system (contrary to other lesions) in inflammation and angiogenesis (in which this lesion requires high-frequency devices, such as catheter-based intravascular ultrasound (IVUS)).81 Several studies have explored atherosclerotic lesions, mostly with a focus on determining the activated platelets and evaluating inflammation for the early detection of plaque progression; therefore, they utilize the glycoproteins on platelets bonded MBs to monitor plaque progression or for an assessment of the response to treatment intervention.82,83 Conversely, other studies have applied inflammatory markers, such as vascular cell adhesion molecule-1 (VCAM1) and ICAM1, to identify the atheroma plaque situation.84
In one study, magnetic nanoparticle microswarms (MNP micSW) were used for the real-time investigation of swarm formation in blood vessels under ultrasound Doppler imaging. As shown in Fig. 4, the rotating micSW disrupted the normal flow of blood cells. Moreover, US waves emitted to micSW, providing an ultrasound Doppler imaging modality for medical imaging-guided SW navigation in the blood vascular system (Fig. 5).85
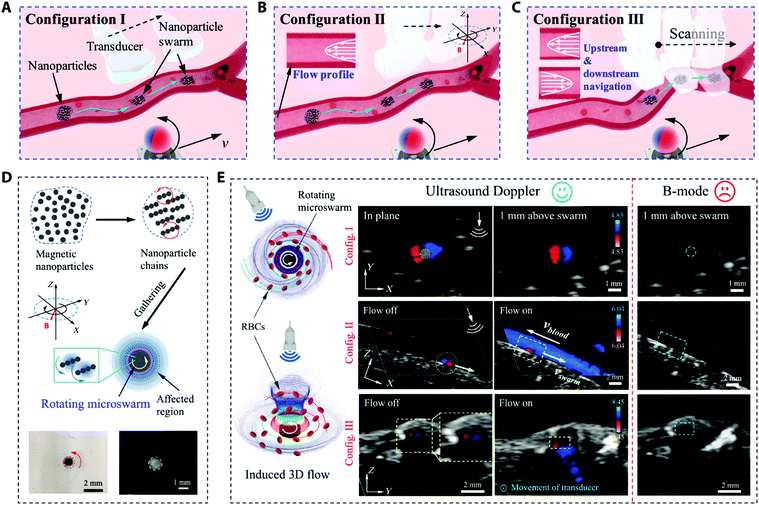 | ||
| Fig. 5 (A–C) Schematic of the US Doppler image-guided swarm (SW) formation and motion in blood vessels. (D) SW formation process, and the magnetic and hydrodynamic interactions of the rotating NPs chains that cause the assembly of the nanoparticles to be a rotating microswarm (micSW). Left (light microscope image) and right panels (B-mode US image) illustrating a micSW in water–glycerol solution and porcine whole blood, respectively. (E) The US Doppler signal around a rotating micSW in blood. Blue dashed lines signify the theoretical position of the micSW in the B-mode US images (right column). Adapted from ref. 85. | ||
In clinical practice, Doppler ultrasound and echocardiography have been used to demonstrate extensive or moderate deep venous thrombosis (DVT) and intracardiac clots, respectively; however, these techniques cannot display the microvascular clots, especially in carotid and coronary arteries. On the other hand, it is essential to distinguish acute thrombosis from chronic thrombosis for providing proper treatment promptly. The use of a targeted contrast agent in ultrasound is recommended, such as for thrombosis using thrombin-responsive NBs.86 The application of USCAs in cardiovascular imaging is summarized in Table 4.
| Lesion type | Types of USCAs | Size of USCAs | Targeting moieties | Lesion site | Ref. |
|---|---|---|---|---|---|
| Inflammation | MBs | 2.2 μm | P-selectin | Abdominal aorta | 87 |
| 3.4 μm | E-selectin | Myocardium | 88 | ||
| <8 μm | P&E selectin | Myocardium | 78 | ||
| 1.5 μm | VCAM1 | Aorta | 89 | ||
| 1 μm | αvβ3 | Carotid arteries | 90 | ||
| Atherosclerosis | MBs | <400 nm | ICAM1-VEGFR | Aorta | 91 |
| NA | VCAM1 | Thoracic aorta | 92 | ||
| 10 μm | Glycoprotein | Carotid Arteries | 93 | ||
| NBs | 200 nm | VEGFR2 | Abdominal aorta | 22 | |
| Myocardial ischemia | MBs | 3.4 μm | E-selectin | Myocardium | 88 |
| <8 μm | Dual(P&E) selectin | Myocardium | 78 | ||
| HMSN | 372.6 nm | — | Myocardium | 59 | |
| Angiogenesis | MBs | 2.8 μm | VEGFR2 | — | 94 |
| NA | αvβ3 | Carotid | 95 | ||
| Thrombosis | MBs | 1.6 μm | Thrombin | — | 96 |
| NA | E-selectin | Iliac vein | 97 | ||
| PFP | 256.6 nm | Fibrin | Inferior vena cava | 98 |
4.2. USCAs in cancer imaging
Ultrasound contrast agents as markers of vascularity and microcirculation for the early recognition of oncologic cases have been developed rapidly, typically employing different targeted ligands. In this context, angiogenesis is an essential process in demonstrating the severity of the invasion and metastasis of tumors and for delineating the extent of tissue adaptation to chronic ischemia. As mentioned in the previous section, the following target moieties markers are used to detect angiogenesis: VEGFR, αvβ7 integrin, monoclonal antibody, and arginine–glycine–aspartic acid (RGD). The most targeted uses of USCAs are related to breast, colon, prostate, and ovarian cancers, respectively (Table 5). Accordingly, based on the type of ligand with a specific application, they are divided into a number of separate categories representing the oncologic applications, as discussed below.| Body system | Organ | Types of USCAs | Targeting moieties | Size | Ref. |
|---|---|---|---|---|---|
| Urogenital cancers | Renal | MBs | VEGFR2 | — | 99 |
| VEGFR-FSHR | — | 100 | |||
| Prostate | NBs | PSMA | 533 nm | 34 | |
| RGD | 210 nm | 101 | |||
| ICAM1 | 683 nm | 102 | |||
| CDCP1 | 172 nm | 103 | |||
| Carbon | PSCA | 75 nm | 104 | ||
| PEG- PLA | — | 2.5 μm | 105 | ||
| MSN | — | 161 nm | 106 | ||
| Gynecological cancers | Breast | MBs | B7-H3 | 1–4 μm | 107 and 108 |
| iRGD-αvβ3 | 1–4 μm | 109 | |||
| Gold-PLGA MBs | VEGFR2-P53 | 277 nm | 110 | ||
| PFP-based NBs | — | 362 nm | 111 | ||
| PLGA-PEG NBs | mAbCAIX | — | 112 | ||
| AnnexinV | 635 nm | 113 | |||
| NBs | Aptamer AS1411 | 533 nm | 34 | ||
| NB-Affibody | HER2 | 478 nm | 20 | ||
| Au | Poly Dopamine | 27.5 nm | 114 | ||
| Ovarian | NBs | Pro GRP | 378 nm | 115 | |
| CA-125 | 75 nm | 116 | |||
| Pt(IV)/PLGA-PEG | cRGD | 43 nm | 117 | ||
| PLGA-SA/PFP | SA | 383 nm | 118 | ||
| NBs | VEGFR2 | — | 119 | ||
| Au | Peptide-Gly3 | 230 nm | 120 | ||
| Cervix | MBs | anti-PD-L1 ab | 939 nm | 121 | |
| Respiratory cancers | Larynx | MBs | RGD | 2.3 μm | 122 |
| Lung | NBs | Liposome | 378 nm | 115 | |
| Gastrointestinal cancers | Bowel | MBs | P&E selectin | 1–3 μm | 123 |
| MBs | VEGFR2 | — | 124 | ||
| PFC-PDA | — | 255 nm | 125 | ||
| Liver | BR55 | VEGFR2 | 1–4 μm | 126 | |
| MBs | VEGFR2 | 1–4 μm | 127 | ||
| Nanogels | — | — | 128 | ||
| Pancreas | MBs | VEGFR2 | 1.6 μm | 129 | |
| VEGF | — | 130 | |||
| Thy1 | 1–3 μm | 131 |
Prostate cancer is the fourth most common cancer in men. The diagnosis of this cancer in the first stage can reduce the mortality rate. In early attempts, researchers focused on evaluating prostate malignancy's angiogenesis employing targeted MBs that express VEGFR2, ICAM1, and αvβ3-integrin. In the next step, a new ligand PMSA aptamer was conjugated with NBs in a mice model, which reduced previous studies' limitations and increased the ultrasound imaging specificity. In the clinical phase, a group of researchers used BR55 (KBR integrated with MBs to provide an appropriate assessment of prostate cancer's malignant stages by targeted ultrasound imaging.132 In another study, PSMA-targeted-NBs were developed as a biomarker of PSMA-negative PC3flu and PSMA-positive PC3pip for simultaneously imaging in a mouse model (Fig. 6). The results proved the active targeting and tumor accumulation by the PSMA-targeted-NBs.21
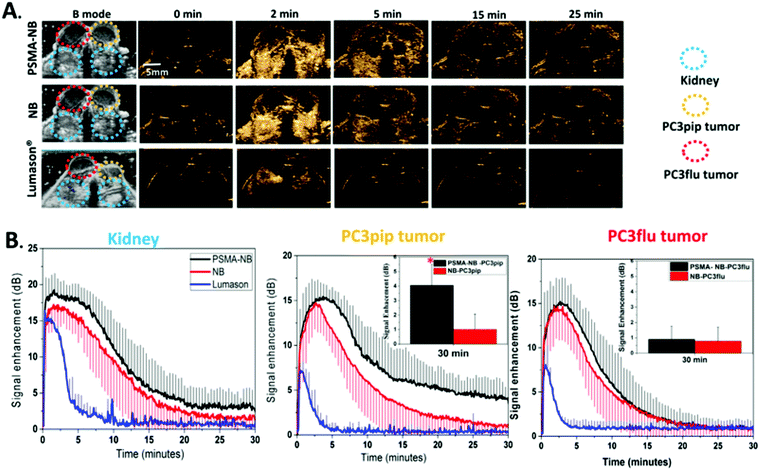 | ||
| Fig. 6 (A) Comparison of ultrasound images of prostate cancer and kidney between PSMA-NB, NB, and Lumason USCAs in consecutive times. (B) Quantitative analysis in terms of the time–intensity curve (TIC) using USCAs in the kidney (left) and for two types of prostate cancer (middle and right). Adapted with permission from ref. 133. | ||
Since ovarian cancer may have no symptoms, it is hard to diagnose this type of cancer in the early stages. However, it remains the most lethal of all gynecological cancers. In the evaluation of ovarian carcinoma, several targeted USCAs, including CA-125 targeted NBs (αvβ3) integrated Sonazoid (MBs-based), have been developed, which accumulate in the ovarian tumor and increase the ultrasound signal intensity significantly. In the human phase, BR55 targeting KDR has also been considered to detect ovarian cancer.136
In one study, MUC16 conjugated bismuth-coated mesoporous silica NPs (MSBi@MUC16-NPs) were used as dual modal US/CT contrast agents for the targeted diagnosis of cervical cancer in an animal model (Fig. 7). The results revealed that by employing MSBi@MUC16-NPs, the ultrasound echo intensity was enhanced for the targeted NPs, both at the cellular level and in a HeLa tumor-bearing animal model.137
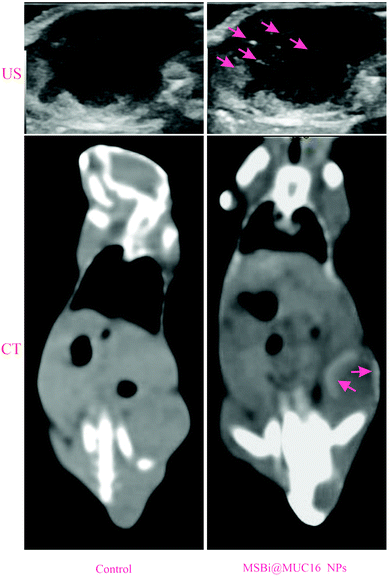 | ||
| Fig. 7 US/CT targeted images of MSBi@MUC16 NPs in HeLa tumor-bearing BALB/c mice pre and post-injection of contrast agents. The contrast enhancement of the tumor site is apparent due to a high uptake of the targeted NPs. Adapted from ref. 137. | ||
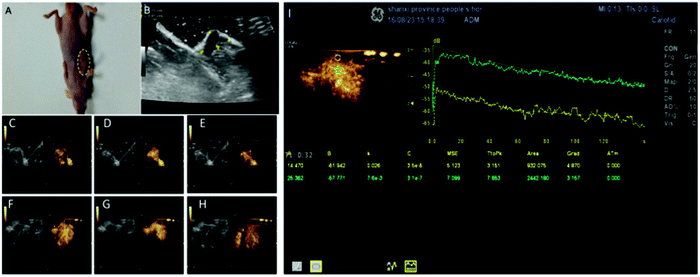 | ||
| Fig. 8 (A) The tumor size was 1.18 ± 0.12 cm. (B–H) Quantitative analysis of the SCLC xenograft tumor in the early and late stage using targeted USCAs. (I) TIC related to normal and cancerous tissues. Adapted from ref. 115. | ||
Also, the CEUS of a tumor before UTMD indicated that the echo of the tumor site was enhanced after the injection of targeted MBs (Fig. 9).
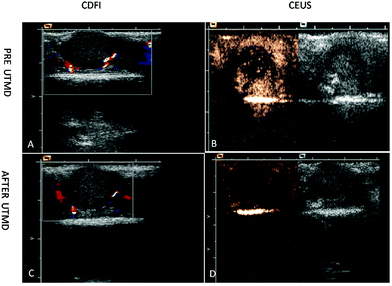 | ||
| Fig. 9 CDFI and CEUS images of intrahepatic tumors in mice after 7 days of injection. (A and C) CDFI of the tumors before and after UTMD treatment. There was a strip blood flow signal around the tumor site when using CDFI. CEUS of tumor (B) before UTMD and (D) after UTMD (MBs in the tumor disappeared). Adapted with permission from ref. 141. | ||
One of the most common pancreatic cancers is ductal adenocarcinoma; and though it does not have a good prognosis, its detection and accurate staging can improve the patient's condition. In clinical practice, ultrasound imaging, the initial technique in substantial clinical applications, enabled pancreatitis detection, and allowed displaying the lesions and appearance staging of the adenocarcinoma. Ultrasound of the pancreas can be performed in various ways, including transabdominal, endoscopic ultrasound, intraoperative ultrasound (IOUS), and contrast-enhanced ultrasound (CEUS). Even by using targeted MBs conjugated with different ligands, intraoperative ultrasound can facilitate the differential diagnosis of pancreatic malignancy and tiny lesions of tumors. To achieve this goal, for displaying angiogenesis of a tumor and visualization of the neo-vasculature, several ligands (VEGFR-2, integrin, endoglin, and Thy-1) integrating MBs have been tested in mice models or clinical trials.112
5. Challenges and future outlook
There are still some fundamental challenges to designing ideal USCAs. One controversial debate for utilizing USCAs is in designing and controlling the USCAs’ size in the preparation steps. When contrast agents enter the body, circulate through the vascular and are distributed, they can interact with plasma proteins, which can lead to a change in the surface charge of the agents and also to their small size, giving rise to larger sizes. In such a way, regardless of the basis of USCAs, whether gas, liquid, or solid based, they are mainly eliminated by the reticulo-endothelial system (RES). Even if they succeed in avoiding clearance by the RES system, they cannot escape the vascular space. This phenomenon will be more significant if the ultrasound contrast materials are gas based or gas convertible materials with a larger size. In this situation, their circulation appears faster and they cannot extravagate into the tumor site, and consequently, their ability to target tumors is reduced significantly. As a reminder, some of the microbubbles are destroyed over time by the high ultrasound acoustic pressure.In contrast, small-sized materials have a weak signal echo intensity, and are almost entirely removed by glomerular filtration by the kidneys. Hence, it can be said that the both clearance and distribution of USCAs are mainly determined by their size, and ultimately both approaches (large and small sizes) have confined limits in their detection capabilities. Therefore, a balance must be struck between the signal intensity and their size. It is expected in the near future, that some effort will be focused on synthesizing nano-sized drug-delivery systems engineered using gas-based USCAs to preserve the appropriate signal echo intensity.
With respect to the USCAs’ size, solid-based ultrasound contrast agents tend to be ductile materials with favorable degradability and greater resistance to acoustic output compared to bubbles, which were the focus of the earliest attempts to provide novel synthesis methods with a simplified design of non-gaseous materials as ultrasound contrast agents, i.e., as an alternative approach to gas-based USCAs. In this regard, the most current research approach is the development of solid-based USCAs by targeting different ligands to assist in reducing the amount of contrast agents to be applied. In this manner, researchers aim to pave the way toward the development of theranostatic materials to facilitate the drug-delivery process and to provide more effective treatment through ultrasound-guided imaging. The optimization of the imaging platform is required to increase the spatial resolution and enhance CNR, followed by the development of further desirable therapeutic strategies. With advances in technology in the fabrication and design of transducers, novel image processing techniques, and a fundamental change in pulse sequences, it is highly expected that cardiovascular diseases and oncologic conditions will be evaluated more accurately by ultrasound imaging and early-stage disease detection will be achieved.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences, Tabriz, Iran, and Department of Medical Physics and Biomedical Engineering, Tehran University of Medical Sciences, Tehran, Iran (grant number: 43096-30-02-98).References
- P. Lancellotti, M.-L. Nguyen Trung, C. Oury and M. Moonen, Eur. Heart J., 2021, 42, 110–112 Search PubMed.
- A. Tarighatnia, G. Johal, A. Aghanejad, H. Ghadiri and N. Nader, Front. Biomed. Technol., 2021, 8(3), 226–235 Search PubMed.
- A. Tarighatnia, M. R. Fouladi, M. R. Tohidkia, G. Johal, N. D. Nader, A. Aghanejad and H. Ghadiri, J. Drug Delivery Sci. Technol., 2021, 66, 102895 CrossRef CAS.
- N. Lassau, Molecular Imaging in Oncology, Springer, 2020, pp. 765–771 Search PubMed.
- N. Vahidfar, A. Aghanejad, H. Ahmadzadehfar, S. Farzanehfar and E. Eppard, Int. J. Mol. Sci., 2021, 22(9), 4597 CrossRef CAS PubMed.
- A. Aghanejad, A. R. Jalilian, S. Maus, H. Yousefnia, P. Geramifar and D. Beiki, Iran. J. Nucl. Med., 2016, 24(1), 29–36 CAS.
- K. Rajamanickam, Arch. Intern. Med. Res., 2020, 3, 032–043 Search PubMed.
- C. Lau, P. Hess, T. Shreves and M.-S. Lee, J. Diagn. Med. Sonograph., 2020, 36, 479–487 CrossRef.
- E. Di Naro, L. Raio, A. Basso and M. R. Catalano, Pick Up and Oocyte Management, Springer, 2020, pp. 49–72 Search PubMed.
- S. Siemer, D. Wünsch, A. Khamis, Q. Lu, A. Scherberich, M. Filippi, M. P. Krafft, J. Hagemann, C. Weiss and G.-B. Ding, Nanomaterials, 2020, 10, 383 CrossRef CAS PubMed.
- J. Baier, A. Rix and F. Kiessling, Molecular Imaging in Oncology, Springer, 2020, pp. 509–531 Search PubMed.
- A. Aghanejad, A. R. Jalilian, Y. Fazaeli, D. Beiki, B. Fateh and A. Khalaj, J. Radioanal. Nucl. Chem., 2014, 299(3), 1635–1644 CrossRef CAS.
- P. N. Nabi, N. Vahidfar, M. R. Tohidkia, A. A. Hamidi, Y. Omidi and A. Aghanejad, Int. J. Biol. Macromol., 2021, 174, 185–197 CrossRef CAS PubMed.
- J. Kadkhoda, M. Akrami-Hasan-Kohal, M. R. Tohidkia, S. Khaledi, S. Davaran and A. Aghanejad, Int. J. Biol. Macromol., 2021, 185, 664–678 CrossRef CAS PubMed.
- A. L. Klibanov, Invest. Radiol., 2021, 56, 50–61 CrossRef CAS PubMed.
- M. Salih, S. M. Ali, N. Jena and K. Ananthasubramaniam, Fut. Cardiol., 2020, 17, 197–214 CrossRef PubMed.
- A. Kosareva, L. Abou-Elkacem, S. Chowdhury, J. R. Lindner and B. A. Kaufmann, Ultrasound Med. Biol., 2020, 46(3), 479–497 CrossRef PubMed.
- X. Gao, D. Guo, X. Mao, X. Shan, X. He and C. Yu, Nanoscale, 2021, 13, 5333–5343 RSC.
- M. L. Johansen, R. Perera, E. Abenojar, X. Wang, J. Vincent, A. A. Exner and S. M. Brady-Kalnay, Int. J. Mol. Sci., 2021, 22(4), 1983 CrossRef CAS PubMed.
- H. Yang, W. Cai, L. Xu, X. Lv, Y. Qiao, P. Li, H. Wu, Y. Yang, L. Zhang and Y. Duan, Biomaterials, 2015, 37, 279–288 CrossRef CAS PubMed.
- R. H. Perera, A. de Leon, X. Wang, Y. Wang, G. Ramamurthy, P. Peiris, E. Abenojar, J. P. Basilion and A. A. Exner, Nanomedicine, 2020, 28, 102213 CrossRef CAS PubMed.
- X. Zhang, M. Wu, Y. Zhang, J. Zhang, J. Su and C. Yang, Colloids Surf., B, 2020, 189, 110861 CrossRef CAS PubMed.
- S. Nimmagadda and M.-F. Penet, Front. Oncol., 2020, 9, 1537 CrossRef PubMed.
- D. V. B. Batchelor, F. J. Armistead, N. Ingram, S. A. Peyman, J. R. McLaughlan, P. L. Coletta and S. D. Evans, Curr. Opin. Colloid Interface Sci., 2021, 54, 101456 CrossRef CAS.
- S. M. Demos, H. Alkan-Onyuksel, B. J. Kane, K. Ramani, A. Nagaraj, R. Greene, M. Klegerman and D. D. McPherson, J. Am. Coll. Cardiol., 1999, 33, 867–875 CrossRef CAS.
- R. Wang, L. Wang, Y. Chen, Y. Xie, M. He, Y. Zhu, Z. Han, L. Xu, D. Chen and L. Zhang, Curr. Med. Chem., 2022, 29(8), 1316–1330 CrossRef PubMed.
- A. Farhadi, G. Ho, M. Kunth, B. Ling, A. Lakshmanan, G. J. Lu, R. W. Bourdeau, L. Schröder and M. G. Shapiro, AIChE J., 2018, 64, 2927–2933 CrossRef CAS PubMed.
- A. Lakshmanan, G. J. Lu, A. Farhadi, S. P. Nety, M. Kunth, A. Lee-Gosselin, D. Maresca, R. W. Bourdeau, M. Yin and J. Yan, Nat. Protoc., 2017, 12, 2050–2080 CrossRef CAS PubMed.
- M. G. Shapiro, P. W. Goodwill, A. Neogy, M. Yin, F. S. Foster, D. V. Schaffer and S. M. Conolly, Nat. Nanotechnol., 2014, 9, 311–316 CrossRef CAS PubMed.
- C. Kang, W. Cho, M. Park, J. Kim, S. Park, D. Shin, C. Song and D. Lee, Biomaterials, 2016, 85, 195–203 CrossRef CAS PubMed.
- H. S. Min, S. Son, D. G. You, T. W. Lee, J. Lee, S. Lee, J. Y. Yhee, J. Lee, M. H. Han and J. H. Park, Biomaterials, 2016, 108, 57–70 CrossRef CAS PubMed.
- Y. Zhu, Y. Liu, Z. Xie, T. He, L. Su, F. Guo, G. Arkin, X. Lai, J. Xu and H. Zhang, Nanophotonics, 2021, 10(12), 3339–3358 CrossRef.
- R. H. Perera, X. Wang, Y. Wang, G. Ramamurthy, P. Peiris, E. Abenojar, J. P. Basilion and A. A. Exner, Nanomedicine, 2020, 102213 CrossRef CAS PubMed.
- K. Fang, L. Wang, H. Huang, M. Lan, D. Shen, S. Dong and Y. Guo, Pharm. Res., 2020, 37, 1–13 CrossRef PubMed.
- K. D. Buchanan, S. Huang, H. Kim, R. C. MacDonald and D. D. McPherson, J. Pharm. Sci., 2008, 97, 2242–2249 CrossRef CAS PubMed.
- J. Osborn, J. E. Pullan, J. Froberg, J. Shreffler, K. N. Gange, T. Molden, Y. Choi, A. Brooks, S. Mallik and K. Sarkar, Nanoscale Adv., 2020, 2, 3411–3422 RSC.
- M. L. P. Vidallon, A. M. Douek, A. Quek, H. McLiesh, J. Kaslin, R. F. Tabor, A. I. Bishop and B. M. Teo, Part. Part. Syst. Charact., 2020, 37, 1900471 CrossRef CAS.
- S. Niu, G. R. Williams, J. Wu, J. Wu, X. Zhang, X. Chen, S. Li, J. Jiao and L. M. Zhu, J. Nanobiotechnol., 2019, 17, 95 CrossRef PubMed.
- R. Guo, N. Xu, Y. Liu, G. Ling, J. Yu and P. Zhang, Ultrasound Med. Biol., 2021, 47(8), 2064–2079 CrossRef PubMed.
- F. Eklund, M. Alheshibri and J. Swenson, Curr. Opin. Colloid Interface Sci., 2021, 53, 101427 CrossRef CAS.
- D. Qin, Q. Zou, S. Lei, W. Wang and Z. Li, Ultrason. Sonochem., 2021, 105608 CrossRef CAS PubMed.
- D. A. Fernandes, S. Appak-Baskoy, E. Berndl and M. C. Kolios, RSC Adv., 2021, 11, 4906–4920 RSC.
- Y.-S. Chen, Y. Zhao, C. Beinat, A. Zlitni, E.-C. Hsu, D.-H. Chen, F. Achterberg, H. Wang, T. Stoyanova, J. Dionne and S. S. Gambhir, Nat. Nanotechnol., 2021, 16, 717–724 CrossRef CAS PubMed.
- B. L. Helfield, K. Yoo, J. Liu, R. Williams, P. S. Sheeran, D. E. Goertz and P. N. Burns, Ultrasound Med. Biol., 2020, 46, 2861–2870 CrossRef PubMed.
- Y. Liang, H. Yang, Q. Li, P. Zhao, H. Li, Y. Zhang, W. Cai, X. Ma and Y. Duan, Cancer Chemother. Pharmacol., 2020, 1–14 Search PubMed.
- Y. Xu, C. Niu, S. An, S. Tang, P. Xiao, Q. Peng and L. Wang, RSC Adv., 2017, 7, 40791–40802 RSC.
- L. Yang, J. Cheng, Y. Chen, S. Yu, F. Liu, Y. Sun, Y. Chen and H. Ran, Sci. Rep., 2017, 7, 45213 CrossRef CAS PubMed.
- S. Lin, A. Shah, J. Hernández-Gil, A. Stanziola, B. I. Harriss, T. O. Matsunaga, N. Long, J. Bamber and M.-X. Tang, Photoacoustics, 2017, 6, 26–36 CrossRef PubMed.
- N. S. Awad, V. Paul, N. M. AlSawaftah, G. ter Haar, T. M. Allen, W. G. Pitt and G. A. Husseini, ACS Pharmacol. Transl. Sci., 2021, 4, 589–612 CrossRef CAS PubMed.
- K. Ovejero Paredes, D. Díaz-García, V. García-Almodóvar, L. Lozano Chamizo, M. Marciello, M. Díaz-Sánchez, S. Prashar, S. Gómez-Ruiz and M. Filice, Cancers, 2020, 12, 187 CrossRef PubMed.
- P. Frinking, T. Segers, Y. Luan and F. Tranquart, Ultrasound Med. Biol., 2020, 46, 892–908 CrossRef PubMed.
- A. Baeza and M. Vallet-Regí, Pharmaceutics, 2020, 12(10), 957 CrossRef CAS PubMed.
- M. Du, Y. Chen, J. Tu, C. Liufu, J. Yu, Z. Yuan, X. Gong and Z. Chen, ACS Biomater. Sci. Eng., 2020, 6, 2904–2912 CrossRef CAS PubMed.
- L.-Q. Zhou, P. Li, X.-W. Cui and C. F. Dietrich, Cancer Letters, 2020, 470, 204–219 CrossRef CAS PubMed.
- S.-G. Moslem, J. Soo and K. Hyock-Ju, Nano Futures, 2021, 5(2) DOI:10.1088/2399-1984/abfebc.
- D. Qin, L. Zhang, H. Zhu, J. Chen, D. Wu, A. Bouakaz, M. Wan and Y. Feng, Int. J. Nanomed., 2021, 16, 3105–3119 CrossRef PubMed.
- Y. Hadadian, J. H. Uliana, A. A. O. Carneiro and T. Z. Pavan, IEEE Trans. Biomed. Eng., 2021, 68, 68–77 Search PubMed.
- A. Milgroom, M. Intrator, K. Madhavan, L. Mazzaro, R. Shandas, B. Liu and D. Park, Colloids Surf., B, 2014, 116, 652–657 CrossRef CAS PubMed.
- M. Guo, W. Du, N. Lyu, X. Chen, Y. Du, H. Wang, D. Yang, S. Wu, J. Liang and Y. Pan, Adv. Healthcare Mater., 2020, 9, 1901155 CrossRef CAS PubMed.
- R. A. Barmin, P. G. Rudakovskaya, O. I. Gusliakova, O. A. Sindeeva, E. S. Prikhozhdenko, E. A. Maksimova, E. N. Obukhova, V. S. Chernyshev, B. N. Khlebtsov and A. A. Solovev, Nanomaterials, 2021, 11, 415 CrossRef CAS PubMed.
- L. G. Delogu, G. Vidili, E. Venturelli, C. Ménard-Moyon, M. A. Zoroddu, G. Pilo, P. Nicolussi, C. Ligios, D. Bedognetti and F. Sgarrella, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 16612–16617 CrossRef CAS PubMed.
- Y. Zhang, Y. Dong, H. Fu, H. Huang, Z. Wu, M. Zhao, X. Yang, Q. Guo, Y. Duan and Y. Sun, Biomaterials, 2020, 120478 Search PubMed.
- V. Schwarze, C. Marschner, G. N. de Figueiredo, J. Rübenthaler and D.-A. Clevert, Eur. J. Ultrasound, 2020, 41, 29–35 Search PubMed.
- M. Bruce, A. Hannah, R. Hammond, Z. Z. Khaing, C. Tremblay-Darveau, P. N. Burns and C. P. Hofstetter, IEEE Trans. Ultrason. Eng., 2020, 67(9), 1776–1784 Search PubMed.
- W. K. Chong, V. Papadopoulou and P. A. Dayton, Abdominal Radiol., 2018, 43, 762–772 CrossRef PubMed.
- A. L. Emanuel, R. I. Meijer, E. van Poelgeest, P. Spoor, E. H. Serné and E. C. Eringa, Microcirculation, 2020, 27, e12588 CrossRef PubMed.
- S. Turco, P. Frinking, R. Wildeboer, M. Arditi, H. Wijkstra, J. R. Lindner and M. Mischi, Ultrasound Med. Biol., 2020, 46(3), 518–543 CrossRef PubMed.
- Z. Zhang, C. Huang, L. Zhang, Q. Guo, Y. Qin, F. Fan, B. Li, B. Xiao, D. Zhu and L. Zhang, Acta Pharm. Sin. B, 2020, 11(2), 520–533 CrossRef PubMed.
- K. G. Brown, D. Ghosh and K. Hoyt, IEEE Trans. Ultrason. Eng., 2020 DOI:10.1109/ULTSYM.2019.8926282.
- R. R. Wildeboer, C. K. Mannaerts, R. J. van Sloun, L. Budäus, D. Tilki, H. Wijkstra, G. Salomon and M. Mischi, Eur. Radiol., 2020, 30, 806–815 CrossRef PubMed.
- K. A. Stewart, S. M. Navarro, S. Kambala, G. Tan, R. Poondla, S. Lederman, K. Barbour and C. Lavy, Int. J. MCH AIDS, 2020, 9, 103–120 CrossRef PubMed.
- R. C. Wang, A. E. Kornblith, J. Grupp-Phelan, R. Smith-Bindman, L. S. Kao and J. Fahimi, Am. J. Roentgenol., 2020, 216, 200–208 CrossRef PubMed.
- M. A. Averkiou, M. F. Bruce, J. E. Powers, P. S. Sheeran and P. N. Burns, Ultrasound Med. Biol., 2020, 46, 498–517 CrossRef PubMed.
- V. Rafailidis, D. Y. Huang, G. T. Yusuf and P. S. Sidhu, Ultrasonography, 2020, 39, 22 CrossRef PubMed.
- A. Ajoolabady, A. Aghanejad, Y. Bi, Y. Zhang, H. Aslkhodapasandhukmabad, A. Abhari and J. Ren, Biochim. Biophys. Acta Rev. Cancer, 2020, 1874(1), 188366 CrossRef CAS PubMed.
- H. W. West and C. Antoniades, Antioxid. Redox Signa., 2020, 34(15), 1217–1243 CrossRef PubMed.
- Q. Jin, C.-Y. Lin, S.-T. Kang, Y.-C. Chang, H. Zheng, C.-M. Yang and C.-K. Yeh, Ultrason. Sonochem., 2017, 36, 262–269 CrossRef CAS PubMed.
- A. Luong, D. Smith, C.-H. Tai, B. Cotter, C. Luo, M. Strachan, A. DeMaria and J. J. Rychak, Ultrasound Med. Biol., 2020, 46, 690–702 CrossRef PubMed.
- C. Lau, M. Rivas, J. Dinalo, K. King and V. Duddalwar, J. Ultrasound Med., 2020, 39, 19–28 CrossRef PubMed.
- J. R. Eisenbrey and F. Forsberg, Eur. J. Nucl. Med. Mol. Imaging, 2010, 37, 138–146 CrossRef PubMed.
- R. Pala, S. Pattnaik, S. Busi and S. M. Nauli, Pharmaceutics, 2021, 13(3), 348 CrossRef CAS PubMed.
- A. Rix, A. Curaj, E. Liehn and F. Kiessling, Semin. Thromb. Hemost., 2020, 46, 545–552 CrossRef PubMed.
- G. Cismaru, T. Serban and A. Tirpe, Biomedicines, 2021, 9(4), 418 CrossRef CAS PubMed.
- S. Feinstein and A. K. Rao, in Therapeutic Lipidology, ed. M. H. Davidson, P. P. Toth and K. C. Maki, Springer International Publishing, Cham, 2021, pp. 605–614 DOI:10.1007/978-3-030-56514-5_32.
- Q. Wang, F. Chan Kai, K. Schweizer, X. Du, D. Jin, H. Yu Simon Chun, J. Nelson Bradley and L. Zhang, Sci. Adv., 2021, 7(9), eabe5914 CrossRef CAS PubMed.
- S. Zhang, W. Chu, H. Wang, Y. Liang, Y. Fan, H. Liu and G. Wei, J. Int. Med. Res., 2020, 48, 0300060520942098 CAS.
- W. Wu, X. Feng, Y. Yuan, Y. Liu, M. Li, J. Bin, Y. Xiao, W. Liao, Y. Liao and W. Zhang, Mol. Imaging Biol., 2017, 19, 183–193 CrossRef CAS PubMed.
- X. Leng, J. Wang, A. Carson, X. Chen, H. Fu, S. Ottoboni, W. R. Wagner and F. S. Villanueva, Mol. Imaging, 2014, 13, 7290 CrossRef.
- K. Thayse, N. Kindt, S. Laurent and S. Carlier, Biology, 2020, 9(11), 368 CrossRef CAS PubMed.
- A. Rix, S. Fokong, S. Heringer, R. Pjontek, L. Kabelitz, B. Theek, M.-A. Brockmann, M. Wiesmann and F. Kiessling, Invest. Radiol., 2016, 51, 767–775 CrossRef CAS PubMed.
- K. E. Hitchcock, D. N. Caudell, J. T. Sutton, M. E. Klegerman, D. Vela, G. J. Pyne-Geithman, T. Abruzzo, P. E. Cyr, Y.-J. Geng and D. D. McPherson, J. Controlled Release, 2010, 144, 288–295 CrossRef CAS PubMed.
- F. Moccetti, C. C. Weinkauf, B. P. Davidson, J. T. Belcik, E. R. Marinelli, E. Unger and J. R. Lindner, Ultrasound Med. Biol., 2018, 44, 1155–1163 CrossRef PubMed.
- A. Maier, P. Plaza-Heck, F. Meixner, F. Guenther, B. A. Kaufmann, M. Kramer, T. Heidt, A. Zirlik, I. Hilgendorf and J. Reinöhl, Atherosclerosis, 2017, 267, 68–77 CrossRef CAS PubMed.
- F. Chen, M. Ma, J. Wang, F. Wang, S.-X. Chern, E. R. Zhao, A. Jhunjhunwala, S. Darmadi, H. Chen and J. V. Jokerst, Nanoscale, 2017, 9, 402–411 RSC.
- V. Daeichin, K. Kooiman, I. Skachkov, J. G. Bosch, T. L. Theelen, K. Steiger, A. Needles, B. J. Janssen, M. J. Daemen and A. F. van der Steen, Ultrasound Med. Biol., 2016, 42, 2283–2293 CrossRef PubMed.
- J. Lux, A. M. Vezeridis, K. Hoyt, S. R. Adams, A. M. Armstrong, S. R. Sirsi and R. F. Mattrey, ACS Appl. Mater. Interfaces, 2017, 9, 37587–37596 CrossRef CAS PubMed.
- D. Myers, P. Lester, R. Adili, A. Hawley, L. Durham, V. Dunivant, G. Reynolds, K. Crego, Z. Zimmerman, S. Sood, R. Sigler, W. Fogler, J. Magnani, M. Holinstat and T. Wakefield, J. Vasc. Surg.: Venous Lymphat. Disord., 2020, 8, 268–278 Search PubMed.
- A. Yang, B. Qiao, E. M. Strohm, J. Cao, Z. Wang, X. Yuan, Y. Luo and Y. Sun, Biomater. Sci., 2020, 8, 4545–4558 RSC.
- S. Wei, N. Fu, Y. Sun, Z. Yang, L. Lei, P. Huang and B. Yang, Ultrasound Med. Biol., 2014, 40, 1250–1259 CrossRef PubMed.
- A. Ingels, I. Leguerney, P.-H. Cournède, J. Irani, S. Ferlicot, C. Sébrié, B. Benatsou, L. Jourdain, S. Pitre-Champagnat and J.-J. Patard, Sci. Rep., 2020, 10, 1–8 CrossRef PubMed.
- X.-M. Guo, J.-L. Chen, B.-H. Zeng, J.-C. Lai, C.-Y. Lin and M.-Y. Lai, RSC Adv., 2020, 10, 39348–39358 RSC.
- P. Li, L. Jin, L. Feng, Y. Wang and R. Yang, 2021 DOI:10.21203/rs.3.rs-400253/v1.
- M. Zhao, Y. Zhu, Y. Zhang, X. Yang, Y. Duan, Y. Chen and Y. Sun, Clinical Hemorheology and Microcirculation, 2020, pp. 1–11, Preprint Search PubMed.
- H. Wu, H. Shi, H. Zhang, X. Wang, Y. Yang, C. Yu, C. Hao, J. Du, H. Hu and S. Yang, Biomaterials, 2014, 35, 5369–5380 CrossRef CAS PubMed.
- L. J. Delaney, J. R. Eisenbrey, D. Brown, J. R. Brody, M. Jimbo, B. E. Oeffinger, M. Stanczak, F. Forsberg, J.-B. Liu and M. A. Wheatley, Acta Biomater., 2021, 130, 385–394 CrossRef CAS PubMed.
- K. Singh, D. S. Chopra, D. Singh and N. Singh, Arabian J. Chem., 2020, 13, 9034–9046 CrossRef CAS.
- S. V. Bachawal, K. C. Jensen, K. E. Wilson, L. Tian, A. M. Lutz and J. K. Willmann, Cancer Res., 2015, 75, 2501–2509 CrossRef CAS PubMed.
- R. Bam, P. S. Lown, L. A. Stern, K. Sharma, K. E. Wilson, G. R. Bean, A. M. Lutz, R. Paulmurugan, B. J. Hackel and J. Dahl, Clin. Cancer Res., 2020, 26, 2140–2150 CrossRef CAS PubMed.
- Y. Liu, Y. Zhou, J. Xu, H. Luo, Y. Zhu, X. Zeng, F. Dong, Z. Wei, F. Yan and H. Zheng, Biomater. Sci., 2021, 9, 2454–2466 RSC.
- L. Xu, J. Du, C. Wan, Y. Zhang, S. Xie, H. Li, H. Yang and F. Li, Int. J. Nanomed., 2018, 13, 1791 CrossRef CAS PubMed.
- D. Sheng, L. Deng, P. Li, Z. Wang and Q. Zhang, ACS Biomater. Sci. Eng., 2021, 7, 605–616 CrossRef CAS PubMed.
- X. Z. Li, J. Song, Z. X. Sun, Y. Y. Yang, Y. Q. Lin and H. Wang, J. Ultrasound Med., 2020, 39(9), 1687–1694 CrossRef PubMed.
- T. Zhou, W. Cai, H. Yang, H. Zhang, M. Hao, L. Yuan, J. Liu, L. Zhang, Y. Yang and X. Liu, J. Controlled Release, 2018, 276, 113–124 CrossRef CAS PubMed.
- B. Shang, X. Zhang, R. Ji, Y. Wang, H. Hu, B. Peng and Z. Deng, Mater. Sci. Eng.: C, 2020, 106, 110174 CrossRef CAS PubMed.
- J.-P. Wang, X.-L. Zhou, J.-P. Yan, R.-Q. Zheng and W. Wang, Oncotarget, 2017, 8, 78153 CrossRef PubMed.
- Y. Gao, C. Hernandez, H.-X. Yuan, J. Lilly, P. Kota, H. Zhou, H. Wu and A. A. Exner, Nanomedicine, 2017, 13, 2159–2168 CrossRef CAS PubMed.
- Y. Zhang, Y. Dong, H. Fu, H. Huang, Z. Wu, M. Zhao, X. Yang, Q. Guo, Y. Duan and Y. Sun, Biomaterials, 2021, 269, 120478 CrossRef CAS PubMed.
- H. Zhou, J. Fu, Q. Fu, Y. Feng, R. Hong, P. Li, Z. Wang, X. Huang and F. Li, PeerJ, 2021, 9, e11486 CrossRef PubMed.
- X. Duan and M. Hou, Revista Cientifica-Facultad de Ciencias Veterinarias, 2020, 30(1), 320–330 Search PubMed.
- Q. Dongying, L. Lan and D. Qian, Process Biochem., 2020, 98, 51–58 CrossRef.
- Y. Liu, J. Jiang, C. Liu, W. Zhao, Y. Ma, Z. Zheng, Q. Zhou and Y. Zhao, Am. J. Transl. Res., 2021, 13, 988–1005 CAS.
- Q. Hu, X.-Y. Wang, L.-K. Kang, H.-M. Wei, C.-M. Xu, T. Wang and Z.-H. Wen, PLoS One, 2016, 11, e0149075 CrossRef PubMed.
- H. Wang, S. A. Felt, S. Machtaler, I. Guracar, R. Luong, T. Bettinger, L. Tian, A. M. Lutz and J. K. Willmann, Radiology, 2015, 276, 809–817 CrossRef PubMed.
- T. Payen, A. Dizeux, C. Baldini, D. Le Guillou-Buffello, M. Lamuraglia, E. Comperat, O. Lucidarme and S. L. Bridal, Ultrasound Med. Biol., 2015, 41, 2202–2211 CrossRef PubMed.
- J. Zhu, Z. Wang, X. Xu, M. Xu, X. Yang, C. Zhang, J. Liu, F. Zhang, X. Shuai and W. Wang, Mol. Pharmaceutics, 2020, 17, 817–826 CrossRef CAS PubMed.
- A. Helbert, M. Von Wronski, D. Colevret, C. Botteron, F. Padilla, T. Bettinger, I. Tardy and J.-M. Hyvelin, Invest. Radiol., 2020, 55(10), 657–665 CrossRef CAS PubMed.
- C. Qiu, T. Sha, T. Yin, W. Zhang, X. Chen, X. Miao, R. Zheng, X. Shuai and J. Ren, Biomater. Sci., 2021, 9(17), 5802–5811 RSC.
- Q. Wu, Q. Zhang, T. Yu, X. Wang, C. Jia, Z. Zhao and J. Zhao, ACS Appl. Bio Mater., 2021, 4, 4244–4253 CrossRef CAS PubMed.
- N. Shimamoto, M. Ito, M. Chiba, S. Honma, H. Imazu and K. Sumiyama, Hepatobil. Pancreat. Dis. Int., 2020, 19, 478–485 CrossRef PubMed.
- M. Lamuraglia, G. Barrois, D. Le Guillou-Buffello, M. Santin, A. Kerbol, E. Comperat, A. Coron, O. Lucidarme and S. L. Bridal, Technol. Cancer Res. Treat., 2020, 19, 1533033819886896 CAS.
- R. Bam, I. Daryaei, L. Abou-Elkacem, J. G. Vilches-Moure, E. J. Meuillet, A. Lutz, E. R. Marinelli, E. C. Unger, S. S. Gambhir and R. Paulmurugan, Invest. Radiol., 2020, 55(11), 711–721 CrossRef CAS PubMed.
- G. Köse, M. Darguzyte and F. Kiessling, Nanomaterials, 2020, 10(10), 1935 CrossRef PubMed.
- R. H. Perera, X. Wang, Y. Wang, G. Ramamurthy, P. Peiris, E. Abenojar, J. P. Basilion and A. A. Exner, Nanomedicine, 2020, 28, 102213 CrossRef CAS PubMed.
- E. Heer, A. Harper, N. Escandor, H. Sung, V. McCormack and M. M. Fidler-Benaoudia, Lancet Global Health, 2020, 8, e1027–e1037 CrossRef PubMed.
- A. A. Berlin, M. Young, A. E. Kaffas, S. Gambhir, A. Lutz, M. L. Storto and J. Willmann, 2020, arXiv preprint arXiv:2006.11993.
- Y. Zhang, Y. Li, M. Wu, F. Zhang, G. Shao and Q. Wang, J. Med. Imaging Health Inform., 2021, 11, 981–987 CrossRef.
- A. Tarighatnia, M. H. Abdkarimi, N. D. Nader, T. Mehdipour, M. R. Fouladi, A. Aghanejad and H. Ghadiri, New J. Chem., 2021, 45, 18871–18880 RSC.
- P. Tomà, Pediatr. Radiol., 2020, 50, 314–320 CrossRef PubMed.
- M. A. Zamzam, A. A. Abd El-Aziz, I. I. El-Mahallway, G. A. AbdeLaal, M. K. Abd El-Mageed and H. A. Eid, Egyptian J. Chest Dis. Tuberculos., 2020, 69, 183 CrossRef.
- X. Yu, Y. Yang and J. Li, Eur. J. Inflamm., 2020, 18, 2058739220961194 Search PubMed.
- Y. Wu, T. Sun, J. Tang, Y. Liu and F. Li, Ultrasound Med. Biol., 2020, 46, 679–689 CrossRef PubMed.
- M. B. Toaldo, V. Salvatore, S. Marinelli, C. Palamà, M. Milazzo, L. Croci, L. Venerandi, M. Cipone, L. Bolondi and F. Piscaglia, Mol. Imaging Biol., 2015, 17, 29–37 CrossRef PubMed.
- L. Barghi, A. Aghanejad, H. Valizadeh, J. Barar and D. Asgari, Adv. Pharm. Bull, 2012, 2(1), 119–122 Search PubMed.
- D. Asgari, A. Aghanejad and J. S. Mojarrad, Bull. Korean Chem. Soc., 2011, 32, 909–914 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |