Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Research progress on nano-sensitizers for enhancing the effects of radiotherapy
Yuan
Zhang†
a,
Xiao
Han†
a,
Yuan
Liu
a,
Shuang
Wang
a,
Xianlin
Han
*b and
Cui
Cheng
 *a
*a
aCollege of Biological Science and Engineering, Fuzhou University, Fuzhou 350108, China. E-mail: ibptcc@fzu.edu.cn
bDepartment of General Surgery, Peking Union Medical College Hospital, Beijing, 100730, P. R. China. E-mail: hanxianlin@pumch.cn
First published on 25th March 2022
Abstract
Radiotherapy (RT) is local control of tumors using radiation, including external irradiation (EBRT) and internal irradiation (RIT). Cancer radiotherapy based on external beams is a major clinical treatment for cancer, which has been widely used in the treatment of more than one-third of local solid tumors. However, due to the tumor's insensitivity to radiation, low absorption rate of radiation by tumors, large radiation doses are often needed during radiotherapy, causing serious damage to normal tissues near the tumors. The most important is that the extent of cancer cell damage caused by radiotherapy and the radiosensitivity of tumors is mainly determined by the concentration of oxygen. The above two aspects severely limit the effect of radiotherapy. Nano-sensitizers can effectively accumulate radiation doses and enhance radiation effects, thereby improving the efficacy of radiotherapy. In addition, nano-sensitizers can also increase tumor sensitivity to radiation through reactive oxygen species (ROS). Therefore, this article reviews the latest progress of nano-sensitizers for radiotherapy, which is focused on precious metal-based nano-sensitizers, rare earth metal-based nano-sensitizers, semiconductor metal-based nano-sensitizers, other metal- and nonmetal-based nano-sensitizers. And the recent literature reports and applications of the nano-sensitizers are also discussed.
1. Introduction
According to data from the World Health Organization (WHO), the number of cancer-related deaths each year is expected to increase by 45% from 2007 to 2030.1 About 17.5 million new cancer cases were reported worldwide, with 8.7 million cancer-related deaths in 2015.2 Sadly, it is estimated that the number of new cancer cases reached 19.3 million globally, and nearly 10 million people died from cancer in 2020, according to the updated estimates of cancer incidence and mortality at the end of 2020 from the GLOBOCAN 2020.3 Cancer is still the main cause of death for people in most countries in the world.Radiation therapy (RT) is one of the mainstream tumor treatments alongside chemotherapy and surgery.4,5 In the current clinical cancer treatment, about half of the cancer patients will use radiotherapy or combine it with other treatment methods in the process of cancer treatment.6 Radiotherapy is a key method in the treatment of malignant tumors, and its role and status are becoming increasingly prominent. The treatment principle of RT is that high-energy ionizing radiation (such as γ-rays and X-rays) directly interacts with the cell DNA, causing DNA damage (DNA is the main target that determines radiobiological effects),7 or indirectly reacts with water molecules to produce ROS to damage DNA or other cellular components, inducing apoptosis and necrosis.8 Radiotherapy mainly includes internal radioisotope therapy (RIT) and external radiation therapy (EBRT). RIT uses a minimally invasive method to introduce therapeutic radioisotopes into tumors to induce cancer cell death. EBRT uses high-energy X-beams, electron beams, or proton beams from outside the body to directly irradiate tumors.9
Although the role of radiotherapy has become increasingly prominent, its therapeutic effect is not ideal. The hypoxia problem in most solid tumors hinders the effect of radiotherapy to a large extent, and oxygen is critical to increase radiation-induced DNA damage.10 In the 1930s, Crabtree and Cramer11 showed that molecular oxygen is a key determinant of the cell's response to radiation. In addition, since the therapeutic effect of radiotherapy depends on the patients’ radiosensitivity, its complete cure rate is very low.12 In order to effectively destroy cancer cells and inhibit tumor growth, large doses of X-rays are usually required, especially when treating deep-seated tumors; however, the radiation resistance caused by the tumor's hypoxic environment cannot be alleviated by high-dose radiotherapy, and it can also severely damage normal tissues and cause toxic side effects.13 Therefore, it is necessary to seek a high-efficiency, low-toxicity radiosensitizer to improve the tumors’ radiosensitivity and reduce the damage to the surrounding normal cells.
In the past ten years, the rapid development of emerging advanced nanomaterials, nanobiotechnology and nanomedicine has provided a good opportunity for tumor radiosensitization,6 because nanomaterials have the following excellent physical and chemical properties: good biocompatibility, inherent radiosensitization activity, high loading of multiple drugs, enhanced tumor tissue permeability and retention (EPR) effects, etc. Nanomaterials have been widely studied and applied in improving the effect of radiotherapy.14 In recent years, new nano-radiosensitizers and methods of radiosensitization have been continuously proposed.15 The types of nanomaterials are not limited to precious metals (silver (Ag), gold (Au), and platinum (Pt)); some nanomaterials that are based on rare earth metals (gadolinium (Gd), hafnium (Hf), etc.), semiconductor metals (bismuth (Bi)), and other metals (titanium (Ti), etc.) and non-metal nano-sensitizers are also widely used. The classification of main element-based nanomaterials as radiosensitizers is shown in Fig. 1. Therefore, this article systematically summarizes the classification and research results of nano-radiosensitizers as it is very necessary to make a new summary of the latest developments in this field. On the basis of studying its existing research results, it is more important to promote further research and development in this field.
2. Precious metal-based nano-radiosensitizers
In the past, precious metal-based nano-radiosensitizers have been the most researched. Due to the strong X-ray attenuation ability of precious metal elements, they can accumulate the radiation dose on cancer cells, thereby achieving the effect of radiotherapy sensitization.16 The multiple advantages of two precious metals Au and Ag make them superior to other materials in the preparation of nanomaterials, including low toxicity, easy preparation, easy surface functionalization, high chemical stability, good biocompatibility, controllable size and morphology.17The underlying mechanism of the radiation sensitization of gold-based nanostructures is as follows: due to the high X-ray absorption coefficient of gold nanoparticles (GNPs), secondary electrons (such as Compton electrons, photoelectrons, Auger electrons) and fluorescent photons are emitted under X-ray irradiation, which will lead to ionization of water molecules or intracellular components and increase the local radiation dose.18 In addition, GNPs have attracted the interest of researchers due to their low toxicity, easy to achieve surface modification and wide photoelectric cross section. Ma et al.18 prepared gold nanospikes (GNSs) with different surface functionalizations (TAT–GNSs NH2–GNSs, FA–GNSs) and evaluated their radiation sensitization effects. In vitro studies have demonstrated that the ionizing radiation effects of these GNSs have a good correlation with their cell uptake, and the ratio of the sensitization enhancement (SER) of TAT–GNSs reaches 2.30 at a radiation dose of 4 Gy, showing a significant radiation sensitization effect. Ma et al.19 also synthesized GNSs, GNPs and gold nanorods (GNRs) with different shapes but closed average particle diameters (∼50 nm), and modified them with nano-polyethylene glycol (PEG) molecules. The cell uptake ability increased in the order of GNPs > GNSs > GNRs, and their SER values were 1.62, 1.37, and 1.21, respectively, indicating that the shape of gold-based nanomaterials also had a very important effect on tumor radiotherapy. It is noteworthy that previous studies have assumed that spherical cells are located in the central nucleus. However, tumor cells are usually not spherical, but have complex shapes. Then, Sung et al.20 evaluated the biological effects of GNPs on cells with different geometric shapes. Human breast cancer cells and rat glioma cells were used as models, when the nucleus was close to the cell membrane, the SER value increased to 1.2 times. Furthermore, the interaction between GNPs and low-energy photons at the nanoscale was simulated using a microcomputer, and the results were applied to biological models to quantify the dependence of GNP radiosensitization on cell geometry. The dose was less than 1% of the surface dose at a distance of about 100 nm from GNPs, indicating that the shape, size and other geometric parameters and position of cells and nuclei were very important for evaluating GNP-mediated radiosensitization. It also proved that the use of low energy photons could effectively enhance the feasibility of radiosensitization therapy for GNP-mediated superficial tumors such as breast cancer and glioma close to the skull. Particularly, for glioma and glioblastoma, GNPs could be injected directly at the surgical site, which dramatically reduces the complications associated with GNP transmission across the BBB to the target. This is the first time that the dependence of GNP radiosensitization on cell geometry was testified. Jia et al.21 synthesized atomic precision gold nanoclusters (Au8NCs) with a diameter of about 2 nm, as showed in Fig. 2a. When X-ray irradiated Au8NCs, they produced ROS, leading to irreversible apoptosis (Fig. 2b). X-ray irradiation (4 Gy) in conjunction with Au8NCs reduced the cell survival rate to 2.7% and showed a tumor inhibition rate of 74.2% (Fig. 2c and d). The nano-radiosensitizer established in this study not only reduced the X-ray doses, but also reduced the side effects of radiation on normal tissues. As an atomically accurate radiosensitizer, the success of Au8NCs provided a prospect for the design of a radiosensitizer at the atomic level. Similarly, Kamkaew et al.22 designed polyethylene glycol (PEG) functionalized nanoparticles (Au–Pt NPs) which were composed of metallic elements Au and Pt. Au–Pt nanoparticles had anti-enzyme activity, could catalyze the conversion of H2O2 to O2, and had the synergistic radiosensitization effect in tumor treatment, which could enhance the deposition of X-ray energy. Compared with the control group, Au–Pt nanoparticles could significantly inhibit tumor growth by reducing tumor hypoxia under X-ray irradiation. Yang et al.23 also prepared polyethylene glycol (PEG) functionalized nanoparticles composed of metallic elements Au and Pt to improve their co-radiosensitivity. Liu et al.24 prepared pegylated Au@Pt nanodendrites. The shape of nanomaterials was different from that mentioned in the previous studies of Yang et al.23 and Liu et al.,24 but they still had good radiation sensitization. Shi et al.25 enhanced radiotherapy of HCT116 human colon cancer cells by tiopronin-coated GNPs (Tio–GNPs) in combination with low-energy X-ray, and found that intratumoral injection of nanoparticles resulted in 94 times more radiation accumulation than intravenous injection, suggesting that GNPs are indeed an effective radiosensitizer. Bhattarai et al.26 evaluated the effects of pegylated gold nanotriangles (PAuNTs) on uptake, cytotoxicity, biodistribution and radiosensitization of human glioblastoma multiforme (GBM) cells. Despite extensive literature on the effectiveness of metal nanoparticles as radiosensitizers, much remains unknown regarding the definition of the ideal shape, size, therapeutic cell type and shape of nanoparticles to improve the efficacy of radiotherapy.
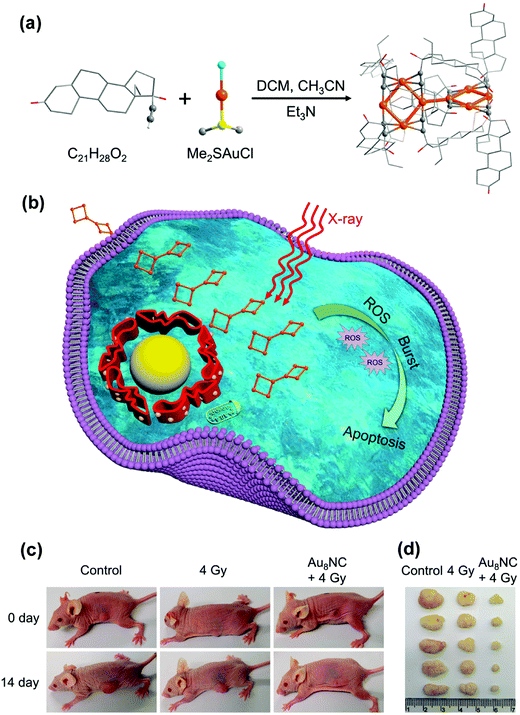 | ||
| Fig. 2 (a) Synthetic schematic of Au8NCs; (b) Au8NCs for cancer radiotherapy via the ROS burst; (c) representative images of mice treated under various conditions at days 0 and 14; (d) images of dissected tumors. Copyright © 2019 American Chemical Society. Reprinted from ref. 21 with permission. | ||
Silver nanoparticles (AgNPs) have also attracted great attention due to their common and excellent radiation sensitization.27 Xu et al.28 reported that AgNPs had a radiosensitization effect on glioma cells. AgNPs of different particle sizes (20 nm, 50 nm and 100 nm) had different sensitization effects to radiation. The radiosensitization effect of AgNPs reduced with the increasing particle size. Subsequently, Swanner et al.29 demonstrated that AgNPs exhibited radiosensitization in breast cancer tumor cells. And then, more researchers experimentally proved that AgNPs have therapeutic effects on other tumor cells, such as liver cancer, lung cancer and leukemia.30–32 As a novel nano-radiosensitizer, AgNPs have shown good radiosensitization performance in radiotherapy, but their ability to efficiently enter and accumulate in tumor cells remains to be improved. Hence, targeted modification of AgNPs was aimed to solve this problem. Habiba et al.33 synthesized PGAgNPs by modifying AgNPs with pegylated graphene quantum dots (GQDs), which showed good intracellular uptake and radiation sensitization in radiation-resistant HT29 colorectal cancer cells. At 10 Gy of X-ray radiation, nanoparticles have significantly reduced the growth of tumors and prolonged survival compared to radiotherapy alone. Similarly, Zhao et al.34 synthesized AgNPs modified with polyethylene glycol (PEG) and aptamer AS1411 (AsNPs). AsNPs have been shown to specifically target C6 glioma cells without entering normal human microvascular endothelial cells. Results also showed that AsNPs had better radiation sensitization than AgNPs and PEGylated AgNPs (PNPs) and induced a higher apoptosis rate. Meanwhile, Zhao et al.35 also designed AgNPs coated with AS1411, verapamil (VRP) and bovine serum albumin (BSA) (AgNPs@BSA–AS–VRP). The results showed that the mixture of AgNPs@BSA–AS and AgNPs@BSA–AS–VRP (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) could significantly accumulate in tumor cells, and the corresponding SER value was 1.55, which significantly improved the therapeutic effect of radiotherapy. As a highly effective nanometer radiosensitizer, it had great potential in the radiotherapy of glioma. Similarly, Liu et al.36 confirmed that the in vivo 50% inhibitory concentration (IC50) values of AgNPs on hypoxic U251 cells and C6 cells were 30.32 l g mL−1 and 27.53 l g mL−1, respectively. SER indicated that the radiosensitization of AgNPs to hypoxia cells was significantly increased than that of normoxic cells.
1) could significantly accumulate in tumor cells, and the corresponding SER value was 1.55, which significantly improved the therapeutic effect of radiotherapy. As a highly effective nanometer radiosensitizer, it had great potential in the radiotherapy of glioma. Similarly, Liu et al.36 confirmed that the in vivo 50% inhibitory concentration (IC50) values of AgNPs on hypoxic U251 cells and C6 cells were 30.32 l g mL−1 and 27.53 l g mL−1, respectively. SER indicated that the radiosensitization of AgNPs to hypoxia cells was significantly increased than that of normoxic cells.
Although GNPs and AgNPs have excellent radiosensitization, which one has better radiosensitization effects has aroused intense attention. Liu et al.37 have assessed and compared the effect of GNPs and AgNPs on glioma in vitro and in vivo. The results confirmed that AgNPs had a stronger radiosensitization ability than GNPs at the same mass and molar concentration, which causes a higher apoptosis rate. In addition, AgNPs + radiation significantly increased autophagy levels compared with GNPs + radiation. In conclusion, the sensitization effect of gold nanoparticles depends on the shape and size of nanoparticles, the type of the surface modifier and the shape of tumor cells. However, AgNPs are not as inert as GNPs; their biological mechanism of radiation sensitization and synergistic effect may be more complex.38
3. Rare earth metal-based nano-radiosensitizers
Rare earth elements with high Z values (57–71) are significant to improve the application of radiotherapy.39Gd is a lanthanide element commonly used as a positive contrast agent for magnetic resonance imaging (MRI),40 and Gd-based nanoparticles (GdNPs) are also attractive due to their radiation-enhancing properties.41,42 Gd as a promising radiosensitizer could be used in radiosensitizing therapy because of its high X-ray photon capture cross-section and Compton scattering effect.43 Li et al.44 prepared gadolinium oxide nanocrystalline (GON) and found that the SER at 10% survival level was correlated with the concentration of Gd in NSCLC cells, the maximum SER values of GONs at 10% cell survival fraction (SF10) were 1.10, 1.11, and 1.20 for A549, NH1299 and NH1650 cells under carbon ion irradiation. Wu et al.45 designed hyaluronic acid (HA) modified Gd2O3 nanoparticles with targeting and radiosensitization functions to overcome inherent radioresistance and inaccurate tumor localization. Similarly, Andoh et al.46 used gadolinium-loaded chitosan nanoparticles (Gd–Nano CPs) to treat melanoma cells. Zangeneh et al.47 doped Gd into ZnO nanoparticles to prepare Gd-doped ZnO NPs, in which Gd acted as a radiosensitizer. SER values of 10 and 20 μg mL−1 nanoparticles were 1.47 and 1.61 under 6 mV X-ray radiation, which exhibited a dose-dependent manner. Huang et al.48 constructed a kind of nanoparticle coordination polymer (H@Gd–NCPs) based on Gd–heme chloride to perform X-ray deposition and glutathione depletion simultaneously. As shown in Fig. 3, H@Gd–NCPs could effectively enhance X-ray absorption and produce more ROS, especially hydroxyl radical within tumor tissues. Hemin encapsulated in H@Gd–NCPs could enhance peroxidase-like properties to utilize overexpressed H2O2 in the tumor microenvironment to deplete GSH. The integration of ROS enhancement and GSH depletion eventually amplified irradiation mediated oxidative stress and induced ICD. Sun et al.49 designed Gd-rose bengal coordination polymer nanodots (GRDs), which have better X-ray absorption than bengal roses alone. Both Lee et al.50 and Ma et al.51 synthesized Gd@C-dots using different methods and achieved a good radiosensitization effect. Most importantly, Dufort et al.52 prepared Gd nanoparticles (AGuIX NPs) with ultra-small particle sizes (3.0 ± 1.0 nm) for radiosensitization. Du et al.53 studied the sensitization and therapeutic effect of AGuIX NPs in radiotherapy of H1299 non-small cell lung cancer cells. It showed that AGuIX NPs exhibit a high absorption of the photons emitted by the radiation beam, which enhanced the local dose deposition. In a further study, Verry et al.54 prepared AGuIX NPs containing a polysiloxane nucleus surrounded by a ring ligand of Gd. The in vitro experiments showed that the addition of AGuIX (from 0.1 mm to 1.0 mm) increased the radiation efficiency by 1.1∼2.5 times, and the sensitization effect was depended on the cell line and the photon beam energy (6 mV∼50 kV). AGuIX was also demonstrated as a radiosensitizer in 6 animal tumor models. In a rat model of intracranial glioma, the overall survival rate of the AGuIX injection group was 2 times higher than that of radiotherapy alone. AGuIX combined with WBRT significantly improved survival by 25% in mouse models of multiple melanoma brain metastases (B16F10). In a pancreatic tumor model (CAPAN-1), sensitization therapy significantly reduced tumor growth by 50% compared with radiation alone. AGuIX had good toxicity in rats and even non-human primates, could be directly administered, intravenously and possessed imaging and radiosensitization properties, which prompted its use in patients. This is also the first time that it has been injected into humans. Meanwhile, AGuIX was also reported to have sensitization therapy effects to squamous cell carcinoma of head and neck SQ20B,55 glioblastoma U-87 MG,56 cervical cancer HeLa,57 pancreatic cancer CAPAN-11858 and melanoma B16F10.59 Afterwards, Lux et al.60 summarized preclinical evidence supporting AGuIX's transfer to the first human clinical evaluation. Sancey et al.61 translated AGuIX into a phase I clinical evaluation for the treatment of brain metastases and advanced cervical cancer.
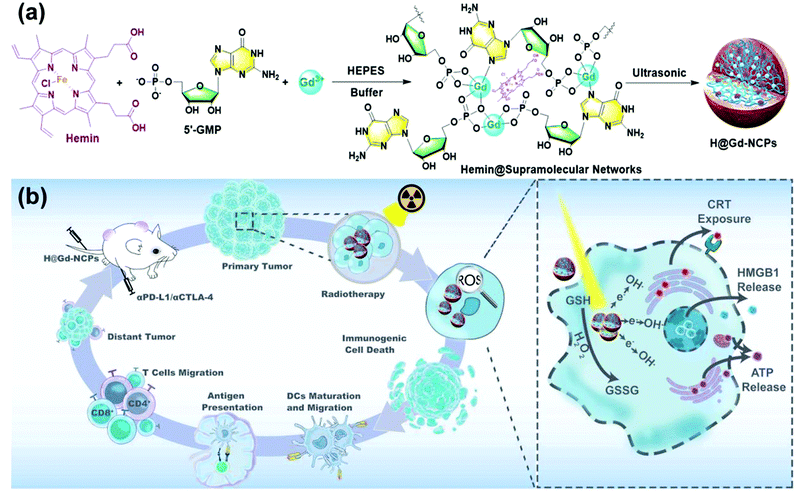 | ||
| Fig. 3 (a) Synthetic schematic of H@Gd-NCPs; (b) The mechanism of H@Gd-NCPs for radiosensitization via amplifying intracellular oxidative stress to potentiate checkpoint blockade immunotherapies. Copyright © The Author(s) 2021. Reprinted from ref. 48 with permission. | ||
Hf metal, a rare earth metal, has often been used in the X-ray manufacturing industry because of its ability of electron emission. It is a material which plays a crucial role in the atomic energy industry and has also been used in medical research and use.62 Hf-based nanomaterials have strong X-ray attenuation capabilities, and are widely studied not only for their physical effects, but also for their chemical and biological effects (negligible toxicity and chemical inertia).63 Hf element could not only enhance the effect of RT by absorbing X-ray energy, but also transform H2O and O2 into some ROS and induce cell apoptosis. Chen et al.64 designed enzyme-like, folic acid modified, Hf-based manganoporphyrin metal–organic framework nanoparticles (MnTCPP–Hf–FA MOF NPs) for targeted tumor radiotherapy and overcoming radiation resistance induced by hypoxia. Hf could absorb X-ray energy, transform H2O and O2 into some ROS and induce cell apoptosis. Hence, the nanoparticles could effectively inhibit the growth of melanoma and prevent tumor recurrence after a single X-ray irradiation with intravenous injection. Gong et al.65 constructed Hf–nMOFs functioned by Fe3+ (Hf–BPY–Fe). Under radiographic radiation, Hf4+ produced a huge number of high-energy electrons, some of which converted H2O to ˙OH, positioning the cell cycle in the radiation-sensitive G2/M phase and down-regulating DNA repair related proteins to reduce DNA self-repair. The calculated sensitization enhancement ratios by using the model of multi-target single-hit for Hf–BPY and Hf–BPY–Fe were 1.41 and 1.74, which almost achieved ideal radiotherapy results. Liu et al.66 prepared coordination polymer nanoparticles with strong RT and RDT effects by using aggregation induced luminescent materials (AIE) and Hf nanoparticles. Hf could not only absorb X-rays as a nano-radiosensitizer to enhance RT storage of radiation energy, but also acted as an intermediary to transfer X-ray energy for RDT. Hf–AIE–PEG significantly enhanced tumor growth inhibition compared to RT/PDT alone in the control group. Results also showed that Hf–AIE–PEG–DBCO could generate effective ˙OH and radiosensitization under X-ray irradiation, realizing most anticancer efficiency by synchronous RDT and RT.
As a member of the most abundant rare natural element in the earth's crust, cerium (Ce) is also a high Z metal which has been widely used in the medical field. Zhong et al.67 firstly reported the cerium (Ce)-doped NaCeF4:Gd, Tb scintillating nanoparticles (SCNPs), which had good radiosensitization through the photoelectric effect and synchronous RT/RDT could be achieved to significantly inhibit tumor growth. Sun et al.68 constructed cisplatin loaded LiLuF4:Ce3+ scintillation nanoparticles (NPs + Cis) to enhance tumor radiosensitivity. Besides, the newly synthesized Ce oxide nanoparticles (CONPs) were also studied. The researchers have investigated the inherent toxicity of CONPs to cancer cells of various origins, including alveolar epithelial cancer cells,69 hepatocellular carcinoma cells70 and pancreatic carcinoma cells.71 Goushbolagh et al.72 studied the radiation dose reduction factors (DRFs) of CONPs in MRC-5 human lung fibroblasts and MCF-7 breast cancer cells. Wason et al.73 demonstrated the production of ROS in CONP treated cancer cells for RT. The mechanism of CONPs in specifically killing cancer cells was that ROS drove the oxidation of thioredoxin 1 (TRX1) and activated c-Jun terminal kinase (JNK), resulting in the activation of apoptosis signaling kinase 1 (ASK1) to induce apoptosis. After this, Ce oxide based nanoparticles were supported as a novel tumor tissue sensitizer. Jiang et al.74 prepared a kind of spindle-shaped CuS@CeO2 NPs made up of mixed Ce elements (Ce(IV) and Ce(III)) and CuS NPs. CeO2 could function as a nanoenzyme to catalyze endogenous H2O2 in the tumor tissue into O2, which remodeled the hypoxic microenvironment into the one susceptible to RT. At the same time, it could also combine self-supplied oxygen, photothermal capacity and RT sensitivity for cancer treatment. Zhou et al.75 fixed CeO2 nanoparticles with two-dimensional graphite acetylene (GDY) to form a GDY–CeO2 nanocomposite material, which could alleviate tumor hypoxia, promote radiation-induced DNA damage, and ultimately inhibit tumor growth in vivo.
Except for Gd, Hf and Ce based nano-radiosensitizers, some other rare earth elements such as europium (Eu) and yttrium (Y) containing nanoparticles were also used for radiosensitization. Ghaemi et al.76 doped high Z elements Eu and Gd into zinc oxide (ZnO) nanoparticles. The results showed that the therapeutic effect of 20 μg mL−1 of nanoparticles at 2 Gy of X-ray dose was the same as that of untreated cells under 6 Gy X-ray irradiation. The efficiency of intracellular X-ray was improved effectively. Porosnicu et al.77 studied the radiotherapy effect of Y2O3 nanoparticles combined with X-ray irradiation on A375 melanoma cells. The DNA damage of cells exposed to 50 μg mL−1 of Y2O3 nanoparticles was more severe than that of 6 Gy X-ray dose irradiation alone. Notably, Liu et al.78 constructed a multifunctional nano-radiosensitizer with the under conversion nanoparticles (UCNPs) as the core. The UCNP core was used as a radiation dose amplifier, a multifunctional matrix for bioimaging, and a near-infrared control/MR monitoring of drug release. It has the potential to be further developed into a powerful platform for future multi-modal imaging-guided therapy.
4. Semiconductor metal-based nano-radiosensitizers
The conductivity of a semiconductor is between an insulator and a conductor, and it may greatly change the stimulation under external light and heat. Therefore, semiconductor materials have great potential in the application of radiotherapy sensitization. Semiconductor nanomaterials with photocatalytic function could be activated by light to produce free radicals and enhance the radiation effect.78The metal element Bi has the highest atomic number among all non-radioactive elements and possesses excellent radiosensitization.79 Bi-containing semiconductors such as Bi2S3, Bi2Se3 and Bi2O3 have the advantages of small carrier effective masses, long Fermi wavelength, and small band overlap energy, are popular materials in the field of radiosensitization.80–83 In addition, studies have shown that Bi is not only an excellent photothermal material, but can also be used for computed tomography (CT) as a radiosensitizer and a contrast agent.84 Bi-based nanomaterials could utilize the high-Z element Bi to block X-rays and interact with and deplete GSH, thereby increasing X-ray deposition at tumor sites and enhancing RT.85,86 Yu et al.10 constructed Bi–LyP-1 NPs based on peptide (LyP-1) labeled ultra-small Bi NPs with a diameter of about 3.6 nm. The ability of Bi element to absorb ionizing radiation and radiation under the second near-infrared laser (NIR-II 1064 nm) enabled the Bi–LyP-1 NPs to perform dual-mode photoacoustic/CT imaging and highly coordinated NIR-II tumor photothermal-/radio-therapy. The survival score of cells treated with Bi–LyP-1 NPs s at 4 Gy was about 0.107, which improves the efficacy of radiotherapy significantly. In addition, Bi–LyP-1 NPs could be completely eliminated from the mouse's body via urine and faeces after 30 days. This is the first report on the photothermal and radiation properties of Bi NPs and their applications in biomedical and multimodal imaging. However, the easy oxidation of Bi nanocrystals hindered their development. To solve this problem, Yu et al.87 used the chemical reduction method to coat Bi NPs with thiol ligands and modified them with polyethylene glycol phospholipids on their surfaces. Because the adsorption energy between metal and sulfur was enhanced, thiol ligands on the surface of Bi–SR–PEG were able to remarkably avoid the nuclear oxidation of Bi. Importantly, under the irradiation of 4 Gy X-ray dose, the survival rate of tumor cells in the injected nanoparticle group was half of that in the blank control group, indicating Bi NPs have a significant photosensitization effect and could absorb and concentrate the radiation dose. Cheng et al.81 prepared Bi2S3 nanoagents; both in vivo and in vitro experiments confirmed that Bi2S3 nanoagents could improve the anti-tumor effect of RT by enhancing the local radiation dose and photothermal effect, thus increasing the lethal effect of radiation. In addition, these nanoagents also have the ability to act as contrast agents for X-ray, CT and photoacoustic imaging. Subsequently, Ren et al.88 doped ultra-small Bi2S3 quantum dots into hollow mesoporous Prussian blue (HMPB) nanocubes to amplify tumor oxidative stress and enhance light/radiotherapy. Ultra-small Bi2S3 quantum dots could not only be used in CT and RT with obvious therapeutic effects, but also prolong blood circulation and reduce systemic toxicity of kidney metabolism. Du et al.89 used a hydrothermal method to synthesize hyaluronic acid functionalized bismuth oxide nanoparticles (HA–Bi2O3 NPs) for targeted CT imaging and radiosensitization of tumors. Song et al.90 prepared PEG–Bi2Se3@PFC@O2 nanoparticles. Experiment showed the cancer cells treated with PEG–Bi2Se3 + RT exhibited remarkably enhanced DNA damage compared to those treated with RT and PEG–Bi2Se3 alone. In addition, further enhanced DNA damage was induced by PEG–Bi2Se3@PFC@O2 + RT, to a level even higher than that achieved by PEG–Bi2Se3 + RT. On the one hand, Bi as a high-Z element can effectively concentrate a greater local radiation dose within the tumor, thus enhancing the RT efficacy to cancer. On the other hand, PFC loaded inside these hollow nanoparticles could be used as an oxygen carrier to moderately improve tumor oxygenation during NIR laser irradiation and further overcome the hypoxia-associated radio-resistance of tumors. As shown in Fig. 4, Yao et al.80 used bovine serum albumin as a template to prepare Bi2Se3–MnO2 nanocomposites. The nanocomposites were used as radiosensitizers to increase the local radiation dose, and showed excellent performance in CT and MRI. Similarly, Liu et al.91 encapsulated bismuth (Bi)-based nanospheres in the MnO2 layer to form a core–shell-structured radiosensitizer (Bi@Mn), and then loaded docetaxel (DTX). The biodegradable composite Bi@Mn–DTX–PFA could simultaneously modulate TME and achieve multimodal treatment (RT/CDT/CHT) for hypoxic tumors. Song et al.92 designed MnSe@Bi2Se3 core–shell nanostructures by a partial cation exchange method. The Bi2Se3 shell endowed the nanostructure with strong absorbance of both X-rays and NIR light, which was useful for computed tomography (CT) imaging, enhanced RT, as well as PTT. A remarkably enhanced DNA damage level induced by X-rays (4 Gy) was observed on cancer cells treated with MnSe@Bi2Se3–PEG in comparison to the PBS control; the SER of MnSe@Bi2Se3–PEG was calculated to be 1.16, suggesting the strong RT enhancement effect of the Bi-containing nanoparticles. In recent years, Bi element based heterostructures also attracted much attention. Wang et al.93 proposed a metal–semiconductor heterostructure based on the Schottky barrier (Au–Bi2S3 HNSCs). As a radiosensitizer, it could not only deposit a higher radiation dose in the tumor in the form of high-energy electrons, but also generate a large number of low-energy electron–hole pairs triggered by X-rays. Moreover, it could use the catalytic reaction triggered by X-rays to effectively decompose H2O2 over-expressed in the tumor microenvironment into highly toxic ˙OH to enhance the effect of selective radiotherapy in hypoxic tumors. Under X-ray irradiation, the γ-H2AX fluorescent spots of cells treated with Au–Bi2S3 HNSCs increased significantly, which was 5.65 times that of cells treated with X-rays alone. Moreover, colonies formed by Au–Bi2S3 HNSCs treated HeLa cells decreased sharply from 80% to 15% under X-ray irradiation, reflecting the high efficiency of Au–Bi2S3 HNSCs as radiosensitizers. The catalytic process of Au–Bi2S3 HNSCs triggered by X-rays did not require oxygen and provided an easy and effective method to produce non-oxygen dependent free radicals in hypoxic tumors, which provided a new idea for the rational design of effective radiosensitizers.
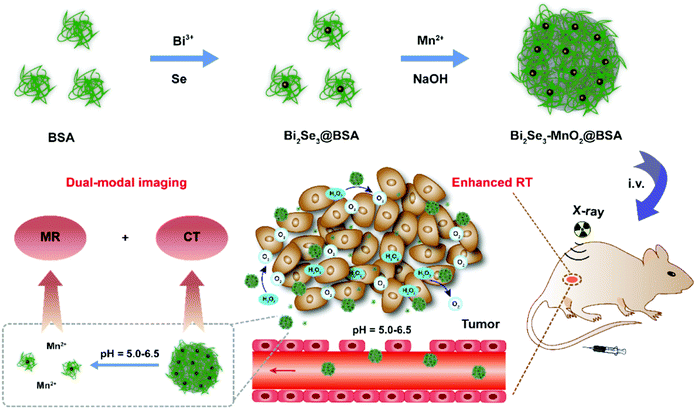 | ||
| Fig. 4 Synthetic schematic of Bi2Se3-MnO2@BSA NPs and their application in enhanced RT. Copyright © 2021 American Chemical Society. Reprinted from ref. 80 with permission. | ||
It is important to note that Bi is more suitable for in vivo applications due to its low toxicity, good biocompatibility, and higher cost-effectiveness than other high-altitude ordinal elements.83,94 In addition, Bi also has good reactivity and solubility. The size and shape of its particles in the process of synthesis are easy to control and they can be easily eliminated from the body.95 They are also used in off-the-shelf drugs (e.g. Pepto-Bismol),96 which confirmed their relative safety. Therefore, Bi NPs are a very promising multi-mode nanoplatform for dual-mode CT/PA guided combination therapy. Zang et al.97 synthesized polyvinylpyrrolidone (PVP) modified Bi2WO6 nanosheets with good biocompatibility; it was also the first time to be used in radiotherapy as a radiosensitizer. The high-Z elements Bi (Z = 83) and W (Z = 74) endowed PVP–Bi2WO6 with better X-ray energy deposition performance, thereby enhancing the radiation damage. Besides, the Bi2WO6 semiconductor exhibited obvious photocurrent and photocatalytic radiation catalytic activity under X-ray irradiation, leading to the effective separation of electron/hole pairs, thereby promoting the production of ROS and ˙OH. PVP–Bi2WO6 nanosheets displayed excellent enhancement of radiotherapy efficacy in animal models, and could be used as an excellent contrast agent for X-ray CT imaging. These findings may provide another nanotechnology strategy for simultaneous radiation energy deposition and radiocatalytic tumor radiosensitization.
Being nontoxic and bio-inert, tantalum (Ta) has been widely used in clinical implants, artificial joints, and stents.98–100 Ta is known to strongly absorb X-rays, so radiation energy can be deposited within the tumor to sensitize RT.101 Many research studies focused on investigating the radiosensitization of tantalum oxide (TaOx).102,103 Chen et al.104 fabricated mesoporous tantalum oxide (mTa2O5) nanoparticles with PEG modification to allow efficient loading of doxorubicin (DOX). Since Ta possessed high X-ray attenuation coefficient, mTa2O5–PEG/DOX nanoparticles could offer an intrinsic radiosensitization effect to increase X-ray-induced DNA damage during radiotherapy. The nanoparticles could not only offer a significant radiosensitization effect, but also show dramatically reduced systemic toxicity compared to conventional chemoradiotherapy using free DOX. Song et al.105 developed a simple and mild method to encapsulate catalase into hollow TaOx nanospheres (TaOx@Cat–PEG) as bio-nanoreactors. TaOx@Cat–PEG exhibited a RT enhancement effect, which was attributed to the factors that: (1) the Ta element could enable the deposition of radiation energy within the tumor to sensitize RT; (2) the catalase loaded inside TaOx nanospheres could effectively improve tumor oxygenation by decomposing endogenic H2O2 in the tumor microenvironment, further overcoming the hypoxia-associated radio-resistance of tumors. Moreover, Song et al.106 fabricated polyethylene glycol (PEG) stabilized perfluorocarbon (PFC) nano-droplets decorated with TaOx nanoparticles (TaOx@PFC–PEG), and Gong et al.107 prepared the core–shell TaOx@MnO2 nanostructures for RT enhancement. The two studies also improved radiosensitivity through the accumulation of TaOx in X-rays and the increase of oxygen content in the tumor microenvironment. Furthermore, Peng et al.108 constructed an oxygen-carrying nanoplatform based on polyethylene glycol TaOx (HMTCP@PFP) for triple sensitized tumor RT. O2 would release when HMTCP@PFP was triggered by a near-infrared laser, which would improve the efficiency of radiotherapy. Meanwhile, radiant energy would be deposited inside the tumor by the Ta element, resulting in the reduction of the survival fractions of 4T1 cells after combined treatment (HMTCP@PFP@O2 + RT) to 23.4%.
5. Other metal-based nano-radiosensitizers
Ti is considered to be a rare metal that is widely used in the medical field, especially in surgical applications, such as dental repair, human bone and tissue transplantation, etc.109–111 Ti dioxide (TiO2) has been widely used in cancer treatment due to its high ionization energy conversion efficiency, larger surface area, low cytotoxicity and ultraviolet radiation absorption ability.112,113 Studies have shown that TiO2-NPs could generate free radicals after irradiation, promoting the spontaneous production of ROS, thereby destroying nucleic acids (e.g., DNA).114–116 Youkhana et al.117 proved that TiO2-NPs are cytocompatible to cells, even at very high concentrations. Incubation of prostate cancer and keratinocyte cell lines with TiO2-NPs could achieve significant radiosensitization. Pan et al.118 developed nuclear targeted mesoporous TiO2 nanoparticles (MTiO2(SN-38)–TAT–RGD), in which TiO2 acted as a radiosensitizer to control the cancer cell cycle in the G2/M phase and enhance the lethality of radiation therapy to cancer cells. Followed by this, Pan's group119 modified the core–shell structure of TiO2@MnO2 with glucose oxidase (Gox), which could also effectively prevent the formation of lung metastases and prolong the survival rate of mice. Later, Morita et al.120 designed the polyacrylic acid modified nano-titanium dioxide nanoparticles (PAA–TiOx NPs), which could strengthen the therapeutic effect of X-ray irradiation when used for local injection of tumors. PAA–TiOx NPs could also serve as carriers of H2O2 to transport and continuously release H2O2 in cells for at least 7 hours to keep H2O2 at a high level. Thus, the radiosensitivity of tumor cells to X-rays was improved. Hou et al.121 synthesized a nano-titanium dioxide composited polyurethane/polyacrylamide (TPU/PAAM) hydrogel and made it into pills. The results showed that the dose distribution of the TPU/PAAM group in the target area was much better than that in the commercial injection group, and sufficient dose was located at the lesion site. TPU/PAAM also had an antibacterial effect, which could produce better curative effects on superficial tumors.Other common metal types with nano-radiosensitivity include iron (Fe) based and copper (Cu) based nano-radiosensitizers, which could also catalyze the H2O2 substrate to produce ROS. The sensitization mechanism of Cu-based nanoparticles is as follows:122 Cu2+ + H2O2 → Cu+ + HOO· + H+ (1); Cu+ + H2O2 → Cu2+ + HO· + OH− (2). Zhang et al.123 designed an intelligent radiosensitizer based on Cu2(OH)PO4 nanocrystalline (Cu2(OH)PO4@ PAAS NCs), which could respond to both exogenous stimuli (X-rays) and endogenous stimuli (H2O2). After X-ray irradiation, Cu2(OH)PO4 nanocrystals would undergo photoelectron transfer to generate Cu1 positions. Under Fenton reaction, Cu1 sites triggered by X-rays play a role as a catalyst to effectively decompose H2O2 overexpressed in TME into highly toxic hydroxyl radicals, which ultimately induced tumor cell apoptosis and necrosis. This ensured that the radiosensitization process was performed only in the hypoxic tumor and not in normal cells, thus effectively reducing the damage to the surrounding healthy tissue. Similarly, Fan et al.124 proposed a nanoplatform (G5.NHAc–Pyr/Cu(II)) based on the complexation of pyridine (Pyr) and 5th generation (G5) polyamide (PAMAM) dendrimers with Cu(II) (Fig. 5a), which could effectively enhance radiotherapy. After 22 days of treatment, the relative volume of tumor showed in Fig. 5b and c. The order of tumor size was G5.NHAc-Pyr/Cu(II) + RT (2.91 ± 0.63 times) < G5.NHAc-Pyr/Cu(II) (4.66 ± 0.59 times) < NS + RT (8.19 ± 1.12 times) < NS (10.56 ± 0.57 times). This is the first report of PAMAM dendrimers-coordinated Cu(II) complexes for tumor nanotherapy and metastasis. In order to maximize the multimodal imaging and therapeutic effects of nanomaterials, a variety of ways have been designed. Among them, the formation of heterostructures was of great interest, because the heterostructures not only exhibited the characteristics of individual components, but also had synergistic properties. Huang et al.125 designed dumbbell-shaped multiphase nanocrystalline copper selenide gold (CSA), which could be used as an effective radiosensitizer, and this heterogeneous structure showed significant radiosensitization. Studies have showed that CuO nanoparticles are able to generate oxygen after microwave radiation, which obviously improves the oxygen concentration and oxygen pressure in TME, allowing tumor hypoxic cells to reoxygenize.126 Chen et al.127 reported that microwave (MW)-excitated IL-Quercetin–CuO–SiO2@ZrO2–PEG nanoparticles (IQuCS@Zr–PEG NSPs) could uninterruptedly produce oxygen after microwave irradiation. After 20 min of microwave irradiation, the oxygen concentration produced was 3.10 times that of bare solution (phosphate buffered brine, PBS), improving the reoxygenation ability of the tumor, thereby enhancing the combined effect of radiotherapy and microwave hyperthermia.
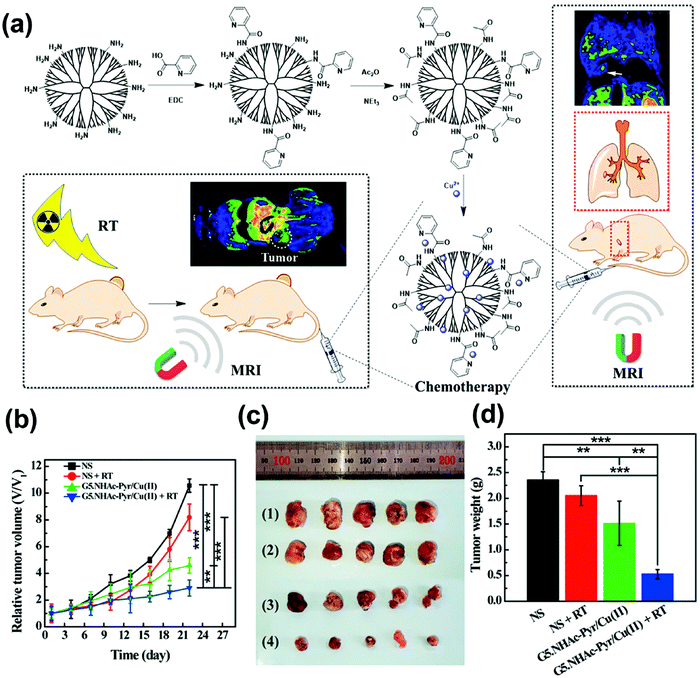 | ||
| Fig. 5 (a) Synthetic schematic of Cu(II) complexes with Pyr- functionalized PAMAM dendrimers for the RT-enhanced T1 MR imaging and chemotherapy of tumors and tumor metastasis; (b) the relative tumor volumes in 22 days after various treatments (n = 5 in each group); (c) representative photographs of tumor tissues in (1) NS group, (2) NS plus RT group, (3) G5.NHAc-Pyr/Cu(II) group, and (4) G5.NHAc-Pyr/Cu(II) plus RT group; and (d) the average tumor weight. Copyright © 2019 American Chemical Society. Reprinted from ref. 124 with permission. | ||
Some iron (Fe)-based nano-radiosensitizers have also been reported. The sensitization mechanism of Fe-based NPs is as follows:128 Fe2+ + H2O2 → Fe3+ + HO· + OH− (1); Fe3+ + H2O2 → Fe2+ + HOO· + H+ (2). Yang et al.129 developed a multifunctional hyperthermia system (Ge11–PDA–Pt@USPIOs) by wrapping ultra-small and super-paramagnetic iron oxide nanoparticles with polyacrylic acid. It exhibited synergistic therapeutic effects of radiotherapy and chemotherapy under low-temperature conditions in vitro. This study was also the first one to demonstrate that USPIO could alleviate tumor hypoxia and enhance tumor sensitivity to radiotherapy. Meidanchi et al.130 used a hydrothermal reaction method to prepare superparamagnetic spinel zinc ferrite nanoparticles (ZnFe2O4 NPs) as a radiosensitizer for tumor treatment. External radiotherapy of ZnFe2O4 NPs on human prostate cancer cells (as a model of highly radiation-resistant cells) under γ ray irradiation showed that their killing rate for highly radiation-resistant cells was 17 times higher than that of radiotherapy alone. The highly biocompatible ZnFe2O4 NPs (at a concentration of 100 μg mL−1) had a synergistic effect in radiotherapy and were a reliable radiosensitizer. Shetake et al.131 prepared iron oxide nanoparticles with oleic acid function (MN–OA). In MN–OA and radiation-treated cells, long lasting DNA damage could always be observed in the form of γ-H2AX lesions. Results showed that the cytotoxicity of the combination therapy (MN–OA + 2 Gy) was 3–5 times stronger than that of 2 Gy of radiation alone. The mechanism and effect of MN–OA induced radiosensitization was also verified in immunoactive mouse fibrosarcoma models. Hauser et al.128 proved that iron oxide nanoparticles could be utilized to enhance the effect of radiation via ROS. Fakhimikabir et al.132 prepared folic acid-conjugated polyglycerol coated iron oxide nanoparticles (FA–PG–SPIONs). Results revealed that higher concentrations of the FA–PG–SPIONs (200 μg mL−1) in combination with 6 MeV electron beams could enhance radiosensitization of HeLa cells. Jafari et al.133 studied the radiosensitization of polyglycerol coated superparamagnetic iron oxide nanoparticles (PG–SPIONs) on U87-MG cancer cells. The results showed that compared with radiotherapy alone, the survival rate of U87-MG cells was significantly decreased by PG–SPIONs + 6 MV X-rays. Studies have shown that clinically relevant radiotherapeutic isotopes (such as (223)Ra, (213)Bi, (177)Lu, (90)Y, (89)Zr, (67)Cu and (64)Cu)) marked superparamagnetic iron oxide nanoparticles could lead to enhanced localized submicron radiation damage with up to 20% increase in radiation dose.134
Tungsten (W) is able to emit photoelectrons, scattered photons, Compton electrons, negative electron pairs and positron, and Auger electrons under high energy irradiation to produce radiochemicals (free radicals and ionization) that kill tumor cells.1 Wang et al.135 demonstrated that tungsten sulfide quantum dots (WS2 QDs) could be used for photothermal therapy (PTT) and RT. Dong et al.136 designed semiconductor heterojunction structured WO2.9–WSe2–PEG nanoparticles. Under X-ray irradiation, the nanosystem could catalyze the high expression of H2O2 in TME to produce oxygen-independent ROS. The results showed that local RT/PTT under low radiation dose and mild temperature was able to efficiently inhibit tumor metastasis, ablate local tumors, and prevent recurrence of tumors. At the same time, the nanosystem could also induce high temperature under near-infrared irradiation to enhance RT results.
Molybdenum (Mo) has a high Z number and has also been used as a radiosensitizer in radiotherapy. Wang et al.137 synthesized MoS2@PANI multifunctional nanomaterials, which could effectively enhance radiation sensitivity and improve radiotherapy. Kirakci et al.138 reported a new generation of RSs based on octahedral molybdenum cluster complexes (Mo6) that could directly produce O2(1Δg) after exposure to X-rays. And it had evident radiotoxicity towards human cervix carcinoma HeLa and human MRC fibroblast cells. Another study by their group also confirmed that Mo6 with iodine inner ligands could be efficiently quenched by oxygen to produce O2 (1Δg) during X-ray irradiation, and exhibited a noticeable radiotoxic effect against cancerous Hep-2 cells but negligible radiotoxic effect against normal MRC-5 cells.139
6. Non-metal-based nano-radiosensitizers
Black phosphorus (BP) is known as a supermaterial, which not only attracts wide attention in the fields of transistors, optoelectronic devices, catalysis, energy and so on, but also shines in the application in biological materials.140 BP nanosheets were able to induce overproduction of 1O2 during X-ray irradiation, creating damage and apoptosis of nearby cancerous cells.141 However, BP is easily oxidized into PxOy species at room temperature, which greatly limits its application prospects.142 Zhang et al.143 synthesized Pt@BP through surface coordination, and maintained the surface morphology and performance of BP nanosheets for more than 24 h at room temperature. Pt@BP showed good cell uptake rates compared to unmodified cisplatin. This study was the first attempt to stabilize BP with cationic cisplatin, providing a new way to alleviate the oxidation of BP. Huang et al.142 synthesized the BP/Bi2O3 heterostructure by the in situ growth method as a highly effective biocompatible sensitizer for tumor synergistic radiotherapy. The Bi2O3 modification inhibited the rapid degradation of BP nanosheets and made the BP/Bi2O3 heterojunction exhibit good stability in water. The synergistic effect of Bi2O3 and BP triggered the excessive production of 1O2, improving efficient X-ray photodynamic therapy effect, blocking cell cycle and inducing apoptosis. Similarly, Chan et al.144 designed a nanosystem (PLGA–SS–D@BPQDs) based on poly (lactic-co-glycolic acid) and ultrasmall black phosphorus quantum dots (BPQDs) for accurate tumor radiosensitization (Fig. 6). The singlet oxygen efficacy of PLGA–SS–D@BPQDs increased from 100% to 178% after X-ray irradiation. Therefore, the relative tumor volume on the 21st day after PLGA–SS–D@BPQDs and PLGA–SS–D@BPQDs + X-ray treatment reduced to 2168% and 1220%, while the relative tumor volume on the 21st day after the saline and X-ray treatment increased to 4279% and 3866%. These research studies indicated that there is great potential and research space for the application of materials containing BP nanostructures in biomedicine.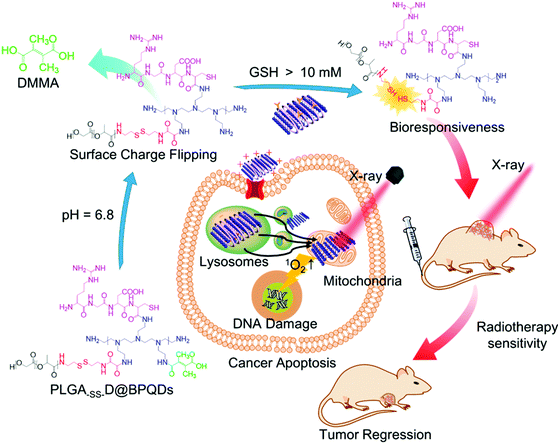 | ||
| Fig. 6 Rational design and application of PLGA-SS-D@BPQDs to tumor radiotherapy. Copyright © 2018 American Chemical Society. Reprinted from ref. 144 with permission. | ||
Selenium (Se) is an essential trace element in the human body.145 In the past, Se has shown interesting radioprotective properties as a low toxic and potent antioxidant agent.146–150 In recent years, Se has also been found to have radiosensitization,151 and it exhibited a differential effect on the tumor and normal cells.152 Cruz et al.153 synthesized Se nanoparticles (SeNPs). MTT assay manifested that the synergistic effect of the SeNPs + X-ray played a key role on increasing cell killing through remarkably elevating caspase-3 activity to induce apoptosis and cell cycle arresting. SeNPs also showed potent cytotoxicity effect on cancer cells, but relatively less toxic effect on normal healthy cells. Furthermore, Chen et al.154 evaluated the therapeutic effect of nano-Se as a novel radiosensitizer. Nano-Se was applied in combination with radiation against McF-7 breast cancer cells. Results showed that nano-Se could enhance the toxic function on radiation, resulting in higher mortality than when used alone. Hence, nano-Se was expected to be used as an adjunct drug to increase the sensitivity of cancer cells to the toxic effects of radiation, thereby reducing the damage to nearby normal tissues. Later, Gao et al.155 constructed a series of ionizing radiation-responsive NPs using Se-containing block co-polymers (PSeR/DOX). In vitro simulation experiments showed that when treated with PSeR NPs/5 Gy radiation, a remarkable reduction in the GSH/GSSG level was observed in MDA-MB-231 cells, the expression of catalase was increased and the intracellular ROS level was up-regulated from 55.3% to 85%. This treatment also down-regulated the expression of HLA-E and enhanced the NK cell-mediated cytotoxicity, demonstrating that Se-containing NPs not only had a sensitive response to radiation stimuli but also possessed potential anticancer effects and immune checkpoint inhibitor activity with radiotherapy. Similarly, Farhood et al.152 also discussed the radiomitigative and radioprotective effects of selenium on normal cells/tissues, and its radiosensitive effect on cancer cells.
Quercetin is one of the main flavonoids, a secondary metabolite of plants, and a traditional Chinese medicine used for asthma, anti-allergic, antihypertensive and tumor treatments.156 It has been reported that quercetin played an important role in tumor radiosensitivity, which can improve the radiosensitive effect by inhibiting the ATM mediated pathway whether in vitro or in vivo.157 Huang et al.157 used quercetin-loaded mesoporous silica nanoparticles as a radiosensitizer. The results showed that the nanosystem could promote the apoptosis process in tumor cell and inhibit tumor growth whether in vitro or in vivo. However, the poor solubility of quercetin is an urgent problem to be solved. Ma et al.158 proposed metal–organic framework (Zr–MOF–QU) nanoparticles based on quercetin (QU) modification, in which QU acted as a radiosensitizer, and Zr–MOF acted as both the raw material for the production of carbonic anhydrase IX (CAIX) inhibitor and the nano-carrier. QU was embedded in the Zr–MOF structure to increase the absorption of radiation energy and reduce the hypoxia conditions of tumor, so as to achieve synergic dual sensitization therapy. RT experimental results showed that the composite nanomaterial could reduce the resistance of tumor tissue to radiation injury and enhance the sensitivity of tumor tissue to radiation injury.
Imidazole compounds, especially nitroimidazole, are a kind of hypoxic cell radiosensitizing agent.159 A variety of nitroimidazole radiotherapy sensitizers have been developed and used in clinical practice, such as pemonidazole, nimoprazole, and glycididazole sodium.160 Liu et al.161 combined the hypoxic radiosensitizer nitroimidazole with lipid molecules with hydrolyzable ester bonds to form MDH, and then mixed it with DSPE–PEG2000 and cholesterol to prepare MLP liposomes. The hypoxia radiosensitizer nitroimidazole increased the radiosensitivity of radiation-tolerant hypoxia cells through electron affinity and caused the DNA damage by ionizing radiation. Masunaga et al.162 compared the radiosensitizing effect of the radiosensitizing agents on three hypoxic cells (SCC VII, SAS/neo and SAS/mp53 tumors) under aerobic and hypoxic conditions. The radiosensitization and repair inhibition of α-ray irradiation under aerobic and hypoxia conditions were as follows: Nimorazole < SR-2514 < misonidazole. The combination of radiosensitizer and conventional radiotherapy showed good radiosensitization and repair inhibition in controlling radio-resistant Q tumor cells and p53 mutant tumor cells, but the toxicity of the radiosensitizing agents to normal tissues needed to be further studied.
Some other nonmetallic nanomaterials also have good radiosensitization effect. For example, SiO2 layers can enhance radiation sensitization by increasing ROS production. Fathy et al.163 prepared silica coated magnetic iron oxide nanoparticles (SiO–MnPs), which were a promising engineering nanoagent for enhancing the radiosensitivity of breast cancer. This was the first study to evaluate the radiosensitization effect of silica coated iron oxide nanoparticles. But it lacked in vivo testing. Ruan et al.13 prepared graphene quantum dots (GQDs) with a high oxidation degree, which were used for the first time in radiotherapy of colorectal cancer. Results showed that the synergistic effect of GQDs and ionizing radiation could improve G2/M phase arrest greatly, repress cell proliferation and promote cell apoptosis. It also led to excessive production of ROS, mitochondrial damage of tumor cells, activation of apoptosis-related regulatory proteins, and ultimately resulted in the apoptosis of tumor cells. 5-Iodine-2-deoxyuracil nucleoside (IUdR) has been shown to play an important role in radiosensitization of glioblastoma.164 DNA double-strand repair inhibitor (DSBRI) KU55933 was once deemed to be one of the most prospective drugs for improving radiotherapy, but its clinical application still encountered some problems due to its latent poisonous nature to normal tissues, inability to optionally enter tumor cells and poor solubility.165
7. Conclusions and prospects
The rapid development and application of nanomaterials in the biomedical field provide a good opportunity to improve the efficiency of radiotherapy. Through this review, we have comprehensively summarized the recent advances in nanotechnology in improving radiation therapy for cancers. Due to their unique properties, nanomaterials can play a key role in tumor radiation therapy in a number of different ways to overcome radiation resistance and enhance radiation response. According to the physical and chemical properties of their main elements, they mainly include nanomaterials containing precious metals; nanomaterials containing semiconductor metal elements; nanomaterials containing rare earth metal elements; nanomaterials containing other metal elements; and nanomaterials containing non-metallic elements (Table 1). Precious metal-based nano-radiosensitizers such as GNPs and AgNPs were reported to have low toxicity. However, the sensitization effect of GNPs depends on the shape and size of nanoparticles, the type of surface modifier and the shape of tumor cells. And the radiation sensitization mechanism of AgNPs may be more complex. Therefore, the potential for clinical translation of the GNPs and AgNPs needs more investigations. Rare earth metal-based nano-radiosensitizers (Gd, Hf, Ce etc.) have been widely used in biomedical fields due to their non-toxicity and good biocompatibility. In particular Gd-based radiosensitizers have been used in a variety of animals (including non-human primates) and tested in a variety of tumors, and they have great significance and potential for clinical translation. Semiconductor metal based nano-radiosensitizers such as Bi containing radiosensitizers have also been widely focused due to their low toxicity, good biocompatibility, excellent photothermal conversion ability and radiosensitization ability. But, the easy oxidation of Bi nanocrystals and the lack of in-depth research on Bi make their clinical transformation requiring further study. The toxicity of other metal elements (Ti, Cu, Fe, Ta etc.) and non-metal elements (BP, Se etc.) is also studied. Surprisingly, Se element based radiosensitizers exhibit differential effect on the tumor and normal cells and show interesting radioprotective properties and radiosensitization. We believe that the clinical application of selenium-based nano-radiosensitizers also should receive more attention.| Main types | Main elements | Nano-sensitizers | Type and dose of radiation | SER value | Tumor cells | Ref. |
|---|---|---|---|---|---|---|
| Precious metal | Au | NH2–GNSs, FA–GNSs, TAT–GNSs | X-rays 4 Gy | 2.30 (TAT–GNSs) | KB cancer cells | 18 |
| GNPs, GNSs, GNRs | X-rays 4 Gy | 1.62, 1.37, 1.21 | KB cancer cells | 19 | ||
| Au–Pt NPs | X-rays 4 Gy | 22–24 | ||||
| PAuNTs | X-rays 250 kVp | U87-MG cells | 26 | |||
| Ag | AgNPs | X-rays 0–8 Gy | Glioma, Breast cancer, U251, C6 cells, et al. | 28–32 | ||
| AsNPs | X-rays 0–8 Gy | C6 glioma cells | 34 | |||
| AgNPs@BSA–AS–VRP | X-rays 4 Gy | 1.55 | U251 cells | 35 | ||
| Rare earth metal | Gd | GONs | Carbon ion radiation | 1.10 (A549), 1.11 (NH1299), 1.20 (NH1650) | A549 cells | 44 |
| Gd-doped ZnO NPs | X-rays 6 mV | 1.47 (10 μg mL−1), 1.61 (20 μg mL−1) | SKLC-6 cancer | 47 | ||
| AGuIXNPs | X-rays 2–8 Gy | H1299, U87-MG, HeLa cells, et al. | 52–61 | |||
| Hf | MnTCPP–Hf–FA MOF NPs | X-rays 4 Gy | B16-F10 cells | 64 | ||
| Hf–BPY–Fe NPs | X-rays 4 Gy | 1.41 (Hf–BPY), 1.74 (Hf–BPY–Fe) | A549, RM-1 cells | 65 | ||
| Semiconductor metal | Bi | Bi–LyP-1NPs | X-rays 4 Gy | 1.218 (SERD0), 1.248 (SER10) | 4T1 tumor cells | 10 |
| Bi–SR–PEG | X-rays 4 Gy | 4T1 tumor cells | 87 | |||
| Bi2S3 Nanoagents | X-rays 4 Gy | 4T1 tumor cells | 81 | |||
| Ta | mTa2O5–PEG/DOX | X-rays 6 or 8 Gy | 4T1 tumor cells | 104 | ||
| TaOx@Cat–PEG | X-rays 0–8 Gy | 4T1 tumor cells | 105 | |||
| TaOx@MnO2 | X-rays 0–8 Gy | 4T1 tumor cells | 107 | |||
| Other metals | Ti | MTiO2(SN-38)–TAT–RGD NPs | X-rays | 4T1-Luc cells | 118 | |
| PAA–TiOxNPs | X-rays 5 Gy | BxPC3 cells | 120 | |||
| Cu | Cu2(OH)PO4@PAAS NCs | X-ray irradiation (voltage:50 KV, current: 75 μA) | HeLa cells | 123 | ||
| G5.NHAc–Pyr/Cu(II) | X-rays 4 Gy or 6 MeV | 4T1, KB cells | 124 | |||
| Fe | GE11-PDA–Pt@USPIOs | γ-rays (Cs137, 662 keV) 4 and 6 Gy | H1299, MCF-7 cells | 129 | ||
| PG–SPIONs | X-rays 6 MV | U87-MG cells | 133 | |||
| Non-metal | Se | SeNPs | X-rays | Lung cancer | 153 | |
| BP | PLGA–SS–D@BPQDs | X-rays 2 or 4 Gy | A375, HeLa cells | 144 |
To sum up, the effect of radiosensitization is affected by the size, shape, and modification method of the nanoparticles. The sensitization effect of the same nanomaterial to different cells under the same dose of radiation and the sensitization effect of the same cell under different doses are different. Therefore, there is a need to design nanoparticles with the best sensitization effect and corresponding size and shape for different tumor cells, as well as the specific preparation method of synthetic nanoparticles. Moreover, nanomaterials have enough time to function in the body due to their slow biodegradation ability, low metabolic rate and long-time retention. However, these properties also tend to cause them to accumulate in the organs of the body, especially in the liver and kidneys, thereby leading to health hazards. Therefore, aiming for clinical translation, it is necessary to improve the metabolic rate of nanomaterials in the body and design green, easily degradable nanomaterials with non-toxic and harmless degradation products.
Conflicts of interest
There is no potential conflict of interest to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (81901896) and Young and Middle-aged Backbone Personnel Training Project of Fujian Health and Family Planning Commission (2021GGA043).References
- J. F. Hainfeld, F. A. Dilmanian, D. N. Slatkin and H. M. Smilowitz, J. Pharm. Pharmacol., 2008, 60, 977–985 CrossRef CAS PubMed.
- C. Global Burden of Disease Cancer, C. Fitzmaurice, C. Allen, R. M. Barber, L. Barregard, Z. A. Bhutta, H. Brenner, D. J. Dicker, O. Chimed-Orchir, R. Dandona, L. Dandona, T. Fleming, M. H. Forouzanfar, J. Hancock, R. J. Hay, R. Hunter-Merrill, C. Huynh, H. D. Hosgood, C. O. Johnson, J. B. Jonas, J. Khubchandani, G. A. Kumar, M. Kutz, Q. Lan, H. J. Larson, X. Liang, S. S. Lim, A. D. Lopez, M. F. MacIntyre, L. Marczak, N. Marquez, A. H. Mokdad, C. Pinho, F. Pourmalek, J. A. Salomon, J. R. Sanabria, L. Sandar, B. Sartorius, S. M. Schwartz, K. A. Shackelford, K. Shibuya, J. Stanaway, C. Steiner, J. Sun, K. Takahashi, S. E. Vollset, T. Vos, J. A. Wagner, H. Wang, R. Westerman, H. Zeeb, L. Zoeckler, F. Abd-Allah, M. B. Ahmed, S. Alabed, N. K. Alam, S. F. Aldhahri, G. Alem, M. A. Alemayohu, R. Ali, R. Al-Raddadi, A. Amare, Y. Amoako, A. Artaman, H. Asayesh, N. Atnafu, A. Awasthi, H. B. Saleem, A. Barac, N. Bedi, I. Bensenor, A. Berhane, E. Bernabe, B. Betsu, A. Binagwaho, D. Boneya, I. Campos-Nonato, C. Castaneda-Orjuela, F. Catala-Lopez, P. Chiang, C. Chibueze, A. Chitheer, J. Y. Choi, B. Cowie, S. Damtew, J. das Neves, S. Dey, S. Dharmaratne, P. Dhillon, E. Ding, T. Driscoll, D. Ekwueme, A. Y. Endries, M. Farvid, F. Farzadfar, J. Fernandes, F. Fischer, G. H. TT, A. Gebru, S. Gopalani, A. Hailu, M. Horino, N. Horita, A. Husseini, I. Huybrechts, M. Inoue, F. Islami, M. Jakovljevic, S. James, M. Javanbakht, S. H. Jee, A. Kasaeian, M. S. Kedir, Y. S. Khader, Y. H. Khang, D. Kim, J. Leigh, S. Linn, R. Lunevicius, H. M. A. El Razek, R. Malekzadeh, D. C. Malta, W. Marcenes, D. Markos, Y. A. Melaku, K. G. Meles, W. Mendoza, D. T. Mengiste, T. J. Meretoja, T. R. Miller, K. A. Mohammad, A. Mohammadi, S. Mohammed, M. Moradi-Lakeh, G. Nagel, D. Nand, Q. Le Nguyen, S. Nolte, F. A. Ogbo, K. E. Oladimeji, E. Oren, M. Pa, E. K. Park, D. M. Pereira, D. Plass, M. Qorbani, A. Radfar, A. Rafay, M. Rahman, S. M. Rana, K. Soreide, M. Satpathy, M. Sawhney, S. G. Sepanlou, M. A. Shaikh, J. She, I. Shiue, H. R. Shore, M. G. Shrime, S. So, S. Soneji, V. Stathopoulou, K. Stroumpoulis, M. B. Sufiyan, B. L. Sykes, R. Tabares-Seisdedos, F. Tadese, B. A. Tedla, G. A. Tessema, J. S. Thakur, B. X. Tran, K. N. Ukwaja, B. S. C. Uzochukwu, V. V. Vlassov, E. Weiderpass, M. Wubshet Terefe, H. G. Yebyo, H. H. Yimam, N. Yonemoto, M. Z. Younis, C. Yu, Z. Zaidi, M. E. S. Zaki, Z. M. Zenebe, C. J. L. Murray and M. Naghavi, JAMA Oncol., 2017, 3, 524–548 CrossRef PubMed.
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal and F. Bray, Ca-Cancer J. Clin., 2021, 71, 209–249 CrossRef PubMed.
- Z. Li, Y. Gao, W. Li, Y. Li, H. Lv, D. Zhang, J. Peng, W. Cheng, L. Mei and H. Chen, Smart Materials in Medicine, 2022, 3, 243–253 CrossRef.
- K. Haume, S. Rosa, S. Grellet, M. A. Smialek, K. T. Butterworth, A. V. Solov'yov, K. M. Prise, J. Golding and N. J. Mason, Cancer Nanotechnol., 2016, 7, 8 CrossRef PubMed.
- A. Z. Wang and J. E. Tepper, J. Clin. Oncol., 2014, 32, 2879–2885 CrossRef CAS PubMed.
- M. A. Hill, Int. J. Radiat. Biol., 2018, 94, 759–768 CrossRef CAS PubMed.
- R. Schulte, V. Bashkirov, G. Garty, C. Leloup, S. Shchemelinin, A. Breskin, R. Chechik, J. Milligan and B. Grosswendt, Australas. Phys. Eng. Sci. Med., 2003, 26, 149–155 CrossRef CAS PubMed.
- G. Song, L. Cheng, Y. Chao, K. Yang and Z. Liu, Adv. Mater., 2017, 29(32), 1700996 CrossRef PubMed.
- X. Yu, A. Li, C. Zhao, K. Yang, X. Chen and W. Li, ACS Nano, 2017, 11, 3990–4001 CrossRef CAS PubMed.
- S. Rockwell, I. T. Dobrucki, E. Y. Kim, S. T. Marrison and V. T. Vu, Curr. Mol. Med., 2009, 9(4), 442–458 CrossRef CAS PubMed.
- H. Huang, C. Zhang, X. Wang, J. Shao, C. Chen, H. Li, C. Ju, J. He, H. Gu and D. Xia, Nano Lett., 2020, 20, 4211–4219 CrossRef CAS PubMed.
- J. Ruan, Y. Wang, F. Li, R. Jia, G. Zhou, C. Shao, L. Zhu, M. Cui, D.-P. Yang and S. Ge, ACS Appl. Mater. Interfaces, 2018, 10, 14342–14355 CrossRef CAS PubMed.
- E. K. Lim, T. Kim, S. Paik, S. Haam, Y. M. Huh and K. Lee, Chem. Rev., 2015, 115, 327–394 CrossRef CAS PubMed.
- Y. Yang, W. Zeng, P. Huang, X. Zeng and L. Mei, View, 2021, 2, 20200042 CrossRef CAS.
- M. Hernandez-Rivera, I. Kumar, S. Y. Cho, B. Y. Cheong, M. X. Pulikkathara, S. E. Moghaddam, K. H. Whitmire and L. J. Wilson, ACS Appl. Mater. Interfaces, 2017, 9, 5709–5716 CrossRef CAS PubMed.
- X. Yang, M. Yang, B. Pang, M. Vara and Y. Xia, Chem. Rev., 2015, 115, 10410–10488 CrossRef CAS PubMed.
- N. Ma, P. Liu, N. He, N. Gu, F.-G. Wu and Z. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 31526–31542 CrossRef CAS PubMed.
- N. Ma, F.-G. Wu, X. Zhang, Y.-W. Jiang, H.-R. Jia, H.-Y. Wang, Y.-H. Li, P. Liu, N. Gu and Z. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 13037–13048 CrossRef CAS PubMed.
- W. Sung, S.-J. Ye, A. L. McNamara, S. J. McMahon, J. Hainfeld, J. Shin, H. M. Smilowitz, H. Paganetti and J. Schuemann, Nanoscale, 2017, 9, 5843–5853 RSC.
- T. T. Jia, G. Yang, S. J. Mo, Z. Y. Wang, B. J. Li, W. Ma, Y. X. Guo, X. Chen, X. Zhao, J. Q. Liu and S. Q. Zang, ACS Nano, 2019, 13, 8320–8328 CrossRef CAS PubMed.
- A. Kamkaew, F. Chen, Y. Zhan, R. L. Majewski and W. Cai, ACS Nano, 2016, 10, 3918–3935 CrossRef CAS PubMed.
- S. Yang, G. Han, Q. Chen, L. Yu, P. Wang, Q. Zhang, J. Dong, W. Zhang and J. Huang, Int. J. Nanomed., 2021, 16, 239–248 CrossRef PubMed.
- X. Liu, X. Zhang, M. Zhu, G. Lin, J. Liu, Z. Zhou, X. Tian and Y. Pan, ACS Appl. Mater. Interfaces, 2017, 9, 279–285 CrossRef CAS PubMed.
- M. Shi, B. Paquette, T. Thippayamontri, L. Gendron, B. Guerin and L. Sanche, Int. J. Nanomed., 2016, 11, 5323–5333 CrossRef CAS PubMed.
- S. R. Bhattarai, P. J. Derry, K. Aziz, P. K. Singh, A. M. Khoo, A. S. Chadha, A. Liopo, E. R. Zubarev and S. Krishnan, Nanoscale, 2017, 9, 5085–5093 RSC.
- P. Liu, Z. Huang, Z. Chen, R. Xu, H. Wu, F. Zang, C. Wang and N. Gu, Nanoscale, 2013, 5(23), 11829–11836 RSC.
- R. Xu, J. Ma, X. Sun, Z. Chen, X. Jiang, Z. Guo, L. Huang, Y. Li, M. Wang, C. Wang, J. Liu, X. Fan, J. Gu, X. Chen, Y. Zhang and N. Gu, Cell Res., 2009, 19, 1031–1034 CrossRef CAS PubMed.
- R. Singh, J. Swanner, J. Mims, S. Akman, C. Furdui, S. Torti and D. Carroll, Int. J. Nanomed., 2015, 10, 3937–3953 CrossRef PubMed.
- R. G. Saratale, H. S. Shin, G. Kumar, G. Benelli, D. S. Kim and G. D. Saratale, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 211–222 CrossRef CAS PubMed.
- S. N. Sunil Gowda, S. Rajasowmiya, V. Vadivel, S. Banu Devi, A. Celestin Jerald, S. Marimuthu and N. Devipriya, Toxicol. In Vitro, 2018, 52, 170–177 CrossRef CAS PubMed.
- L. Zhu, D. Guo, L. Sun, Z. Huang, X. Zhang, W. Ma, J. Wu, L. Xiao, Y. Zhao and N. Gu, Nanoscale, 2017, 9, 5489–5498 RSC.
- K. Habiba, K. Aziz, K. Sanders, C. M. Santiago, L. S. K. Mahadevan, V. Makarov, B. R. Weiner, G. Morell and S. Krishnan, Sci. Rep., 2019, 9(1), 1–9 CAS.
- J. Zhao, P. Liu, J. Ma, D. Li, H. Yang, W. Chen and Y. Jiang, Int. J. Nanomed., 2019, 14, 9483–9496 CrossRef CAS PubMed.
- J. Zhao, D. Li, J. Ma, H. Yang, W. Chen, Y. Cao and P. Liu, Nanotechnology, 2021, 32(14), 145102 CrossRef CAS PubMed.
- Z. Liu, H. Tan, X. Zhang, F. Chen, Z. Zhou, X. Hu, S. Chang, P. Liu and H. Zhang, Artif. Cells, Nanomed., Biotechnol., 2018, 46, S922–S930 CrossRef CAS PubMed.
- P. D. Liu, H. Jin, Z. Guo, J. Ma, J. Zhao, D. Li, H. Wu and N. Gu, Int. J. Nanomed., 2016, 11, 5003–5014 CrossRef CAS PubMed.
- Y. Liu, P. Zhang, F. Li, X. Jin, J. Li, W. Chen and Q. Li, Theranostics, 2018, 8, 1824–1849 CrossRef CAS PubMed.
- W. Sun, Z. Zhou, G. Pratx, X. Chen and H. Chen, Theranostics, 2020, 10, 1296–1318 CrossRef CAS PubMed.
- Z. Li, Y. Yang, H. Wei, X. Shan, X. Wang, M. Ou, Q. Liu, N. Gao, H. Chen and L. Mei, J. Controlled Release, 2021, 338, 719–730 CrossRef CAS PubMed.
- A. Ku, V. J. Facca, Z. Cai and R. M. Reilly, EJNMMI Radiopharm. Chem., 2019, 4, 27 CrossRef PubMed.
- R. Mueller, M. Moreau, S. Yasmin-Karim, A. Protti, O. Tillement, R. Berbeco, J. Hesser and W. Ngwa, Nanomaterials, 2020, 10(11), 2249 CrossRef CAS PubMed.
- S. Dufort, A. Bianchi, M. Henry, F. Lux, G. Le Duc, V. Josserand, C. Louis, P. Perriat, Y. Crémillieux and O. Tillement, Small, 2015, 11, 215–221 CrossRef CAS PubMed.
- F. Li, Z. Li, X. Jin, Y. Liu, P. Li, Z. Shen, A. Wu, X. Zheng, W. Chen and Q. Li, Nanoscale Res. Lett., 2019, 14, 328 CrossRef PubMed.
- C. Wu, R. Cai, T. Zhao, L. Wu, L. Zhang, J. Jin, L. Xu, P. Li, T. Li, M. Zhang and F. Du, Nanoscale Res. Lett., 2020, 15, 94 CrossRef CAS PubMed.
- T. Andoh, Y. Nakatani, M. Suzuki, Y. Sakurai, T. Fujimoto and H. Ichikawa, Appl. Radiat. Isot., 2020, 164, 109270 CrossRef CAS PubMed.
- M. Zangeneh, H. A. Nedaei, H. Mozdarani, A. Mahmoudzadeh and M. Salimi, Mater. Sci. Eng., C, 2019, 103, 109739 CrossRef CAS PubMed.
- Z. Huang, Y. Wang, D. Yao, J. Wu, Y. Hu and A. Yuan, Nat. Commun., 2021, 12(1), 1–18 CrossRef PubMed.
- W. Sun, L. Luo, Y. Feng, Y. Qiu, C. Shi, S. Meng, X. Chen and H. Chen, Adv. Mater., 2020, 32, e2000377 CrossRef PubMed.
- C. Lee, X. Liu, W. Zhang, M. A. Duncan, F. Jiang, C. Kim, X. Yan, Y. Teng, H. Wang and W. Jiang, Nanoscale, 2021, 13, 9252–9263 RSC.
- X. Ma, C. Lee, T. Zhang, J. Cai, H. Wang, F. Jiang, Z. Wu, J. Xie, G. Jiang and Z. Li, J. Nanobiotechnol., 2021, 19, 1–10 CrossRef PubMed.
- S. Dufort, G. Appelboom, C. Verry, E. L. Barbier, F. Lux, E. Brauer-Krisch, L. Sancey, S. D. Chang, M. Zhang, S. Roux, O. Tillement and G. Le Duc, J. Clin. Neurosci., 2019, 67, 215–219 CrossRef CAS PubMed.
- Y. Du, H. Sun, F. Lux, Y. Xie, L. Du, C. Xu, H. Zhang, N. He, J. Wang, Y. Liu, G. Leduc, T. Doussineau, K. Ji, Q. Wang, Z. Lin, Y. Wang, Q. Liu and O. Tillement, ACS Appl. Mater. Interfaces, 2020, 12, 56874–56885 CrossRef CAS PubMed.
- C. Verry, L. Sancey, S. Dufort, G. Le Duc, C. Mendoza, F. Lux, S. Grand, J. Arnaud, J. L. Quesada, J. Villa, O. Tillement and J. Balosso, BMJ Open, 2019, 9(2), e023591 CrossRef PubMed.
- I. Miladi, M. T. Aloy, E. Armandy, P. Mowat, D. Kryza, N. Magne, O. Tillement, F. Lux, C. Billotey, M. Janier and C. Rodriguez-Lafrasse, Nanomedicine, 2015, 11, 247–257 CrossRef CAS PubMed.
- P. Mowat, A. Mignot, W. Rima, F. Lux, O. Tillement, C. Roulin, M. Dutreix, D. Bechet, S. Huger, L. Humbert, M. Barberi-Heyob, M. T. Aloy, E. Armandy, C. Rodriguez-Lafrasse, G. Le Duc, S. Roux and P. Perriat, J. Nanosci. Nanotechnol., 2011, 11, 7833–7839 CrossRef CAS PubMed.
- M. Luchette, H. Korideck, M. Makrigiorgos, O. Tillement and R. Berbeco, Nanomedicine, 2014, 10, 1751–1755 CrossRef CAS PubMed.
- A. Detappe, S. Kunjachan, L. Sancey, V. Motto-Ros, D. Biancur, P. Drane, R. Guieze, G. M. Makrigiorgos, O. Tillement, R. Langer and R. Berbeco, J. Controlled Release, 2016, 238, 103–113 CrossRef CAS PubMed.
- S. Kotb, A. Detappe, F. Lux, F. Appaix, E. L. Barbier, V. L. Tran, M. Plissonneau, H. Gehan, F. Lefranc, C. Rodriguez-Lafrasse, C. Verry, R. Berbeco, O. Tillement and L. Sancey, Theranostics, 2016, 6, 418–427 CrossRef CAS PubMed.
- F. Lux, V. L. Tran, E. Thomas, S. Dufort, F. Rossetti, M. Martini, C. Truillet, T. Doussineau, G. Bort, F. Denat, F. Boschetti, G. Angelovski, A. Detappe, Y. Cremillieux, N. Mignet, B. T. Doan, B. Larrat, S. Meriaux, E. Barbier, S. Roux, P. Fries, A. Muller, M. C. Abadjian, C. Anderson, E. Canet-Soulas, P. Bouziotis, M. Barberi-Heyob, C. Frochot, C. Verry, J. Balosso, M. Evans, J. Sidi-Boumedine, M. Janier, K. Butterworth, S. McMahon, K. Prise, M. T. Aloy, D. Ardail, C. Rodriguez-Lafrasse, E. Porcel, S. Lacombe, R. Berbeco, A. Allouch, J. L. Perfettini, C. Chargari, E. Deutsch, G. Le Duc and O. Tillement, Br. J. Radiol., 2019, 92, 20180365 Search PubMed.
- L. Sancey, F. Lux, S. Kotb, S. Roux, S. Dufort, A. Bianchi, Y. Cremillieux, P. Fries, J. L. Coll, C. Rodriguez-Lafrasse, M. Janier, M. Dutreix, M. Barberi-Heyob, F. Boschetti, F. Denat, C. Louis, E. Porcel, S. Lacombe, G. Le Duc, E. Deutsch, J. L. Perfettini, A. Detappe, C. Verry, R. Berbeco, K. T. Butterworth, S. J. McMahon, K. M. Prise, P. Perriat and O. Tillement, Br. J. Radiol., 2014, 87, 20140134 CrossRef CAS PubMed.
- M. H. Chen, N. Hanagata, T. Ikoma, J. Y. Huang, K. Y. Li, C. P. Lin and F. H. Lin, Acta Biomater., 2016, 37, 165–173 CrossRef CAS PubMed.
- L. R. H. Gerken, K. Keevend, Y. Zhang, F. H. L. Starsich, C. Eberhardt, G. Panzarasa, M. T. Matter, A. Wichser, A. Boss, A. Neels and I. K. Herrmann, ACS Appl. Mater. Interfaces, 2019, 11, 437–448 CrossRef CAS PubMed.
- Y. Chen, H. Zhong, J. Wang, X. Wan, Y. Li, W. Pan, N. Li and B. Tang, Chem. Sci., 2019, 10, 5773–5778 RSC.
- T. Gong, Y. Li, B. Lv, H. Wang, Y. Liu, W. Yang, Y. Wu, X. Jiang, H. Gao, X. Zheng and W. Bu, ACS Nano, 2020, 14, 3032–3040 CrossRef CAS PubMed.
- J. Liu, F. Hu, M. Wu, L. Tian, F. Gong, X. Zhong, M. Chen, Z. Liu and B. Liu, Adv. Mater., 2021, 33(9), 2007888 CrossRef CAS PubMed.
- X. Zhong, X. Wang, G. Zhan, Y. a. Tang, Y. Yao, Z. Dong, L. Hou, H. Zhao, S. Zeng, J. Hu, L. Cheng and X. Yang, Nano Lett., 2019, 19, 8234–8244 CrossRef CAS PubMed.
- L. Sun, C. Jiang, W. Li, Z. He, G. Wang, C. Cheng, F. Chen, X. Fu, H. Jiang and Q. Sun, J. Biomed. Nanotechnol., 2020, 16, 1482–1494 CrossRef CAS PubMed.
- S. Singh, A. Kumar, A. Karakoti, S. Seal and W. T. Self, Mol. BioSyst., 2010, 6, 1813–1820 RSC.
- G. Cheng, W. Guo, L. Han, E. Chen, L. Kong, L. Wang, W. Ai, N. Song, H. Li and H. Chen, Toxicol. In Vitro, 2013, 27, 1082–1088 CrossRef CAS PubMed.
- M. S. Wason, J. Colon, S. Das, S. Seal, J. Turkson, J. Zhao and C. H. Baker, Nanomedicine, 2013, 9, 558–569 CrossRef CAS PubMed.
- N. Abdi Goushbolagh, R. Abedi Firouzjah, K. Ebrahimnejad Gorji, M. Khosravanipour, S. Moradi, A. Banaei, A. Astani, M. Najafi, M. H. Zare and B. Farhood, Artif. Cells, Nanomed., Biotechnol., 2018, 46, S1215–S1225 CrossRef CAS PubMed.
- M. S. Wason, H. Lu, L. Yu, S. K. Lahiri, D. Mukherjee, C. Shen, S. Das, S. Seal and J. Zhao, Cancers, 2018, 10(9), 303 CrossRef PubMed.
- W. Jiang, X. Han, T. Zhang, D. Xie, H. Zhang and Y. Hu, Adv. Healthcare Mater., 2020, 9, e1901303 CrossRef PubMed.
- X. Zhou, M. You, F. Wang, Z. Wang, X. Gao, C. Jing, J. Liu, M. Guo, J. Li, A. Luo, H. Liu, Z. Liu and C. Chen, Adv. Mater., 2021, 33, e2100556 CrossRef PubMed.
- B. Ghaemi, O. Mashinchian, T. Mousavi, R. Karimi, S. Kharrazi and A. Amani, ACS Appl. Mater. Interfaces, 2016, 8, 3123–3134 CrossRef CAS PubMed.
- I. Porosnicu, C. M. Butnaru, I. Tiseanu, E. Stancu, C. V. A. Munteanu, B. I. Bita, O. G. Duliu and F. Sima, Molecules, 2021, 26(11), 3403 CrossRef CAS PubMed.
- Y. Liu, Y. Liu, W. Bu, Q. Xiao, Y. Sun, K. Zhao, W. Fan, J. Liu and J. Shi, Biomaterials, 2015, 49, 1–8 CrossRef CAS PubMed.
- F. Zhang, S. Liu, N. Zhang, Y. Kuang, W. Li, S. Gai, F. He, A. Gulzar and P. Yang, Nanoscale, 2020, 12, 19293–19307 RSC.
- Y. Yao, P. Li, J. He, D. Wang, J. Hu and X. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 28650–28661 CrossRef CAS PubMed.
- X. Cheng, Y. Yong, Y. Dai, X. Song, G. Yang, Y. Pan and C. Ge, Theranostics, 2017, 7, 4087–4098 CrossRef CAS PubMed.
- G. Song, C. Liang, X. Yi, Q. Zhao, L. Cheng, K. Yang and Z. Liu, Adv. Mater., 2016, 28, 2716–2723 CrossRef CAS PubMed.
- M. A. Shahbazi, L. Faghfouri, M. P. A. Ferreira, P. Figueiredo, H. Maleki, F. Sefat, J. Hirvonen and H. A. Santos, Chem. Soc. Rev., 2020, 49, 1253–1321 RSC.
- H. Xie, M. Liu, B. You, G. Luo, Y. Chen, B. Liu, Z. Jiang, P. K. Chu, J. Shao and X. F. Yu, Small, 2020, 16, e1905208 CrossRef PubMed.
- R. Zhou, H. Wang, Y. Yang, C. Zhang, X. Dong, J. Du, L. Yan, G. Zhang, Z. Gu and Y. Zhao, Biomaterials, 2019, 189, 11–22 CrossRef CAS PubMed.
- Y. Li, Y. Sun, T. Cao, Q. Su, Z. Li, M. Huang, R. Ouyang, H. Chang, S. Zhang and Y. Miao, Nanoscale, 2017, 9, 14364–14375 RSC.
- N. Yu, Z. Wang, J. Zhang, Z. Liu, B. Zhu, J. Yu, M. Zhu, C. Peng and Z. Chen, Biomaterials, 2018, 161, 279–291 CrossRef CAS PubMed.
- C. Ren, Y. Cheng, W. Li, P. Liu, L. Yang, Q. Lu, M. Xu, F. Tan, J. Li and N. Li, Biomater. Sci., 2020, 8, 1981–1995 RSC.
- F. Du, J. Lou, R. Jiang, Z. Fang, X. Zhao, Y. Niu, S. Zou, M. Zhang, A. Gong and C. Wu, Int. J. Nanomed., 2017, 12, 5973–5992 CrossRef CAS PubMed.
- G. Song, C. Liang, X. Yi, Q. Zhao, L. Cheng, K. Yang and Z. Liu, Adv. Mater., 2016, 28, 2716–2723 CrossRef CAS PubMed.
- J. Liu, J. Zhang, K. Song, J. Du, X. Wang, J. Liu, B. Li, R. Ouyang, Y. Miao and Y. Sun, Small, 2021, 17, 2101015 CrossRef CAS PubMed.
- G. Song, C. Liang, H. Gong, M. Li, X. Zheng, L. Cheng, K. Yang, X. Jiang and Z. Liu, Adv. Mater., 2015, 27, 6110–6117 CrossRef CAS PubMed.
- X. Wang, C. Zhang, J. Du, X. Dong, S. Jian, L. Yan, Z. Gu and Y. Zhao, ACS Nano, 2019, 13, 5947–5958 CrossRef CAS PubMed.
- X. Ren, S. Yang, N. Yu, A. Sharjeel, Q. Jiang, D. K. Macharia, H. Yan, C. Lu, P. Geng and Z. Chen, J. Colloid Interface Sci., 2021, 591, 229–238 CrossRef CAS PubMed.
- J. Deng, S. Xu, W. Hu, X. Xun, L. Zheng and M. Su, Biomaterials, 2018, 154, 24–33 CrossRef CAS PubMed.
- D. W. Bierer, Rev. Infect. Dis., 1990, 12(Suppl 1), S3–S8 CrossRef CAS PubMed.
- Y. Zang, L. Gong, L. Mei, Z. Gu and Q. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 18942–18952 CrossRef CAS PubMed.
- E. Koshevaya, E. Krivoshapkina and P. Krivoshapkin, J. Mater. Chem. B, 2021, 9, 5008–5024 RSC.
- C. Kang, L. Wei, B. Song, L. Chen, J. Liu, B. Deng, X. Pan and L. Shao, Int. J. Nanomed., 2017, 12, 4323 CrossRef CAS PubMed.
- M. Lu, S. Xu, Z.-X. Lei, D. Lu, W. Cao, M. Huttula, C.-H. Hou, S.-H. Du, W. Chen and S.-W. Dai, Chin. Med. J., 2019, 132, 51–62 CrossRef PubMed.
- D. Ding, D. Zhang, F. He, G. Xie and Z. Chen, Mater. Sci. Eng., C, 2020, 110, 110700 CrossRef CAS PubMed.
- N. Lee, H. R. Cho, M. H. Oh, S. H. Lee, K. Kim, B. H. Kim, K. Shin, T.-Y. Ahn, J. W. Choi and Y.-W. Kim, J. Am. Chem. Soc., 2012, 134, 10309–10312 CrossRef CAS PubMed.
- G. Song, Y. Chao, Y. Chen, C. Liang, X. Yi, G. Yang, K. Yang, L. Cheng, Q. Zhang and Z. Liu, Adv. Funct. Mater., 2016, 26, 8243–8254 CrossRef CAS.
- Y. Chen, G. Song, Z. Dong, X. Yi, Y. Chao, C. Liang, K. Yang, L. Cheng and Z. Liu, Small, 2017, 13, 1602869 CrossRef PubMed.
- G. Song, Y. Chen, C. Liang, X. Yi, J. Liu, X. Sun, S. Shen, K. Yang and Z. Liu, Adv. Mater., 2016, 28, 7143–7148 CrossRef CAS PubMed.
- G. Song, C. Ji, C. Liang, X. Song, X. Yi, Z. Dong, K. Yang and Z. Liu, Biomaterials, 2017, 112, 257–263 CrossRef CAS PubMed.
- F. Gong, J. Chen, X. Han, J. Zhao, M. Wang, L. Feng, Y. Li, Z. Liu and L. Cheng, J. Mater. Chem. B, 2018, 6, 2250–2257 RSC.
- C. Peng, Y. Liang, Y. Chen, X. Qian, W. Luo, S. Chen, S. Zhang, Q. Dan, L. Zhang and M. Li, ACS Appl. Mater. Interfaces, 2019, 12, 5520–5530 CrossRef PubMed.
- S. El Hakim, T. Chave and S. I. Nikitenko, Ultrason. Sonochem., 2021, 70, 105336 CrossRef CAS PubMed.
- J. Jakubowicz, Materials, 2020, 13(7), 1696 CrossRef CAS PubMed.
- N. E. Putra, M. J. Mirzaali, I. Apachitei, J. Zhou and A. A. Zadpoor, Acta Biomater., 2020, 109, 1–20 CrossRef CAS.
- W. Pan, B. Cui, P. Gao, Y. Ge, N. Li and B. Tang, Chem. Commun., 2020, 56, 547–550 RSC.
- S. Cesmeli and C. Biray Avci, J. Drug Targeting, 2019, 27, 762–766 CrossRef CAS PubMed.
- J. J. Yin, J. Liu, M. Ehrenshaft, J. E. Roberts, P. P. Fu, R. P. Mason and B. Zhao, Toxicol. Appl. Pharmacol., 2012, 263, 81–88 CrossRef CAS PubMed.
- M. Babaei and M. Ganjalikhani, BioImpacts, 2014, 4, 15–20 CAS.
- H. E. Townley, J. Kim and P. J. Dobson, Nanoscale, 2012, 4, 5043–5050 RSC.
- E. Q. Youkhana, B. Feltis, A. Blencowe and M. Geso, Int. J. Med. Sci., 2017, 14, 602–614 CrossRef CAS PubMed.
- W. Pan, S. Gong, J. Wang, L. Yu, Y. Chen, N. Li and B. Tang, Chem. Commun., 2019, 55, 8182–8185 RSC.
- W. Pan, B. Cui, P. Gao, Y. Ge, N. Li and B. Tang, Chem. Commun., 2020, 56, 547–550 RSC.
- K. Morita, Y. Nishimura, S. Nakamura, Y. Arai, C. Numako, K. Sato, M. Nakayama, H. Akasaka, R. Sasaki, C. Ogino and A. Kondo, Colloids Surf., B, 2021, 198, 111451 CrossRef CAS PubMed.
- Y. Hou, Y. Song, X. Sun, Y. Jiang, M. He, Y. Li, X. Chen and L. Zhang, J. Mater. Chem. B, 2020, 8, 2627–2635 RSC.
- Y. Huang, X. Ran, Y. Lin, J. Ren and X. Qu, Chem. Commun., 2015, 51, 4386–4389 RSC.
- C. Zhang, L. Yan, X. Wang, X. Dong, R. Zhou, Z. Gu and Y. Zhao, Nano Lett., 2019, 19, 1749–1757 CrossRef CAS PubMed.
- Y. Fan, J. Zhang, M. Shi, D. Li, C. Lu, X. Cao, C. Peng, S. Mignani, J. P. Majoral and X. Shi, Nano Lett., 2019, 19, 1216–1226 CrossRef CAS PubMed.
- Q. Huang, S. Zhang, H. Zhang, Y. Han, H. Liu, F. Ren, Q. Sun, Z. Li and M. Gao, ACS Nano, 2019, 13(2), 1342–1353 CAS.
- C. Lin, Y. Yu, H. G. Zhao, A. Yang, H. Yan and Y. Cui, Radiother. Oncol., 2012, 104, 395–400 CrossRef CAS PubMed.
- Z. Chen, W. Guo, Q. Wu, L. Tan, T. Ma, C. Fu, J. Yu, X. Ren, J. Wang, P. Liang and X. Meng, Theranostics, 2020, 10, 4659–4675 CrossRef CAS PubMed.
- A. K. Hauser, M. I. Mitov, E. F. Daley, R. C. McGarry, K. W. Anderson and J. Z. Hilt, Biomaterials, 2016, 105, 127–135 CrossRef CAS PubMed.
- C. Yang, X. Mi, H. Su, J. Yang, Y. Gu, L. Zhang, W. Sun, X. Liang and C. Zhang, Biomater. Sci., 2019, 7, 2076–2090 RSC.
- A. Meidanchi, O. Akhavan, S. Khoei, A. A. Shokri, Z. Hajikarimi and N. Khansari, Mater. Sci. Eng., C, 2015, 46, 394–399 CrossRef CAS PubMed.
- N. G. Shetake, A. Kumar and B. N. Pandey, Biochim. Biophys. Acta, Gen. Subj., 2019, 1863, 857–869 CrossRef CAS PubMed.
- H. Fakhimikabir, M. B. Tavakoli, A. Zarrabi, A. Amouheidari and S. Rahgozar, J. Photochem. Photobiol., B, 2018, 182, 71–76 CrossRef CAS PubMed.
- S. Jafari, M. Cheki, M. B. Tavakoli, A. Zarrabi, K. Ghazikhanlu Sani and R. Afzalipour, J. Biomed. Phys. Eng., 2020, 10, 15–24 Search PubMed.
- Y. H. Gholami, R. Maschmeyer and Z. Kuncic, Sci. Rep., 2019, 9(1), 1–13 CAS.
- J. Wang, X. Wu, P. Shen, J. Wang, Y. Shen, Y. Shen, T. J. Webster and J. Deng, Int. J. Nanomed., 2020, 15, 1903–1914 CrossRef CAS PubMed.
- X. Dong, R. Cheng, S. Zhu, H. Liu, R. Zhou, C. Zhang, K. Chen, L. Mei, C. Wang, C. Su, X. Liu, Z. Gu and Y. Zhao, ACS Nano, 2020, 14, 5400–5416 CrossRef CAS PubMed.
- J. Wang, X. Tan, X. Pang, L. Liu, F. Tan and N. Li, ACS Appl. Mater. Interfaces, 2016, 8, 24331–24338 CrossRef CAS PubMed.
- K. Kirakci, J. Zelenka, M. Rumlova, J. Martincik, M. Nikl, T. Ruml and K. Lang, J. Mater. Chem. B, 2018, 6, 4301–4307 RSC.
- K. Kirakci, T. N. Pozmogova, A. Y. Protasevich, G. D. Vavilov, D. V. Stass, M. A. Shestopalov and K. Lang, Biomater. Sci., 2021, 9, 2893–2902 RSC.
- W. Liu, A. Dong, B. Wang and H. Zhang, Adv. Sci., 2021, 8, 2003033 CrossRef CAS PubMed.
- L. Chan, X. Chen, P. Gao, J. Xie, Z. Zhang, J. Zhao and T. Chen, ACS Nano, 2021, 15, 3047–3060 CrossRef CAS PubMed.
- H. Huang, L. He, W. Zhou, G. Qu, J. Wang, N. Yang, J. Gao, T. Chen, P. K. Chu and X.-F. Yu, Biomaterials, 2018, 171, 12–22 CrossRef CAS PubMed.
- J. Zhang, Y. Ma, K. Hu, Y. Feng, S. Chen, X. Yang, J. Fong-Chuen Loo, H. Zhang, F. Yin and Z. Li, Bioconjugate Chem., 2019, 30, 1658–1664 CrossRef CAS PubMed.
- L. Chan, P. Gao, W. Zhou, C. Mei, Y. Huang, X. F. Yu, P. K. Chu and T. Chen, ACS Nano, 2018, 12, 12401–12415 CrossRef CAS PubMed.
- H. Xu, W. Cao and X. Zhang, Acc. Chem. Res., 2013, 46, 1647–1658 CrossRef CAS PubMed.
- K. E. McColl, M. J. Brodie, R. Whitesmith, K. F. Gray and T. J. Thomson, Acta Hepatogastroenterol., 1979, 26, 407–412 CAS.
- I. M. Puspitasari, C. Yamazaki, R. Abdulah, M. Putri, S. Kameo, T. Nakano and H. Koyama, Oncol. Lett., 2017, 13, 449–454 CrossRef CAS PubMed.
- C. Borek, J. Nutr., 2004, 134, 3207S–3209S CrossRef CAS PubMed.
- S. O. Evans, P. F. Khairuddin and M. B. Jameson, Anticancer Res., 2017, 37, 6497–6509 CAS.
- D. Schilling, B. Herold, S. E. Combs and T. E. Schmid, Radiat. Environ. Biophys., 2019, 58, 433–438 CrossRef CAS PubMed.
- E. Handa, I. M. Puspitasari, R. Abdulah, C. Yamazaki, S. Kameo, T. Nakano and H. Koyama, J. Trace Elem. Med. Biol., 2020, 62, 126653 CrossRef CAS PubMed.
- B. Farhood, K. Mortezaee, E. Motevaseli, H. Mirtavoos-Mahyari, D. Shabeeb, A. Eleojo Musa, N. S. Sanikhani, M. Najafi and A. Ahmadi, J. Cell. Biochem., 2019, 120, 18559–18571 CrossRef CAS PubMed.
- L. Y. Cruz, D. Wang and J. Liu, J. Photochem. Photobiol., B, 2019, 191, 123–127 CrossRef CAS PubMed.
- F. Chen, X. H. Zhang, X. D. Hu, P. D. Liu and H. Q. Zhang, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 937–948 CrossRef CAS PubMed.
- S. Gao, T. Li, Y. Guo, C. Sun, B. Xianyu and H. Xu, Adv. Mater., 2020, 32, e1907568 CrossRef PubMed.
- U. Shabbir, M. Rubab, E. B. Daliri, R. Chelliah, A. Javed and D. H. Oh, Nutrients, 2021, 13(1), 206 CrossRef CAS PubMed.
- C. Huang, T. Chen, D. Zhu and Q. Huang, Front. Chem., 2020, 8, 225 CrossRef CAS PubMed.
- T. Ma, Y. Liu, Q. Wu, L. Luo, Y. Cui, X. Wang, X. Chen, L. Tan and X. Meng, ACS Nano, 2019, 13, 4209–4219 CrossRef CAS PubMed.
- J. P. Kelly, T. W. Hannam and G. R. Giles, Cancer Treat. Rev., 1979, 6(Suppl), 53–61 CrossRef PubMed.
- S. Chen, S. Yu, Z. Du, X. Huang, M. He, S. Long, J. Liu, Y. Lan, D. Yang, H. Wang, S. Li, A. Chen, Y. Hao, Y. Su, C. Wang and S. Luo, J. Med. Chem., 2021, 64, 3381–3391 CrossRef CAS PubMed.
- H. Liu, Y. Xie, Y. Zhang, Y. Cai, B. Li, H. Mao, Y. Liu, J. Lu, L. Zhang and R. Yu, Biomaterials, 2017, 121, 130–143 CrossRef CAS PubMed.
- S. Masunaga, Y. Uto, H. Nagasawa, H. Hori, K. Nagata, M. Suzuki, Y. Kinashi and K. Ono, Anticancer Res., 2006, 26, 1261–1270 CAS.
- M. M. Fathy, H. M. Fahmy, O. A. Saad and W. M. Elshemey, Life Sci., 2019, 234, 116756 CrossRef CAS PubMed.
- S. Shirvalilou, S. Khoei, S. Khoee, S. R. Mahdavi, N. J. Raoufi, M. Motevalian and M. Y. Karimi, J. Photochem. Photobiol., B, 2020, 205, 111827 CrossRef CAS PubMed.
- Y. Xie, Y. Han, X. Zhang, H. Ma, L. Li, R. Yu and H. Liu, Front. Oncol., 2021, 11, 855 Search PubMed.
Footnote |
| † Yuan Zhang and Xiao Han contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |