Open Access Article
Open Access ArticleSinglet oxygen formation from photoexcited P3HT:PCBM films applied in oxidation reactions†
Aleksandra
Nyga
 ab,
Agata
Blacha-Grzechnik
*ab,
Przemysław
Podsiadły
b,
Alicja
Duda
ab,
Agata
Blacha-Grzechnik
*ab,
Przemysław
Podsiadły
b,
Alicja
Duda
 b,
Kinga
Kępska
a,
Maciej
Krzywiecki
b,
Kinga
Kępska
a,
Maciej
Krzywiecki
 c,
Radosław
Motyka
c,
Radosław
Motyka
 b,
René A. J.
Janssen
b,
René A. J.
Janssen
 d and
Przemysław
Data
d and
Przemysław
Data
 *a
*a
aCentre for Organic and Nanohybrid Electronics, Silesian University of Technology, Konarskiego 22b, 44-100 Gliwice, Poland. E-mail: przemyslaw.data@polsl.pl; agata.blacha@polsl.pl
bFaculty of Chemistry, Silesian University of Technology, Strzody 9, 44-100 Gliwice, Poland
cInstitute of Physics – CSE, Silesian University of Technology, Konarskiego 22b, 44-100 Gliwice, Poland
dMolecular Materials and Nanosystems & Institute for Complex Molecular Systems, Eindhoven University of Technology P.O. Box 513, 5600 MB, Eindhoven, The Netherlands
First published on 13th January 2022
Abstract
Poly(3-hexylthiophene) thin films containing carbon-based nanostructures, i.e. fullerenes such as buckminsterfullerene (C60) or phenyl-C61-butyric acid methyl ester (PCBM), or single-walled carbon nanotubes, were investigated as heterogeneous photosensitizers producing singlet oxygen (1O2) in aerated organic solvents. Thin films were deposited on borosilicate glass using spin coating and characterized by profilometry, UV-vis, Raman and XPS. Photogeneration of 1O2 was confirmed by photooxidation of 1,3-diphenylisobenzofuran and by reaction of 1,5-dihydroxynaphthalene to juglone. The photochemical efficiency of the blends was found to depend on the carbon-based photosensitizer and can be increased by varying its concentration in the poly(3-hexylthiophene) matrix.
1. Introduction
Blends of conjugated polymers and carbon nanostructures, such as fullerenes and carbon nanotubes (CNTs), have been under high scientific interest for application in organic photovoltaic (OPV) devices1–3 and have been extensively studied for their photophysical properties. In polymer:fullerene blends two competitive processes have been identified to occur from the initially-formed interfacial charge-transfer state: charge separation and charge recombination into a triplet state.4 The efficiencies of these processes not only depend on the individual properties of the donor and acceptor in the blend, but also on their ratio and the layer morphology.5,6 Triplet generation is considered as a significant drawback in OPV, because it reduces the short-circuit current density, open-circuit voltage, and power conversion efficiency, and in the presence of oxygen may lead to the formation of singlet oxygen (1O2) which is highly reactive and destructive for the photoactive layers.7,8Though the formation of 1O2 may be unfavorable for organic electronic devices, it received much scientific attention as an efficient oxidative agent in fine-chemicals synthesis, wastewater treatment, and in photodynamic therapy (PDT).9–13 The direct optical excitation of triplet ground state oxygen to the singlet excited state is spin-forbidden, but 1O2 formation is possible using photosensitizers. In such a process, a photosensitizer absorbs light, forming a singlet-excited state (S1) and converts to a triplet-excited state (T1) via intersystem crossing (ISC) that can subsequently transfer its energy in a spin-allowed reaction to ground state triplet oxygen (3O2) resulting in formation of singlet-state oxygen and the photosensitizer in the ground state.9,13
The most commonly studied photoactive molecules are organic dyes, transition metal complexes, and inorganic oxides.9,10,13 In recent years, carbon-based photosensitizers have been shown to produce 1O2 in good yields,14 but practical applications are limited because these materials mainly absorb in the high-energy region. To circumvent this problem, additional organic chromophores can be introduced.15–19 The high reactivity of 1O2 causes its lifetime to be very short and it thus must be produced in situ, using either homogenous or heterogeneous photocatalysts. Immobilization of photosensitizers generally results in a decrease in their activity but may be beneficial for commercial applications.10,20
Reactive oxygen species (ROS), such as singlet oxygen, exhibit strong antimicrobial properties acting in a versatile way on bacteria, viruses, and fungi. The main advantage of photodynamic antimicrobial chemotherapy (PACT) is the absence of microbial resistance towards ROS, and that it does not cause the spread of drug-resistant bacteria.19,21,22 High attention is put nowadays on the introduction of antimicrobial coatings in health-related areas to decrease the number of patients gaining nosocomial infections.23 The introduction of photoactive antimicrobial coatings would allow also reduce the use of chlorinated, toxic disinfectants. Various approaches for the immobilization of photoactive molecules, mainly dyes, have been explored, e.g. the non-covalent immobilization in a polymer matrix, like cellulose acetate,24 or covalent binding at a surface.25 In the first case a high antimicrobial activity has been reported, however such materials may possess low stability, due to leaching of the photoactive molecule from the blend.24,26 The covalent binding of dyes, e.g. Rose Bengal, to a polymer matrix, like polystyrene, polyamide, or poly(methyl methacrylate), can be achieved via chemical reaction between the matrix functional groups and the dyes.27,28 The main disadvantage, however, is a more complicated multistep procedure.
In this study, we investigate the possibility of applying poly(3-hexylthiophene) (P3HT) layers containing carbon nanostructures, as a heterogeneous source of singlet oxygen. P3HT has been selected because it absorbs strongly in the visible region and its blends with carbon nanostructures can be easily deposited on solid supports. Moreover, their photophysical properties are well characterized in the literature. It has been shown that energy transfer from P3HT to fullerenes and carbon nanotubes occurs in solution29,30 and in the solid-state,31,32 and that such blends can produce singlet oxygen.20 Here, P3HT is assumed to act both as support for the carbon-based photosensitizers and as a visible-light antenna. The blend layers were characterized with various spectroscopic techniques. Singlet oxygen photogeneration was investigated with 1,3-diphenylisobenzofuran (DPBF) in methanol under excitation with green light, which allowed for the determination of quantum yields of the photoprocess. On the other hand, oxidation of 1,5-dihydroxynaphthalene (DHN) to juglone in acetonitrile under white light illumination was demonstrated as an example of fine-chemical synthesis. The influence of the type of carbon photosensitizer and its content on the photoactive properties of the layer was studied.
2. Experimental
2.1. Materials
C60 (purity 99.9%) was purchased from Acros Organics. [6,6]-Phenyl-C61-butyric acid methyl ester (PCBM) (purity 99.0%) and single-walled carbon nanotubes (SWCNTs) were obtained from Osilla Ltd. Regiorandom P3HT was synthesized following a well-established procedure (ESI†). Chlorobenzene (>99%, Acros Organics) was applied as a solvent for layer preparation. Sodium dodecyl sulfate (>98%, Sigma-Aldrich), isopropanol (99.5%), and acetone (95%) (both Acros Organics) were used for cleaning glass slides. 1,3-Diphenylisobenzofuran (DPBF, >97%) dissolved in methanol (99.9%, both Across Organic) was used as a singlet oxygen scavenger. The quantum yield of 1O2 photogeneration was determined with Rose Bengal (Acros Organics) as a reference. Photooxidation under white light was tested with 1,5-dihydroxynaphthalene (DHN, 97%) in acetonitrile (≥99.9% both from Sigma-Aldrich).2.2. Photoactive layers deposition and characterization
P3HT layers containing carbon nanostructures as photosensitizers were formed on borosilicate glass slides (1 × 1 cm2 or 3 × 3 cm2, Präzisions Glas & Optik GmbH, PG&O) via spin coating (Laurell spin-coater, WS-650 M2-23). Before layer deposition, the glass substrates were cleaned with sodium dodecyl sulfate aqueous solution and then sonicated in acetone, pure water, and finally isopropanol. Carbon nanostructures and P3HT were dispersed in chlorobenzene in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mass ratio, and additionally in 1
2 mass ratio, and additionally in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratios in case of PCBM, and sonicated for 15 min. 30 mm3 of the solution was dropped on the glass slide and spin coated for 30 s at a spinning rate of 2000 rpm.
1 mass ratios in case of PCBM, and sonicated for 15 min. 30 mm3 of the solution was dropped on the glass slide and spin coated for 30 s at a spinning rate of 2000 rpm.
A Veeco, Dektak 150 profilometer was used to determination the layer thickness, employing 1200 μm scanning length, a needle with a diameter of 12.5 μm, and a pressure force of 5.00 mg.
UV-vis spectra of the films were recorded with a HP 8452A spectrometer Raman spectra were recorded using Renishaw inVia Raman Microscope (Renishaw, Inc., New Mills, UK) equipped with a 514 nm diode laser, a 2400 line per mm grating, and a 50× objective. All spectra were smoothened and the baseline was subtracted utilizing Renishaw software.
X-ray photoelectron spectroscopy (XPS) analysis was done with PREVAC EA15 hemispherical electron energy analyzer with 2D multi-channel plate detector. Al-Kα X-ray source (PREVAC dual-anode XR-40B, 1486.6 eV) was used for sample irradiation. The measurements were conducted under 9 × 10−9 Pa base pressure. Pass energy was equal to 200 eV pass energy for survey spectra (scanning step 0.9 eV) and 100 eV (scanning step 0.05 eV) for high-resolution spectra acquisition. The binding energy scale was calibrated with respect to C–C component in the C1s region (284.8 eV).33 The spectra were analyzed applying CASA XPS® software. Shirley function was used as a background and the product of Gaussian and Lorentzian functions were used for components fitting.
2.3. Singlet oxygen photogeneration
The effectiveness of the photoactive layers containing carbon-based nanomaterials in the process of singlet oxygen photogeneration was determined using a 0.06 mM solution of DPBF as specific 1O2 quencher in methanol.34,35 The reaction progress was monitored with a Hewlett Packard 8452A UV-vis spectrometer as the change in the DPBF absorbance at 410 nm. The process was conducted in situ in a standard 10 mm × 4 mm quartz cuvette (Hellma Analytics) under 532 nm laser irradiation (Oxxius, LCX-532L-150-CSB-PPA model having 150 mW maximum power reduced to 50 mW).35 The quantum efficiency of the light-induced 1O2 production was determined with the DPBF method and Rose Bengal as a standard having ΦRB equal to 0.80 in CH3OH.36–392.4. Material photooxidation of DHN
Selected P3HT-fullerene layers deposited on borosilicate glass slides were applied as a source of singlet oxygen in the oxidation of DHN. In situ measurements were conducted in the set-up as for DPBF tests with a 100 W xenon lamp acting as a source of light. The initial concentration of DHN in acetonitrile was equal to 0.14 mM. The reaction with 1O2 was followed by monitoring the decrease in the absorbance of DHN at 298 nm and the increase of the absorbance of the oxygen adduct, juglone, at 406 nm.Photooxidation of DHN was also done in a 100 ml photoreactor. Nine glass slides (9 cm2 each) covered with P3HT:PCBM were introduced into the photoreactor filled with a 0.0146 M solution of DHN in acetonitrile and illuminated with a xenon lamp. During the reaction, the mixture was magnetically stirred and bubbled with oxygen. After 4 h the reaction mixture was evaporated and the crude product was purified by column chromatography with dichloromethane as eluent. The structure of the product was confirmed by 1H-NMR spectroscopy (Varian Unity Inova 300 MHz Spectrometer, CDCl3).
3. Results and discussion
3.1. Deposition and characterization of photoactive layers
P3HT layers containing various carbon-based photosensitizers were deposited on glass substrates by spin coating. The spin-coating parameters were optimized, i.e. various rotation speeds were tested in the range between 500 and 4000 rpm, to obtain layers with ca. 35 nm thickness. The average thickness of the deposited layers is given in Table S1 (ESI†). Deposited layers were not subjected to heat treatment, i.e. thermal annealing, to minimize phase segregation and crystallization of fullerene and polymer,40,41 that is known to assist charge separation between donor and acceptor units.5,42–44Deposited layers were first characterized by UV-vis spectroscopy (Fig. 1). For P3HT a broad absorption band is observed between 400 and 600 nm with a maximum at ca. 510 nm attributed to its π–π* transition42 and a shoulder at ca. 605 nm assigned to inter-chain stacking of P3HT and thus polymer ordering.42,45,46 For fullerene-containing layers, the distinct fullerene absorption is visible at 340 nm and 334 nm for P3HT:C60 and P3HT:PCBM, respectively.47 The maximum of the P3HT π–π* absorption is blue-shifted to ca. 490 nm for P3HT:PCBM, and further to 455 nm for P3HT:C60. Moreover, the significant decrease in the absorption of the shoulder at 605 nm is observed in the latter case suggesting less inter-chain interactions within P3HT upon addition of C60.48,49 On the other hand, the UV-vis spectrum of the P3HT:SWCNT film almost completely coincides with P3HT-only spectrum, indicating that at this concentration of SWCNTs the polymeric inter-chain interactions remain dominant over interactions of P3HT with SWCNTs.31,48 The presence of carbon nanotubes in the blend is confirmed by weak absorption peak appearing close to 700 nm.31
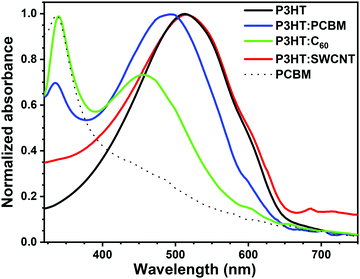 | ||
Fig. 1 UV-vis spectra of P3HT, P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (2 PCBM (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P3HT 1), P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C60 (2 C60 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P3HT 1), P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SWCNT (2 SWCNT (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and PCBM layers deposited on borosilicate glass. 1) and PCBM layers deposited on borosilicate glass. | ||
The chemical composition of the layers was also analyzed with Raman spectroscopy (Fig. 2). For all spectra, characteristic bands of P3HT are observed. The deformation vibration of the C–S–C bond arises at ca. 720 cm−1, the C–C skeletal stretching at 1379 cm−1, while the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations occur at 1450 cm−1.43 The latter is broadened and slightly shifted to higher wavenumbers for fullerene-containing layers, which is due to the additional contribution of the “pentagonal pinch” Ag mode vibrations of C60 spheres50 and may suggest the lower order and crystallinity of the P3HT.51 Raman spectra of P3HT:SWCNT coating exhibit additional signal typically observed for CNTs, so-called G band at 1593 cm−1.2 However, in this case, the C
C stretching vibrations occur at 1450 cm−1.43 The latter is broadened and slightly shifted to higher wavenumbers for fullerene-containing layers, which is due to the additional contribution of the “pentagonal pinch” Ag mode vibrations of C60 spheres50 and may suggest the lower order and crystallinity of the P3HT.51 Raman spectra of P3HT:SWCNT coating exhibit additional signal typically observed for CNTs, so-called G band at 1593 cm−1.2 However, in this case, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration band of thiophene ring, and thus order of polymeric matrix seems unaffected by the introduction of carbon nanotubes,51 which is in agreement with above-mentioned UV-vis results.
C stretching vibration band of thiophene ring, and thus order of polymeric matrix seems unaffected by the introduction of carbon nanotubes,51 which is in agreement with above-mentioned UV-vis results.
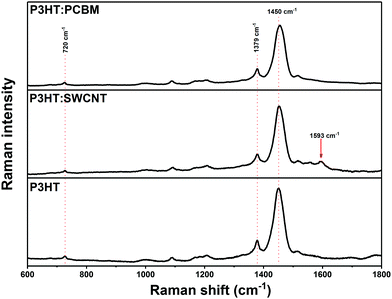 | ||
Fig. 2 Raman spectra of P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (2 PCBM (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), P3HT 1), P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SWCNT (2 SWCNT (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and P3HT photoactive layers deposited on borosilicate glass. 1) and P3HT photoactive layers deposited on borosilicate glass. | ||
A XPS survey spectrum recorded for the P3HT:PCBM film (Fig. S1a, ESI†) confirms full coverage of the glass substrate. Moreover, basing on survey spectra and C1s region (see Fig. S1c, ESI†), no signals of impurities coming from the solvent or reagents used for P3HT synthesis are observed. The position of S2p3/2 component in S2p high-resolution spectrum (Fig. S1b, ESI†) suggests that polythiophene exists in its neutral form.52,53
3.2. Photogeneration of singlet oxygen
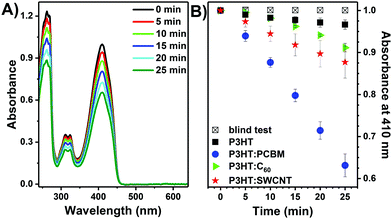
The photoactive layers were tested as a heterogeneous source of singlet oxygen in photooxidation reactions. As mentioned, P3HT plays a double role. First of all, it is used as a matrix for carbon-based photosensitizers and second, it may enhance absorption of visible light for 1O2 production.First, the process of singlet oxygen photogeneration was investigated with DPBF, which is a specific 1O2 quencher.34 The UV-vis spectra of a DPBF solution in methanol in contact with a P3HT:PCBM layer recorded during illumination with a green laser are shown in Fig. 3A. A clear decrease in the DPBF absorbance at 410 nm with time is observed, indicating that it is oxidized by singlet oxygen generated by irradiation of the P3HT:PCBM photoactive thin film.17 This is further confirmed by control experiments, in which almost no drop in DPBF absorption is observed when the bare glass is illuminated or when the photoactive layer is in contact with the solution but not illuminated (Fig. 3B).
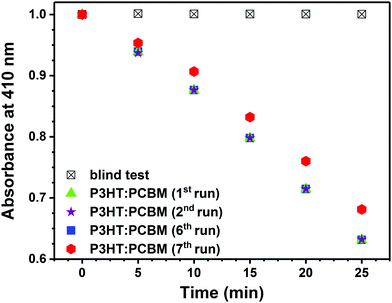
Fig. 3B shows the change of DPBF absorbance at 410 nm vs. time when various layers were illuminated. The largest drop in DPBF concentration after 25 min was observed for the P3HT layer containing PCBM. Under the applied conditions, P3HT itself exhibits poor photosensitizing properties.20 Importantly, since no additional bands arise in the recorded UV-vis spectra in the course of the process (Fig. 3), the release of the fullerene into reaction mixture can be excluded. As shown in Fig. 4, the P3HT:PCBM photoactive layer retains its photoactivity towards singlet oxygen production in consecutive DPBF-tests, indicating that it can be effectively re-used. Ca. 10% decrease in the effectiveness of DPBF photooxidation was observed in the 7th run.
The quantum yield of singlet oxygen photogeneration (Φ) can be determined with respect to the well-known reference photosensitizers.39,54 Here Rose Bengal was chosen. For P3HT:PCBM layers the quantum yield of singlet oxygen photogeneration was equal to 1.1% at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio and increased to 4.2% at a 1
1 mass ratio and increased to 4.2% at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mass ratio (Table 1). Since the quantum yield of singlet oxygen photogeneration by the pristine PCBM layer (1.9%) is lower than that of P3HT
2 mass ratio (Table 1). Since the quantum yield of singlet oxygen photogeneration by the pristine PCBM layer (1.9%) is lower than that of P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (1
PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), energy transfer from P3HT to PCBM (which acts as the photosensitizer in the 1O2 formation) is suggested.17,31,32,55 We note that the energy of charge-separated state in P3HT:PCBM blends is at 1.14 eV and thus lower than the triplet energy of PCBM at 1.5 eV,6,56 indicating that charge separation process is energetically favored. Nevertheless, it seems that in P3HT:PCBM blends energy transfer from P3HT to PCBM is efficient enough to form singlet the excited state of PCBM, which yields 3PCBM* via intersystem crossing that reacts with 3O2.
2), energy transfer from P3HT to PCBM (which acts as the photosensitizer in the 1O2 formation) is suggested.17,31,32,55 We note that the energy of charge-separated state in P3HT:PCBM blends is at 1.14 eV and thus lower than the triplet energy of PCBM at 1.5 eV,6,56 indicating that charge separation process is energetically favored. Nevertheless, it seems that in P3HT:PCBM blends energy transfer from P3HT to PCBM is efficient enough to form singlet the excited state of PCBM, which yields 3PCBM* via intersystem crossing that reacts with 3O2.
| Photoactive layer | Φ CH3OH [%] |
|---|---|
P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C60 (2 C60 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
<0.5 |
P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SWCNT (2 SWCNT (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
<0.5 |
P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (2 PCBM (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.1 |
P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (1 PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2.0 |
P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (1 PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
4.2 |
| Pristine PCBM | 1.9 |
The drop in the absorbance of DPBF after 25 min and the corresponding quantum yields of singlet oxygen photogeneration are significantly lower for both P3HT:SWCNT and P3HT:C60 (Fig. 3B and Table 1), which is probably related to the low solubility of C60 and SWCNT and the thus higher tendency of the two to form agglomerates and clusters during deposition process.57–59 It has already been shown that the lifetime of the triplet-excited state of the photosensitizer, and consequently the 1O2 photogeneration efficiency, can be significantly reduced due to agglomeration. Moreover, it has been shown that carbon nanotubes can also effectively quench 1O2, thus lowering its overall production yield.60
Taking the above into account, the P3HT:PCBM composite was further investigated as a heterogeneous source of singlet oxygen. Since PCBM possesses significantly higher solubility than unmodified C60, it is possible to increase its concentration in the polymeric matrix, while avoiding its aggregation.
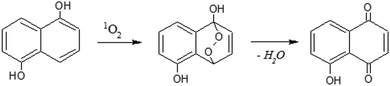
3.3. Photooxidation of 1,5-dihydroxynaphthalene
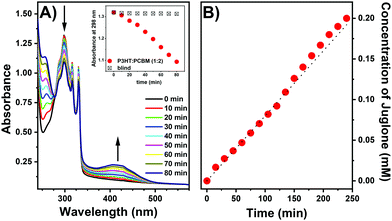
1,5-Dihydroxynaphthalene (DHN) is a commonly used substrate in fine chemical reactions in the production of juglone (5-hydroxy-1,4-naphthoquinone) an anthelmintic drug (Scheme 1), which naturally occurs in plants, especially in black walnut.61,62 Oxidation of DHN to juglone was used as a proof of concept for singlet oxygen generation by direct measurement of the formed species. The progress of DHN oxidation can be easily monitored by UV-vis spectroscopy as changes in absorbance at 298 and 406 nm.63,64The DHN photooxidation was conducted in situ applying photoexcitation of P3HT:PCBM layers to generate singlet oxygen. A xenon lamp was used for illumination, to excite the P3HT:PCBM films. Fig. 5A presents a set of UV-vis spectra of a DHN solution collected during illumination of the P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (1
PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) layer. The absorbance of DHN at 298 nm decreases with time, indicating its reaction with 1O2 to produce juglone, also indicated by the appearance of the specific absorption band with a maximum at ca. 406 nm that is gradually increasing as the reaction proceeds. Almost no decrease in DHN concentration is observed when the bare glass is illuminated (Fig. 5A inset). As in the case of the DPBF test, the dissolution of the layer in the reaction mixture can be excluded, since neither characteristic absorption bands of PCBM nor P3HT have been recorded.
2) layer. The absorbance of DHN at 298 nm decreases with time, indicating its reaction with 1O2 to produce juglone, also indicated by the appearance of the specific absorption band with a maximum at ca. 406 nm that is gradually increasing as the reaction proceeds. Almost no decrease in DHN concentration is observed when the bare glass is illuminated (Fig. 5A inset). As in the case of the DPBF test, the dissolution of the layer in the reaction mixture can be excluded, since neither characteristic absorption bands of PCBM nor P3HT have been recorded.
Similar sets of UV-vis spectra were collected for P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM layers with 1
PCBM layers with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The rate constants of DHN oxidation by singlet oxygen, which is pseudo-zero order reaction under applied conditions, are given in Table 2. As mentioned, during illumination of the photoactive layers with a xenon lamp also the PCBM photosensitizer is excited directly. As expected, an increase in PCBM content increases the value of rate constant of DHN oxidation. The relation is not linear and the trend corresponds to the quantum yield of 1O2 generation (Table 1). The rate constant for DHN oxidation with pristine PCBM layer is about 3-times lower than for P3HT
1 ratio. The rate constants of DHN oxidation by singlet oxygen, which is pseudo-zero order reaction under applied conditions, are given in Table 2. As mentioned, during illumination of the photoactive layers with a xenon lamp also the PCBM photosensitizer is excited directly. As expected, an increase in PCBM content increases the value of rate constant of DHN oxidation. The relation is not linear and the trend corresponds to the quantum yield of 1O2 generation (Table 1). The rate constant for DHN oxidation with pristine PCBM layer is about 3-times lower than for P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (1
PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), suggesting that P3HT absorbing in the visible region plays an additional role in the formation of singlet oxygen, either directly or indirectly by energy transfer to form C60 triplet excited state.
2), suggesting that P3HT absorbing in the visible region plays an additional role in the formation of singlet oxygen, either directly or indirectly by energy transfer to form C60 triplet excited state.
| Photoactive layer | k × 107 (mol dm−3 min−1) |
|---|---|
P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (2 PCBM (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.90 |
P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (1 PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.20 |
P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (1 PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
3.25 |
| Pristine PCBM | 1.07 |
In a final step, DHN photooxidation was conducted in a self-constructed photoreactor equipped with a xenon lamp as illumination source using P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (1
PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) covered glass slides with a total active area of 81 cm2. The reaction was carried out for 4 h and samples were taken every 15 min. and analyzed by UV-vis spectroscopy. Fig. 5B shows the increase in the concentration of juglone in the reaction mixture, calculated based on the absorbance at 406 nm. The structure of the reaction product juglone was further confirmed by 1H-NMR spectroscopy (ESI†). The steady increase in the absorbance of juglone with time, confirms that P3HT:PCBM photoactive layers retain activity under prolonged illumination, and thus can be effectively applied as a heterogeneous source of singlet oxygen.
2) covered glass slides with a total active area of 81 cm2. The reaction was carried out for 4 h and samples were taken every 15 min. and analyzed by UV-vis spectroscopy. Fig. 5B shows the increase in the concentration of juglone in the reaction mixture, calculated based on the absorbance at 406 nm. The structure of the reaction product juglone was further confirmed by 1H-NMR spectroscopy (ESI†). The steady increase in the absorbance of juglone with time, confirms that P3HT:PCBM photoactive layers retain activity under prolonged illumination, and thus can be effectively applied as a heterogeneous source of singlet oxygen.
4. Conclusions
Photoactive layers based on a P3HT matrix containing carbon nanostructures as photosensitizers were investigated as photosensitizers to produce singlet oxygen. In these blends P3HT acts as a visible-light absorber and transfers energy to the carbon nanostructure, which is the actual photosensitizer that produces singlet oxygen. Singlet oxygen formation was monitored spectroscopically in situ via the oxidation of DPBF in methanol and was used synthetically to form juglone from DHN in acetonitrile. The efficiency of 1O2 production depends on the photosensitizer and is significantly lower for blends of P3HT with C60 and single-walled carbon nanotubes than for blends of P3HT with PCBM. For P3HT:PCBM-based thin films, the quantum efficiency of 1O2 photogeneration can be tuned by varying the PCBM concentration in the P3HT layer. The results show that such easily-fabricated fullerene–polymer blends can be considered for singlet oxygen generation using visible-light and applied for fine chemicals synthesis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Center, Poland (Grand number: 2016/21/D/ST5/01641). The synthesis of P3HT was performed due to the financial support from the National Science Centre, Poland under the PRELUDIUM 11 program (Grant number: 2016/21/N/ST8/01871). A. N. acknowledges the support from the Silesian University of Technology (04/040/BKM21/0179). A. N., A. D. & P. D. kindly acknowledge the support received from the First Team program of the Foundation for Polish Science co-financed by the European Union under the European Regional Development Fund (Project number: First TEAM POIR.04.04.00-00-4668/17-00). Authors are grateful to the networking action funded from the European Union's Horizon 2020 research and innovation program under grant agreement no. 691684. Authors acknowledges the supporting actions from EU's Horizon 2020 ERA-Chair project ExCEED, grant agreement no. 952008.References
- S. Ren, M. Bernardi, R. R. Lunt, V. Bulovic, J. C. Grossman and S. Gradecak, Nano Lett., 2011, 11, 5316–5321 CrossRef CAS.
- V. C. Tung, J.-H. Huang, J. Kin, A. J. Smith, C.-W. Chu and J. Huang, Energy Environ. Sci., 2012, 5, 7810–7818 RSC.
- R. M. Williams, H. Chen, D. Di Nuzzo, S. C. J. Meskers and R. A. J. Janssen, J. Spectrosc., 2017, 2017, 6867507 Search PubMed.
- A. J. Gillett, A. Privitera, R. Dilmurat, A. Karki, D. Qian, A. Pershin, G. Londi, W. K. Myers, J. Lee, J. Yuan, S. J. Ko, M. K. Riede, F. Gao, G. C. Bazan, A. Rao, T. Q. Nguyen, D. Beljonne and R. H. Friend, Nature, 2021, 597, 666–671 CrossRef CAS PubMed.
- J. J. Benson-Smith, H. Ohkita, S. Cook, J. R. Durrant, D. D. C. Bradley and J. Nelson, Dalton Trans., 2009, 10000–10005 RSC.
- D. Di Nuzzo, A. Aguirre, M. Shahid, V. S. Gevaerts, S. C. J. Meskers and R. A. J. Janssen, Adv. Mater., 2010, 22, 4321–4324 CrossRef CAS PubMed.
- S. Cook, H. Ohkita, J. R. Durrant, Y. Kim, J. J. Benson-smith, J. Nelson and D. D. C. Bradley, Appl. Phys. Lett., 2006, 89, 101128 CrossRef.
- A. Distler, P. Kutka, T. Sauermann, H.-J. Egelhaaf, D. M. Guldi, D. Di Nuzzo, S. C. J. Meskers and R. A. J. Janssen, Chem. Mater., 2012, 24, 4397–4405 CrossRef CAS.
- M. C. DeRosa and R. J. Crutchley, Coord. Chem. Rev., 2002, 233–234, 351–371 CrossRef CAS.
- J. Wahlen, D. E. De Vos, P. A. Jacobs and P. L. Alsters, Adv. Synth. Catal., 2004, 346, 152–164 CrossRef CAS.
- I. Pibiri, S. Buscemi, A. Palumbo Piccionello and A. Pace, ChemPhotoChem, 2018, 2, 535–547 CrossRef CAS.
- A. A. Ghogare and A. Greer, Chem. Rev., 2016, 116, 9994–10034 CrossRef CAS PubMed.
- P. R. Ogilby, Chem. Soc. Rev., 2010, 39, 3181–3209 RSC.
- P. Dallas, G. Rogers, B. Reid, R. A. Taylor, H. Shinohara, G. A. D. Briggs and K. Porfyrakis, Chem. Phys., 2016, 465–466, 28–39 CrossRef CAS.
- Q. Li, H. Guo, X. Yang, S. Zhang and H. Zhang, Tetrahedron, 2017, 73, 6632–6636 CrossRef CAS.
- S. Guo, H. Zhang, L. Huang, Z. Guo, G. Xiong and J. Zhao, Chem. Commun., 2013, 49, 8689–8691 RSC.
- L. Huang, X. Cui, B. Therrien and J. Zhao, Chem. – Eur. J., 2013, 19, 17472–17482 CrossRef CAS.
- A. Blacha-Grzechnik, M. Krzywiecki, R. Motyka and M. Czichy, J. Phys. Chem. C, 2019, 123, 25915–25924 CrossRef CAS.
- E. Reynoso, A. M. Durantini, C. A. Solis, L. P. Macor, L. A. Otero, M. A. Gervaldo, E. N. Durantini and D. A. Heredia, RSC Adv., 2021, 11, 23519–23532 RSC.
- M. Bregnhøj, M. Prete, V. Turkovic, A. U. Petersen, M. B. Nielsen, M. Madsen and P. R. Ogilby, Methods Appl. Fluoresc., 2020, 8, 014001 CrossRef.
- M. Wainwright, Photodiagn. Photodyn. Ther., 2009, 6, 167–169 CrossRef PubMed.
- N. E. Grammatikova, L. George, Z. Ahmed, N. R. Candeias, N. A. Durands and A. Efimov, J. Mater. Chem. B, 2019, 7, 4379–4384 RSC.
- S. Noimark, C. W. Dunnill and I. P. Parkin, Adv. Drug Delivery Rev., 2013, 65, 570–580 CrossRef CAS PubMed.
- V. Decraene, J. Pratten and M. Wilson, Appl. Environ. Microbiol., 2006, 72, 4436–4439 CrossRef CAS PubMed.
- C. Piccirillo, S. Perni, J. Gil-Thomas, P. Prokopovich, M. Wilson, J. Pratten and I. P. Parkin, J. Mater. Chem., 2009, 19, 6167–6171 RSC.
- G. B. Hwang, E. Allan and I. P. Parkin, ACS Appl. Mater. Interfaces, 2016, 8, 15033–15039 CrossRef CAS PubMed.
- M. Krouit, R. Granet and P. Krausz, Eur. Polym. J., 2009, 45, 1250–1259 CrossRef CAS.
- C. Ringot, V. Sol, M. Barrière, N. Saad, P. Bressollier, R. Granet, P. Couleaud, C. Frochot and P. Krausz, Biomacromolecules, 2011, 12, 1716–1723 CrossRef CAS PubMed.
- E. A. Lukina, I. P. Pozdnyakov, A. S. Mereshchenko, M. N. Uvarov and L. V. Kulik, J. Photochem. Photobiol., A, 2015, 311, 193–198 CrossRef CAS.
- R. A. J. Janssen, N. S. Sariciftci and A. J. Heeger, J. Chem. Phys., 1994, 100, 8641–8645 CrossRef CAS.
- A. J. Ferguson, J. L. Blackburn, J. M. Holt, N. Kopidakis, R. C. Tenent, T. M. Barnes, M. J. Heben and G. Rumbles, J. Phys. Chem. Lett., 2010, 1, 2406–2411 CrossRef CAS.
- A. R. S. Kandada, G. Grancini, A. Petrozza, S. Perissinotto, D. Fazzi, S. S. K. Raavi and G. Lanzani, Sci. Rep., 2013, 3, 2073 CrossRef PubMed.
- G. Beamson and D. Briggs, XPS of Organic Polymers, John Wiley & Sons, Chichester, 1992 Search PubMed.
- M. Wainwright, Dyes Pigm., 2007, 73, 7–12 CrossRef CAS.
- K. Piwowar, A. Blacha-Grzechnik, R. Turczyn and J. Zak, Electrochim. Acta, 2014, 141, 182–188 CrossRef CAS.
- F. Lv, Y. Yu, E. Hao, C. Yu, H. Wang and L. Jiao, Org. Biomol. Chem., 2019, 17, 1–44 RSC.
- C. R. Lambert and I. E. Kochevar, J. Am. Chem. Soc., 1996, 118, 3297–3298 CrossRef CAS.
- M. I. Burguete, F. Galindo, R. Gavara, S. V. Luis, M. Moreno and D. A. Russell, Photochem. Photobiol. Sci., 2009, 8, 37–44 CrossRef CAS PubMed.
- N. Epelde-Elezcano, V. Martinez-Martinez, E. Pena-Cabrera, C. F. A. Gomez-Duran, I. L. Arbeloa and S. Lacombe, RSC Adv., 2016, 6, 41991–41998 RSC.
- R. A. Marsh, J. M. Hodgkiss, S. Albert-Seifried and R. H. Friend, Nano Lett., 2010, 10, 923–930 CrossRef CAS PubMed.
- U. Zhokhavets, T. Erb, G. Gobsch, M. Al-Ibrahim and O. Ambacher, Chem. Phys. Lett., 2006, 418, 347–350 CrossRef CAS.
- B. Kadem, A. Hassan and W. Cranton, J. Mater. Sci.: Mater. Electron., 2016, 27, 7038–7048 CrossRef CAS.
- F. Otieno, B. K. Mutuma, M. Airo, K. Ranganathan and R. Erasmus, Thin Solid Films, 2017, 625, 62–69 CrossRef CAS.
- Y.-C. Huang, Y.-C. Liao, S.-S. Li, M.-C. Wu, C.-W. Chen and W.-F. Su, Sol. Energy Mater. Sol. Cells, 2009, 93, 888–892 CrossRef CAS.
- B. Y. Kadem, M. K. Al-hashimi and A. K. Hassan, Energy Procedia, 2014, 50, 237–245 CrossRef CAS.
- Y. Yang, S. Feng, M. Li, Z. Wu, X. Fang, F. Wang, D. Geng, T. Yang, X. Li, B. Sun and X. Gao, ACS Appl. Mater. Interfaces, 2015, 7, 24430–24437 CrossRef CAS PubMed.
- R. V. Bensasson, E. Bienvenue, M. Dellinger, S. Leach and P. Seta, J. Phys. Chem., 1994, 98, 3492–3500 CrossRef CAS.
- P. J. Goutam, D. K. Singh and P. K. Iyer, J. Phys. Chem. C, 2012, 116, 8196–8201 CrossRef CAS.
- J. U. Lee, A. Cirpan, T. Emrick, T. P. Russell and W. H. Jo, J. Mater. Chem., 2009, 19, 1483–1489 RSC.
- H. Chadli, A. Rahmani and J.-L. Sauvajol, J. Phys.: Condens. Matter, 2010, 22, 145303 CrossRef CAS PubMed.
- C.-Y. Su, A.-Y. Lu, Y.-L. Chen, C.-Y. Wei, P.-C. Wang and C.-H. Tsai, J. Mater. Chem., 2010, 20, 7034–7042 RSC.
- A. P. P. Alves, J. P. C. Trigueiro, H. D. R. Calado and G. G. Silva, Electrochim. Acta, 2011, 209, 111–120 CrossRef.
- G. J. H. Melvin, Q.-Q. Ni, Y. Suzuki and T. Natsuki, J. Mater. Sci., 2014, 49, 5199–5207 CrossRef CAS.
- W. Li, L. Li, H. Xiao, R. Qi, Y. Huang, Z. Xie, X. Jing and H. Zhang, RSC Adv., 2013, 3, 13417–13421 RSC.
- M. Narutaki, K. Takimiya, T. Otsubo, Y. Harima, H. Zhang, Y. Arki and O. Ito, J. Org. Chem., 2006, 71, 1761–1768 CrossRef CAS PubMed.
- K. Vandewal, K. Tvingstedt, A. Gadisa, O. Inganäs and J. V. Manca, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 125204 CrossRef.
- S. Wang, R. Gao, F. Zhou and M. Selke, J. Mater. Chem., 2004, 487–493 RSC.
- R. S. Ruoff, D. S. Tse, R. Malhotra and D. C. Lorents, J. Phys. Chem., 1993, 97, 3379–3383 CrossRef CAS.
- S. Berson, R. De Bettignies, S. Bailly, S. Guillerez and B. Jousselme, Adv. Funct. Mater., 2007, 17, 3363–3370 CrossRef CAS.
- N. Gandra, P. L. Chiu, W. Li, Y. R. Anderson, S. Mitra, H. He and R. Gao, J. Phys. Chem. C, 2009, 113, 5182–5185 CrossRef CAS PubMed.
- S. Cosmulescu, I. Trandafir, G. Achim and A. Baciu, Not. Bot. Horti Agrobot. Cluj-Napoca, 2011, 39, 237–240 CrossRef CAS.
- S. Sugie, K. Okamoto, K. M. W. Rahman, T. Tanaka, K. Kawai, J. Yamahara and H. Mori, Cancer Lett., 1998, 127, 177–183 CrossRef CAS PubMed.
- S. Takizawa, R. Aboshi and S. Murata, Photochem. Photobiol. Sci., 2011, 10, 895–903 CrossRef CAS PubMed.
- M. Luiz, A. T. Soltermann, A. Biasutti and N. A. Garcia, Can. J. Chem., 2006, 74, 49–54 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ma01151k |
| This journal is © The Royal Society of Chemistry 2022 |