Open Access Article
Open Access ArticleHigh water adsorption features of graphene oxide: potential of graphene oxide-based desert plantation
Md. Saidul
Islam
ab,
Junya
Yagyu
b,
Yoshihiro
Sekine
 bc,
Shinichiro
Sawa
*bd and
Shinya
Hayami
bc,
Shinichiro
Sawa
*bd and
Shinya
Hayami
 *abd
*abd
aInstitute of Industrial Nanomaterials (IINa), Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan. E-mail: hayami@kumamoto-u.ac.jp
bDepartment of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan. E-mail: sawa@kumamoto-u.ac.jp
cPriority Organization for Innovation and Excellence, Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
dInternational Research Center for Agricultural and Environmental Biology (IRCAEB), 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
First published on 9th March 2022
Abstract
Herein, we have demonstrated the potential of graphene oxide (GO) in the plantation process. In a typical case study, two sets of tomato plants were allowed to grow with and without GO in the surrounding environment. The current results suggested the possibilities of GO-aided plantation under a limited supply of water.
Deserts are considered as one of the major land-cover types, covering approximately one-third of the terrestrial area of the earth.1,2 The widescale implementation of desert greening/desert forests are very important to maintain an optimum ecosystem in the associated areas. Moreover, desert greening (farm or forests) can play a positive role in decreasing the recent concern of global warming while reducing the amount of CO2 from the environment and releasing O2 in the photosynthesis process. However, the major barriers to desert greening include the water supply associated with the survival and/or proper growth of plants. The source of water for plants in desert areas mainly consists of artificial external water, groundwater and soil adsorbed water. Among them, the artificially supplied water and groundwater must be limited to the most limited amount, and a significant focus should be given to the water adsorbed by the dry and sandy soil of the desert for a sustainable design of forest greening. In particular, only a very limited portion of the supplied water can be adsorbed by the desert sandy soil. As a result, the planted trees are facing a lack of water which significantly affects their growth process. Therefore, the improvement in the water adsorption ability of the desert soil can be a potential route to the development of the forest greening process. In fact, there is still a wide range of research space in this field; in particular, the introduction of new material technology is worthy of attention to improve the water adsorption ability of the surrounding soil.
On the other hand, the ever-increasing development of nanotechnology arises from the unique feature of nanomaterials, which are serving society with the implementation of them in different fundamental challenges. Graphene oxide (GO) and its chemical modified hybrids have been extensively studied during the last few decades in almost all areas of science, primarily due to their extraordinary physicochemical properties, non-toxic nature, and low cost.3–8 Significantly, GO possesses a large ratio of oxygen-containing functional groups, including epoxy, hydroxyl, and carboxyl groups in both sides of the backbone carbon skeleton.9 These functional groups allow them to adsorb the water molecules in the humidified/water environment. Previously, we utilized this water adsorption nature of GO for the proton conductive membrane in proton exchange membrane fuel cells.10–13 We believe that this intrinsic water adsorption property of GO can be utilized in other applications where water is required for better performance of the process.
Water is attributed as the lifeblood of plants. Specifically, water is taken up by the roots of the plant and serves the plant in many ways including (i) cooling through evaporation maintains plant leaves at an equable temperature, (ii) it acts as a substrate for a multitude of biochemical reactions that sustain plant growth, and (iii) the movement of water past the reactive surfaces of soil particles brings to the plant, via the roots, dissolved chemicals that serve either as nutrients for plant production or toxins that curtail growth.14 Herein, we have demonstrated the potential of GO in the plantation process while GO is used as an additive in the soil during the soil hydrolysis process before plantation. In a typical case study, we select two sets of tomato plants and grow them in a GO surrounded environment in the seedbed. The water supply was stopped after a certain period of time, and the growth of the tomato plants was monitored. The results were compared with the growth of another set of tomato plants in exactly the same environment except for the use of the GO dispersion during soil hydrolysis. Interestingly, the GO surrounded tomato plants grow nicely and healthy when compared with the tomato plants that were grown in the soil without GO. Moreover, we have estimated the role of the supplied GO amount for the process. As the presence/absence of GO in the soil was the only difference between the growth of tomato plants, we propose that the high-water adsorption ability of GO facilitates the soil to adsorb more water which is responsible for the better growth of tomato plants in the GO surrounded soil.
Tomato seeds (Pritz, Kaneko seeds) were sown on jiffy pots (Jiffy seven, Sakata Seed) and grown at 28 °C for 7 days with continuous light conditions. Seedlings were then transplanted to pots with soil. Each pot was prepared with 50 g of soil (Cell soil, Latec) plus 50 mL of 0.1 mg mL−1 or 1 mg mL−1 GO-dispersed water, such that the soil is thoroughly hydrated. The pots were placed on a plastic tray (20 pots in each tray). The seedlings were watered every other day from the tray such that minimal excess water remains in the tray with continuous light conditions. Watering was stopped 14 days after germination (7 days after transplant), and the plants were grown for another 8 days without water. Another set of plants was prepared in the same process except using GO in the soil hydration for comparison. Both sets of plants were allowed to grow in the same environment and the difference 15 days after plantation was recorded. The GO was purchased from Nippon Shokubai Co and used after several times washing with water. GO dispersions of two different concentrations of 0.10 mg mL−1 and 1.00 mg mL−1 were prepared.
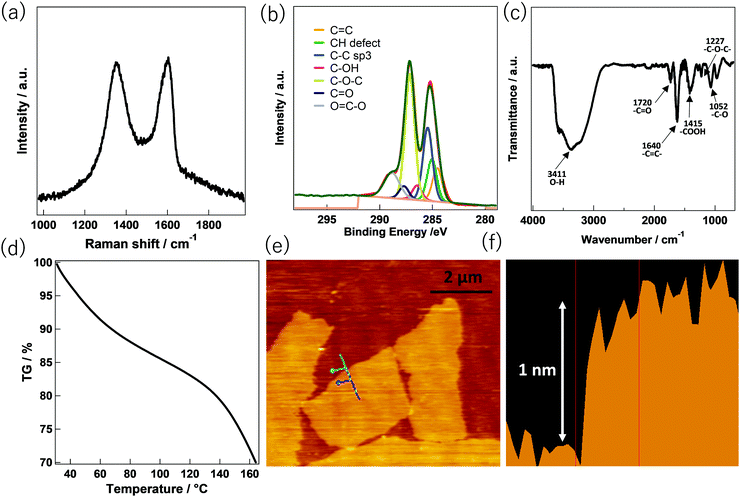
Fig. 1 shows the chemical properties and morphology of GO. The Raman spectrum in Fig. 1a, conducted at an excitation laser of 532 nm, shows two characteristic peaks, the “D band” and the “G band” which is in line with the reported literature. The D band, located at ∼1350 cm−1, was assigned to the respiratory mode (a1g) representing the sp3 domain. On the other hand, the G band located at ∼1580 cm−1 has been reported to be associated with the in-plane bond stretching motion (e2g) of the sp2 carbon atom pair mode.15 The ratio of the peak heights of the D band and the G band (ID/IG) was calculated to be 0.98. This indicates that oxidation (to convert graphene oxide from graphite) has significantly increased the number of sp3 domains. The existence of oxygen-containing functional groups in GO can be confirmed from the XPS and Fourier transform infrared (FT-IR) spectra. The C1s XPS spectrum of GO in Fig. 1b represents the abundance of oxygenated functional groups. Among various oxygenated sites, the epoxy (C–O–C) group is predominant, with a peak at approximately 286.8–287.0 eV. Carbonyl (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and carboxyl (–COOH) peaks occur at 287.8–288.0 eV and 289.0–289.3 eV, respectively.16 FT-IR spectroscopy of GO (Fig. 1c) also supports the XPS spectra regarding the existence of oxygenated functional groups in the GO. In particular, the oxygenated functional groups give rise to a C
O) and carboxyl (–COOH) peaks occur at 287.8–288.0 eV and 289.0–289.3 eV, respectively.16 FT-IR spectroscopy of GO (Fig. 1c) also supports the XPS spectra regarding the existence of oxygenated functional groups in the GO. In particular, the oxygenated functional groups give rise to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration peak at ∼1727 cm−1, a C–O stretching peak at ∼1052 cm−1, a carboxyl peak at ∼1415 cm−1, and an epoxy peak at ∼1226 cm−1. Moreover, a strong peak at ∼1640 cm−1 due to the aromatic C
O stretching vibration peak at ∼1727 cm−1, a C–O stretching peak at ∼1052 cm−1, a carboxyl peak at ∼1415 cm−1, and an epoxy peak at ∼1226 cm−1. Moreover, a strong peak at ∼1640 cm−1 due to the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and a broad peak characteristic of O–H group vibrations at ∼3410 cm−1 are observed. The adsorption of water by GO can be confirmed using thermogravimetric analysis (TGA) analysis. Fig. 1d shows the observed weight loss (% TG) of GO with increasing temperature. The weight loss up to 100 °C represents the adsorbed water content in the GO. Herein, the weight loss of 16.1% up to 100 °C indicates significant water adsorbed by GO.17 The morphological characteristics of GO deposited on the silicon substrates using atomic force microscopy (AFM) (Fig. 1e and f) were also measured. Exfoliation of the GO destroys the van der Waals interaction between the GO layers during the ultrasonic treatment. The surface of the GO nanosheet was shown to be a monolayer and has a flat sheet structure due to the mutual repulsion of the negative surface charges derived from the oxygenated functional groups. As shown in the AFM image in Fig. 1e, the width and the length of the individual GO flakes are ∼2 μm and ∼4 μm, respectively. The exfoliated GO sheet was observed to have a thickness of about 1 nm in keeping with it being a monolayer and thus being highly dispersed in an aqueous solution (Fig. 1f).
C and a broad peak characteristic of O–H group vibrations at ∼3410 cm−1 are observed. The adsorption of water by GO can be confirmed using thermogravimetric analysis (TGA) analysis. Fig. 1d shows the observed weight loss (% TG) of GO with increasing temperature. The weight loss up to 100 °C represents the adsorbed water content in the GO. Herein, the weight loss of 16.1% up to 100 °C indicates significant water adsorbed by GO.17 The morphological characteristics of GO deposited on the silicon substrates using atomic force microscopy (AFM) (Fig. 1e and f) were also measured. Exfoliation of the GO destroys the van der Waals interaction between the GO layers during the ultrasonic treatment. The surface of the GO nanosheet was shown to be a monolayer and has a flat sheet structure due to the mutual repulsion of the negative surface charges derived from the oxygenated functional groups. As shown in the AFM image in Fig. 1e, the width and the length of the individual GO flakes are ∼2 μm and ∼4 μm, respectively. The exfoliated GO sheet was observed to have a thickness of about 1 nm in keeping with it being a monolayer and thus being highly dispersed in an aqueous solution (Fig. 1f).
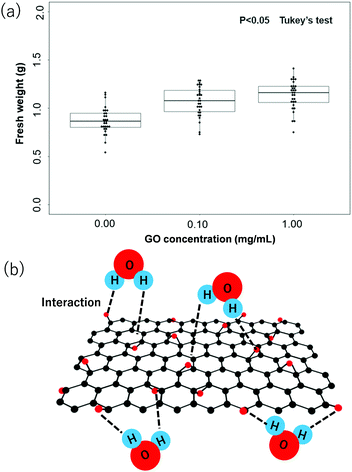
Fig. 2 represents the difference between the growth of tomato plants in the current study due to the addition of the GO dispersion during the soil hydration process. Fig. 2a–c show the optical photographs of the tomato plants after 15 days under the experimental conditions without the use of GO dispersion, with the use of GO dispersion (0.1 mg mL−1), and with the use of GO dispersion (1.0 mg mL−1), respectively. Clearly, a visible difference is observed between plants grown with GO addition during soil hydrolysis. The tomato plants in Fig. 2a, soil without GO, are significantly drier and rougher when compared to the tomato plants in Fig. 2b and c where GO was added to the soil during hydrolysis. The tomato plants with larger green leaves and more healthy-looking show the trend of 2c > 2b > 2a. Furthermore, Fig. 3a represents the quantitative analysis of the fresh weight of tomato plants with respect to the different concentrations of GO used in the soil hydrolysis. The statistical significance was assessed through Tukey's test (P < 0.05). Obviously, the average (mean) weight of tomato plants increases with increasing the concentration of GO dispersion during the soil hydrolysis process. The average fresh weights of tomato plants are 0.92, 1.12 and 1.20 g for the concentration of the GO dispersion of 0.00, 0.10 and 1.00 mg mL−1, respectively. The above results clearly demonstrate the benefit of GO dispersion in the tomato plantation during soil hydrolysis.
Clearly, the above results confirm a better growth of tomato plants in the GO surrounding soil under limited water-supplied conditions. It is well established that trees/plants absorb water from the soil through their roots. In particular, plant roots are covered by tiny hairs with benefit fungi growing on them that draw water into the roots by osmosis. Once the water is sucked into the roots via the root hairs, it gets into a sort of botanical pipeline in the tree's inner bark that carries the water up the tree. Therefore, in a limited water supply condition, any additives that ensure more adsorbed water in the soil are expected to improve the plantation process. The remarkable water adsorption ability of GO has been well documented during the last few decades.18,19 Using experiments and theoretical analysis, Lian et al. demonstrated that the expandable 2D porous laminated structure of GO is responsible for the high-water uptake capacity. The observed high water uptake capacity is up to 0.58 grams of water per gram of GO under high humidity conditions.18
Moreover, the fast water adsorption/desorption ability of GO was attributed to the existence of wrinkle-like water tunnels. In particular, hydrophilic GO sheets can attract water molecules via strong hydrogen bonding between water molecules and oxygen-containing polar groups, and monolayers or bilayers consisting of water molecules can be trapped in the GO interlayer space, depending on the relative humidity and temperature. On the other hand, the amount of adsorbed water molecules by GO in liquid water is much higher than in a highly humidified atmosphere. For example, Y. H. Cho and co-worker studied the water adsorption ability of GO in high humidified conditions and water dispersion conditions using the XRD data in the wet state and water uptake measurements.19 They observed that when compared with the interlayer spacing of 0.8 nm of a GO membrane dried at ambient conditions (50% RH), the interlayer spacing of a fully hydrated GO membrane increased up to ∼1.3 nm, indicating that a few layers of water would form in the interlayer space of the GO membranes in liquid water.19 Therefore, in water dispersion conditions, GO membranes can absorb a relatively larger amount of water molecules (120 wt% in water) than in a humidified atmosphere.19 As GO contains oxygenated functional groups on both sides of the backbone carbon skeleton, and also at the edge of the nanosheets, the water adsorption takes place from all directions, responsible for the higher water uptake by the GO. A typical interaction of hydrophilic functional groups of GO and H2O is shown in Fig. 3b. The water adsorption and desorption of GO membranes are fully reversible;19 therefore, GO might appear as an ideal additive for improving the hydration ability of soil. In addition, GO is unlikely to be adsorbed by the plant roots or penetrate into the plant, which can be attributed to (i) the GO sheets having a flake structure of micro-meter size in length that does not allow them to pass through the nanopores in the cytoderm of the plant, (ii) the soil environment is not conducive to the dissolution and movement of GO, which limits the movement of GO sheets and (iii) the roots of vegetables can secrete plenty of mucilage, which carries abundant negative charges and coats the root surface. The GO sheets might have been prevented from contacting the root surface by the electrostatic repulsion from the mucilage.20–22
In the present study, we presented the preliminary results that we have obtained from tomato plantation using GO modified soil while supplying limited water in the plantation process. However, it is difficult to conclude the quality of the fruits in the current stage. The detailed study of GO-aided plantation for different kinds of plants in real desert conditions is ongoing by our research group and we have a plan to include it in our future works. Furthermore, As the GO-aided soil hydrolysis does not require analytical grade (highly pure) GO, it is expected that the GO-aided plantation technology will be economical for large-scale applications.
In summary, we have demonstrated the effect of GO in the hydration of soil for tomato plantations. The soil that contains GO showed a better growth of tomato plants compared to that of bare soil, which might be attributed to the water adsorption ability of GO. The oxygenated functional groups of GO facilitated the adsorption of water in the soil, thereby improving the overall water adsorption of the soil. The results from the current findings can be a future guide for GO-assisted plantation in low water supply areas and/or desert plantations. Moreover, the outcome might be applied for the cultivation of other plants while GO can be utilized for better hydration of the soil.
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. A. Mabbutt, Desert landforms, Australian National University Press, 1977 Search PubMed.
- L. M. Bidak, S. A. Kamal, M. W. A. Halmy and S. Z. Heneidy, Glob. Ecol. Conserv., 2015, 3, 433 CrossRef.
- M. S. Islam, Y. Shudo and S. Hayami, Bull. Chem. Soc. Jpn., 2022, 95, 1 CrossRef CAS.
- M. Fukuda, M. S. Islam, R. Shimizu, H. Nassar, N. N. Rabin, Y. Takahashi, Y. Sekine, L. F. Lindoy, T. Fukuda, T. Ikeda and S. Hayami, ACS Appl. Nano Mater., 2021, 4, 11881 CrossRef CAS.
- Y. Shudo, M. S. Islam, H. Zenno, M. Fukuda, M. Nakaya, N. N. Rabin, Y. Sekine, L. F. Lindoy and S. Hayami, Phys. Chem. Chem. Phys., 2021, 23, 24233 RSC.
- M. R. Karim, M. S. Islam, N. N. Rabin, H. Takehira, K. Wakata, M. Nakamura, R. Ohtani, K. Toda and S. Hayami, ChemistrySelect, 2017, 2, 4248 CrossRef CAS.
- M. S. Islam, H. Ohmagari, M. A. Rahman, M. Fukuda, Y. Shudo, J. Yagyu, Y. Sekine, L. F. Lindoy and S. Hayami, Mater. Adv., 2021, 2, 5649 Search PubMed.
- M. Fukuda, M. S. Islam, Y. Sekine, T. Shinmei, L. F. Lindoy and S. Hayami, ChemistrySelect, 2021, 6, 3399 CrossRef CAS.
- M. R. Karim, M. S. Islam, K. Hatakeyama, M. Nakamura, R. Ohtani, M. Koinuma and S. Hayami, J. Phys. Chem. C, 2016, 120, 21976 CrossRef CAS.
- J. Yagyu, Md. S. Islam, Y. Shudo, M. Fukuda, H. Ushijima, J. Ohyama, S. Ida, L. F. Lindoy and S. Hayami, ACS Appl. Energy Mater., 2021, 4, 6296 CrossRef CAS.
- M. Fukuda, M. S. Islam, Y. Shudo, J. Yagyu, L. F. Lindoy and S. Hayami, Chem. Commun., 2020, 56, 4364 RSC.
- M. R. Karim, M. S. Islam, N. N. Rabin, R. Ohtani, M. Nakamura, M. Koinuma and S. Hayami, Aust. J. Chem., 2017, 70, 642 CrossRef CAS.
- K. Wakata, M. S. Islam, M. R. Karim, K. Hatakeyama, N. N. Rabin, R. Ohtani, M. Nakamura, M. Koinuma and S. Hayami, RSC Adv., 2017, 7, 21901–21905 RSC.
- B. E. Clothier and S. R. Green, Soil Sci., 1997, 162, 534 CrossRef CAS.
- M. Pimenta, G. Dresselhaus, M. Dresselhaus, L. Cancado and A. Jorio, Phys. Chem. Chem. Phys., 2007, 9, 1276 RSC.
- M. Koinuma, H. Tateishi, K. Hatakeyama, S. Miyamoto, C. Ogata, A. Funatsu, T. Taniguchi and Y. Matsumoto, Chem. Lett., 2013, 42, 924 CrossRef CAS.
- K. Wakata, M. R. Karim, M. S. Islam, R. Ohtani, M. Nakamura, M. Koinuma and S. Hayami, Chem. – Asian J., 2017, 12, 194 CrossRef CAS PubMed.
- B. Lian, S. De Luca, Y. You, S. Alwarappan, M. Yoshimura, V. Sahajwalla, S. C. Smith, G. Lesliea and R. K. Joshi, Chem. Sci., 2018, 9, 5106 RSC.
- Y. H. Cho, H. W. Kim, H. D. Lee, J. E. Shin, B. M. Yoo and H. B. Park, J. Membr. Sci., 2017, 544, 425 CrossRef CAS.
- Y. He, R. Hu, Y. Zhong, X. Zhao, Q. Chen and H. Zhu, Nano Res., 2018, 11, 1928 CrossRef CAS.
- D. H. Lin and B. S. Xing, Environ. Sci. Technol., 2008, 42, 5580 CrossRef CAS PubMed.
- Z. Y. Zhang, X. He, H. F. Zhang, Y. H. Ma, P. Zhang, Y. Y. Ding and Y. L. Zhao, Metallomics, 2011, 3, 816 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |