Open Access Article
Open Access ArticlePtSe2 on a reduced graphene oxide foil for the alkaline hydrogen evolution reaction†
Filipa M.
Oliveira
 a,
Iryna
Danylo
a,
Iryna
Danylo
 b,
Vlastimil
Mazánek
a,
Martin
Veselý
b,
Vlastimil
Mazánek
a,
Martin
Veselý
 b,
Rui
Gusmão
b,
Rui
Gusmão
 *a and
Zdeněk
Sofer
*a and
Zdeněk
Sofer
 *a
*a
aDepartment of Inorganic Chemistry, Faculty of Chemical Technology, University of Chemistry and Technology Prague, Technická 5, 166 28 Prague 6, Czech Republic. E-mail: rui.gusmao@vscht.cz; zdenek.sofer@vscht.cz
bDepartment of Organic Technology, Faculty of Chemical Technology, University of Chemistry and Technology Prague, Technická 5, 166 28 Prague 6, Czech Republic
First published on 11th April 2022
Abstract
Reducing energy consumption during the hydrogen evolution reaction (HER) in electrocatalytic water splitting for hydrogen production is still a challenge. Among the transition metal dichalcogenide family, platinum diselenide (PtSe2) exhibits good air stability, a low band-gap, and semimetallic properties, rendering it a promising catalyst for the HER. Herein, the selenization of predeposited Pt on a reduced graphene oxide (rGO) substrate forming an 88 ± 6 nm PtSe2 thin layer results in a freestanding, efficient HER electrocatalyst yielding a competitive performance in an alkaline medium, achieving a low overpotential of 95 mV at −10 mA cm−2. Furthermore, the PtSe2/rGO foil used in a two-electrode cell configuration demonstrates good long-term stability, up to 18 hours. The performance of the PtSe2/rGO foil results from the distribution of PtSe2 over the rGO substrate, which is maintained after the electrochemical reactions as attested by Raman mapping, and complemented by scanning electron microscopy, inductively coupled plasma optical emission spectrometry, and X-ray photoelectron spectroscopy characterization.
Introduction
In the last few decades, society has revealed a growing concern about the use of sustainable and clean energy supplies. However, a lack of balance in the implementation of sustainable alternatives and the management of available resources, namely, traditional fossil fuels, is causing an environmental crisis. Hydrogen (H2) has proven to be a green alternative to substitute fossil fuels and its production by water-splitting reactions has been thoroughly investigated.1–3As part of the large family of two-dimensional (2D) materials,4–6 transition metal dichalcogenides (TMDs) with a general formula of MX2, where M stands for a transition metal (e.g., Mo, Co, W, or Pt) atom sandwiched by X chalcogen atoms (S, Se or Te),7 have great potential for realizing highly performing electrocatalysts for H2 production through the hydrogen evolution reaction (HER).8–10 Research on TMDs as electrocatalysts is mostly focused on Group 6 TMDs, namely, MoS211,12 and WS2.13,14 However, limitations concerning electrical conductivity and available active sites often require extra procedures for their modification in order to improve their catalytic activity. For instance, in a recent study, Pt nanocrystals were deposited on the surface of MoS2 edge-exposed nanoplatelets for enhancing HER performance in acidic media.15 To overcome these limitations, and considering the multiple possibilities in the TMD family, a significant amount of research is ongoing regarding Pt dichalcogenides (PtX2), belonging to Group 10 TMDs.16–18 In particular, the good air stability,19,20 low band-gap in the range of 0.21–1.2 eV,21,22 and semi-metallic behavior21,23 of platinum diselenide (PtSe2) have incited research groups to explore its performance as a HER electrocatalyst.23–26 It is extensively reported that a narrow band-gap is beneficial to improving the conductivity of a given material and higher carrier transmission rate, which is crucial for improving electrocatalytic performance. PtSe2 can be prepared by mechanical exfoliation,19,27 chemical vapor transport,25,28 chemical vapor deposition,23,29–31 selenization of Pt atoms21 or Pt films24 and molecular beam epitaxy,19,32 to name a few. However, all these techniques then require the deposition of PtSe2 onto an electrode support such as glassy carbon to assess its electrochemical performance. As the substrate may influence the properties of electrocatalysts due to capacitance, synergetic effects from the support, or other electrochemical features,33 it is important to find a suitable strategy for acquiring reliable results from the electrochemical measurements. Metal-foam or metal-foil-based substrates are other approaches to support an active catalyst; however, drawbacks, such as oxidation sensitivity, limitations on large-scale production, and degradation after long-term operation are found to exist in such avenues. Therefore, the synthesis of PtSe2 directly on a given substrate, thus avoiding the drop-casting technique to modify the active electrode area, could be an efficient approach to overcome the above-mentioned limitations on electrocatalyst preparation. The selection of a stable substrate such as reduced graphene oxide (rGO) could favor the production of an electrocatalyst with a scalable area, thus allowing the achievement of more reliable performance for commercial applications. In addition, the use of a flexible substrate like rGO allows easy handling during the electrocatalyst production and, later, during the HER measurements.
Hence, we produced a flexible and freestanding PtSe2/rGO foil by direct selenization of a Pt film previously deposited on an rGO flexible substrate and report for the first time the HER performance in alkaline medium of PtSe2 synthesized on a carbon substrate. Scanning electron microscopy (SEM) coupled with energy dispersive spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), X-ray diffraction (XRD), and Raman spectroscopy are used to investigate the structure and morphology of the PtSe2/rGO foil. The PtSe2/rGO foil is directly used for the HER in alkaline media, revealing an exceptional performance. Structural characterization after long-term stability tests shows that the overall integrity of the PtSe2/rGO foil is sustained which encourages the design of Pt dichalcogenides for high-performance HER catalysis.
Materials and methods
Chemicals and materials
Platinum (99.99%) was obtained from K. J. Lesker, USA, and selenium (99.999%) was obtained from STREM, Germany. Potassium hydroxide, sulfuric acid (99.999%), and platinum nanoparticles supported on carbon with 10 and 20 wt% loading (10% Pt/C and 20% Pt/C, respectively) were purchased from Sigma-Aldrich, Czech Republic. Ruthenium(IV) oxide (RuO2) (99.95% metal basis, Ru typically 74%) and graphite powder (2–15 μm, 99.9995%) were purchased from Alfa Aesar, Germany. A polycarbonate (PC) membrane (90 mm diameter, pore size 400 nm) was purchased from Sterlitech, USA. N,N-Dimethylformamide (DMF), phosphoric acid (85%), potassium permanganate (99%), hydrogen peroxide (30%), nitric acid (HNO3), and hydrochloric acid (HCl) were purchased from Penta, Czech Republic. Nickel mesh (Ni mesh) (>99.8%, porosity 97%, areal density 350 g m−2) and carbon paper (Cpaper) (100%, porosity 75%, density 0.78 g m−3) were purchased from Xiamen TOB New Energy Technology Co. Ltd, China. EpoFix hardener and EpoFix resin were purchased from Struers GmbH, Czech Republic.Preparation of PtSe2/reduced graphene oxide foil
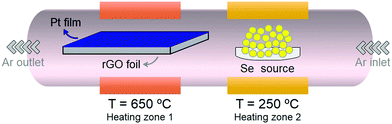
The rGO foil was prepared by thermal reduction of graphene oxide (GO) prepared by assembling on a support membrane. First, the GO powder was prepared by the Tour method34 and following the same procedure reported previously,35 in which 3 g of graphite was stirred in a solution of concentrated sulfuric acid (98 wt%, 360 mL) and phosphoric acid (85 wt%, 40 mL). The mixture was allowed to cool to 0 °C, and afterward, 18 g of potassium permanganate, KMnO4, was added to the reaction mixture, whereafter the mixture was stirred for 1 h and allowed to reach room temperature. Then, the reaction mixture was heated to 50 °C for 6 h and left to cool to room temperature and stirred for 12 h. Subsequently, the reaction mixture was poured into a flask containing 400 g of ice and 10 mL of hydrogen peroxide (H2O2). The unreacted KMnO4 and manganese dioxide were removed by adding 20 mL of H2O2. The reaction mixture was allowed to settle and then decanted. Finally, the GO was purified by repeated centrifugation and multiple washes in deionized water followed by a final vacuum drying. Afterward, the obtained GO was dispersed in water (1 mg mL−1), of which 100 mL was filtered through a PC membrane, and dried in an oven at 60 °C for 24 h. The GO foil was mechanically removed from the PC template and reduced by heating at 800 °C in a hydrogen (H2) atmosphere (heating rate of 1 °C min−1, a duration of 2 h, H2 gas flux of 100 mL min−1). For the synthesis of PtSe2 on the rGO foil, a 60 nm thick layer of platinum (Pt) was first evaporated (at a pressure of 1 × 10−5 mbar) onto an rGO foil using an e-gun vacuum evaporator. Subsequently, the Pt/rGO foil was placed in a two-zone furnace. First, the Pt/rGO foil was heated at 650 °C in an argon (Ar) gas flow (99.9999%, 250 mL min−1), and afterward, the selenium (Se) source was heated in the second zone at 250 °C. The treatment proceeded for 2 hours and the furnace was freely cooled to room temperature. A schematic illustration of the synthesis of PtSe2 on the rGO foil is shown in Scheme 1.Structural and morphological characterization
The morphology of the materials was investigated using SEM with a FEG electron source (Tescan Lyra dual-beam microscope). The measurements were carried out using 8 and 10 kV accelerating voltages, depending on the samples: 8 kV for surface analysis of the PtSe2/rGO foil and 10 kV for cross-section analysis of the resin mold. EDS was used for elemental composition and mapping using an 80 mm2 SDD detector (Oxford Instruments) and AZtecEnergy software. The measurements were carried out using 8, 10, or 20 kV accelerating voltages, depending on the samples. For SEM and EDS analyses of the surface morphology and respective elemental composition, the samples were directly placed on carbon tape. For cross-section analysis by SEM and EDS, the already measured PtSe2/rGO foil by SEM was fastened to a plastic holder in such a way that the microstructure along the foil thickness and length was accessible for imaging. The assembly was put into a plastic cup, serving as a container for epoxy resin, with an inner diameter of 25 mm. The container volume was filled with the resin prepared prior to the filling by mixing EpoFix Resin and EpoFix Hardener in the weight ratio of 25 to 3, respectively. After 24 h of hardening at a temperature of 60 °C, the resin block was removed from the plastic cup, and the surface of the hardened block was ground and polished. Before the SEM analysis, the polished surface was coated with a 5 nm gold layer to ensure electrical conductivity.High-resolution (HR) XPS (HR-XPS) was performed using an ESCAProbeP spectrometer (Omicron Nanotechnology Ltd, Germany) with a monochromatic Al X-ray radiation source (1486.7 eV). The samples were placed on conductive carbon tape and HR scans of Pt 4f and Se 3d were collected. A low-energy e-gun was used to eliminate sample charging during the measurement (1–5 V).
TEM was performed using an EFTEM Jeol 2200 FS microscope (Jeol, Japan) at an acceleration voltage of 200 keV, assisted by the selected area electron diffraction (SAED) technique. Pictures were taken using an SIS MegaView III digital camera (Soft Imaging Systems) and analyzed using AnalySIS v. 2.0 software. Elemental maps were acquired using an X-MaxN 80 TS SDD detector from Oxford Instruments (England). For sample preparation, the material was lightly scraped from the as-prepared PtSe2/rGO foil on the PtSe2 side and dispersed in DMF. The suspension was drop-cast on a TEM grid (Cu, 200 mesh, Formvar/carbon from TED PELLA, Inc.) and dried overnight in a vacuum oven at 50 °C.
An InVia Raman microscope (Renishaw, England) was used for Raman spectroscopy measurements in backscattering geometry with a CCD detector. A Nd:YAG laser (532 nm wavelength) and a 20× objective were used for data collection. Instrument calibration was achieved using a silicon reference, which gave the peak position at 520 cm−1 and a resolution down to 1 cm−1. The laser power used for these measurements was 1 mW. Mapping scans were collected with pixel sizes of 6.2 μm × 6.2 μm and the individual Raman spectra were batch-processed using our routine written in MATLAB. For every single spectrum, routine (I) smooths the raw spectrum using a nonparametric method removing noise with preserving signal intensity, routine (II) identifies and corrects the baseline of the smoothened spectrum, routine (III) identifies the number of peaks in the range of Raman shift between 140 and 260 cm−1, and routine (IV) fits the baseline-corrected spectrum with a number of Gaussian peaks, which equals the number of identified peaks. Then, the fitted peaks around the Raman shift of 180 cm−1 and 209 cm−1 were used to plot maps showing the homogeneity of the PtSe2/rGO foil on the PtSe2 side for peak intensity, peak intensity ratio, and exact Raman shifts. Details of the used routine are included in the ESI.†
XRD measurements were carried out using a Bruker D8 Discoverer (Bruker, Germany) diffractometer in Bragg–Brentano parafocusing geometry under Cu Kα radiation (λ = 0.15418 nm, U = 40 kV, I = 40 mA). The diffraction patterns were collected at room temperature for 2θ values from 10 to 60°. The data were evaluated using HighScore Plus 4.9 software.
Electrothermal vaporization (ETV) coupled with inductively coupled plasma optical emission spectrometry (ICP-OES) was used to determine the wt% Pt elemental composition of the as-prepared PtSe2/rGO foil and PtSe2/rGO foil after a long-term stability test. The measurements were carried out using a Spectro ARCOS spectrometer (SPECTRO Analytical Instruments, Germany). For sample preparation, a representative 4 to 5 mg of sample was dissolved in 4 mL of a mixture of concentrated HNO3 and HCl in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 volume ratio using a hot plate. After the dissolution process, the dissolute was cooled, filtered, diluted with deionized water, and transferred to a clean vial prior to analysis.
3 volume ratio using a hot plate. After the dissolution process, the dissolute was cooled, filtered, diluted with deionized water, and transferred to a clean vial prior to analysis.
Electrochemical characterization
Electrochemical measurements were done at room temperature using an Autolab PGSTAT204 (Eco Chemie, Utrecht, The Netherlands) controlled by NOVA Version 2.1.4 software. Glassy carbon (GC) electrodes with a diameter of 3 mm (CH Instruments, Texas, USA), and PtSe2/rGO foil, rGO foil, carbon paper (Cpaper), and Ni mesh, all of them with an area of 0.9 cm2, were used as working electrodes (WE); a carbon rod was used as a counter electrode and Ag/AgCl/Sat. KCl was used as a reference electrode (CH Instruments, Texas, USA). Suspensions of 10% Pt/C (1.5 mg mL−1 in DMF), 20% Pt/C (1.5 mg mL−1 in DMF), and RuO2 (1 mg mL−1 in deionized water) were used for WE modification. The Cpaper and Ni mesh were modified with 10% Pt/C and RuO2, respectively. For 10% Pt/C@Cpaper, the modification was done by drop-casting 20 μL of 10% Pt/C suspension onto both surfaces (20 × 2 μL, total mass loading of 66.7 μg cm−2) of the Cpaper and drying under air for 3 h. The RuO2@Ni mesh catalyst was prepared by following the same process by drop-casting 30 μL of RuO2 suspension onto both surfaces (30 × 2 μL, total mass loading of 66.7 μg cm−2) of the Ni mesh. The modification of the GC WE was done by drop-casting 3 μL of 10% Pt/C (total mass loading of 63.4 μg cm−2) and 20% Pt/C (total mass loading of 63.4 μg cm−2) on it. The assembly of PtSe2/graphene foil, Cpaper, and Ni mesh as WEs was carried out as follows: the WEs were cut into rectangular pieces with a dimension of 0.5 × 1.5 cm. For each WE, the respective rectangular piece was attached to a platinum plate electrode clamp (Gaoss Union, China) and then the active surface area corresponding to 0.9 cm2 was immersed into the electrolyte. For the modification of Cpaper and Ni mesh, the 10% Pt/C and RuO2 suspensions were drop-cast on the corresponding 0.9 cm2 active area.Double-layer capacitance (Cdl) measurements were carried out in a non-faradaic region. The scan rate varied within the range of 20 <ν < 200 mV s−1, and 0.9 < E < 1.3 V vs. reversible hydrogen electrode (RHE). The Cdl values of each electrode were extracted from the slope of the dependence of the current density (ΔIa – Ic) on the scan rate, at a fixed potential of +1.1 V vs. RHE (for further details see the ESI†). The electrochemical impedance spectroscopy (EIS) response of each electrode was measured at −0.08 V vs. RHE at a frequency ranging from 0.1 Hz to 1 MHz and an amplitude of 10 mV.
Linear sweep voltammetry (LSV) measurements were performed in a three-electrode cell configuration at 2 mV s−1 and the potentials were converted to the RHE using the Nernst equation, ERHE(V) = EAg/AgCl + 0.059pH + E0Ag/AgCl, in which E0Ag/AgCl = 0.197 V vs. standard hydrogen electrode (SHE). Cyclic voltammograms (CVs) of the PtSe2/rGO foil were obtained at a scan rate of 100 mV s−1 between η20 and +0.3 V vs. RHE, for 100, 1000, and 3000 cycles. The long-term stability experiments were conducted in: (i) a three-electrode cell configuration with the PtSe2/rGO foil for 120 min at a constant current density of −10 mA cm−2. The rGO foil was tested under the same conditions for comparison; and in (ii) a two-electrode cell configuration with the PtSe2/rGO foil as the cathode and Ni mesh-RuO2 as the anode for 18 h at a constant current density of 10 mA cm−2. For comparison, the same conditions were used for Cpaper (cathode)‖Ni mesh (anode) and 10% Pt/C@Cpaper (cathode)‖RuO2@Ni (anode) cell configurations. All electrochemical experiments were carried out in 1.0 M KOH solution without iR compensation.
Results and discussion
Morphological and structural characterization of the PtSe2/rGO foil
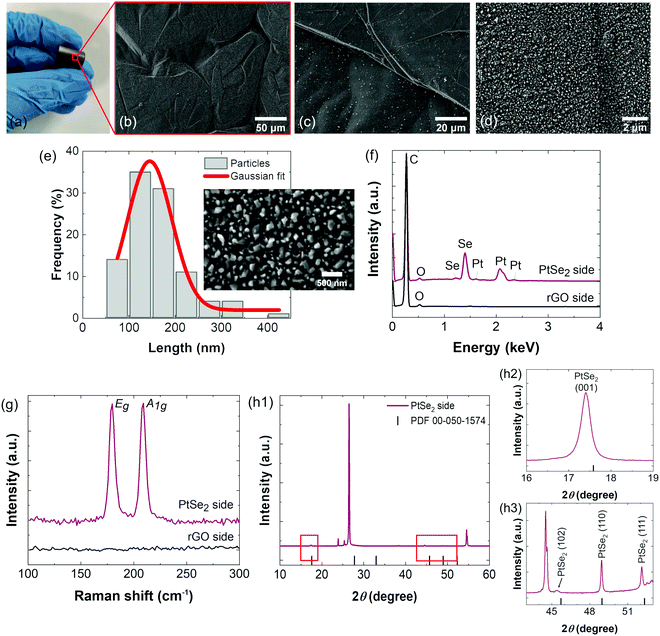
The PtSe2/rGO foil was prepared by the direct selenization of Pt, which was previously deposited on an rGO foil by physical vapor deposition as illustrated and described in the Experimental section. Fig. 1(a) shows the visual aspect of the PtSe2/rGO foil, capable of sustaining torsion. From the SEM micrographs at low magnification, it is observable that the surface of the foil on the PtSe2 side is filled with irregularities, wrinkles, and plateaus from the foil (Fig. 1b and c). As shown in Fig. 1(d), at higher magnification, the PtSe2 side is abundant in submicron particles with an average size of 145.0 ± 48.1 nm. Fig. 1(e) shows a histogram of the particle size distribution fitted by a Gaussian curve on the PtSe2 side. The ratio of each element was measured by EDS, as shown in Fig. 1(f) and Table S1 (ESI†), confirming the successful selenization of Pt as the expected stoichiometry was obtained, that is ≈1 for the metal (Pt) and ≈2 for the chalcogenide (Se). From the elemental mapping by EDS (Fig. S1, ESI†), a homogeneous distribution of the Pt and Se elements on the PtSe2 side is observed, additional evidence of the effectiveness of the method used in the production of the PtSe2/rGO foil. The EDS spectrum (Fig. 1f) reveals the energy signals associated with Pt and Se, whereas the rGO side attests that only carbon and oxygen are present. Morphological analysis was also performed by TEM and SAED, coupled with EDS elemental mapping (Fig. S2, ESI†). The TEM image (Fig. S2a, ESI†) shows clusters of PtSe2 particles, and from the details of the PtSe2 surface by HR-TEM (Fig. S2b, ESI†) regular lattice fringes are observed with a d-spacing of 0.5385 nm (Fig. S2c, ESI†), thus suggesting the formation of a layered structure. The corresponding SAED pattern (Fig. S2d, ESI†) evidenced the crystalline structure of the as-synthesized PtSe2 on rGO foil. The EDS elemental mapping (Fig. S2e, ESI†) revealed that both Pt and Se elements are homogeneously distributed, and non-coincidental with the mapping of the C element.In the Raman spectrum of the PtSe2 side of the foil (Fig. 1g), two prominent peaks with similar intensity are observed at 179.7 and 209.0 cm−1 assigned, respectively, to Eg and A1g modes.36 These values are in agreement with previous studies on Raman characterization of PtSe2 materials.28,37,38 From the Raman spectrum on the rGO side (Fig. 1g), it is evident that only carbon is present, as the vibrational modes of PtSe2 were not observed. The full Raman spectrum of the rGO side is shown in Fig. S3(a) (ESI†), with a weak D band at 1339 cm−1 and a very intense G band at 1582 cm−1, indicating a high level of crystallinity of the graphene formed by the reduction of the GO foil. A magnification of the spectrum is shown in Fig. S3(b) (ESI†). Included in Fig. S5 (ESI†) is the Raman spectrum of the GO foil with D- and G-bands at 1344 and 1598 cm−1, respectively. By comparing the GO and rGO foil spectra, it is noticed that there is a significant decrease in the D band intensity in the rGO foil due to the highly effective reduction of GO during the heat treatment at 800 °C. Such a reduction removed the oxygen groups in the GO, thus increasing the surface area of rGO.
The XRD diffractogram of the PtSe2 film on the PtSe2 side is shown in Fig. 1(h1–h3), and the crystallographic data agree with the hexagonal structure of PtSe2 with P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1 (164) space group and lattice constants of a = b = 3.728 Å and c = 5.081 Å.16 According to the powder diffraction file (PDF) no. 00-050-1574, the peaks at 17.4, 45.4, 48.9 and 52.1° can be ascribed, respectively, to the (001), (102), (110) and (111) crystal planes of PtSe2. The magnification of Fig. 1(h1) in the 16–19° (Fig. 1h2) and 43–53° (Fig. 1h3) 2θ ranges shows these peaks more clearly. The peaks at 26.4° (002), 44.5° (101) and 54.6° (004) are associated with the rGO substrate together with corresponding Kβ diffraction peaks at 23.9 and 25.4°.
m1 (164) space group and lattice constants of a = b = 3.728 Å and c = 5.081 Å.16 According to the powder diffraction file (PDF) no. 00-050-1574, the peaks at 17.4, 45.4, 48.9 and 52.1° can be ascribed, respectively, to the (001), (102), (110) and (111) crystal planes of PtSe2. The magnification of Fig. 1(h1) in the 16–19° (Fig. 1h2) and 43–53° (Fig. 1h3) 2θ ranges shows these peaks more clearly. The peaks at 26.4° (002), 44.5° (101) and 54.6° (004) are associated with the rGO substrate together with corresponding Kβ diffraction peaks at 23.9 and 25.4°.
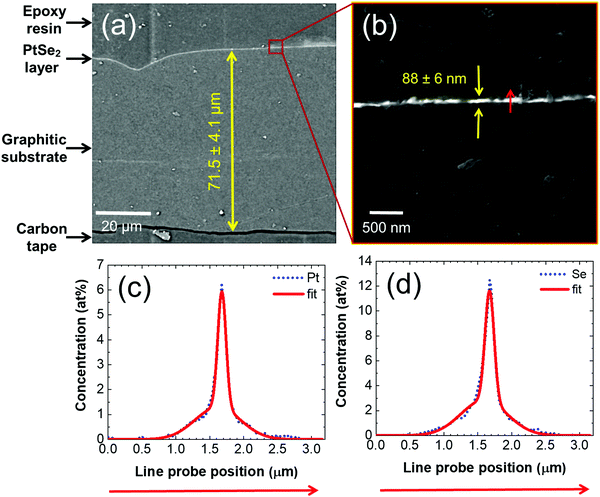
To characterize the cross-section of the PtSe2/rGO foil, we embedded the material in epoxy resin, let the epoxy harden, and grounded and polished the epoxy block. The complete cross-section shown in Fig. 2(a) reveals the entire thickness of the prepared foil of 71.5 ± 4.1 μm, as well as the thickness of the PtSe2 layer of 88 ± 6 nm, which is shown in detail in Fig. 2(b). Fig. 2(b) further shows the red arrow, alongside which an EDS elemental mapping was acquired. The elemental profiles show clear Gaussian distributions with full width at half maximum (FWHM) values of 109 nm and 136 nm for Pt and Se, respectively (Fig. 2c and d), which confirms the thin character of the PtSe2 layer at the top of the foil. Furthermore, Pt and Se evince a high positive spatial correlation with a Pearson coefficient of 0.986. This infers that a spot with a high Pt concentration also has a high Se concentration. Complementary to the Pt and Se profiles, carbon was measured as well. The spatial correlation of Pt–C and Se–C evinces negative correlation coefficients of −0.8978 and −0.9026, respectively. The value means that where the concentration of Pt or Se is high, the concentration of C is low, and vice versa. Furthermore, the atomic ratio of Pt/Se alongside the whole line probe is constantly close to the value of 0.5, which also equals the stoichiometry of PtSe2 on the PtSe2 side.
PtSe2/rGO as an electrocatalyst for the HER
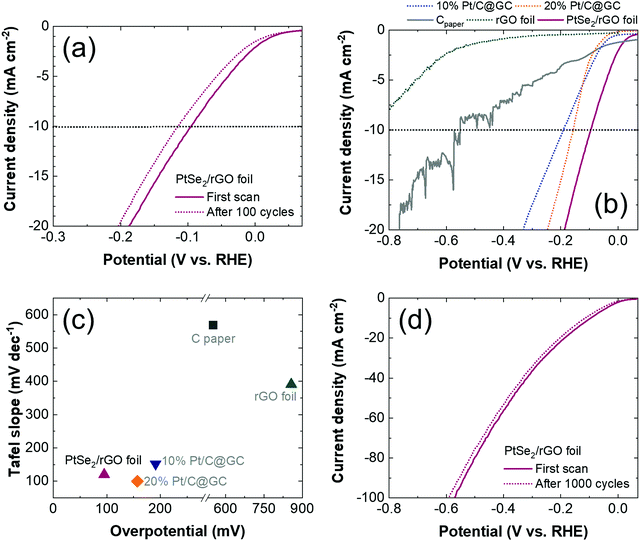
To evaluate the HER performance, the PtSe2/rGO foil was initially tested in a three-electrode cell in an alkaline (1.0 M KOH) electrolyte by LSV at a sweep rate of 2 mV s−1. The LSV polarization curves are shown in Fig. 3(a) and it is seen that in the first LSV, an overpotential of 95 mV was necessary to reach the current density of −10 mA cm−2 (η10). After 100 continuous cyclic voltammograms (CVs) at 100 mV s−1, a difference of only 19 mV in the η10 was observed (Fig. 3a).As these results indicate that the PtSe2/rGO foil has excellent performance for the HER under alkaline conditions, its electrocatalytic activity was further evaluated and compared with that of the commercial benchmark catalyst 10% Pt/C deposited on GC (10% Pt/C@GC). In Fig. S4 (ESI†), an optical image of Cpaper (Fig. S4a, on the left, ESI†) and its morphology by SEM (Fig. S4b, ESI†) are shown. The typical LSV curves are illustrated in Fig. 3(b) and the numeric results are included in Table S2 (ESI†). Unmodified Cpaper and rGO are included for comparison and they were revealed to be unstable in alkaline media, reaching a η10 of 552 and 850 mV, respectively, in the first scan. The LSV curves of the WEs modified with 10% Pt/C show their typical performance as HER electrocatalysts in alkaline media. However, the η10 at 191 mV for 10% Pt/C@GC, represents a value of 96 mV, higher compared to that of the fabricated PtSe2/rGO foil. Moreover, the commercial 20% Pt/C deposited on GC (20% Pt/C@GC) yielded an η10 value of 157 mV (Fig. 3b and Table S2, ESI†), which confirms the superior catalytic activity of the PtSe2/rGO foil.
To further assess the performance of the PtSe2/rGO foil, the HER kinetics was evaluated based on the Tafel slopes determined from the corresponding LSV curves, shown in Fig. S5a (ESI†) and summarized in Table S2 (ESI†). The Tafel slope for the PtSe2/rGO foil is 120 mV dec−1, which is lower than that of 10% Pt/C@GC (152 mV dec−1). On the other hand, the Tafel slope of 20% Pt/C@GC was 100 mV dec−1 (Fig. S5a and Table S2, ESI†). Despite these lower Tafel slope values compared to those of the PtSe2/rGO foil, a favorable kinetic process on the PtSe2/rGO foil can be stated as it requires less energy for water dissociation. Moreover, it is suggested that the adsorption equilibria follow a Volmer–Heyrovský pathway as the Tafel slope value is in the range of 40–120 mV dec−1.10
Moreover, a correlation between the Tafel slope and η10 is shown in Fig. 3(c), and it is seen that the direct selenization of Pt is advantageous for the HER activity because the PtSe2/rGO foil has a comparable kinetics process to that of 10% Pt/C@GC but requires a lower η10 (Fig. 3 and Table S2, ESI†), the recommended parameter for evaluating the HER efficiency. As mentioned above, the PtSe2/rGO foil has a higher Tafel slope value compared to that of 20% Pt/C@GC (Fig. 3 and Table S2, ESI†), but since these three materials fall within a similar rate-determining step (RDS) i.e., the Volmer–Heyrovský mechanism, improvements are expected in the catalytic activity of the PtSe2/rGO foil.
The Pt mass activity was determined within the potential range studied, as shown in Fig. S5c (ESI†), reaching a value of 48 mA mgPt−1 @ −0.10 V vs. RHE. The turnover frequency (TOF)39 was also determined for the different potentials, as shown in Fig. S5d (ESI†), reaching a maximum of 0.45 s−1 @ −0.57 V vs. RHE.
The general trend in the catalytic performance of the materials under study is maintained, i.e., PtSe2/rGO foil > 20% Pt/C@GC > 10% Pt/C@GC > Cpaper. Also included in Table S2 (ESI†) is the electrocatalytic performance of some metal diselenide and platinum catalysts for the HER in KOH reported in the literature. As observed, the HER performance of the PtSe2/rGO foil in alkaline media yields competitive values compared to those of 10% Pt/C and other Pt-based materials.
Furthermore, the electrocatalytic stability of the PtSe2/rGO foil was assessed before and after 1000 CVs, aiming to determine the overpotential at a current density of −100 mA cm−2 (η100). In addition to the PtSe2/rGO foil remaining stable until reaching the desired current (−100 mA cm−2), again, there is a slight shift of about 20 mV in the η100 after 1000 CVs, stabilizing at 591 mV, as shown in Fig. 3(d). However, worse PtSe2/rGO foil performance was verified with additional cycling, after 3000 CVs; the η10 increased to 280 mV and the η100 reached 725 mV, as shown in Fig. S5b (ESI†).
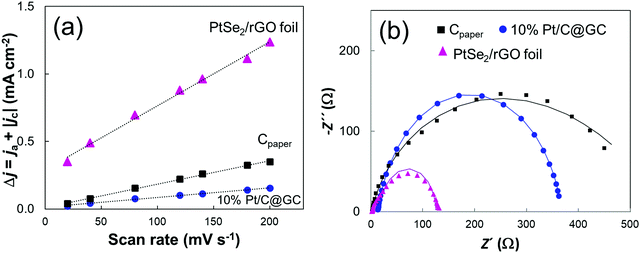
To further understand the role of PtSe2 in the improved HER performance of the PtSe2/rGO foil, the electrochemical double-layer capacitance (Cdl) and charge transfer resistance (Rct) were evaluated. The Cdl value is obtained by plotting the capacitive currents from the cyclic voltammetry (CV) curve as a function of the scan rates at a selected potential,40 in a non-faradaic region, as shown in Fig. 4(a) and respective CVs in Fig. S6(a–c), with further details given in the ESI.† The CV measurements were performed at various scan rates, from 20 to 200 mV s−1, with the calculated Cdl values of PtSe2/rGO at 1.1 V vs. RHE being much higher than those of the other materials (Fig. S6d, ESI†), thus implying a larger electrochemically accessible surface area and higher concentration of active sites.
EIS measurements were performed to estimate electrode kinetics, with lower Rct being associated with a faster reaction rate. The respective Nyquist plots of the materials are shown in Fig. 4(b), as well the fitted curves from which Rct values were determined. The Rct value of PtSe2/rGO of 105 Ω still was lower than that of Cpaper and 10% Pt/C@GC.
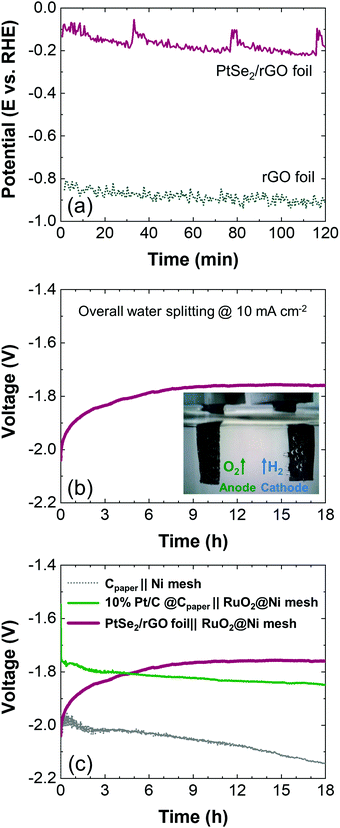
Next, the long-term stability of the PtSe2/rGO foil was investigated in the chronopotentiometric mode by maintaining a constant current density of −10 mA cm−2 for 120 min. The corresponding response is shown in Fig. 5(a) and it is compared with that of the rGO foil. The PtSe2/rGO foil performs better when compared to the rGO foil after 120 min as the first one requires an average potential of −162 mV vs. RHE while −883 mV vs. RHE is necessary for the latter in order to sustain −10 mA cm−2. This improvement in stability in the PtSe2/rGO foil suggests greater robustness of this electrode conferred by the selenization procedure.
To obtain a complete profile of the stability of the PtSe2/rGO foil, long-term tests were performed by chronopotentiometry for 18 h at a fixed current of 10 mA cm−2, as the current density of −10 mA cm−2 is accepted as the benchmark to compare electrocatalytic materials for the HER.41 Instead of a conventional three-electrode configuration, its performance was tested in a two-electrode cell, as it is the configuration used commercially. The PtSe2/rGO foil was used as the cathode and for the anode, RuO2, a benchmark catalyst for the OER,42 was deposited on Ni mesh (RuO2@Ni mesh) as described in the Experimental section. Fig. S7(a) (ESI†) shows the LSV curves towards the OER for Ni mesh and RuO2@Ni mesh samples, and the chronopotentiometric response of the RuO2@Ni mesh sample at a fixed current of −10 mA cm−2 over 18 h in a three-electrode configuration is presented in Fig. S7(b) (ESI†). In Fig. S4(a) (ESI†) (on the right) an optical image of the Ni mesh and its morphology is shown by SEM (Fig. S4f, ESI†) as well as the morphological and elemental characterization after modification with RuO2 (Fig. S4g–i, ESI†). A comparison with Cpaper after modification with 10% Pt/C as the cathode is also included, and its morphological and elemental characterization is shown in Fig. S4(c–e) (ESI†).
The corresponding chronopotentiometric curve in a two-electrode configuration is shown in Fig. 5(b), and the inset exhibits gas bubbling as evidence of electrolysis. The PtSe2/rGO foil reaches a plateau, being stable at a voltage of −1.76 V by the end of the stability test (18 h). By comparing with other alkaline electrolyzers in the same two-electrode configuration (Fig. 5c), the setup with the benchmarks Pt/C and RuO2 (10% Pt/C@Cpaper‖RuO2@Ni mesh) revealed a stable performance over time but requires a higher voltage (−1.85 V) to sustain the current. The stability curve of the configuration with the unmodified electrodes, i.e., Cpaper‖Ni mesh, shows major instability over time and decreased performance, and therefore, a higher voltage is required (−2.14 V).
Structural characterization after the HER
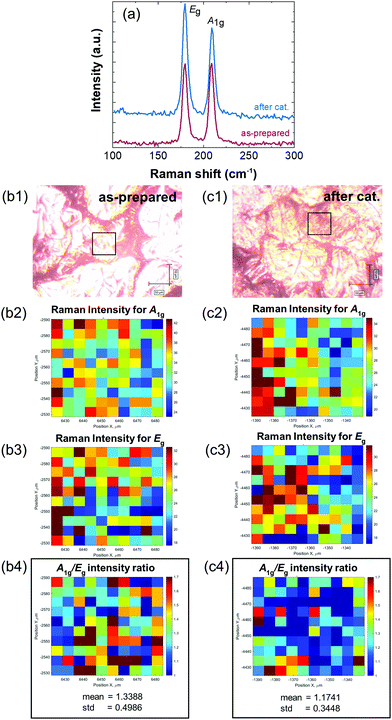
To assess the integrity of the PtSe2/rGO foil on the PtSe2 side after electrochemical tests, it was subject to characterization by Raman spectroscopy, SEM, ICP-OES, and XPS. Fig. 6(a) compares the Raman spectra of the PtSe2/rGO foil before and after long-term stability tests. Both Eg and A1g vibration modes can be observed with no Raman shift deviations, i.e., the two peaks are centered at 179.7 and 209.0 cm−1. A visual comparison between the as-prepared and the used PtSe2/rGO foil on the PtSe2 side is shown in Fig. S8(a and b) (ESI†), respectively. From Fig. S8(b) (ESI†) it can be noticed that there is some loss of the active material in restricted sections of the film, but more importantly, holes or punctures are absent, thus the overall integrity of the foil is sustained. This evidence that the PtSe2 foil maintains its integrity after electrolysis was confirmed by SEM after long-term stability tests, and is shown in Fig. S8(c1–c3) (ESI†). The micrographs of the resulting EDS elemental mapping (Fig. S8e, ESI†) show that the distribution along the surface of the Pt and Se elements is maintained.The PtSe2 side of the foil, both for as-prepared and used (after electrochemistry), was mapped using a Raman microscope in the area of 50 × 50 μm (Fig. 6b1–c4) to determine the spatial distribution of Raman A1g and Eg vibrational modes of PtSe2. Intensities of these vibrational modes are dependent on the number of layers in the exfoliated PtSe2,32 where A1g intensity denotes the out-of-plane stretching of Se atoms. Therefore, the A1g/Eg intensity ratio value increases with the number of layers in PtSe2. Thus, Raman mapping provides information about the degree of exfoliation within the explored area and the exfoliation spatial homogeneity. To access these quantities, individual Raman spectra were independently processed to smoothen the signal (Fig. S9a, ESI†), detect and remove the spectrum background (Fig. S9b, ESI†) and fit the smoothed and background-corrected spectrum with a set of Gaussian functions (Fig. S9c, ESI†). After this, the Gaussian parameters describing the A1g and Eg vibrational modes were used for the determination of the peak position and peak intensity. Both A1g and Eg intensity values are very homogeneous within the mapped area. However, the A1g intensity values, which denote the out-of-plane stretching of Se atoms, reach for the foil after the long-term stability test around 80% of A1g intensity values for the as-prepared foil, although the Eg intensity values remain almost the same for both foils. As a result of map combination, the A1g/Eg intensity ratio for the foil after the long-term stability test (Fig. 6c4) shows homogeneously distributed lower values than those of the as-prepared foil (Fig. 6b4).
The Cdl of the used PtSe2/rGO foil after catalysis was also monitored, as shown in Fig. S5(f) (ESI†) in which a decrease of ca. 9% was obtained. Thus, the foil subjected to prolonged HER did not have a significant loss in its electrochemical surface area.
By ICP-OES analysis a Pt content of about 0.087 wt% was measured, which is further evidence of the stability of the catalyst as the value measured before the long-term stability test was 0.088 wt%.
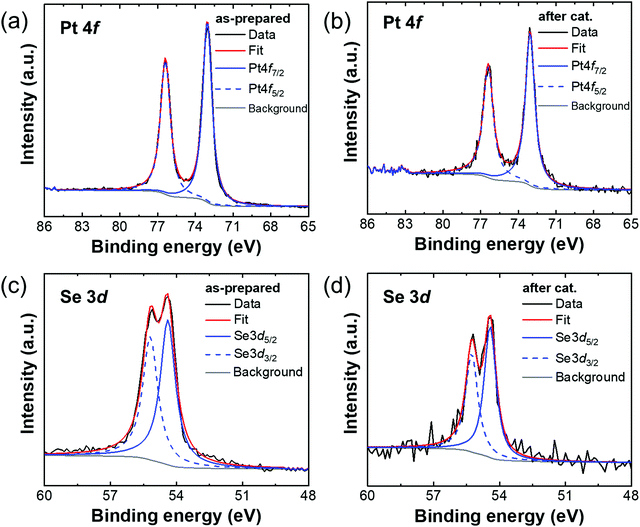
Hence, XPS spectra (Fig. 7) were measured to monitor the chemical stability of the PtSe2/rGO foil before and after the long-term stability test. Pt 4f (Fig. 7a and b) was deconvoluted with a pair of peaks for PtSe2 at a binding energy of 73 eV for the Pt 4f7/2 component and with a spin–orbit splitting of 3.33 eV. The core level Se 3d (see Fig. 7c and d) was also deconvoluted with pair of peaks representing selenide at binding energies of 54.4 and 55.2 eV for Se 3d5/2 and Se 3d3/2 components, respectively. Except for components assigned to PtSe2, no other bonding states can be observed for the as-prepared and used PtSe2/rGO foil, which further confirms its stability during the long-term experiment.
Conclusions
In this paper, we have reported the successful selenization of predeposited Pt on a freestanding rGO foil. The produced PtSe2/rGO foil behaves as an efficient and stable electrocatalyst for the hydrogen evolution reaction in alkaline media as it reached an η10 of 95 mV, having a rate-determining step related to the Volmer–Heyrovský mechanism. In the long-term stability test, a voltage of −1.76 V was sustained at the end of 18 h when tested in a two-electrode cell at 10 mA cm−2. After the long-term stability tests, no Raman shift deviations were noticed for the characteristic vibration modes of PtSe2, confirming its stability as a HER catalyst in an alkaline medium. Furthermore, ICP-OES results reveal that the amount of Pt is not significantly reduced in the used PtSe2/rGO foil, with the XPS also pointing to no major shifts in the binding energies and the oxidation state of the Pt and Se elements.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This project was supported by Czech Science Foundation (GACR No. 20-16124J). M. V. acknowledges the European Structural and Investment Funds, OP RDE-funded project 'CHEMFELLS IV' (No. CZ.02.2.69/0.0/0.0/20_079/0017899) for support. I. D. acknowledges the University of Chemistry and Technology Prague for specific research grant No. A2_FCHT_2021_053.References
- M. Zeng and Y. Li, J. Mater. Chem. A, 2015, 3, 14942–14962 RSC.
- Q. Lu, Y. Yu, Q. Ma, B. Chen and H. Zhang, Adv. Mater., 2016, 28, 1917–1933 CrossRef CAS PubMed.
- R. Gusmão, M. Veselý and Z. Sofer, ACS Catal., 2020, 10, 9634–9648 CrossRef.
- Z. Wang, H. H. Wu, Q. Li, F. Besenbacher, Y. Li, X. C. Zeng and M. Dong, Adv. Sci., 2020, 7, 1901382 CrossRef CAS PubMed.
- T. Qin, Z. Wang, Y. Wang, F. Besenbacher, M. Otyepka and M. Dong, Nano-Micro Lett., 2021, 13, 1–34 CrossRef PubMed.
- F. M. Oliveira, N. Antonatos, V. Mazánek, D. Sedmidubský, Z. Sofer and R. Gusmão, FlatChem, 2022, 32, 100334 CrossRef CAS.
- S. Manzeli, D. Ovchinnikov, D. Pasquier, O. V. Yazyev and A. Kis, Nat. Rev. Mater., 2017, 2, 17033 CrossRef CAS.
- C. (Rose) Zhu, D. Gao, J. Ding, D. Chao and J. Wang, Chem. Soc. Rev., 2018, 47, 4332–4356 RSC.
- H. Jin, C. Guo, X. Liu, J. Liu, A. Vasileff, Y. Jiao, Y. Zheng and S. Z. Qiao, Chem. Rev., 2018, 118, 6337–6408 CrossRef CAS PubMed.
- Z. Chen, X. Duan, W. Wei, S. Wang and B.-J. Ni, J. Mater. Chem. A, 2019, 7, 14971–15005 RSC.
- Z. Chen, D. Cummins, B. N. Reinecke, E. Clark, M. K. Sunkara and T. F. Jaramillo, Nano Lett., 2011, 11, 4168–4175 CrossRef CAS PubMed.
- H. Li, C. Tsai, A. L. Koh, L. Cai, A. W. Contryman, A. H. Fragapane, J. Zhao, H. S. Han, H. C. Manoharan, F. Abild-Pedersen, J. K. Nørskov and X. Zheng, Nat. Mater., 2016, 15, 48–53 CrossRef CAS PubMed.
- D. Voiry, H. Yamaguchi, J. Li, R. Silva, D. C. B. Alves, T. Fujita, M. Chen, T. Asefa, V. B. Shenoy, G. Eda and M. Chhowalla, Nat. Mater., 2013, 12, 850–855 CrossRef CAS PubMed.
- L. Cheng, W. Huang, Q. Gong, C. Liu, Z. Liu, Y. Li and H. Dai, Angew. Chem., Int. Ed., 2014, 53, 7860–7863 CrossRef CAS PubMed.
- S. Li, J. K. Lee, S. Zhou, M. Pasta and J. H. Warner, Chem. Mater., 2019, 31, 387–397 CrossRef CAS.
- E. Chen, W. Xu, J. Chen and J. H. Warner, Mater. Today Adv., 2020, 7, 100076 CrossRef.
- K. Lasek, J. Li, S. Kolekar, P. M. Coelho, L. Guo, M. Zhang, Z. Wang and M. Batzill, Surf. Sci. Rep., 2021, 76, 100523 CrossRef CAS.
- S. Supriya, N. Antonatos, J. Luxa, R. Gusmão and Z. Sofer, FlatChem, 2021, 29, 100280 CrossRef CAS.
- Y. Zhao, J. Qiao, Z. Yu, P. Yu, K. Xu, S. P. Lau, W. Zhou, Z. Liu, X. Wang, W. Ji and Y. Chai, Adv. Mater., 2017, 29, 1604230 CrossRef PubMed.
- Y. Ma, X. Shao, J. Li, B. Dong, Z. Hu, Q. Zhou, H. Xu, X. Zhao, H. Fang, X. Li, Z. Li, J. Wu, M. Zhao, S. J. Pennycook, C. H. Sow, C. Lee, Y. L. Zhong, J. Lu, M. Ding, K. Wang, Y. Li and J. Lu, ACS Appl. Mater. Interfaces, 2021, 13, 8518–8527 CrossRef CAS PubMed.
- Y. Wang, L. Li, W. Yao, S. Song, J. T. Sun, J. Pan, X. Ren, C. Li, E. Okunishi, Y.-Q. Wang, E. Wang, Y. Shao, Y. Y. Zhang, H. Yang, E. F. Schwier, H. Iwasawa, K. Shimada, M. Taniguchi, Z. Cheng, S. Zhou, S. Du, S. J. Pennycook, S. T. Pantelides and H.-J. Gao, Nano Lett., 2015, 15, 4013–4018 CrossRef CAS PubMed.
- R. A. B. Villaos, C. P. Crisostomo, Z.-Q. Huang, S.-M. Huang, A. A. B. Padama, M. A. Albao, H. Lin and F.-C. Chuang, npj 2D Mater. Appl., 2019, 3, 2 CrossRef.
- J. Shi, Y. Huan, M. Hong, R. Xu, P. Yang, Z. Zhang, X. Zou and Y. Zhang, ACS Nano, 2019, 13, 8442–8451 CrossRef CAS PubMed.
- S. Lin, Y. Liu, Z. Hu, W. Lu, C. H. Mak, L. Zeng, J. Zhao, Y. Li, F. Yan, Y. H. Tsang, X. Zhang and S. P. Lau, Nano Energy, 2017, 42, 26–33 CrossRef CAS.
- D. Hu, T. Zhao, X. Ping, H. Zheng, L. Xing, X. Liu, J. Zheng, L. Sun, L. Gu, C. Tao, D. Wang and L. Jiao, Angew. Chem., Int. Ed., 2019, 58, 6977–6981 CrossRef CAS PubMed.
- L.-J. Ma and H. Shen, Appl. Surf. Sci., 2021, 545, 149013 CrossRef CAS.
- A. Ciarrocchi, A. Avsar, D. Ovchinnikov and A. Kis, Nat. Commun., 2018, 9, 919 CrossRef PubMed.
- B. M. Szydłowska, O. Hartwig, B. Tywoniuk, T. Hartman, T. Stimpel-Lindner, Z. Sofer, N. McEvoy, G. S. Duesberg and C. Backes, 2D Mater., 2020, 7, 45027 CrossRef.
- A. Boudenne, L. Ibos, M. Fois, J. C. Majesté and E. Géhin, Composites, Part A, 2005, 36, 1545–1554 CrossRef.
- Z. Wang, Q. Li, F. Besenbacher and M. Dong, Adv. Mater., 2016, 28, 10224–10229 CrossRef CAS PubMed.
- H. Xu, H. Zhang, Y. Liu, S. Zhang, Y. Sun, Z. Guo, Y. Sheng, X. Wang, C. Luo, X. Wu, J. Wang, W. Hu, Z. Xu, Q. Sun, P. Zhou, J. Shi, Z. Sun, D. W. Zhang and W. Bao, Adv. Funct. Mater., 2019, 29, 1805614 CrossRef.
- M. Yan, E. Wang, X. Zhou, G. Zhang, H. Zhang, K. Zhang, W. Yao, N. Lu, S. Yang, S. Wu, T. Yoshikawa, K. Miyamoto, T. Okuda, Y. Wu, P. Yu, W. Duan and S. Zhou, 2D Mater., 2017, 4, 45015 CrossRef.
- J. D. Benck, B. A. Pinaud, Y. Gorlin and T. F. Jaramillo, PLoS One, 2014, 9, e107942 CrossRef PubMed.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- D. Bouša, K. Friess, K. Pilnáček, O. Vopička, M. Lanč, K. Fónod, M. Pumera, D. Sedmidubský, J. Luxa and Z. Sofer, Chem. – Eur. J., 2017, 23, 11416–11422 CrossRef PubMed.
- E. Chen, W. Xu, J. Chen and J. H. Warner, Mater. Today Adv., 2020, 7, 100076 CrossRef.
- M. O’Brien, N. McEvoy, C. Motta, J.-Y. Zheng, N. C. Berner, J. Kotakoski, K. Elibol, T. J. Pennycook, J. C. Meyer, C. Yim, M. Abid, T. Hallam, J. F. Donegan, S. Sanvito and G. S. Duesberg, 2D Mater., 2016, 3, 21004 CrossRef.
- C. Yim, K. Lee, N. McEvoy, M. O’Brien, S. Riazimehr, N. C. Berner, C. P. Cullen, J. Kotakoski, J. C. Meyer, M. C. Lemme and G. S. Duesberg, ACS Nano, 2016, 10, 9550–9558 CrossRef CAS PubMed.
- P. Kuang, Y. Wang, B. Zhu, F. Xia, C. W. Tung, J. Wu, H. M. Chen and J. Yu, Adv. Mater., 2021, 33, 2008599 CrossRef CAS PubMed.
- C. M. A. Brett and A. M. O. Brett, Electrochemistry: principles, methods, and applications, Oxford Science Publications, Oxford, 1993 Search PubMed.
- S. Anantharaj, S. R. Ede, K. Karthick, S. Sam Sankar, K. Sangeetha, P. E. Karthik and S. Kundu, Energy Environ. Sci., 2018, 11, 744–771 RSC.
- J. Song, C. Wei, Z. F. Huang, C. Liu, L. Zeng, X. Wang and Z. J. Xu, Chem. Soc. Rev., 2020, 49, 2196–2214 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00190j |
| This journal is © The Royal Society of Chemistry 2022 |