DOI:
10.1039/D2MA00345G
(Paper)
Mater. Adv., 2022,
3, 7865-7871
Synthesis and characterization of all-inorganic (CsPbBr3) perovskite single crystals†
Received
25th March 2022
, Accepted 26th July 2022
First published on 2nd August 2022
Abstract
This work investigates the synthesis of Cesium (Cs) based all-inorganic (CsPbBr3) perovskite single crystals (PSCs) at low temperatures (45 °C) for application in photosensitive devices. The Cs-based PSCs are more environmentally stable in comparison with PSCs based on formamidinium and methylammonium. The crystals are grown using an inverse temperature crystallization method. The structural characterization was conducted using an X-ray diffractometer and confirms that the crystal is highly crystalline and it also shows that it belongs to the {110} family of planes. The optical analysis of the Cs-based single crystal was conducted using photoluminescence spectroscopy. The crystal has a bandgap of 2.18 eV as calculated by using the Tauc formula. Furthermore, a remarkable photocurrent analysis of the responsivity and detectivity parameters was conducted using a visible light laser (532 nm) and revealed values of ∼1 A W−1 and 3.7 × 1011 Jones, respectively. Thus, the all-inorganic PSC, CsPbBr3, will be of benefit to optoelectronic device applications.
1 Introduction
Solution-processed metal halide perovskite materials have gained research interest around the globe after delivering promising results in perovskite solar cells (PSCs). PSCs have reached a power conversion efficiency of up to 25.8%.1 Recently, single crystals based on perovskite materials have been proposed for use in optoelectronic devices. In the field of all-inorganic perovskite materials, the Cs-based perovskites always receive the most interest due to their higher ambient environmental stability.2 Compared with the MA and FA cation, the degradation rate is lower in the ambient environment for Cs-based PSCs devices.3–8 Formamidinium (FA) and methylammonium (MA) contain sensitive hydrocarbons that can easily be affected by humidity and oxygen-containing environments resulting in reduced ambient stability.2,9 Replacing organic–inorganic (MA/FA) hybrid perovskites with all-inorganic Cs-based perovskites gives materials with superior mechanical properties and that are more moisture resistant.6 The benefits of all-inorganic perovskite materials include higher carrier mobilities, longer diffusion lengths, low trap densities, and the direct bandgap of the materials.10–12 These materials also show outstanding optical characteristics, for example, high quantum yields, high absorption coefficients, high luminous efficiencies, and easily tunable luminous wavelength.13,14 These enormous potential benefits have astonishing results in the fields of high luminescence quantum dots (QD), stable solar cells, photodetectors, lasers, and high-energy photon detectors.15–22 The growth of all-inorganic perovskite single crystals is significant for the exploration of their potential in single-crystalline-based optoelectronics devices.23 Moreover, all-inorganic perovskite materials have reached new heights in solar cells and nanocrystal quantum dot applications.18,24–29 CsPbBr3 is a widely applied perovskite compound and is an all-inorganic perovskite material for use in solar cells and single crystal-based optoelectronic devices.30–32 Detectors based on CsPbBr3 are used in an extensive range of applications, such as high-energy radiation detection, medical diagnostics, environmental monitoring, security examinations, space science, etc.4,33–37 High-energy photon detection requires a material with a large atomic number, to block the rays, a bandgap with a range between 1.5–3.0 eV, and high charge carrier mobilities.38–42 In the case of CsPbBr3, it is a semiconductor with a direct bandgap of 2.26 eV and has a high atomic number, thus implying that it has a high absorption radiation coefficient.43,44 The trap densities in polycrystalline perovskite CsPbBr3 are slightly higher than in single crystals.2,45,46 Growth of perovskite single-crystals has been developed using different growth processes. In the past, the Bridgman method was employed to grow CsPbBr3 single crystals and involves reacting CsBr and PbBr2 in equimolar amounts at >600 °C, which is unsuitable for optoelectronics and cost-effective single-crystal synthesis.47–52 Researchers have also applied the vertical Bridgman method for the synthesis of CsPbBr3 large single crystals but, unfortunately, it also requires high temperature processing.53 Thus, low-temperature solution-growth is an attractive method for perovskite single-crystal synthesis as this would be a low-cost method. Recently, the application of CsPbBr3 single crystals as photodetectors has been of interest and it shows promising results in replacing conventional inorganic photodetectors.54–56 Ding et al.57 reported that CsPbBr3 single crystals could be prepared by the antisolvent method, and their steady and transient performances delivered the highest responsivity of 0.028 A W−1. Zhang et al.58 prepared large-size CsPbBr3 single crystals with low trap densities using a modified Bridgman method. The photoresponse measurements showed a high responsivity of 5.83 A W−1 when irradiated by a 532 nm laser. The synthesis of CsPbBr3 single crystals has also been reported via a low-temperature processing method.30,59 Ding et al.57 reported the growth of CsPbBr3 single crystals from saturated solution by dissolving CsBr and PbBr2 in DMSO with continuous stirring at room-temperature and the highest responsivity was 0.028 A W−1. Here we present the growth mechanism of all-inorganic perovskite single crystals using inverse temperature crystallization (ITC) at low temperature to open new avenues for the application of PSCs in optoelectronics devices alongside the benefit of synthesizing cost-effective single crystals. The ITC method grown CsPbBr3 single crystal-based photodetector has been reported in the literature, nevertheless, it has shown low responsivity and detectivity.6,30,60,61
In the present work, we have demonstrated the synthesis of CsPbBr3 single crystals using the ITC method and characterized this single crystal using UV-vis spectroscopy, photoluminescence spectroscopy, X-ray diffraction, and scanning electron microscopy (SEM).
2 Materials
The salts PbBr2 (lead bromide) and CsBr (cesium bromide), as well as the dimethylsulfoxide (DMSO) solvent, were purchased from Sigma-Aldrich. All chemicals were used without any further purification.
3 Growth of CsPbBr3 perovskite single crystal (PSCs)
In general, it is a three-step process, as shown in Fig. 1(a). The first step is preparation of the precursor solution and waiting for growth of the small seed crystals. In the second step, the best small crystal is selected. After that, in the final step, this small seed crystal is used to grow a much larger crystal by putting it into a new precursor solution. Fig. 1(b) shows a schematic illustration of the single-crystal growth process. First, we prepared an equimolar solution of CsBr and PbBr2 in DMSO, stirring at 45 °C until we obtained a clear solution. Next, we filtered the precursor solution over a PTFE filter and stored it in a new vial. We placed this vial without any disturbance in the silicon oil bath to grow the small single crystals. In the next step, we picked the best shaped small seed crystal, made a new precursor solution, and placed this seed at the bottom of the vial. Fig. 1(c and d) show the perovskite structure and the final CsPbBr3 perovskite single crystal.
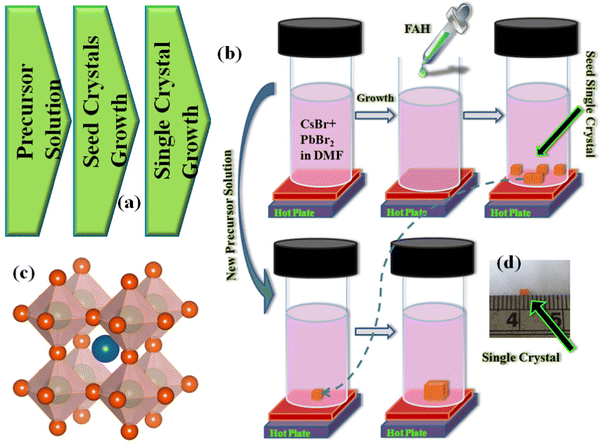 |
| | Fig. 1 (a) A block diagram of the single-crystal synthesis process, (b) step-by-step representation of the CsPbBr3 PSCs synthesis, (c) perovskite structure, and (d) actual image of a CsPbBr3 PSC. | |
4 Characterization and measurements
The characterization was performed under ambient environmental conditions. In the structural characterization, a powder X-ray diffractometer (Rigaku Ultima IV Diffractometer: XRD) was used to analyze the CsPbBr3 single crystal. The pattern was recorded at a speed of 8° min−1 and a step size of 0.02° using a copper Kα target. In the optical characterization, UV-vis (PerkinElmer Lambda 1050) and photoluminescence (Edinburgh FLS-980 d2d2) spectrometers were used for the absorption and photoluminescence studies, respectively.
5 Discussion
5.1 Photo-physical properties of the CsPbBr3 perovskite single crystals
The photoluminescence and absorption properties of the synthesized PSCs are shown in Fig. 2(a and b), respectively. The CsPbBr3 single crystals show an emission peak at 565 nm, which is close to the value reported by Xu et al.37 Also, the calculated band gap of the CsPbBr3 single crystal is ∼2.18 eV, which was calculated by using the Tauc relationship as shown in the inset of Fig. 2(b).
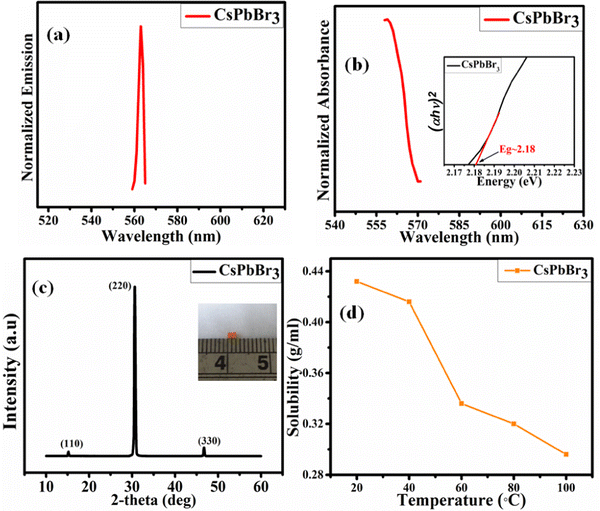 |
| | Fig. 2 (a) The CsPbBr3 PSCs photoemission, and (b) absorption spectra (inset: Tauc plot). (c) The CsPbBr3 crystal XRD pattern (inset: crystal image), and (d) the CsPbBr3 crystal solubility test results. | |
5.2 Structural characterization of the CsPbBr3 perovskite single crystals
Fig. 2(c) shows the X-ray diffraction pattern of the CsPbBr3 perovskite single crystal. The XRD peaks appear at angles with 2θ values of ∼15.2°, ∼30.6°, and ∼47.1°, which denote the (110), (220), and (330) planes.62 The planes show that the sample is crystalline in nature. This was also confirmed by the XRD analysis which shows a Cs-based single crystal that elongated along the {110} unit direction.
5.3 Solubility tests for the CsPbBr3 perovskite single crystals
A solubility test was performed on the all-inorganic PSCs in their respective solution. First, a mortar and pestle were used to grind the CsPbBr3 bulk single crystal. A small amount of CsPbBr3 powder (0.01 g) was mixed in DMSO with continuous stirring and heated until a clear solution was obtained. The mass at the solution saturation point was marked as the maximum weight to be added to the vials. The exact process was then followed over the 20–100 °C temperature range within the same precursor solvent. For CsPbBr3, ∼40 °C is the maximum solubility temperature for crystallization, as shown in Fig. 2(d).
5.4 SEM and energy-dispersive X-ray analysis of the CsPbBr3 PSCs
SEM was used to characterize the morphology of the synthesized crystal and the image is shown in Fig. S3 (ESI†). It shows that the single crystal surface was not smooth over the entire facet. Potential variation in the growth mechanism hindrance ions from attaching to other nearby ions to prevent the growth of a smooth shape. Thus, steps and flat surfaces are seen at the boundaries of the growing truncated single-crystal shapes. The elemental analysis using energy-dispersive X-ray spectroscopy is shown in Fig. S1 (ESI†), and the Pb and Cs content has been estimated to be a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in CsPbBr3.
1 ratio in CsPbBr3.
5.5 CsPbBr3 single-crystal photocurrent analysis
We studied the behavior of the grown PSCs under the illumination of different wavelengths of light. Ag (silver) paste was used for making the electrical contacts, as shown in Fig. 3(a). We also measured the I–V curve under irradiation of two different laser light wavelengths. It is observed that the photoconductivity of the CsPbBr3 perovskite single-crystal gradually increases with irradiation power. It increases up to a few hundred nA compared to the experiments conducted in the dark, which showed single digit increases, as shown in Fig. 3(b).
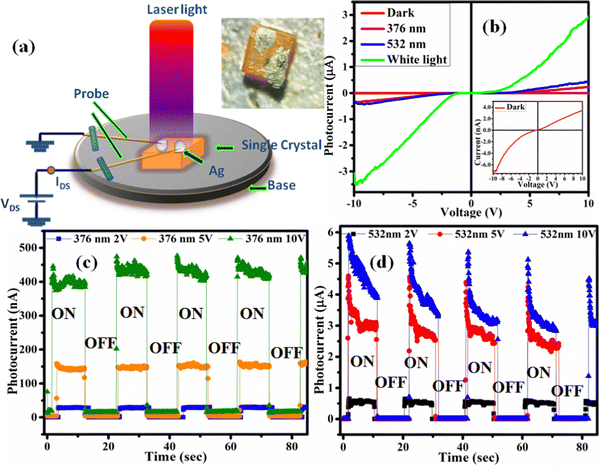 |
| | Fig. 3 (a) Illustration of the setup of the probe station (inset: cesium crystal image), and (b) the CsPbBr3 single-crystal I–V curves. The rise and decay of the photocurrent of CsPbBr3 single crystal, under (c) 376 nm, and (d) 532 nm laser illumination. | |
5.6 Time-dependent photocurrent analysis for the CsPbBr3 PSCs
The photocurrent analysis was conducted by illuminating the PSCs using the 376 nm and 532 nm wavelengths of two different types of laser. Fig. 3(c and d) show the time-dependent photocurrent of the CsPbBr3 PSCs under three (2, 5, and 10 V) different applied voltages. After the incident light hit the single crystal, the photocurrent increased. A photocurrent of approximately 5 μA was achieved using the 532 nm laser (32 mW cm−2) at a bias voltage of 10 V and the resultant curves are shown in Fig. 3(d). The photocurrent recovered rapidly after exposure to light illumination and it offers excellent repeatability, which promises photocurrent stability.58 Fig. S2 (ESI†) shows the extended response under illumination with 376 nm light. The device performance was analyzed by calculating the responsivity, D* (detectivity), rise time, and decay time parameters; the equations used for this are shown in the ESI.† The high detectivity and responsivity values found in response to visible light (532 nm) are 3.73 × 1011 Jones and ∼1 A W−1, respectively, as given in Table 1. Fig. 4(a) depicts the photocurrent response to both the lasers at 5 V. The CsPbBr3 PSCs show a higher response to the 532 nm laser than to the 376 nm laser. As shown in Fig. 4(b), studies on the photocurrent output at different voltages have also been conducted for both lasers and both showed higher photocurrent at 10 V. Furthermore, the photocurrent vs responsivity graph, shown in Fig. 4(d), indicates a high responsivity value under illumination at 532 nm. Table 2 contains a summary of the transient photocurrent response of the CsPbBr3 single crystal at different laser wavelengths and applied voltages. The responsivity of the CsPbBr3 PSCs at different voltages and in response to both wavelength lasers is plotted in Fig. 4(e). It shows that the responsivity of the CsPbBr3 PSCs increases as the voltage also increase. Fig. 4(c and f) show the measured and fitted data for the rise time (55 ms) and decay time (36 ms), respectively. Compared with previous reports on the rise and decay time of CsPbBr3 PSCs, both of the values obtained in our study are much faster. The short response times suggest that the material has low defect density and lower grain boundaries.
Table 1 Perovskite-based detectors and their photocurrent parameters
| No. |
Perovskite lead-based detector |
Responsivity (A W−1) |
Detectivity (Jones) |
Rise time |
Fall time |
Ref. |
| (i) |
CsPbBr3 |
0.02 |
1.7 × 1011 |
|
|
6
|
| (ii) |
MAPbI2.5Br0.5 |
∼0.1 |
∼4 × 1012 |
<10 μs |
<10 μs |
63
|
| (iii) |
MAPbIxBr3−x |
∼0.01 |
— |
2.3 s |
2.7 s |
64
|
| (iv) |
MAPbCl3 |
∼0.05 |
1.2 × 1010 |
24 ms |
62 ms |
65
|
| (v) |
CsPbBr3 |
0.028 |
1.8 × 1011 |
<100 ms |
<100 ms |
57
|
| (vi) |
CsPbBr3 |
∼1.0 |
3.7 × 1011 |
55 ms |
36 ms |
This work |
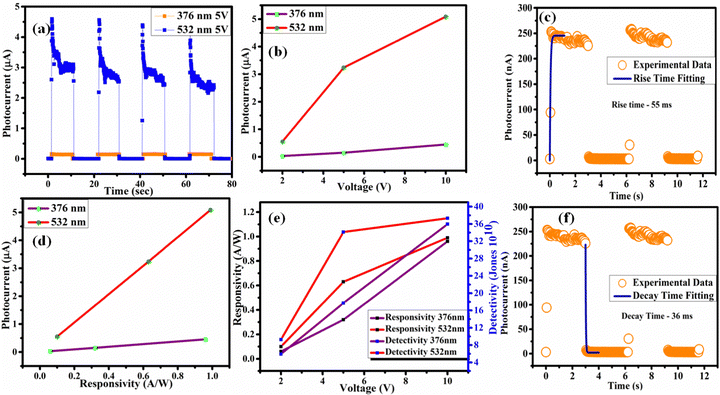 |
| | Fig. 4 Photocurrent analysis in response to illumination with 376 and 532 nm wavelength lasers (a) fixed at 5 V, and (b) at different voltages. (c) Rise time experimental data and fitting. (d) Photocurrent versus responsivity and (e) responsivity versus voltage. (f) Fall time experimental data and fitting. | |
Table 2 Transient photocurrent response for CsPbBr3 PSCs
| Laser wavelength (nm) |
Bias voltages (V) |
Responsivity (A W−1) |
Detectivity (Jones) |
| 376 |
10 |
0.96 |
3.59 × 1011 |
| 5 |
0.32 |
1.77 × 1011 |
| 2 |
0.06 |
5.92 × 1010 |
| 532 |
10 |
0.99 |
3.73 × 1011 |
| 5 |
0.63 |
3.41 × 1011 |
| 2 |
0.10 |
9.27 × 1010 |
6 Conclusions
All-inorganic CsPbBr3 perovskite single-crystals (PSCs) have the ability to work as photodetectors, like high energy photodetectors for X-rays, Gamma rays, Beta rays, alpha rays, etc. This study describes the growth of high quality cesium-based all-inorganic perovskite single crystals under favorable low temperature and solution-processable conditions. A study of the crystallinity as well as the optical properties of the all-inorganic PSCs has been conducted. The XRD peaks at 2θ values of ∼15.2°, ∼30.6°, and ∼46.7° represent the (110), (220), and (330), planes, respectively. The existence of these planes confirms the highly crystalline nature of the material. Additionally, the XRD pattern confirmed that the crystal belongs to the {110} family of planes and showed that it was a single crystal. The peak photocurrent result for the PSCs was 5 μA reached under a 10 V applied bias voltage. The calculated detectivity and responsivity under illumination with a 532 nm laser were 3.71 × 1011 Jones and 1 A W−1, respectively. This study has shown significant improvement in the detectivity and responsivity values of an all-inorganic perovskite-based single-crystal.
Author contributions
Ramashanker Gupta: investigation, methodology, conceptualization, writing – original draft; Ram Datt: writing – review & editing, formal analysis; Swapnil Barthwal: methodology; Harshit Sharma: resources; Animesh Pandey: resources; Ridip Deka: resources; Pranjit Sarkar: resources; Sudhir Husale: resources; Ritu Srivastava: supervision; Vinay Gupta: supervision; Sandeep Arya: writing – review & editing; Sandeep Pathak: conceptualization, visualization, project administration.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The authors would like to thank EPSRC POLARIS HOUSE (EPSRCPH) for financial support under the SUNRISE (Strategic University Network to Revolutionise Indian Solar Energy) grant Number (RP03541). The author (R. G.) also thanks the Council of Scientific and Industrial Research (CSIR) New Delhi, India, for Research Fellowship (31/001(0451)/2016-EMR-I).
References
- H. Min, D. Y. Lee, J. Kim, G. Kim, K. S. Lee, J. Kim, M. J. Paik, Y. K. Kim, K. S. Kim, M. G. Kim, T. J. Shin and S. Il Seok, Nature, 2021, 598, 444–450 CrossRef CAS PubMed.
- L. Z. Jiaoxian Yu, A. G. Liu, C. Chen, Y. Li, M. Xu, T. Wang and G. Zhao, J. Mater. Chem. C, 2020, 8, 6326 RSC.
- J. Leroudier, J. Zaccaro, J. Debray, P. Segonds and A. Ibanez, Cryst. Growth Des., 2013, 13, 3613–3620 CrossRef CAS.
- Y. Feng, L. Pan, H. Wei, Y. Liu, Z. Ni, J. Zhao, P. N. Rudd, L. R. Cao and J. Huang, J. Mater. Chem. C, 2020, 8, 11360–11368 RSC.
- H. Zhao, Y. Fu, Z. Li, S. Yang, B. Xu, X. Liu, J. Xu, S. Liu and J. Yao, J. Mater. Chem. A, 2021, 9, 4922–4932 RSC.
- J. Ding, S. Du, Z. Zuo, Y. Zhao, H. Cui and X. Zhan, J. Phys. Chem. C, 2017, 121, 4917–4923 CrossRef CAS.
- Y. Zhou and Y. Zhao, Energy Environ. Sci., 2019, 12, 1495–1511 RSC.
- Q. Tai, K. Tang and F. Yan, Energy Environ. Sci., 2019, 12, 2375–2405 RSC.
- X. Miao, T. Qiu, S. Zhang, H. Ma, Y. Hu, F. Bai and Z. Wu, J. Mater. Chem. C, 2017, 5, 4931–4939 RSC.
- D. Shi, V. Adinolfi, R. Comin, M. Yuan, E. Alarousu, A. Buin, Y. Chen, S. Hoogland, A. Rothenberger, K. Katsiev, Y. Losovyj, X. Zhang, P. A. Dowben, O. F. Mohammed, E. H. Sargent and O. M. Bakr, Science, 2015, 347, 519–522 CrossRef CAS PubMed.
- Y. Fang, Q. Dong, Y. Shao, Y. Yuan and J. Huang, Nat. Photonics, 2015, 9, 679–686 CrossRef CAS.
- M. I. Saidaminov, A. L. Abdelhady, B. Murali, E. Alarousu, V. M. Burlakov, W. Peng, I. Dursun, L. Wang, Y. He, G. MacUlan, A. Goriely, T. Wu, O. F. Mohammed and O. M. Bakr, Nat. Commun., 2015, 6, 1–6 Search PubMed.
- J. H. Kim, P. W. Liang, S. T. Williams, N. Cho, C. C. Chueh, M. S. Glaz, D. S. Ginger and A. K. Y. Jen, Adv. Mater., 2015, 27, 695–701 CrossRef CAS PubMed.
- G. Tong, L. K. Ono and Y. Qi, Energy Technol., 2020, 1900961, 1–17 Search PubMed.
- Y. Wei and J. Lin, Chem. Soc. Rev., 2019, 48, 310 RSC.
- X. Sheng, G. Chen, C. Wang, W. Wang, J. Hui, Q. Zhang, K. Yu, W. Wei, M. Yi, M. Zhang, Y. Deng, P. Wang, X. Xu, Z. Dai, J. Bao and X. Wang, Adv. Funct. Mater., 2018, 28, 1–9 Search PubMed.
- Q. Zhang and Y. Yin, ACS Cent. Sci., 2018, 4, 668–679 CrossRef CAS PubMed.
- L. Gao and Q. Yan, Sol. RRL, 2019, 1900210, 1–12 Search PubMed.
- X. Cheng, S. Yang, B. Cao, X. Tao and Z. Chen, Adv. Funct. Mater., 2020, 1905021, 1–20 Search PubMed.
- D. Ghosh, M. Y. Ali, D. K. Chaudhary and S. Bhattacharyya, Sol. Energy Mater. Sol. Cells, 2018, 185, 28–35 CrossRef CAS.
- W. Liu, Z. Jiang, W. Fan, Q. Zhang and X. W. Sun, J. Phys. Chem. Lett., 2021, 12, 10197–10203 CrossRef CAS PubMed.
- Y. Hua, F. Cui, P. Zhang, G. Zhang, Q. Zhang and X. Tao, Z. Anorg. Allg. Chem., 2022, e202200025, 1–7 Search PubMed.
- S. Arya, P. Mahajan, R. Gupta, R. Srivastava, N. Kumar Tailor, S. Satapathi, R. R. Sumathi, R. Datt and V. Gupta, Prog. Solid State Chem., 2020, 60, 100286 CrossRef CAS.
- M. Zhang, Z. Zheng, Q. Fu, Z. Chen, J. He, S. Zhang, L. Yan, Y. Hu and W. Luo, CrystEngComm, 2017, 19, 6797–6803 RSC.
- Y. Fu, H. Zhu, C. C. Stoumpos, Q. Ding, J. Wang, M. G. Kanatzidis, X. Zhu and S. Jin, ACS Nano, 2016, 10, 7963–7972 CrossRef CAS PubMed.
- J. Li, H. Zhang, S. Wang, D. Long, M. Li, D. Wang and T. Zhang, Materials, 2018, 11, 1–9 CrossRef PubMed.
- Q. Fu, X. Tang, B. Huang, T. Hu, L. Tan, L. Chen and Y. Chen, Adv. Sci., 2018, 5, 1700387 CrossRef PubMed.
- S. W. Eaton, M. Lai, N. A. Gibson, A. B. Wong, L. Dou, J. Ma, L. W. Wang, S. R. Leone and P. Yang, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 1993–1998 CrossRef CAS PubMed.
- S. Wei, Y. Yang, X. Kang, L. Wang, L. Huang and D. Pan, Chem. Commun., 2016, 52, 7265–7268 RSC.
- Y. Rakita, N. Kedem, S. Gupta, A. Sadhanala, V. Kalchenko, M. L. Böhm, M. Kulbak, R. H. Friend, D. Cahen and G. Hodes, Cryst. Growth Des., 2016, 16, 5717–5725 CrossRef CAS.
- J. Song, Q. Cui, J. Li, J. Xu, Y. Wang, L. Xu, J. Xue, Y. Dong, T. Tian, H. Sun and H. Zeng, Adv. Opt. Mater., 2017, 5, 1–8 Search PubMed.
- Y. Gao, Y. Wu, H. Lu, C. Chen, Y. Liu, X. Bai, L. Yang, W. W. Yu, Q. Dai and Y. Zhang, Nano Energy, 2019, 59, 517–526 CrossRef CAS.
- J. Peng, C. Q. Xia, Y. Xu, R. Li, L. Cui, J. K. Clegg, L. M. Herz, M. B. Johnston and Q. Lin, Nat. Commun., 2021, 12, 1531 CrossRef CAS PubMed.
- Z. Fan, J. Liu, W. Zuo, G. Liu, X. He, K. Luo, Q. Ye and C. Liao, Phys. Status Solidi, 2020, 217, 2000104 CrossRef CAS.
- L. Pan, Y. Feng, P. Kandlakunta, J. Huang and L. R. Cao, IEEE Trans. Nucl. Sci., 2020, 67, 443–449 CAS.
- Y. Zhong, K. Liao, W. Du, J. Zhu, Q. Shang, F. Zhou, X. Wu, X. Sui, J. Shi, S. Yue, Q. Wang, Y. Zhang, Q. Zhang, X. Hu and X. Liu, ACS Nano, 2020, 14, 15605–15615 CrossRef PubMed.
- Q. Xu, X. Wang, H. Zhang, W. Shao, J. Nie, Y. Guo, J. Wang and X. Ouyang, ACS Appl. Electron. Mater., 2020, 2, 879–884 CrossRef CAS.
- Y. Zhou, J. Chen, O. M. Bakr and O. F. Mohammed, ACS Energy Lett., 2021, 6, 739–768 CrossRef CAS.
- H. Wu, Y. Ge, G. Niu and J. Tang, Matter, 2021, 4, 144–163 CrossRef CAS.
- G. J. Matt, I. Levchuk, J. Knüttel, J. Dallmann, A. Osvet, M. Sytnyk, X. Tang, J. Elia, R. Hock, W. Heiss and C. J. Brabec, Adv. Mater. Interfaces, 2020, 7, 1901575 CrossRef CAS.
- L. Pan, Y. Feng, J. Huang and L. R. Cao, IEEE Trans. Nucl. Sci., 2020, 67, 2255–2262 CAS.
- D. N. Dirin, I. Cherniukh, S. Yakunin, Y. Shynkarenko and M. V. Kovalenko, Chem. Mater., 2016, 28, 8470–8474 CrossRef CAS PubMed.
- M. Zhang, Z. Zheng, Q. Fu, Z. Chen, J. He, S. Zhang, C. Chen and W. Luo, J. Cryst. Growth, 2018, 484, 37–42 CrossRef CAS.
- C. A. López, C. Abia, M. C. Alvarez-Galván, B. K. Hong, M. V. Martínez-Huerta, F. Serrano-Sánchez, F. Carrascoso, A. Castellanos-Gómez, M. T. Fernández-Dĺaz and J. A. Alonso, ACS Omega, 2020, 5, 5931–5938 CrossRef PubMed.
- D. Liu, L. Wu, C. Li, S. Ren, J. Zhang, W. Li and L. Feng, ACS Appl. Mater. Interfaces, 2015, 7, 16330–16337 CrossRef CAS PubMed.
- Q. Han, S. H. Bae, P. Sun, Y. T. Hsieh, Y. Yang, Y. S. Rim, H. Zhao, Q. Chen, W. Shi, G. Li and Y. Yeng, Adv. Mater., 2016, 28, 2253–2258 CrossRef CAS PubMed.
- C. C. Stoumpos, C. D. Malliakas, J. A. Peters, Z. Liu, M. Sebastian, J. Im, T. C. Chasapis, A. C. Wibowo, D. Y. Chung, A. J. Freeman, B. W. Wessels and M. G. Kanatzidis, Cryst. Growth Des., 2013, 13, 2722–2727 CrossRef CAS.
- M. Sebastian, J. A. Peters, C. C. Stoumpos, J. Im, S. S. Kostina, Z. Liu, M. G. Kanatzidis, A. J. Freeman and B. W. Wessels, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 235210 CrossRef.
- D. J. Clark, C. C. Stoumpos, F. O. Saouma, M. G. Kanatzidis and J. I. Jang, Phys. Rev. B: Condens. Matter Mater. Phys., 2016, 93, 195202 CrossRef.
- Y. He, Z. Liu, K. M. McCall, W. Lin, D. Y. Chung, B. W. Wessels and M. G. Kanatzidis, Nucl. Instrum. Methods Phys. Res., Sect. A, 2019, 922, 217–221 CrossRef CAS.
- X. Chen, Y. Wang, J. Song, X. Li, J. Xu, H. Zeng and H. Sun, J. Phys. Chem. C, 2019, 123, 10564–10570 CrossRef CAS.
- D. Kim, H. Ryu, S. Y. Lim, K. M. McCall, J. Park, S. Kim, T. J. Kim, J. Kim, Y. S. Kim, M. G. Kanatzidis, H. Cheong and J. I. Jang, Chem. Mater., 2021, 33, 7185–7193 CrossRef CAS.
- P. Zhang, Q. Sun, Y. Xu, X. Li, L. Liu, G. Zhang and X. Tao, Cryst. Growth Des., 2020, 20, 2424–2431 CrossRef CAS.
- J.-H. Cha, J. H. Han, W. Yin, C. Park, Y. Park, T. K. Ahn, J. H. Cho and D.-Y. Jung, J. Phys. Chem. Lett., 2017, 8, 565–570 CrossRef CAS PubMed.
- J. Liu, S. Cristoloveanu and J. Wan, Phys. Status Solidi, 2021, 218, 2000751 CrossRef CAS.
-
Y. Ren and V. Van, in 2019 24th OptoElectronics and Communications Conference (OECC) and 2019 International Conference on Photonics in Switching and Computing (PSC), 2019, pp. 1–3 Search PubMed.
- J. Ding, S. Du, Z. Zuo, Y. Zhao, H. Cui and X. Zhan, J. Phys. Chem. C, 2017, 121, 4917–4923 CrossRef CAS.
- P. Zhang, G. Zhang, L. Liu, D. Ju, L. Zhang, K. Cheng and X. Tao, J. Phys. Chem. Lett., 2018, 9, 5040–5046 CrossRef CAS PubMed.
- Y. Cheng, M. Zhu, F. Wang, R. Bai, J. Yao, W. Jie and Y. Xu, J. Mater. Chem. A, 2021, 9, 27718–27726 RSC.
- R. Gupta, T. B. Korukonda, S. K. Gupta, B. P. Dhamaniya, P. Chhillar, R. Datt, P. Vashishtha, G. Gupta, V. Gupta, R. Srivastava and S. Pathak, J. Cryst. Growth, 2020, 537, 125598 CrossRef CAS.
- R. Gupta, V. Gupta, R. Datt, S. Arya, A. Pandey, A. Singh, S. Husale, R. Srivastava and S. Pathak, Mater. Adv., 2022, 3, 2089–2095 RSC.
- V. I. Yudin, M. S. Lozhkin, A. V. Shurukhina, A. V. Emeline and Y. V. Kapitonov, J. Phys. Chem. C, 2019, 123, 21130–21134 CrossRef CAS.
- D. Arquer, X. Gong, R. P. Sabatini, M. Liu, G. Kim, B. R. Sutherland, O. Voznyy, J. Xu, Y. Pang, S. Hoogland, D. Sinton and E. Sargent, Nat. Commun., 2017, 8, 14757 CrossRef PubMed.
- M. Cao, J. Tian, Z. Cai, L. Peng, L. Yang and D. Wei, Appl. Phys. Lett., 2016, 109, 233303 CrossRef.
- G. Maculan, A. D. Sheikh, A. L. Abdelhady, M. I. Saidaminov, A. Haque, B. Murali, E. Alarousu, O. F. Mohammed, T. Wu and O. M. Bakr, J. Phys. Chem. Lett., 2015, 6, 3781–3787 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2022 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article ad,
Swapnil
Barthwal
c,
Harshit
Sharma
ab,
Animesh
Pandey
ab,
Ridip
Deka
a,
Pranjit
Sarkar
a,
Sudhir
Husale
ab,
Ritu
Srivastava
*ab,
Vinay
Gupta
ad,
Swapnil
Barthwal
c,
Harshit
Sharma
ab,
Animesh
Pandey
ab,
Ridip
Deka
a,
Pranjit
Sarkar
a,
Sudhir
Husale
ab,
Ritu
Srivastava
*ab,
Vinay
Gupta
 *e,
Sandeep
Arya
*e,
Sandeep
Arya
 f and
Sandeep
Pathak
*c
f and
Sandeep
Pathak
*c

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in CsPbBr3.
1 ratio in CsPbBr3.



