Open Access Article
Open Access ArticleEvaluating the thermal behaviour of benzimidazolylidene sources for thin-film applications†
Alex J.
Veinot‡
 a,
Matthew B. E.
Griffiths‡
a,
Matthew B. E.
Griffiths‡
 b,
Ishwar
Singh
b,
Ishwar
Singh
 a,
Joseph A.
Zurakowski
b,
Paul A.
Lummis
a,
Joseph A.
Zurakowski
b,
Paul A.
Lummis
 a,
Seán T.
Barry
a,
Seán T.
Barry
 *b and
Cathleen M.
Crudden
*b and
Cathleen M.
Crudden
 *ac
*ac
aDepartment of Chemistry, Queen's University, 90 Bader Lane, Kingston, Ontario K7L 3N6, Canada
bDepartment of Chemistry, Carleton University, 1125 Colonel By Drive, K1S 5B6, Ottawa, Ontario, Canada
cInstitute of Transformative Bio-Molecules, ITbM-WPI, Nagoya University, Nagoya, Chikusa 464-8601, Japan
First published on 9th June 2022
Abstract
We show that the N-heterocyclic carbene precursor employed has a significant influence on the purity of the resulting films prepared by vapour-phase deposition. 1,3-Diisopropylbenzimidazolylidene is stable up to 363 K, before undergoing thermal decomposition to 1,2-diisopropylbenzimidazole as the major product. Various minor products arising from wing-tip loss are also observed. Kinetic and thermochemical analyses indicate that this reaction is second-order with respect to the carbene and proceeds through a transient tetraazafulvalene.
N-Heterocyclic carbenes (NHCs, I) are an important class of ligands in molecular chemistry with emerging applications in materials chemistry.1 As exceptional σ-donors, NHCs are capable of binding strongly to gold and other metal surfaces and the resulting monolayers possess high chemical and thermal stability.2 Self-assembled monolayers (SAMs) of NHCs have been formed on Cu,3 Au,1,2 Ag,3a Pt,4 and Mg surfaces,5 and have shown promise in applications such as biosensing,6 surface patterning,7 and metal oxide etching.8 Among NHC SAMs published to date, 1,3-diisopropylbenzimidazolylidene (benzNHCiPr) has received considerable attention. Flanking isopropyl groups (wing-tip groups) reinforce an upright binding mode and promote long-range order,9 essential properties for NHC SAMs to be effective in material science applications.
Vapour deposition methods are commonly used to prepare NHC monolayers and overlayers, and enable the use of ultra-high vacuum (UHV) surface science analytics.3,4a,8,10a Furthermore, vapour-phase deposition can be advantageous for high-volume manufacturing (HVM) applications since it removes the need to recycle or replace a solvent system, leading to lower production costs and environmental impact.
While preparing these films, the free NHC I can be employed after preparation via deprotonation of the salt II, but is more commonly generated in situ thermally from either carboxylate III or hydrogen carbonate salt IV (Scheme 1).10 It is generally assumed that differences between NHC sources are minimal such that the resulting SAMs formed during volatilisation are equivalent. While preparing NHC overlayers with benzNHCiPr and its precursor hydrogen carbonate salt benzNHCiPr·H2CO3, it became apparent from X-ray photoelectron spectroscopy (XPS) that this was not true, prompting us to study the thermochemistry of several important NHC SAM precursors by thermogravimetric analysis (TGA). Herein, we provide a comprehensive thermochemical study on benzNHCiPr, its precursor hydrogen carbonate salt benzNHCiPr·H2CO3 and the related tetraazafulvalene (benzNHCEt)2. The results shed light on optimal precursor choice, decomposition products, likely mechanisms of decomposition and the effect of decomposition products on surface analysis.
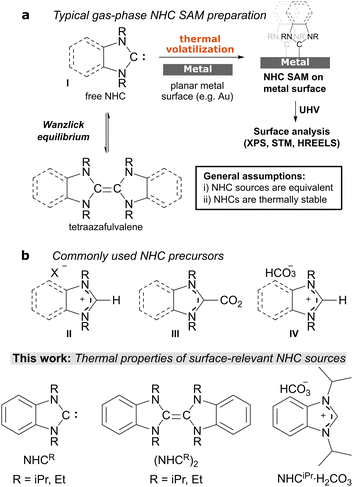 | ||
| Scheme 1 (a) Typical workflow for studying NHC SAMs on metallic surfaces. (b) Common and surface-relevant NHC precursors and NHC sources examined here. | ||
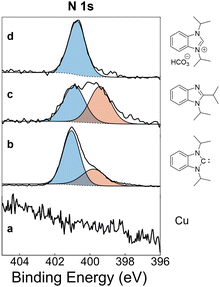
Carbene benzNHCiPr was prepared following a known literature procedure,11 and purified by vacuum distillation to remove trace iodide contaminants. To examine films prepared from the free carbene, neat benzNHCiPr prepared and purified as above was sublimed at 373 K onto a copper wafer. Decomposition of the residual NHC (distillant) was evident from a colour change to orange from colourless, and from 1H NMR spectroscopic analysis. Analysis of the resulting NHC-modified copper surface using XPS indicated that although a benzNHCiPr overlayer was present, decomposition products were also transferred to the copper surface, evident by a broad peak with bimodal distribution in the N 1s region (Fig. 1b). This prompted further study of the thermal stability of the isolated carbene benzNHCiPr.
To gain further insights, we carried out a bulk thermolysis reaction of benzNHCiPr. Using a sand bath heated between 453–483 K, neat benzNHCiPr was thermolysed over 4 h in a sealed vessel (Scheme 2). The resulting dark brown-coloured oil was purified by column chromatography, yielding 1,2-diisopropylbenzimidazole (39%, 1a) and 1-isopropyl-benzimidazole (21%, 1b) as the major and minor products, respectively. The structures of benzNHCiPr and 1a were determined using single crystal X-ray diffraction (Scheme 2, see ESI† for details). Benzimidazole 1a is a structural isomer of benzNHCiPr and was also characterised using 1H and 13C {1H} NMR spectroscopy and elemental analysis (see ESI†). Benzimidazole 1b has been previously described,11 arising from the loss of a wing-tip group from benzNHCiPr. Its structure was verified using 1H NMR spectroscopy.
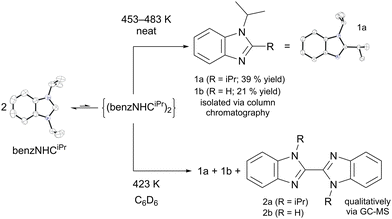 | ||
| Scheme 2 Proposed thermal decomposition of benzNHCiPr through its transient tetraazafulvalene. For full crystallographic details, see ESI.† | ||
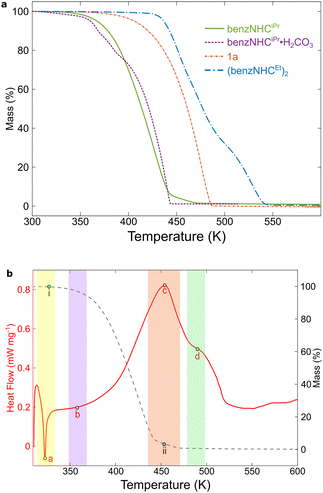
The thermolysis reaction of benzNHCiPr was repeated in a C6D6 solution at 423 K, and subsequent qualitative analysis using GC-MS revealed that in addition to 1a and 1b, 2,2′-bibenzimidazoles 2a and 2b are produced. Lappert and coworkers previously demonstrated the conversion of 1,2-dibenzylbenzimidazole to bibenzimidazoles,12a so 1a was examined by TGA to test for further decomposition (Fig. 3a).
Using a 10 mM 1,2-dichloroethane solution, 1a was deposited onto a copper surface and studied using XPS. In the N 1s XPS region, 1a exhibits two peaks each fit using a single component at 399.4 eV and 400.9 eV for amine and imine moieties, respectively (Fig. 1c). These peaks are similar in binding energy to the ones observed from vapor deposition of benzNHCiPr, suggesting that benzimidazole contaminants likely co-deposited with free NHC. Due to overlap in the N 1s binding energies for organic compounds, benzimidazole 1b or 2,2′-bibenzimidazoles 2a and 2b cannot be discounted as surface contaminants and will be indistinguishable in these studies.
Similar decomposition reactions are known for NHCs and their heteroatom congeners bearing allyl, imino and benzyl wing-tip groups.12 Radicals and tetraazafulvalenes (or related carbene dimers) have been implicated in these rearrangements, the latter accessible via the Wanzlick equilibrium.13 The dimerisation of benzimidazolylidenes is highly sensitive to the steric properties of the wing-tip substituents,14 such that formation of (benzNHCiPr)2 is unexpected.14c
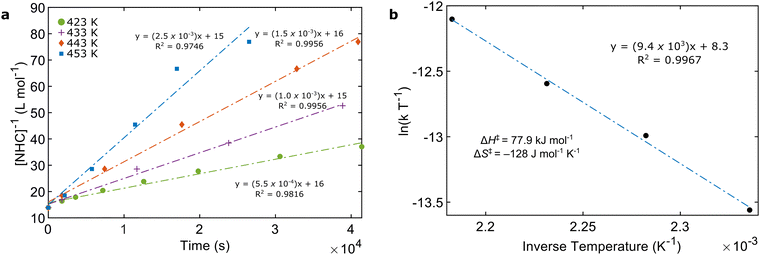
Evidence for the formation of (benzNHCiPr)2 during thermal decomposition was provided by solution-phase kinetics measurements in C6D6 between 423–453 K (Scheme 2, see ESI† for details). The concentration of benzNHCiPr was monitored by 1H NMR spectroscopy and examined using first, second and third-order integrated rate laws. Both second-order (Fig. 2a) and third-order (Fig. S10, ESI†) rate laws yielded linear relationships, suggesting a complicated thermal mechanism. While trimolecular reactions are uncommon, our data may indicate the establishment of a monomer/dimer pre-equilibrium during thermal decomposition.14d,e Assuming that the rate-determining transition state is bimolecular with respect to benzNHCiPr, an Eyring–Polanyi plot (Fig. 2b) yields the activation enthalpy (ΔH‡ = 77.9 kJ mol−1) and entropy (ΔS‡ = −128 J mol−1 K−1). For comparison, the enthalpy (ΔH°) and entropy (ΔS°) of dissociation for the equilibrium between benzNHCEt and its dimer are 57.3 kJ mol−1 and 127 kJ mol−1 K−1, respectively.14b Compared to the dimerisation of benzNHCEt, the sign and magnitude of ΔS‡ supports the formation of (benzNHCiPr)2 during thermal decomposition while sterically larger iPr groups increase ΔH‡ and suppress dimerisation.
Since (benzNHCiPr)2 is fleeting and could not be isolated, (benzNHCEt)2 was prepared instead and studied using TGA (Fig. 3a).14b For (benzNHCEt)2, an onset of mass loss occurs around 453 K, which is 130 K higher than benzNHCiPr and is a consequence of increased molecular weight upon dimerisation. An inflection was observed around 493 K, which is likely related to the conversion of (benzNHCEt)2 to its monomer.14b
For comparison, the thermogram of benzNHCiPr (Fig. 3b) shows an onset of mass loss at 323 K (i), coincident with the melting point of benzNHCiPr (322 K) determined using differential scanning calorimetry (DSC) (point a, yellow region). At 453 K, an inflection is observed (ii) which can be attributed to sample volatilisation. However, DSC measurements indicate that decomposition begins around 363 K (point b, purple region), culminating in a major exothermic event around 453 K (point c, red region), coincident with the observed inflection point ii from TGA, suggesting that volatilisation and decomposition occur over a similar temperature regime. A second thermal inflection is found at 483 K in the DSC curve (point d, green region), suggesting that an ongoing, complicated thermal decomposition continues as the temperature is increased. Using neat samples of benzNHCiPr and (benzNHCEt)2, a thermal scrambling experiment was completed using our thermolysis conditions. The mixture of products obtained could not be resolved by 1H NMR spectroscopy; however, cross-over products containing both ethyl and isopropyl wing-tips were detected by ESI-MS (see ESI†) providing further qualitative evidence that the thermolysis of NHC proceeds bimolecularly.
As an alternative to often air-sensitive free carbenes, azolium hydrogen carbonate salts have been used as effective, air-stable precursors for vapour phase depositions of NHCs onto Cu, Au and Pt surfaces.3b,4a,15 When heated, these salts liberate H2O and CO2, generating free NHCs in the process (eqn (1)).16 To examine the preparation of NHC overlayers with this precursor, we heated benzNHCiPr·H2CO3 to 373 K in the presence of a copper wafer. The successful formation of an NHC overlayer was indicated by a single symmetric peak in the N 1s XPS region, fit using one component centred at 400.7 eV (Fig. 1d). XPS analysis indicated that films prepared using benzNHCiPr·H2CO3 had a clean monomodal distribution in the N 1s region, unlike the bimodal distribution for free benzNHCiPr.
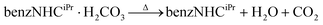 | (1) |
TGA was performed for benzNHCiPr·H2CO3 to understand these differences (Fig. 3a, purple). The thermogram shows that water and carbon dioxide are lost by 395 K indicating the in situ formation of free benzNHCiPr.16a
With increasing temperature, a smooth exponential decay to zero residual mass was observed, consistent with clean volatilisation, unlike free benzNHCiPr, which has an inflection before coming to zero residual mass. The thermogram of benzNHCiPr·H2CO3 was insensitive to heating rate or added KI, the latter a possible contaminant in benzNHCiPr arising from its preparation. Co-mixing benzNHCiPr·HI and benzNHCiPr·H2CO3 produced a similar curve as those found in the literature,16b suggesting that incomplete anion exchange may have occurred in previous studies. Co-mixing of benzNHCiPr·HI and benzNHCiPr·H2CO3 also reproduced the TGA inflection observed for free benzNHCiPr, suggesting possible acid-catalysis involved in the decomposition (see ESI†).14d,e Since the thermolysis of benzNHCiPr is likely bimolecular, differences in thermal behaviour between benzNHCiPr and benzNHCiPr·H2CO3 likely arise from the kinetics of the release of carbene. The use of benzNHCiPr·H2CO3 in NHC generation results in a lower flux of benzNHCiPr at any given time, which minimises thermal decomposition.
In summary, TGA and DSC measurements have shown that benzNHCiPr thermally decomposes during volatilisation above 363 K. This reaction proceeds through the transient tetraazafulvalene (benzNHCiPr)2 to primarily afford benzimidazoles 1 and 2. Analysis of N 1s XPS suggests that these decomposition products can persist on copper surfaces and complicate peak fitting. These impurities were not observed in films prepared under similar conditions using benzNHCiPr·H2CO3, illustrating that selecting the right NHC source is an important consideration for thermal applications. As on-surface applications of NHCs are being rapidly discovered, detailed thermochemical analyses of NHCs have become a necessary part of the toolkit for proper qualitative and quantitative surface analysis.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canada Foundation for Innovation (CFI) are thanked for support of this work in terms of operating and equipment grants to C. M. C. and S. T. B. (RGPIN-2019-06213). NSERC is also thanked for a Vanier Scholarship (A. J. V), a CGS-D scholarship to M. B. E. G., and an Undergraduate Student Research Award (J. A. Z.). I. S. acknowledges Queen*s University for the RT Mohan Scholarship and the Ontario government for a Graduate Scholarship.Notes and references
- (a) H. V. Huynh, Chem. Rev., 2018, 118, 9457 CrossRef CAS PubMed; (b) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485 CrossRef CAS PubMed; (c) C. A. Smith, M. R. Narouz, P. A. Lummis, I. Singh, A. Nazemi, C.-H. Li and C. M. Crudden, Chem. Rev., 2019, 119, 4986 CrossRef CAS PubMed; (d) S. Engel, E.-C. Fritz and B. J. Ravoo, Chem. Soc. Rev., 2017, 46, 2057 RSC; (e) A. V. Zhukhovitskiy, M. J. MacLeod and J. A. Johnson, Chem. Rev., 2015, 115, 11503 CrossRef CAS PubMed; (f) T. Weidner, J. E. Baio, A. Mundstock, C. Große, S. Karthäuser, C. Bruhn and U. Siemeling, Aust. J. Chem., 2011, 64, 1177 CrossRef CAS PubMed; (g) A. V. Zhukhovitskiy, M. G. Mavros, T. Van Voorhis and J. A. Johnson, J. Am. Chem. Soc., 2013, 135, 7418 CrossRef CAS PubMed.
- C. M. Crudden, J. H. Horton, I. I. Ebralidze, O. V. Zenkina, A. B. McLean, B. Drevniok, Z. She, H.-B. Kraatz, N. J. Mosey, T. Seki, E. C. Keske, J. D. Leake, A. Rousina-Webb and G. Wu, Nat. Chem., 2014, 6, 409 CrossRef CAS PubMed.
- (a) L. Jiang, B. Zhang, G. Médard, A. P. Seitsonen, F. Haag, F. Allegretti, J. Reichert, B. Kuster, J. V. Barth and A. C. Papageorgiou, Chem. Sci., 2017, 8, 8301 RSC; (b) C. R. Larrea, C. J. Baddeley, M. R. Narouz, N. J. Mosey, J. H. Horton and C. M. Crudden, Chem. Phys. Chem., 2017, 18, 3536 CrossRef CAS PubMed.
- (a) Y. Zeng, T. Zhang, M. R. Narouz, C. M. Crudden and P. H. McBreen, Chem. Commun., 2018, 54, 12527 RSC; (b) S. Dery, S. Kim, G. Tomaschun, T. Berg, D. Feferman, A. Cossaro, A. Verdini, D. Floreano, T. Klüner, F. D. Toste and E. Gross, J. Phys. Chem. Lett., 2019, 10, 5099 CrossRef CAS PubMed.
- L. Stephens, J. D. Padmos, M. R. Narouz, A. Al-Rashed, C.-H. Li, N. Payne, M. Zamora, C. M. Crudden, J. Mauzeroll and J. H. Horton, J. Electrochem. Soc., 2018, 165, G139 CrossRef CAS.
- C. M. Crudden, J. H. Horton, M. R. Narouz, Z. Li, C. A. Smith, K. Munro, C. J. Baddeley, C. R. Larrea, B. Drevniok and B. Thanabalasingam, Nat. Commun., 2016, 7, 12654 CrossRef CAS PubMed.
- (a) D. T. Nguyen, M. Freitag, M. Körsgen, S. Lamping, A. Rühling, A. H. Schaefer, M. H. Siekman, H. F. Arlinghaus, W. G. van der Wiel and F. Glorius, Angew. Chem., Int. Ed., 2018, 57, 11465 CrossRef CAS PubMed; (b) Z. She, M. R. Narouz, C. A. Smith, A. MacLean, H.-P. Loock, H.-B. Kraatz and C. M. Crudden, Chem. Commun., 2020, 56, 1275 RSC.
- A. J. Veinot, A. Al-Rashed, J. D. Padmos, I. Singh, D. S. Lee, M. R. Narouz, P. A. Lummis, C. J. Baddeley, C. M. Crudden and J. H. Horton, Chem. – Eur. J., 2020, 26, 11431 CrossRef CAS PubMed.
- (a) A. Inayeh, R. R. K. Groome, I. Singh, A. J. Veinot, F. Crasto de Lima, R. H. Miwa, C. M. Crudden and A. B. McLean, Nat. Commun., 2021, 12, 4034 CrossRef CAS PubMed; (b) G. Lovat, E. A. Doud, D. Lu, G. Kladnik, M. S. Inkpen, M. L. Steigerwald, D. Cvetko, M. S. Hybertsen, A. Morgante, X. Roy and L. Venkataraman, Chem. Sci., 2019, 10, 930 CAS.
- (a) G. Wang, A. Rühling, S. Amirjalayer, M. Knor, J. B. Ernst, C. Richter, H.-J. Gao, A. Timmer, H.-Y. Gao, N. L. Doltsinis, F. Glorius and H. Fuchs, Nat. Chem., 2017, 9, 152 CrossRef CAS PubMed; (b) L. Delaude, Eur. J. Inorg. Chem., 2009, 1681 CrossRef CAS.
- O. Starikova, G. Dolgushin, L. Larina, T. Komarova and V. Lopyrev, ARKIVOC, 2003, 13, 119 Search PubMed.
- (a) B. Çetinkaya, E. Çetinkaya, J. A. Chamizo, P. B. Hitchcock, H. A. Jasim, H. Küçükbay and M. F. Lappert, J. Chem. Soc., Perkin Trans. 1, 1998, 2047 RSC; (b) C. Holtgrewe, C. Diedrich, T. Pape, S. Grimme and F. E. Hahn, Eur. J. Org. Chem., 2006, 3116 CrossRef CAS; (c) G. Steiner, A. Krajete, H. Kopacka, K.-H. Ongania, K. Wurst, P. Preishuber-Pflügl and B. Bildstein, Eur. J. Inorg. Chem., 2004, 2827 CrossRef CAS.
- (a) M. K. Denk, K. Hatano and M. Ma, Tetrahedron Lett., 1999, 40, 2057 CrossRef CAS; (b) H. W. Wanzlick, Angew. Chem., Int. Ed. Engl., 1962, 1, 75 CrossRef.
- (a) F. E. Hahn, L. Wittenbecher, D. Le Van and R. Fröhlich, Angew. Chem., Int. Ed., 2000, 39, 541 CrossRef CAS; (b) Y. Liu, P. E. Lindner and D. M. Lemal, J. Am. Chem. Soc., 1999, 121, 10626 CrossRef CAS; (c) W. J. Humenny, S. Mitzinger, C. B. Khadka, B. K. Najafabadi, I. Vieira and J. F. Corrigan, Dalton Trans., 2012, 41, 4413 RSC; (d) R. W. Alder, M. E. Blake, L. Chaker, J. N. Harvey, F. Paolini and J. Schütz, Angew. Chem., Int. Ed., 2004, 43, 5896 CrossRef CAS PubMed; (e) J. Messelberger, M. Kumar, S. J. Goodner and D. Munz, Org. Chem. Front., 2021, 8, 6663 RSC.
- A. Inayeh, R. R. K. Groome, I. Singh, A. J. Veinot, F. C. de Lima, R. H. Miwa, C. M. Crudden and A. B. McLean, Nat. Commun., 2021, 12, 4034 CrossRef CAS PubMed.
- (a) M. Fèvre, P. Coupillaud, K. Miqueu, J.-M. Sotiropoulos, J. Vignolle and D. Taton, J. Org. Chem., 2012, 77, 10135 CrossRef PubMed; (b) M. Fèvre, J. Pinaud, A. Leteneur, Y. Gnanou, J. Vignolle, D. Taton, K. Miqueu and J.-M. Sotiropoulos, J. Am. Chem. Soc., 2012, 134, 6776 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental, spectroscopic, and crystallographic details for CCDC 2151982 and 2151983. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ma00413e |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |